Abstract
Low-carbohydrate high-fat (LCHF) diets can be just as effective as high-carbohydrate, lower-fat (HCLF) diets for improving cardiovascular disease risk markers. Few studies have compared the effects of the UK HCLF dietary guidelines with an LCHF diet on lipids and lipoprotein metabolism using high-throughput NMR spectroscopy. This study aimed to explore the effect of an ad libitum 8-week LCHF diet compared to an HCLF diet on lipids and lipoprotein metabolism and CVD risk factors. For 8 weeks, n = 16 adults were randomly assigned to follow either an LCHF (n = 8, <50 g CHO p/day) or an HCLF diet (n = 8). Fasted blood samples at weeks 0, 4, and 8 were collected and analysed for lipids, lipoprotein subclasses, and energy-related metabolism markers via NMR spectroscopy. The LCHF diet increased (p < 0.05) very small VLDL, IDL, and large HDL cholesterol levels, whereas the HCLF diet increased (p < 0.05) IDL and large LDL cholesterol levels. Following the LCHF diet alone, triglycerides in VLDL and HDL lipoproteins significantly (p < 0.05) decreased, and HDL phospholipids significantly (p < 0.05) increased. Furthermore, the LCHF diet significantly (p < 0.05) increased the large and small HDL particle concentrations compared to the HCLF diet. In conclusion, the LCHF diet may reduce CVD risk factors by reducing triglyceride-rich lipoproteins and improving HDL functionality.
1. Introduction
Cardiovascular diseases (CVDs) are the leading cause of death, accounting for approximately 17.8 M deaths globally [1]. In 2019, an estimated 12.7 M new cases of CVD were reported, leading to approximately 113 M people living with CVD across European nations alone [2]. To combat CVDs, global bodies recommend limiting dietary fat (particularly saturated fatty acids (SFA)) but encouraging a high dietary intake of carbohydrates (>50% of energy intake, although low in sugar) [3,4]. However, low-carbohydrate (<26% of energy intake) high-fat (LCHF) diets haveshown to perform at least as well as higher-carbohydrate, lower-fat (HCLF) diets in reducing body fat and improving metabolic profiles even with an increase in SFA intake [5].
One concern with LCHF diets is an increase in low-density lipoprotein cholesterol (LDL-C) compared to HCLF diets [6]. Interestingly, after 12 months, these differences disappeared, which may, however, be a dietary compliance issue [7]. While LDL-C is a risk marker for CVD, it does not discriminate between LDL size and particle number, with small dense LDL (sdLDL) and higher LDL particle numbers being stronger predictors of CVD risk [8,9]. Therefore, it is necessary to distinguish between them to estimate CVD risk. Apolipoprotein B (ApoB), the primary apolipoprotein of LDL, is directly proportional to LDL particle number and is also superior to LDL-C at determining CVD risk [10]. Concerning this, two main phenotypes (A and B) have been described; phenotype A is characterised by the prevalence of large buoyant LDL, whereas phenotype B is characterised by the prevalence of small dense LDL, and the latter is strongly associated with metabolic disease [9,11,12]. Not only are lipoprotein concentrations a key risk factor for CVD but metabolites such as circulating branched-chain amino acids (BCAAs) are also associated with biomarkers of metabolic diseases and CVD risk [13,14]. As diet exerts a myriad of effects on global metabolism [15], the measurement of all lipoproteins with amino acid metabolites may enable a clearer understanding of the association between CVD risk and LCHF diets.
Nuclear magnetic resonance (NMR) spectroscopy is a powerful tool that can accurately quantify lipoprotein subclasses (density, size, and particle number) and their associated lipid species [16]. Higher very-low-density lipoprotein (VLDL) and LDL particle concentrations are associated with an elevated CVD risk [17,18]. In contrast, lipid concentrations within large and medium high-density lipoprotein (HDL) particles are inversely associated with CVD risk [17,18]. Large cohort studies have also demonstrated that healthy eating patterns, characterised by high levels of polyunsaturated fatty acids (PUFA) are associated with lower VLDL and LDL-associated lipids, resulting in lower CVD risk [19]. Similarly, controlled studies have highlighted that replacing SFA with PUFA lowers atherogenic particles of the lipoprotein subclasses of VLDL, intermediate-density lipoprotein (IDL), and LDL [20]. However, an LCHF (<20% carbohydrate) diet with higher levels of both dietary SFA and PUFA still improves the lipoprotein particle concentration in comparison to a high-carbohydrate diet, with no difference between LDL-C [21]. Additionally, the consumption of fatty fish is associated with lower levels of tyrosine and valine levels, indicating that dietary fat intake may also modulate amino acid metabolism and may be associated with altered CVD risk [22]. These results indicate that, in contrast to worldwide recommendations [4], reducing dietary carbohydrates rather than SFA may exert greater reductions in the atherogenic lipoprotein profile and improve CVD risk factors. Specifically, the effect of the current UK dietary guidelines [3] in comparison with an LCHF diet on the lipoprotein profile and CVD risk factors has not been explored using NMR spectroscopy.
Therefore, this study aimed to investigate the impact of an ad libitum 8-week LCHF diet compared to an HCLF diet (current UK guidelines) [3] on the global lipid and amino acid profiles in adults with an elevated metabolic risk. We hypothesise that the LCHF diet modifies lipids and lipoprotein profiles that benefit metabolic health, thereby lowering CVD risk.
2. Materials and Methods
All procedures followed the CONSORT guidelines for reporting randomised trials [23,24].
