Women in Selected Communities of Punjab, India Have a High Prevalence of Iron, Zinc, Vitamin B12, and Folate Deficiencies: Implications for a Multiply-Fortified Salt Intervention
Abstract
1. Introduction
2. Materials and Methods
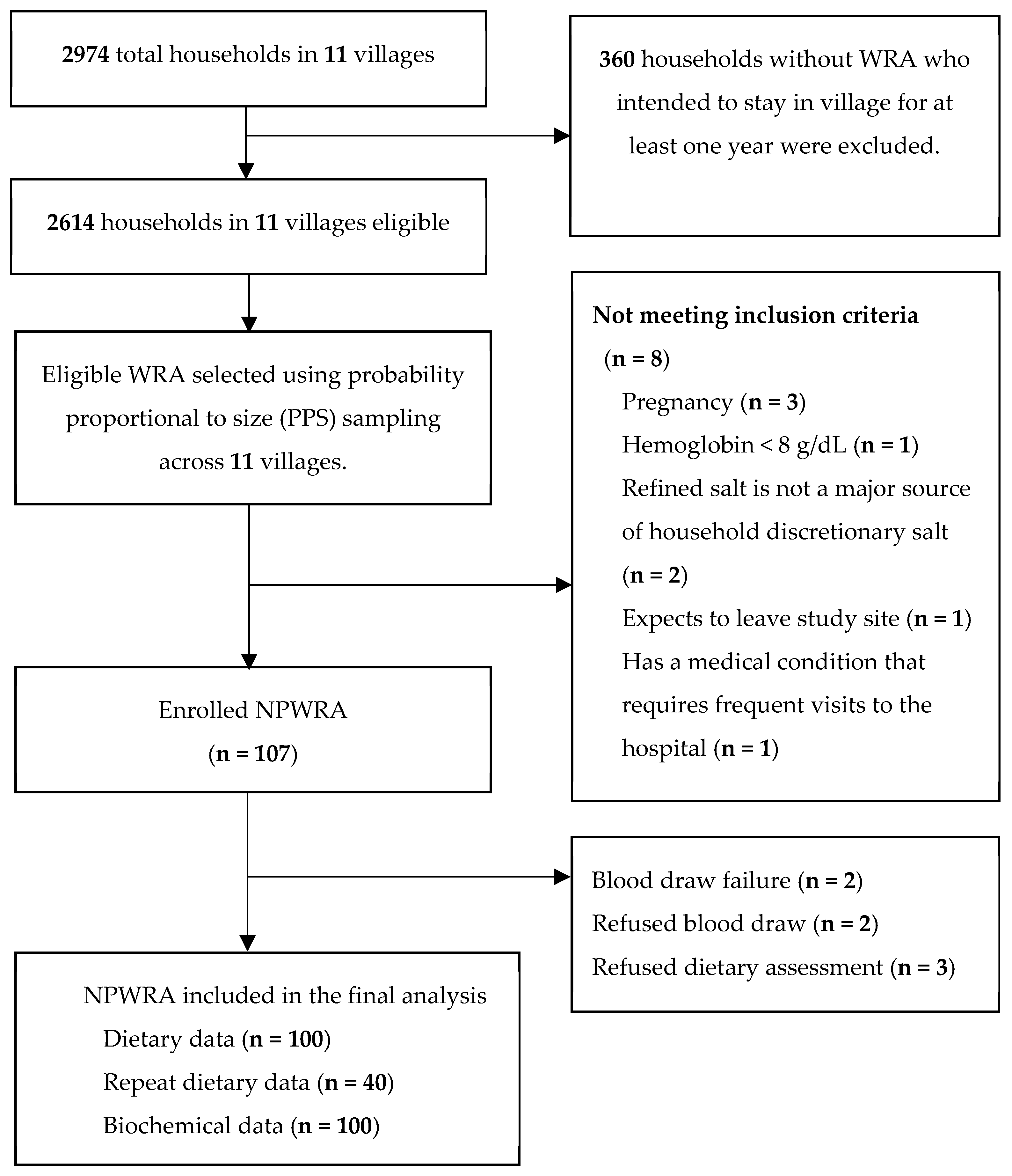
2.1. Study Design and Selection of Study Participants
2.2. Data Collection Procedures
2.2.1. Dietary Assessment
2.2.2. Blood and Urine Specimen Collection and Anthropometry
Processing and Analysis of Biological Specimens
2.3. Definition of Biochemical Outcomes
2.4. Data Analysis
2.4.1. Dietary Data Analysis
2.4.2. Biochemical and Anthropometric Data Analysis
2.4.3. Ethics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, G.A.; Beal, T.; Mbuya, M.N.; Luo, H.; Neufeld, L.M.; Addo, O.Y.; Adu-Afarwuah, S.; Alayón, S.; Bhutta, Z.; Brown, K.H.; et al. Micronutrient Deficiencies among Preschool-aged Children and Women of Reproductive Age Worldwide: A Pooled Analysis of Individual-level Data from population-representative surveys. Lancet Glob. Health 2022, 10, e1590–e1599. [Google Scholar] [CrossRef]
- Das, R.; Duggal, M.; Kaur, M.; Senee, H.K.; Dhanjal, G.S.; Rosenthal, J.; Kumar, A.; Rose, C.; Bhardwaj, S.; Serdula, M.; et al. Folate and Vitamin B12 Status in Women of Reproductive Age in Rural Areas in Haryana, Northern India. Curr. Dev. Nutr. 2019, 3, 3013451. [Google Scholar] [CrossRef]
- Yeung, L.; Duggal, M.; Das, R.; Rosenthal, J.; Bhardwaj, S.; Kankaria, A.; Kaur, M.; Senee, H.; Kumar, A.; Rose, C.; et al. Prevalence of Anemia Among Women of Reproductive Age in Rural Haryana, India. Curr. Dev. Nutr. 2021, 5, 699. [Google Scholar] [CrossRef]
- Ministry of Health and Family Welfare (MoHFW) Government of India, UNICEF, Population Council. Comprehensive National Nutrition Survey (CNNS) National Report. New Delhi. 2019. Available online: https://knowledgecommons.popcouncil.org/departments_sbsr-rh/1541/ (accessed on 14 November 2022).
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P.; Christian, P. Micronutrient Deficiencies in Pregnancy Worldwide: Health Effects and Prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef]
- Scholl, T.O.; Reilly, T. Anemia, Iron, and Pregnancy Outcome. J. Nutr. 2000, 130, 443S–447S. [Google Scholar] [CrossRef]
- Kozuki, N.; Lee, A.C.; Katz, J.; Child Health Epidemiology Reference Group. Moderate to Severe, but not Mild, Maternal Anemia is Associated with Increased Risk of Small-for-Gestational-age Outcomes. J. Nutr. 2012, 142, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Breymann, C. Iron Deficiency Anemia in Pregnancy. Semin. Hematol. 2015, 52, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.; Pfeiffer, C.M.; Georgieff, M.K.; Brittenham, G.; Fairweather-Tait, S.; Hurrell, R.F.; McArdle, H.J.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-Iron Review. J. Nutr. 2018, 148, 1001S–1067S. [Google Scholar] [CrossRef] [PubMed]