2.1. Study Design and Recruitment
Details of the study design were reported previously [24] with ethical approval from the Liverpool John Moores University research ethics committee (REC number: 16/ELS/029). This study is registered as a clinical trial (REF: NCT03257085). Participants were included if they were aged 19–64 years with a BMI of 18.5–29.9 kg/m2 and excluded if they were a smoker, vegan/vegetarian, took dietary supplements, had any known food allergies or intolerances, consumed alcohol above the weekly UK recommendations, were pregnant, suffered from an eating disorder, suffered from current or previous renal impairment, had a history of cardiometabolic diseases or took lipid, blood pressure or blood glucose-lowering medication. Briefly, after the screening, all participants provided written informed consent and were randomly assigned to either an ad libitum HCLF (n = 8) or an LCHF diet (n = 8) for 8 weeks using a computerised random allocation sequence and concealed in envelopes. Participants in the HCLF group were required to consume a diet composed of 50% carbohydrate, 15% protein, and at most 35% fat per day (based on the UK Eatwell Guide) [3]. The LCHF group was instructed to consume a diet consisting of ≤50 g of carbohydrates per day to induce ketosis [25] and increase the amount of fat consumed while consuming similar amounts of protein compared to the HCLF group. At 0, 4, and 8 weeks of the diet, fasting whole blood was collected by trained phlebotomists from the antecubital fossa vein and centrifuged at 3000× g for 15 min at 4 °C to harvest plasma and serum, which were stored at −80 °C until analysis. Anthropometrics, body composition, blood pressure, physical activity, and dietary intake were recorded at each time point.
2.2. NMR Spectroscopy
To quantify lipid, lipoprotein subclasses, fatty acid composition, amino acids, ketone bodies, glycolysis, and Krebs cycle-related metabolites from overnight fasting EDTA plasma, we used the service provided by Nightingale Health Ltd., Helsinki, Finland, which employs a high-throughput proton NMR spectroscopy platform. Lipoprotein subclasses were quantified using lipid concentrations within fourteen subclasses, abundant proteins, and various low-molecular-weight metabolites. The applications and experimentation details of the NMR metabolomics platform were described previously [26,27]. The quantified biomarker measures rather than the NMR spectral data were analysed in relation to clinical/risk factor variables in this study, and examples of spectral annotation were published previously [26,28]. Biomarker quantification was performed in regions where EDTA signals do not overlap, and NMR-based quantification reported comparable results to routine lipid measures and fatty acid measures from gas chromatography [27]. Representative coefficients of variations for the metabolic biomarkers were published previously [29], and all metabolites fell within the range of detection.
2.3. Statistics
All normally distributed data are presented as mean ± SD, whereas non-normally distributed data are presented as median ± interquartile range (IQR). All data were explored for distribution using the Shapiro–Wilks test. Normally distributed data underwent a 2 × 3 mixed ANOVA with 2 between factors (LCHF vs. HCLF) and 3 within factors (baseline vs. interim vs. endpoint) to investigate significant differences for the main and interaction effects. If repeated measures data had a missing value, mixed effects analysis was used instead of ANOVA. Non-normally distributed data were log or square root transformed prior to parametric or non-parametric analyses (Friedman and Kruskal–Wallis test). All p-values were corrected for multiple testing using the Benjamini and Hochberg method [30] and considered significant at p < 0.05. The fold-change percentage from baseline to 8 weeks of the diet was calculated as 100 × (mean C − mean A)/mean A. The GraphPad Prism (San Diego, CA, USA) statistical software was used for statistical analysis. Random forest with a combination of unbiased variable selection framework and repeated double cross-validation was applied using R, version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria), to detect a panel of metabolites representative of the LCHF diet relative to the HCLF diet at the end of the study (week 8). This method fits many classification trees to a data set and then combines the predictions from all trees to present a final predictive model that ranks variables based on their predictive power. The model underwent extensive tuning to optimise its hyperparameters and mitigate overfitting. As a result, it achieved performance metrics, with an R2 value of 0.62 and a Q2 value of 0.64 (an estimate of the predictive ability of the model calculated by cross-validation). Model performance was confirmed via permutation analysis (n = 1000).
3. Results
Participant recruitment, changes in metabolic markers, body composition, and dietary intake were reported previously [24]. The participants consisted of four males and females in the LCHF group (n = 8) and five males and three females in the HCLF group (n = 8). Participants’ mean age was similar between the groups (LCHF, 43.8 ± 10.4; HCLF, 44.6 ± 15.27; p = 0.895). Briefly, no change in dietary intake was reported in the HCLF group during the intervention; however, as reported previously, the percentage of energy derived from fat increased from 34 ± 4 to 61 ± 6%, carbohydrate decreased from 42 ± 9 to 10 ± 4% (both p < 0.001) in the LCHF group, and total energy intake was similar between the groups [24]. Body mass decreased (−3.14 kg) in the LCHF group, but remained unchanged in the HCLF group during the intervention [24].
3.1. Lipid and Lipoprotein Metabolism
Total cholesterol increased from baseline to 8 weeks following the LCHF (5.09 ± 0.76 to 5.50 ± 0.57 mmol/L, p = 0.022) and HCLF (4.55 ± 1.00 to 4.97 ± 1.17 mmol/L, p = 0.032) diets, with differential effects on lipoprotein subclass cholesterol concentrations (Figure 1). The changes in cholesterol concentrations were driven by variations in free and esterified cholesterol levels (Table 1). Triglycerides in very large, large, and medium VLDL were significantly (all p < 0.05) lower in the LCHF group compared to the HCLF diet group throughout the intervention; however, these did not pass multiple testing comparisons (Table 1). Compared to baseline, 4 weeks of the LCHF diet also resulted in a decrease in triglycerides in the medium (0.05 ± 0.02 to 0.03 ± 0.01 mmol/L, p = 0.030) and small (0.05 ± 0.02 to 0.04 ± 0.01 mmol/L, p = 0.029) HDL, but returned to baseline levels by week 8.