- Uriu-Adams, J.Y.; Keen, C.L. Zinc and Reproduction: Effects of Zinc Deficiency on Prenatal and Early Postnatal Development. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2010, 4, 313–325. [Google Scholar] [CrossRef]
- Ota, E.; Mori, R.; Middleton, P.; Tobe-Gai, R.; Mahomed, K.; Miyazaki, C.; Bhutta, Z.A. Zinc Supplementation for Improving Pregnancy and Infant Outcome. Cochrane Database Syst. Rev. 2015, 2, CD000230. [Google Scholar] [CrossRef]
- Molloy, A.M.; Kirke, P.N.; Brody, L.C.; Scott, J.M.; Mills, J.L. Effects of Folate and Vitamin B12 Deficiencies during Pregnancy on Fetal, Infant, and Child Development. Food Nutr. Bull. 2008, 29, S101–S111. [Google Scholar] [CrossRef]
- World Health Organization; De Benoist, B.; Dary, O.; Hurrell, R.L.; Allen, L.H. (Eds.) Guidelines on Food Fortification with Micronutrients; World Health Organization: Geneva, Switzerland, 2006; Volume 126. [Google Scholar]
- Tsang, B.L.; Holsted, E.; McDonald, C.M.; Brown, K.H.; Black, R.; Mbuya, M.N.; Grant, F.; Rowe, L.A.; Manger, M.S. Effects of Foods Fortified with Zinc, Alone or Cofortified with Multiple micronutrients, on Health and Functional Outcomes: A Systematic Review and Meta-analysis. Adv. Nutr. 2021, 12, 1821–1837. [Google Scholar] [CrossRef] [PubMed]
- Pandav, C.; Yadav, K.; Salve, H.; Kumar, R.; Goel, A.; Chakrabarty, A. High National and Sub-national Coverage of Iodised Salt in India: Evidence from the first National Iodine and Salt Intake Survey (NISI) 2014–2015. Public Health Nutr. 2018, 21, 3027–3036. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Luzuriaga, M.J.; Larson, L.M.; Mannar, V.; Martorell, R. Impact of Double-Fortified Salt with Iron and Iodine on Hemoglobin, Anemia, and Iron Deficiency Anemia: A Systematic Review and Meta-Analysis. Adv. Nutr. 2018, 9, 207–218. [Google Scholar] [CrossRef]
- Modupe, O.; Diosady, L.L. Quadruple fortification of salt for the Delivery of Iron, Iodine, Folic acid, and Vitamin B12 to Vulnerable Populations. J. Food Eng. 2021, 300, 110525. [Google Scholar] [CrossRef] [PubMed]
- District SAS Nagar. Available online: https://sasnagar.nic.in/ (accessed on 12 June 2022).
- International Institute for Population Sciences (IIPS) and ICF. National Family Health Survey (NFHS-5), India, 2019–2021; IIPS: Mumbai, India, 2021. [Google Scholar]
- Coates, J.; Swindale, A.; Bilinsky, P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Food Access: Indicator Guide: Version 3; FAO: Washington, DC, USA, 2007. [Google Scholar]
- Food and Agriculture Organization. Dietary Assessment: A Resource Guide to Method Selection and Application in Low Resource Settings; Food and Agriculture Organization: Rome, Italy, 2018. [Google Scholar]
- Casadei, K.; Kiel, J. Anthropometric Measurement. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Forrer, R.; Gautschi, K.; Lutz, H. Simultaneous measurement of the trace elements Al, As, B, Be, Cd, Co, Cu, Fe, Li, Mn, Mo, Ni, Rb, Se, Sr, and Zn in human serum and their reference ranges by ICP-MS. Biol. Trace Elem Res. 2001, 80, 77–93. [Google Scholar] [CrossRef]
- Molloy, A.M.; Scott, J.M. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997, 281, 43–53. [Google Scholar] [CrossRef]
- Windelberg, A.; Arseth, O.; Kvalheim, G.; Ueland, P.M. Automated assay for the determination of methylmalonic acid, total homocysteine, and related amino acids in human serum or plasma by means of methylchloroformate derivatization and gas chromatography-mass spectrometry. Clin. Chem. 2005, 11, 2103–2109. [Google Scholar] [CrossRef]
- Namaste, S.M.; Rohner, F.; Huang, J.; Bhushan, N.L.; Flores-Ayala, R.; Kupka, R.; Mei, Z.; Rawat, R.; Williams, A.M.; Raiten, D.J.; et al. Adjusting ferritin concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106, 359S–371S. [Google Scholar] [CrossRef]
- Rohner, F.; Namaste, S.M.; Larson, L.M.; Addo, O.Y.; Mei, Z.; Suchdev, P.S.; Williams, A.M.; Sakr Ashour, F.A.; Rawat, R.; Raiten, D.J.; et al. Adjusting soluble transferrin receptor concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106, 372S–382S. [Google Scholar] [CrossRef]
- Hotz, C.; Peerson, J.M.; Brown, K.H. Suggested Lower Cut-offs of Serum Zinc Concentrations for Assessing Zinc Status: Reanalysis of the Second National Health and Nutrition Examination Survey data (1976–1980). Am. J. Clin. Nutr. 2003, 78, 756–764. [Google Scholar] [CrossRef]
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-Zinc review. J. Nutr. 2016, 146, 858S–885S. [Google Scholar] [CrossRef]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F., 3rd; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of Nutrition for Development-Folate Review. J. Nutr. 2015, 145, 1636S–1680S. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H.; Miller, J.W.; de Groot, L.; Rosenberg, I.H.; Smith, A.D.; Refsum, H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review. J. Nutr. 2018, 148, 1995S–2027S. [Google Scholar] [CrossRef]
- Rohner, F.; Zimmermann, M.; Jooste, P.; Pandav, C.; Caldwell, K.; Raghavan, R.; Raiten, D.J. Biomarkers of Nutrition for Development-Iodine review. J. Nutr. 2014, 144, 1322S–1342S. [Google Scholar] [CrossRef]