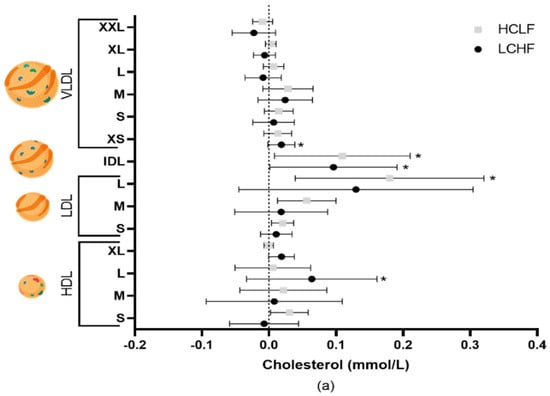
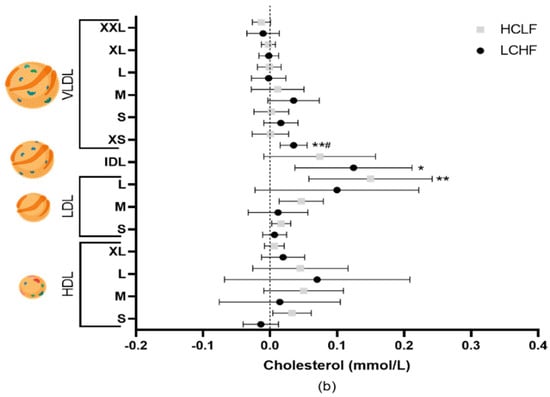
Figure 1.
The effects of LCHF (n = 8) and HCLF diets (n = 8) for 8 weeks on lipoprotein subclass cholesterol concentration. (a) The mean difference ± SD in lipoprotein cholesterol from baseline to 4 weeks following LCHF and HCLF. (b) The mean difference ± SD in lipoprotein cholesterol from baseline to 8 weeks following LCHF and HCLF. Lipoprotein figures were created using BioRender.com. HCLF, high-carbohydrate low-fat diet; LCHF, low-carbohydrate high-fat diet; * p < 0.05, denotes the significant effect of time; ** p < 0.01, denotes the significant effect of time, # p < 0.05, denotes significant differences between diets.

Table 1.
The effect of LCHF (n = 8) and HCLF (n = 8) diets on lipids in lipoprotein subclasses.
Both diets also exerted differential responses in lipoprotein phospholipid content (Table 1). At 4 weeks, the level of small LDL phospholipids increased following the HCLF (p = 0.023) and LCHF (p = 0.013) diets, but IDL phospholipids increased only following the LCHF diet (p = 0.032), and large LDL phospholipids increased following the HCLF (p = 0.021) diet. Similarly, at 8 weeks, the LCHF diet increased IDL (p = 0.010) phospholipids, whereas the HCLF diet increased large (p = 0.026) and small LDL (p = 0.017) phospholipids. Phospholipids in very large HDL increased (p = 0.005) with the LCHF diet compared to the HCLF diet but were not confirmed when corrected for multiple comparisons. Furthermore, sphingomyelins significantly increased from baseline to week 4 (0.48 ± 0.04 to 0.52 ± 0.04 mmol/L, p = 0.004) in the LCHF diet only (Table S1).
ApoB concentrations increased after 4 (p = 0.002) and 8 (p = 0.001) weeks of the HCLF diet, and this increase was significantly greater compared to the LCHF diet (p = 0.0001) (Figure 2). The HCLF diet also increased small HDL particles after 4 (p = 0.019) and 8 (p = 0.025) weeks (Table S1). In contrast, the LCHF diet resulted in increased very small VLDL (p = 0.015) particle concentrations (Table S1). In comparison to HCLF, the LCHF diet resulted in decreased large VLDL (p = 0.050) particles and increased very large (p = 0.031) and large (p = 0.043) HDL particle concentrations (Figure 2) and HDL diameter (p = 0.047); however, after multiple comparisons, the significance was lost.
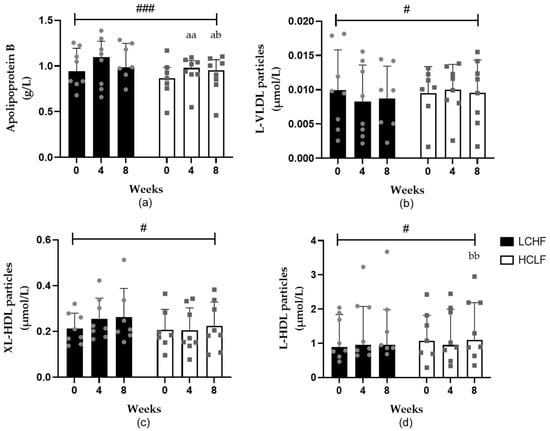
Figure 2.
The effects of LCHF (n = 8) and HCLF diets (n = 8) for 8 weeks on lipoprotein particle concentration. (a) The effect (median ± IQR) of LCHF and HCLF diets on fasting apolipoprotein B concentrations. (b) The effect (mean ± SD) of LCHF and HCLF diets on fasting L-VLDL particle concentrations. (c) The effect (mean ± SD) of LCHF and HCLF diets on fasting XL-HDL particle concentrations. (d) The effect (median ± IQR) of LCHF and HCLF diets on fasting L-HDL particle concentrations. The grey symbols represent individual responses to the LCHF diet (circle) and HCLF diet (square). HCLF, high-carbohydrate low-fat diet; LCHF, low-carbohydrate high-fat diet. # p < 0.05 and ### p < 0.001 denote significant interactions between groups; a p < 0.05, aa p < 0.01 denotes significant difference to week 0; b p < 0.05 and bb p < 0.01 denote significant difference in week 4.
3.2. Amino Acids, Glycolysis, and Fatty Acid-Related Metabolites
Both the LCHF and HCLF diets resulted in some alterations in energy-related metabolites (Table S1). Specifically, glutamine decreased (p = 0.013) after 4 weeks of the LCHF diet (0.052 ± 0.074 mmol/L) and was significantly (p = 0.002) lower compared to HCLF, (0.062 ± 0.067 mmol/L) but returned to baseline by week 8. Histidine also significantly decreased after 8 weeks (0.074 ± 0.007 to 0.067 ± 0.01 mmol/L, p = 0.004) of the LCHF diet. Tyrosine significantly declined following the LCHF diet at 4 and 8 weeks compared to baseline (0.068 ± 0.013 to 0.056 ± 0.015, p = 0.004 to 0.058 ± 0.011 mmol/L, p = 0.004). Total BCAAs were significantly higher in the LCHF diet group compared to the HCLF diet group at week 4 (0.453 ± 0.053 vs. 0.383 ± 0.053 mmol/L, p = 0.021) and week 8 (0.465 ± 0.076 vs. 0.384 ± 0.036 mmol/L, p = 0.033). This was primarily driven by the significantly higher valine concentrations in the LCHF diet compared to the HCLF diet at week 4 (0.258 ± 0.035 vs. 0.214 ± 0.023 mmol/L, p = 0.010) and week 8 (0.259 ± 0.024 vs. 0.217 ± 0.019 mmol/L, p = 0.003). Metabolites of fatty acid oxidation significantly increased with the LCHF diet and were elevated compared to the HCLF diet at 4 weeks (citrate, p = 0.040; acetoacetate, p = 0.009) and 8 weeks (citrate, p = 0.001; acetone, p < 0.001) with the LCHF diet. The ketone body 3-Hydroxybutyrate significantly increased after 4 weeks (median (IQR): 0.07 (0.02) to 0.32 (0.42) mmol/L, p = 0.008) with the LCHF diet and was significantly elevated compared to HCLF at 4 weeks (median (IQR): 0.32 (0.42) vs. 0.07 (0.07) mmol/L, p = 0.007) and 8 weeks (median (IQR): 0.13 (0.18) vs. 0.06 (0.04) mmol/L, p = 0.042). Similarly, albumin also increased from baseline to 4 and 8 weeks (42.11 ± 2.68 to 43.44 ± 2.82, p = 0.022 to 44.05 ± 1.93 mmol/L, p = 0.038) in the LCHF diet group. Furthermore, docosahexaenoic acid significantly increased from baseline to week 4 (0.18 ± 0.03 to 0.25 ± 0.02 mmol/L, p < 0.001) in the LCHF diet group only (Table S1).
3.3. Unbiased Random Forest Analysis
Unbiased random forest analysis revealed 12 metabolites related to lipid, lipoprotein, and amino acid metabolism, thereby distinguishing the LCHF diet from HCLF (Table 2).

Table 2.
Unbiased random forest analysis distinguishing the LCHF diet from the HCLF diet from baseline to 8 weeks.
4. Discussion
Unexpectedly, these pilot data show that both an LCHF and HCLF diet resulted in increased lipoprotein cholesterol, which may indicate an increase in CVD risk. However, the LCHF diet resulted in reduced triglyceride-rich lipoproteins and increased HDL phospholipids and particle numbers, indicating an improvement in HDL functionality. These differential effects on lipid and lipoprotein metabolism in a small cohort (n = 8 per group) provide some insights into how dietary carbohydrate and fat manipulation may affect CVD risk factors in the short term.
Cholesterol concentrations have long been established as key indicators of CVD risk [31], and diet has been shown as an important regulator [32]. In the current study, participants following either an LCHF or an HCLF diet had increased cholesterol levels, which were due to subtle differences in lipoprotein subclass cholesterol concentrations. The LCHF diet increased XS-VLDL-C, IDL-C, and L-HDL-C, whereas the HCLF diet increased only IDL-C and L-LDL-C. These results are perhaps in contrast with previous research that highlights that although cholesterol concentrations do increase with an LCHF diet, they tend to be greater in LDL-C compared to HCLF [5,7]. However, not all studies show an increase in LDL-C with either diet, even in the absence of decreased body mass [33]. Typically, LDL-C is considered to have a causal effect on CVD [34]; however, a recent Mendelian randomisation (MR) study highlighted that elevated VLDL-C and IDL-C are indicative of increased CVD risk independent of LDL-C [35]. Although HDL-C is associated with lower CVD risk [31], there has been much debate on its role, as elevated HDL-C levels do not appear to protect against CVD in MR analysis [36]. However, more recently, an MR study indicated a protective effect of HDL-C on CVD risk, perhaps due to a larger sample size (n = 60,000 vs. n = 12,000) compared to Voight et al. (2012) [35]. Therefore, the increase in L-HDL-C with the LCHF diet may be protective against lipoprotein cholesterol increases, whereas this may not occur with the HCLF diet.