- Longvah, T.; Anantan, I.; Bhaskarachary, K.; Venkaiah, K.; Longvah, T. Indian Food Composition Tables; National Institute of Nutrition, Indian Council of Medical Research: Hyderabad, India, 2017. [Google Scholar]
- Shaheen, N.; Bari, L.; Mannan, M.A. Food Composition Table for Bangladesh. 2013. Available online: https://www.fao.org/fileadmin/templates/food_composition/documents/FCT_10_2_14_final_version.pdf (accessed on 18 May 2021).
- United States Department of Agriculture. USDA Food and Nutrient Database for Dietary Studies 2011–2012; Agricultural Research Service: Washington, DC, USA, 2014.
- Carlsen, M.H.; Andersen, L.F.; Dahl, L.; Norberg, N.; Hjartåker, A. New Iodine Food Composition Database and Updated Calculations of Iodine Intake Among Norwegians. Nutrients 2018, 10, 930. [Google Scholar] [CrossRef] [PubMed]
- Dahdouh, S.; Grande, F.; Espinosa, S.N.; Vincent, A.; Gibson, R.; Bailey, K.; King, J.; Rittenschober, D.; Charrondière, U.R. Development of the FAO/INFOODS/IZINCG Global Food Composition Database for Phytate. J. Food Compost. Anal. 2019, 78, 42–48. [Google Scholar] [CrossRef]
- Vásquez-Caicedo, A.L.; Bell, S.; Hartmann, B. Report on Collection of Rules on Use of Recipe Calculation Procedures Including the Use of Yield and Retention Factors for Imputing Nutrient Values for Composite Foods; European Food Information Resource Network: Brussels, Belgium, 2008. [Google Scholar]
- National Cancer Institute (NCI). Single Regularly-Consumed or Episodically-Consumed Food or Nutrient. NCI Division of Cancer Prevention: Bethesda (MD): 2019. Available online: https://prevention.cancer.gov/research-groups/biometry/measurement-error-impact/software-measurement-error/single-regularly-consumed-or-episodically-consumed-food-or-nutrient (accessed on 22 August 2021).
- Luo, H.; Dodd, K.W.; Arnold, C.D.; Engle-Stone, R. Introduction to the SIMPLE Macro, a Tool to Increase the Accessibility of 24-hour Dietary Recall Analysis and Modeling. J. Nutr. 2021, 151, 1329–1340. [Google Scholar] [CrossRef]
- ICMR-NIN Expert Group on Nutrient Requirement for Indians. Recommended Dietary Allowances (RDA) and Estimated Average Requirements; ICMR-National Institute of Nutrition: Hyderabad, India, 2020. [Google Scholar]
- Ghosh, S.; Sinha, S.; Thomas, T.; Sachdev, H.S.; Kurpad, A.V. Revisiting Dietary Iron Requirement and Deficiency in Indian Women: Implications for Food Iron Fortification and Supplementation. J. Nutr. 2019, 149, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S. Principles of Nutritional Assessment: Evaluation of Nutrient Intakes and Diets. Available online: https://nutritionalassessment.org/evn/index.html (accessed on 6 September 2022).