The LCHF diet resulted in significantly lower triglycerides in the fractions of VLDL compared to the HCLF diet and significantly decreased triglycerides within HDL. A reduction in triglyceride-rich lipoproteins (TRLs) is shown to be associated with reduced CVD risk [37,38]. TRLs can enter the arterial intima similar to LDL, but their larger size may lead to greater preferential retention of cholesterol-enriched remnants [38,39]. TRLs can also undergo direct phagocytosis, leading to foam cell generation, inflammation, and atherosclerotic plaque formation [38,39]. This reduction in TRLs likely contributes to the reduction in HDL triglycerides via reduced cholesteryl ester transfer protein activity [40]. Elevated HDL triglycerides have been shown to be positively associated with the markers of metabolic disease [40], myocardial infarction, and ischaemic stroke, [37,41] indicating a positive effect of an LCHF diet.
The lipoprotein surface is encompassed by phospholipids, primarily phosphatidylcholine and sphingomyelin, and plays a role in lipoprotein functionality. Sphingomyelin is the precursor to ceramides and sphingomyelins significantly increased following 4 weeks of the LCHF diet. However, it is unclear what effect this may have on CVD risk, as an increase in short-chain sphingomyelins and ceramides are associated with increased CVD risk, whereas longer chains are associated with reduced risk [42]. A decrease in sphingomyelin, particularly in HDL, may also be associated with reduced CVD risk; however, this may be confounded by low HDL-C levels [43]. Interestingly, the phospholipids within very large HDL were significantly elevated in the LCHF diet compared to the HCLF diet, again indicating a reduction in CVD risk, perhaps due to improved HDL functionality [43,44]. The process of cholesterol efflux or HDL functionality has been shown to be associated with reduced CVD risk, independent of HDL-C concentrations [45]. The HCLF diet resulted in increased LDL phospholipid content, which, in contrast, has been shown to decrease after following 8 weeks of the cardioprotective Mediterranean diet [46] where increased MUFA was considered responsible [47].
Lipoprotein particle number and size are strong independent risk factors for the development of CVDs [48]. Restricting dietary carbohydrate intake in adults without disease has shown an increase in LDL particle peak size and reduced number, thereby shifting to a lower-risk phenotype (A) [49]. Although there were large differences in carbohydrate intake in the LCHF groups, meta-regression analysis revealed that this was not a factor; however, weight loss may have influenced the findings [49]. Similar to Falkenhain et al. [49], the current results support the hypothesis that carbohydrate restriction improves LDL particle concentrations. The LCHF diet also decreased large VLDL and increased very large and large HDL particle concentrations relative to the HCLF diet, which is consistent with previous research [33,50]. Although HDL particle concentrations still significantly increased following the HCLF diet, this was accompanied by a significant increase in apolipoprotein B levels, which is used as a surrogate for LDL particle number and is positively associated with CVDs [48]. However, the ratio of apolipoprotein B/A1 or total LDL particle number did not change, indicating that perhaps the small changes in apolipoprotein B are of little clinical significance. The improvements in HDL particle concentrations, along with elevated HDL cholesterol, phospholipids, and reduced TRLs following the LCHF diet, highlight how an LCHF diet may reduce CVD risk by improving HDL functionality and metabolism (Figure 3).
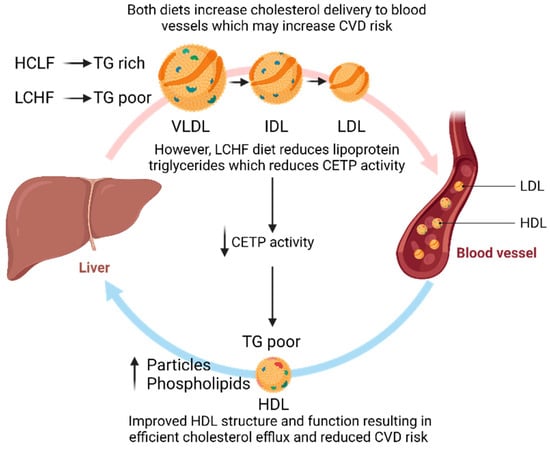
Figure 3.
The potential effect of an LCHF diet on improving HDL functionality to reduce CVD risk. CETP, cholesterol ester transfer protein; HDL, high-density lipoprotein; IDL, intermediate-density lipoprotein; LDL, low-density lipoprotein; TG, triglycerides; VLDL, very low-density lipoprotein. Figures were created using BioRender.com.
Random forest statistical analysis allowed for the unbiased detection of the effects of both diets on multiple metabolites (Table 2). These data also corroborate primary analyses that the LCHF diet results in greater reductions in triglycerides and increased lipids (driven by cholesterol and phospholipids) in HDL compared to the HCLF diet. Additionally, the LCHF diet appears to increase BCAAs and reduce tyrosine and histidine concentrations relative to the HCLF diet. While reductions in tyrosine and histidine may suggest a reduced CVD risk [51], elevated BCAAs have been associated with CVD and type 2 diabetes [41,52]. Increases in BCAAs could be due to increased protein intake and the release of amino acids for gluconeogenesis as a normal part of LCHF adaptations; however, long-term controlled studies are required on these implications.