- Miller, L.V.; Krebs, N.F.; Hambidge, K.M. A Mathematical Model of Zinc Absorption in Humans as a Function of Dietary Zinc and Phytate. J. Nutr. 2007, 137, 135–141. [Google Scholar] [CrossRef]
- Doets, E.L.; In’t Veld, P.H.; Szczecińska, A.; Dhonukshe-Rutten, R.A.; Cavelaars, A.E.; Van’t Veer, P.; Brzozowska, A.; de Groot, L.C. Systematic review on Daily Vitamin B12 Losses and Bioavailability for Deriving Recommendations on Vitamin B12 Intake with the Factorial Approach. Ann. Nutr. Metab. 2013, 62, 311–322. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.M.; Suchdev, P.S.; Krebs, N.F.; Hess, S.Y.; Wessells, K.R.; Ismaily, S.; Rahman, S.; Wieringa, F.T.; Williams, A.M.; Brown, K.H.; et al. Adjusting plasma or serum zinc concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2020, 111, 927–937. [Google Scholar] [CrossRef]
- World Health Organization. WHO Anthro Survey Analyser. Available online: https://nutritionalassessment.org/evn/index.html (accessed on 18 May 2021).
- Goh, Y.E.; Manger, M.S.; Saklani, S.; Agarwal, S.; Budhija, D.; Jamwal, M.; Chauhan, A.; Singh, B.; Dahiya, N.; Duggal, M.; et al. Comparison of methods for estimating discretionary salt intake in field settings. Curr. Dev. Nutr. 2022, 6, 571. [Google Scholar] [CrossRef]
- Kurpad, A.V.; Ghosh, S.; Thomas, T.; Bandyopadhyay, S.; Goswami, R.; Gupta, A.; Gupta, P.; John, A.T.; Kapil, U.; Kulkarni, B.; et al. Perspective: When the Cure might become the Malady: The Layering of Multiple Interventions with Mandatory Micronutrient Fortification of Foods in India. Am. J. Clin. Nutr. 2021, 114, 1261–1266. [Google Scholar] [CrossRef]
- World Health Organization; Food and Agriculture Organization. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Venkatesh, U.; Sharma, A.; Ananthan, V.A.; Subbiah, P.; Durga, R.; CSIR Summer Research Training Team. Micronutrient’s Deficiency in India: A Systematic Review and Meta-analysis. J. Nutr. Sci. 2021, 10, e110. [Google Scholar] [CrossRef]
| Nutrient | Proposed Fortification Level (per Gram of Salt) | Expected Average Amount of Micronutrients Provided by MFS per Day | India Nutrient Reference Values | |||
|---|---|---|---|---|---|---|
| EAR | RDA | UL | % of RDA Met by MFS | |||
| Iron | 1.3 mg | 6.0 mg | 15 mg/day 1 | 29 mg/day | 45 mg/day | 21 |
| Zinc | 1.4 mg | 6.5 mg | 11 mg/day 2 | 13.2 mg/day | 40 mg/day | 49 |
| Vitamin B12 | 0.6 µg | 2.8 µg | 2 µg/day | 2.2 µg/day | --- | 127 |
| Folic acid | 52 µg | 244 µg | 180 µg/day 3 | 220 µg/day 4 | 1000 µg/day | 111 |
| Iodine | 30 µg | 141 µg | 95 µg/day | 140 µg/day | 1100 µg/day | 100 |
| Mean ± SD, Median, IQR; or n (%) | |
|---|---|
| Woman Characteristics (n = 100) | |
| Age, years | 34.5 ± 6.9 |
| BMI, kg/m2 | 26.4 ± 5.5 |
| Underweight < 18.5 kg/m2 | 7 (7.0) |
| Normal 18.5–24.9 kg/m2 | 38 (38.0) |
| Overweight 25.0–29.9 kg/m2 | 31 (31.0) |
| Obese > 30 kg/m2 | 24 (24.0) |
| Mid-upper arm circumference, cm | 29.8 ± 4.4 |
| Number of children under 5 years 2 | 1.9 ± 1.2 |
| Education completed | |
| None | 2 (2.0) |
| Middle/Secondary | 72 (72.0) |
| Diploma/postgraduate | 26 (26.0) |
| Primary Occupation | |
| Homemaker | 79 (79.0) |
| Professional/Clerical | 12 (12.0) |
| Shop owner/Supplier of goods | 9 (9.0) |
| Religion | |
| Sikh | 80 (80.0) |
| Hindu | 18 (18.0) |
| Muslim | 2 (2.0) |
| Marital status | |
| Married | 87 (87.0) |
| Separated/never married | 13 (13.0) |
| Household Characteristics | |
| Monthly income (INR) | 12,000 (10,000, 25,000) |
| Ownership of ration card | 67 (67.0) |
| Category of ration card | |
| Below poverty line | 41 (61.2) |
| Above poverty line | 21 (31.3) |
| Other priority households | 5 (7.5) |
| Household head, female | 17 (17.0) |
| Land ownership, % | 38 (38.0) |
| Number of people living in household 3 | 5.8 ± 2.1 |
| Child Characteristics (n = 34) | |
| Sex, male | 20 (58.8) |
| Age, months | 32.0 ± 14.6 |
| Height-for-age z-score 4 | −0.7 ± 1.0 |
| Weight-for-age z-score | −0.6 ± 1.0 |
| Weight-for-height z-score | −0.3 ± 1.0 |
| Mid-upper arm circumference, cm | |
| 11.5–12.5 cm | 1 (2.9) |
| ≥12.5 cm | 33 (97.1) |
| Nutrients 1 | Mean Intake ± SE | EAR 2 | Inadequate Intake (%) | Excess Intake (%) |
|---|---|---|---|---|
| Iron | ||||
| Iron intake from usual diet, mg/d | 18.8 ± 1.3 | 15 3 | 46.0 ± 5.1 4 | 2.2 ± 1.8 |
| Projected iron intake from usual diet and MFS, mg/day | 25.2 ± 1.4 | 15 3 | 16.9 ± 4.4 | 4.9 ± 3.0 |
| Zinc | ||||
| Zinc intake from usual diet, mg/d | 7.6 ± 0.2 | 11 | 94.5 ± 1.9 | 0.0 ± 0.0 |
| Projected zinc intake from usual diet and MFS, mg/d | 14.1 ± 0.4 | 11 | 18.3 ± 4.03 | 0.0 ± 0.0 |
| Absorbed zinc from usual diet, mg/d 5 | 1.39 ± 0.0 | 3 | 99.9 ± 0.1 | 0.0 ± 0.0 |
| Projected absorbed zinc intake from usual diet and MFS, mg/d | 7.8 ± 0.3 | 3 | 0.3 ± 1.1 | 0.0 ± 0.0 |
| Vitamin B12 | ||||
| Vitamin B12 intake from usual diet, µg/day | 1.3 ± 0.1 | 2 | 82.6 ± 3.5 | - |
| Projected vitamin B12 intake from usual diet and MFS, µg/day | 4.1 ± 0.2 | 2 | 2.0 ± 2.6 | - |
| Absorbed B12 from usual diet, µg/day 6 | 0.5 ± 0.0 | 1 | 93.6 ± 2.6 | - |
| Absorbed B12 from usual diet and MFS, µg/day | 1.5 ± 0.1 | 1 | 11.7 ± 4.1 | - |
| Folate | ||||
| Folate intake from usual diet, µg DFE/d 7 | 200.7 ± 5.8 | 180 | 35.7 ± 4.6 | 0.0 ± 0.0 |
| Projected folate intake from usual and MFS, µg DFE/d | 613.1 ± 20.1 | 180 | 0.01 ± 0.26 | 1.9 ± 1.9 |
| Biomarker | % with Anemia or Evidence of Deficiency |