Like all research, the current study has its limitations, which were previously highlighted [24]. Briefly, the intervention was short, with a small sample size in individuals without CVD; therefore, the results should not be extrapolated to long-term health or generalised to populations with CVD. Although dietary records show good adherence to the LCHF diet, this may not be the case, as ketone markers were highest by week 4 (but below the ketosis threshold of 0.5 mmol/L) and decreased by week 8, which may have reduced the impact of the LCHF diet on biomarkers of CVD risk at this stage. Nonetheless, the objective of this study was to investigate changes in lipid and lipoprotein metabolism, rather than long-term compliance, and these changes were evident. The strengths of this study include the use of high-throughput NMR spectroscopy to identify discrete lipoprotein subclasses that can infer CVD risk and elucidate the regulatory role of diet.
In conclusion, following a short-term LCHF diet may reduce TRLs and improve HDL metabolism and functionality. The potential improvements in HDL functionality may compensate for the increases in VLDL/IDL/LDL cholesterol, but this may not be apparent with an HCLF diet. It is unclear how this may be used to infer the overall CVD risk due to the small sample size and short duration of the study. Furthermore, the LCHF diet increases BCAA levels, which may be associated with an elevated CVD risk; however, this could also be reflective of amino acid release for gluconeogenesis. Long-term studies with large cohorts are warranted to confirm the role of increased dietary fat and carbohydrate restriction in lipoprotein metabolism and CVD risk factors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15133002/s1, Table S1: The effect of LCHF and HCLF diets on markers of cardiovascular disease.
Author Contributions
Conceptualization, I.G.D., C.E.S., K.E.L. and K.J.E.; methodology, D.M., T.H. and M.M.; formal analysis, D.M. and M.M.; writing—original draft preparation, D.M.; writing—review and editing, all authors; supervision, I.G.D., C.E.S., K.E.L., F.A., K.J.E. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
Deaglan McCullough and Tanja Harrison received funding for a PhD studentship from Liverpool John Moores University.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Liverpool John Moores University (REC number: 16/ELS/029 and 14/12/2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 11 April 2023).
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. The Eatwell Guide. Helping You Eat a Healthy Balance Diet; Public Health England: London, UK, 2016. [Google Scholar]
- World Health Organization. Healthy Diet; World Health Organization: Geneva, Switzerland, 2018; pp. 1–6. [Google Scholar]
- Mansoor, N.; Vinknes, K.J.; Veierod, M.B.; Retterstol, K. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors a meta-analysis of randomised controlled trials. Br. J. Nutr. 2016, 115, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Bueno, N.B.; De Melo, I.S.V.; De Oliveira, S.L.; Da Rocha Ataide, T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of Randomised controlled trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef]
- Gjuladin-Hellon, T.; Davies, I.G.; Penson, P.; Baghbadorani, R.A. Effects of carbohydrate-restricted diets on low-density lipoprotein cholesterol levels in overweight and obese adults: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Diffenderfer, M.R.; Schaefer, E.J. The composition and metabolism of large and small LDL. Curr. Opin. Lipidol. 2014, 25, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.A.; Myasoedova, V.A.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. Small Dense Low-Density Lipoprotein as Biomarker for Atherosclerotic Diseases. Oxidative Med. Cell. Longev. 2017, 2017, 1273042. [Google Scholar] [CrossRef] [PubMed]
- Sniderman, A.D.; Toth, P.P.; Thanassoulis, G.; Furberg, C.D. An evidence-based analysis of the National Lipid Association recommendations concerning non-HDL-C and apoB. J. Clin. Lipidol. 2016, 10, 1248–1258. [Google Scholar] [CrossRef]
- Hoogeveen, R.C.; Gaubatz, J.W.; Sun, W.; Dodge, R.C.; Crosby, J.R.; Jiang, J.; Couper, D.; Virani, S.S.; Kathiresan, S.; Boerwinkle, E.; et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1069–1077. [Google Scholar] [CrossRef]
- Fan, J.; Liu, Y.; Yin, S.; Chen, N.; Bai, X.; Ke, Q.; Shen, J.; Xia, M. Small dense LDL cholesterol is associated with metabolic syndrome traits independently of obesity and inflammation. Nutr. Metab. 2019, 16, 7. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, K.; Liu, F.; Lu, X.; Huang, J.; Gu, D. Association of circulating branched-chain amino acids with risk of cardiovascular disease: A systematic review and meta-analysis. Atherosclerosis 2022, 350, 90–96. [Google Scholar] [CrossRef]