|---|---|
| Iron 1 | |
| Hb < 12g/dL | 37 |
| Uadjusted serum ferritin < 15 μg/L | 45 |
| Adjusted serum ferritin < 15 μg/L | 67 |
| Unadjusted soluble transferrin receptor > 8.3 mg/L | 11 |
| Soluble transferrin receptor > 8.3 mg/L | 7 |
| Iron deficiency and anemia | 35 |
| Zinc | |
| Plasma zinc < 70 μg/dL 2 | 34 |
| Vitamin B12 | |
| Serum vitamin B12 < 150 pmol/L | 23 |
| Serum vitamin B12 < 221 pmol/L 3 | 60 |
| Methylmalonic acid > 271 nmol/L | 75 |
| Holotranscobalamin < 35 pmol/L | 46 |
| Plasma homocysteine > 13 μmol/L | 59 |
| Composite vitamin B12 indicator 4 | |
| Elevated levels (cB12 > 1.5) | 3 |
| Adequate levels (−0.5 < cB12 < 1.5) | 34 |
| Low levels (−1.5 < cB12 < −0.5) | 44 |
| Possibly deficient (cB12 < −2.5) | 19 |
| Folate | |
| RBC folate < 748 nmol/L | 70 |
| Iodine | |
| Serum thyroglobulin, μg/L 5 | 13.4 (5.0, 21.3) |
| Urinary iodine < 100 μg/L | 0 |
| I/Cr ratio, μg/g 6 | 409 ± 415 |
| Inflammation | |
| CRP ≥ 5 mg/L | 22 |
| AGP ≥ 1 g/L | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, Y.E.; Manger, M.S.; Duggal, M.; Das, R.; Saklani, S.; Agarwal, S.; Budhija, D.; Jamwal, M.; Singh, B.L.; Dahiya, N.; et al. Women in Selected Communities of Punjab, India Have a High Prevalence of Iron, Zinc, Vitamin B12, and Folate Deficiencies: Implications for a Multiply-Fortified Salt Intervention. Nutrients 2023, 15, 3024. https://doi.org/10.3390/nu15133024
Goh YE, Manger MS, Duggal M, Das R, Saklani S, Agarwal S, Budhija D, Jamwal M, Singh BL, Dahiya N, et al. Women in Selected Communities of Punjab, India Have a High Prevalence of Iron, Zinc, Vitamin B12, and Folate Deficiencies: Implications for a Multiply-Fortified Salt Intervention. Nutrients. 2023; 15(13):3024. https://doi.org/10.3390/nu15133024
Chicago/Turabian StyleGoh, Yvonne E., Mari S. Manger, Mona Duggal, Reena Das, Shipra Saklani, Surbhi Agarwal, Deepmala Budhija, Manu Jamwal, Bidhi L. Singh, Neha Dahiya, and et al. 2023. "Women in Selected Communities of Punjab, India Have a High Prevalence of Iron, Zinc, Vitamin B12, and Folate Deficiencies: Implications for a Multiply-Fortified Salt Intervention" Nutrients 15, no. 13: 3024. https://doi.org/10.3390/nu15133024
APA StyleGoh, Y. E., Manger, M. S., Duggal, M., Das, R., Saklani, S., Agarwal, S., Budhija, D., Jamwal, M., Singh, B. L., Dahiya, N., Luo, H., Long, J. M., Westcott, J., Krebs, N. F., Gibson, R. S., Brown, K. H., & McDonald, C. M. (2023). Women in Selected Communities of Punjab, India Have a High Prevalence of Iron, Zinc, Vitamin B12, and Folate Deficiencies: Implications for a Multiply-Fortified Salt Intervention. Nutrients, 15(13), 3024. https://doi.org/10.3390/nu15133024