- Mangge, H.; Zelzer, S.; Pruller, F.; Schnedl, W.J.; Weghuber, D.; Enko, D.; Bergsten, P.; Haybaeck, J.; Meinitzer, A. Branched-chain amino acids are associated with cardiometabolic risk profiles found already in lean, overweight and obese young. J. Nutr. Biochem. 2016, 32, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Delgado, F.; Katsiki, N.; Lopez-Miranda, J.; Perez-Martinez, P. Dietary habits, lipoprotein metabolism and cardiovascular disease: From individual foods to dietary patterns. Crit. Rev. Food Sci. Nutr. 2021, 61, 1651–1669. [Google Scholar] [CrossRef] [PubMed]
- Aru, V.; Lam, C.; Khakimov, B.; Hoefsloot, H.C.J.; Zwanenburg, G.; Lind, M.V.; Schäfer, H.; van Duynhoven, J.; Jacobs, D.M.; Smilde, A.K.; et al. Quantification of lipoprotein profiles by nuclear magnetic resonance spectroscopy and multivariate data analysis. TrAC-Trends Anal. Chem. 2017, 94, 210–219. [Google Scholar] [CrossRef]
- Würtz, P.; Havulinna, A.S.; Soininen, P.; Tynkkynen, T.; Prieto-Merino, D.; Tillin, T.; Ghorbani, A.; Artati, A.; Wang, Q.; Tiainen, M.; et al. Metabolite profiling and cardiovascular event risk: A prospective study of 3 population-based cohorts. Circulation 2015, 131, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Mora, S.; Otvos, J.D.; Rifai, N.; Rosenson, R.S.; Buring, J.E.; Ridker, P.M. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation 2009, 119, 931–939. [Google Scholar] [CrossRef]
- Akbaraly, T.; Würtz, P.; Singh-Manoux, A.; Shipley, M.J.; Haapakoski, R.; Lehto, M.; Desrumaux, C.; Kähönen, M.; Lehtimäki, T.; Mikkilä, V.; et al. Association of circulating metabolites with healthy diet and risk of cardiovascular disease: Analysis of two cohort studies. Sci. Rep. 2018, 8, 8620. [Google Scholar] [CrossRef]
- Ulven, S.M.; Christensen, J.J.; Nygård, O.; Svardal, A.; Leder, L.; Ottestad, I.; Lysne, V.; Laupsa-Borge, J.; Ueland, P.M.; Midttun, Ø.; et al. Using metabolic profiling and gene expression analyses to explore molecular effects of replacing saturated fat with polyunsaturated fat-a randomized controlled dietary intervention study. Am. J. Clin. Nutr. 2019, 109, 1239–1250. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Knapp, A.; Johnson, A.; Wong, J.M.W.; Greco, K.F.; Ma, C.; Mora, S.; Ludwig, D.S. Effects of a low-carbohydrate diet on insulin-resistant dyslipoproteinemia-a randomized controlled feeding trial. Am. J. Clin. Nutr. 2022, 115, 154–162. [Google Scholar] [CrossRef]
- Hustad, K.S.; Rundblad, A.; Ottestad, I.; Christensen, J.J.; Holven, K.B.; Ulven, S.M. Comprehensive lipid and metabolite profiling in healthy adults with low and high consumption of fatty fish: A cross-sectional study. Br. J. Nutr. 2021, 125, 1034–1042. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ (Online) 2010, 340, 698–702. [Google Scholar] [CrossRef]
- McCullough, D.; Harrison, T.; Boddy, L.M.; Enright, K.J.; Amirabdollahian, F.; Schmidt, M.A.; Doenges, K.; Quinn, K.; Reisdorph, N.; Mazidi, M.; et al. The Effect of Dietary Carbohydrate and Fat Manipulation on the Metabolome and Markers of Glucose and Insulin Metabolism: A Randomised Parallel Trial. Nutrients 2022, 14, 3691. [Google Scholar] [CrossRef] [PubMed]
- Westman, E.C.; Feinman, R.D.; Mavropoulos, J.C.; Vernon, M.C.; Volek, J.S.; Wortman, J.A.; Yancy, W.S.; Phinney, S.D. Low-carbohydrate nutrition and metabolism. Am. J. Clin. Nutr. 2007, 86, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Soininen, P.; Kangas, A.J.; Würtz, P.; Suna, T.; Ala-Korpela, M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ. Cardiovasc. Genet. 2015, 8, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Julkunen, H.; Cichonska, A.; Tiainen, M.; Koskela, H.; Nybo, K.; Makela, V.; Nokso-Koivisto, J.; Kristiansson, K.; Perola, M.; Salomaa, V.; et al. Atlas of plasma NMR biomarkers for health and disease in 118,461 individuals from the UK Biobank. Nat. Commun. 2023, 14, 604. [Google Scholar] [CrossRef]
- Wurtz, P.; Wang, Q.; Soininen, P.; Kangas, A.J.; Fatemifar, G.; Tynkkynen, T.; Tiainen, M.; Perola, M.; Tillin, T.; Hughes, A.D.; et al. Metabolomic Profiling of Statin Use and Genetic Inhibition of HMG-CoA Reductase. J. Am. Coll. Cardiol. 2016, 67, 1200–1210. [Google Scholar] [CrossRef]
- Wurtz, P.; Kangas, A.J.; Soininen, P.; Lawlor, D.A.; Davey Smith, G.; Ala-Korpela, M. Quantitative Serum Nuclear Magnetic Resonance Metabolomics in Large-Scale Epidemiology: A Primer on -Omic Technologies. Am. J. Epidemiol. 2017, 186, 1084–1096. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef]
- Shan, Z.; Li, Y.; Baden, M.Y.; Bhupathiraju, S.N.; Wang, D.D.; Sun, Q.; Rexrode, K.M.; Rimm, E.B.; Qi, L.; Willett, W.C.; et al. Association Between Healthy Eating Patterns and Risk of Cardiovascular Disease. JAMA Intern. Med. 2020, 180, 1090–1100. [Google Scholar] [CrossRef]
- Hyde, P.N.; Sapper, T.N.; Crabtree, C.D.; LaFountain, R.A.; Bowling, M.L.; Buga, A.; Fell, B.; McSwiney, F.T.; Dickerson, R.M.; Miller, V.J.; et al. Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight 2019, 4, e128308. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef]
- Schmidt, A.F.; Joshi, R.; Gordillo-Maranon, M.; Drenos, F.; Charoen, P.; Giambartolomei, C.; Bis, J.C.; Gaunt, T.R.; Hughes, A.D.; Lawlor, D.A.; et al. Biomedical consequences of elevated cholesterol-containing lipoproteins and apolipoproteins on cardiovascular and non-cardiovascular outcomes. Commun. Med. 2023, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Holm, H.; Ding, E.L.; Johnson, T.; et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 2012, 380, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Hou, L.; Chen, X.; Li, W.; Liu, X.; Liu, C.; Li, Y.; Yuan, T.; Li, J.; Wang, B.; et al. Exploring the Causal Roles of Circulating Remnant Lipid Profile on Cardiovascular and Cerebrovascular Diseases: Mendelian Randomization Study. J. Epidemiol. 2022, 32, 205–214. [Google Scholar] [CrossRef]
- Duran, E.K.; Aday, A.W.; Cook, N.R.; Buring, J.E.; Ridker, P.M.; Pradhan, A.D. Triglyceride-Rich Lipoprotein Cholesterol, Small Dense LDL Cholesterol, and Incident Cardiovascular Disease. J. Am. Coll. Cardiol. 2020, 75, 2122–2135. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Varbo, A. Triglycerides and cardiovascular disease. Lancet 2014, 384, 626–635. [Google Scholar] [CrossRef]
- Girona, J.; Amigo, N.; Ibarretxe, D.; Plana, N.; Rodriguez-Borjabad, C.; Heras, M.; Ferre, R.; Gil, M.; Correig, X.; Masana, L. HDL Triglycerides: A New Marker of Metabolic and Cardiovascular Risk. Int. J. Mol. Sci. 2019, 20, 3151. [Google Scholar] [CrossRef]
- Holmes, M.V.; Millwood, I.Y.; Kartsonaki, C.; Hill, M.R.; Bennett, D.A.; Boxall, R.; Guo, Y.; Xu, X.; Bian, Z.; Hu, R.; et al. Lipids, Lipoproteins, and Metabolites and Risk of Myocardial Infarction and Stroke. J. Am. Coll. Cardiol. 2018, 71, 620–632. [Google Scholar] [CrossRef]
- Jensen, P.N.; Fretts, A.M.; Hoofnagle, A.N.; McKnight, B.; Howard, B.V.; Umans, J.G.; Sitlani, C.M.; Siscovick, D.S.; King, I.B.; Sotoodehnia, N.; et al. Circulating ceramides and sphingomyelins and the risk of incident cardiovascular disease among people with diabetes: The strong heart study. Cardiovasc. Diabetol. 2022, 21, 167. [Google Scholar] [CrossRef]
- Ding, M.; Rexrode, K.M. A review of lipidomics of cardiovascular disease highlights the importance of isolating lipoproteins. Metabolites 2020, 10, 163. [Google Scholar] [CrossRef]
- Yancey, P.G.; de la Llera-Moya, M.; Swarnakar, S.; Monzo, P.; Klein, S.M.; Connelly, M.A.; Johnson, W.J.; Williams, D.L.; Rothblat, G.H. High density lipoprotein phospholipid composition is a major determinant of the bi-directional flux and net movement of cellular free cholesterol mediated by scavenger receptor BI. J. Biol. Chem. 2000, 275, 36596–36604. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Zhao, X.; Zhou, Q.; Zhang, Z. High-density lipoprotein cholesterol efflux capacity is inversely associated with cardiovascular risk: A systematic review and meta-analysis. Lipids Health Dis. 2017, 16, 212. [Google Scholar] [CrossRef] [PubMed]
- Rosato, V.; Temple, N.J.; La, C.; Giorgio, V.; Alessandra, C.; Guercio, V. Mediterranean diet and cardiovascular disease: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2019, 58, 173–191. [Google Scholar] [CrossRef]
- Michielsen, C.; Hangelbroek, R.W.J.; Feskens, E.J.M.; Afman, L.A. Disentangling the Effects of Monounsaturated Fatty Acids from Other Components of a Mediterranean Diet on Serum Metabolite Profiles: A Randomized Fully Controlled Dietary Intervention in Healthy Subjects at Risk of the Metabolic Syndrome. Mol. Nutr. Food Res. 2019, 63, e1801095. [Google Scholar] [CrossRef]
- Richardson, T.G.; Sanderson, E.; Palmer, T.M.; Ala-Korpela, M.; Ference, B.A.; Davey Smith, G.; Holmes, M.V. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLoS Med. 2020, 17, e1003062. [Google Scholar] [CrossRef] [PubMed]
- Falkenhain, K.; Roach, L.A.; McCreary, S.; McArthur, E.; Weiss, E.J.; Francois, M.E.; Little, J.P. Effect of carbohydrate-restricted dietary interventions on LDL particle size and number in adults in the context of weight loss or weight maintenance: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2021, 114, 1455–1466. [Google Scholar] [CrossRef]
- Westman, E.C.; Yancy, W.S.; Olsen, M.K.; Dudley, T.; Guyton, J.R. Effect of a low-carbohydrate, ketogenic diet program compared to a low-fat diet on fasting lipoprotein subclasses. Int. J. Cardiol. 2006, 110, 212–216. [Google Scholar] [CrossRef]
- Jauhiainen, R.; Vangipurapu, J.; Laakso, A.; Kuulasmaa, T.; Kuusisto, J.; Laakso, M. The Association of 9 Amino Acids With Cardiovascular Events in Finnish Men in a 12-Year Follow-up Study. J. Clin. Endocrinol. Metab. 2021, 106, 3448–3454. [Google Scholar] [CrossRef]
- Tobias, D.K.; Lawler, P.R.; Harada, P.H.; Demler, O.V.; Ridker, P.M.; Manson, J.E.; Cheng, S.; Mora, S. Circulating Branched-Chain Amino Acids and Incident Cardiovascular Disease in a Prospective Cohort of US Women. Circ. Genom. Precis Med. 2018, 11, e002157. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).