Abstract
The Western diet is a modern dietary pattern characterized by high intakes of pre-packaged foods, refined grains, red meat, processed meat, high-sugar drinks, candy, sweets, fried foods, conventionally raised animal products, high-fat dairy products, and high-fructose products. The present review aims to describe the effect of the Western pattern diet on the metabolism, inflammation, and antioxidant status; the impact on gut microbiota and mitochondrial fitness; the effect of on cardiovascular health, mental health, and cancer; and the sanitary cost of the Western diet. To achieve this goal, a consensus critical review was conducted using primary sources, such as scientific articles, and secondary sources, including bibliographic indexes, databases, and web pages. Scopus, Embase, Science Direct, Sports Discuss, ResearchGate, and the Web of Science were used to complete the assignment. MeSH-compliant keywords such “Western diet”, “inflammation”, “metabolic health”, “metabolic fitness”, “heart disease”, “cancer”, “oxidative stress”, “mental health”, and “metabolism” were used. The following exclusion criteria were applied: (i) studies with inappropriate or irrelevant topics, not germane to the review’s primary focus; (ii) Ph.D. dissertations, proceedings of conferences, and unpublished studies. This information will allow for a better comprehension of this nutritional behavior and its effect on an individual’s metabolism and health, as well as the impact on national sanitary systems. Finally, practical applications derived from this information are made.
1. Introduction to the Western Diet
Like all species, contemporary humans are genetically adapted to the environment of their ancestors, which conditioned their genetic profile. In terms of nutrition, the introduction of agriculture and animal husbandry about 10,000 years ago has lead to profound changes in the diet and lifestyle of these “modern humans” [1].
The problem with this is that these changes have occurred too recently on an evolutionary time scale for the human genome to adapt, thus leading to a discordance between our ancient biology and contemporary lifestyle patterns (Figure 1) [2]. Before agriculture and animal husbandry, hominin diets were limited to minimally processed wild plant and animal foods. However, with the domestication of plants and animals, the nutrient characteristics of these foods changed, which accelerated with advancing technology after the Industrial Revolution [3]. With the introduction of agriculture, novel foods were introduced as staples for which the hominin genome had little evolutionary experience. Furthermore, food processing procedures were developed that allowed for combinations of nutrients and foods not previously encountered in hominin evolution [1]. It is crucial to consider not only the nutrient qualities and the types of foods that would have been consumed by preagricultural hominins but also the types of foods and nutrient qualities that could not have been regularly consumed before the development of agriculture, industrialization, and advanced technology. Additionally, it is worth noting that dairy products, cereals, refined sugars, refined vegetable oils, and alcohol make up 72.1% of the total daily energy consumed by all people in the United States [4], but these types of foods would have contributed little or none of the energy in the typical preagricultural hominin diet [5]. Additionally, processed foods also dominate in a typical Western diet, such as cookies, cake, bakery foods, breakfast cereals, and snack foods [6].
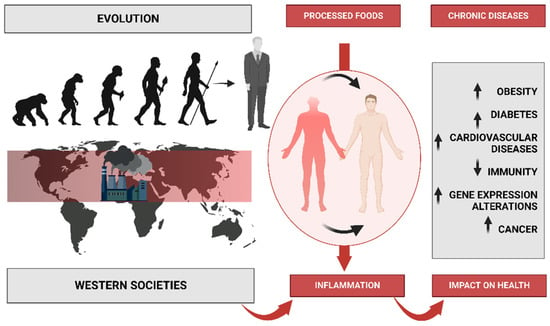
Figure 1.
Description of human evolution and the changes in eating patterns caused by increased industrialization and the marketing of processed foods. This leads to poor habits that cause inflammation, which leads to a variety of diseases.
1.1. Health Consequences, Costs, and Impact
Therefore, there is a dissonance, a change in the food and nutritional model, which reveals a series of epidemiological problems, with an increasing number of many diseases of civilization, including obesity, diabetes, and heart disease [7,8]. In the United States alone, most adults aged 20 and above, around 65%, are either overweight or obese. This has resulted in an estimated 280,184 deaths every year that can be attributed to obesity alone. Cardiovascular diseases (CVD) are prevalent in over 64 million Americans [7], and they remain the leading cause of death, accounting for 38.5% of all deaths in the country [4]. Moreover, 50 million Americans suffer from hypertension and a poor nutritional status, which is a cause of prolonged hospitalization and has a substantial impact on public health cost [9]. Additionally, 11 million have type 2 diabetes and 37 million have high-risk total cholesterol concentrations of 240 mg/dL [9]. In postmenopausal women aged 50 years, around 7.2% have osteoporosis and 39.6% have osteopenia [10]. Additionally, osteoporotic hip fractures have been associated with a 20% excess mortality in the year following the fracture. Cancer is the second leading cause of death in the country, accounting for 25% of all deaths [11]. An estimated one-third of all cancer deaths are due to poor dietary habits and obesity [12]. These figures demonstrate the severe impact of a disruptive diet that moves away from the nutritional model of our ancestors [1], approaching what is known today as the “Western diet”.
Thus, measures must be taken to address these issues, such as promoting healthy eating habits and increasing physical activity. Public health campaigns and initiatives can play a crucial role in educating people about the importance of a balanced diet and an active lifestyle [13]. Policymakers can also implement measures such as food labeling and taxation on unhealthy foods to encourage healthier choices. It is essential to take proactive steps to address these issues and improve the overall health of the nation.
1.2. Scientific Evidence
Indeed, recent clinical trials and interventions have shown that adopting dietary treatments with nutritional characteristics similar to those of pre-agricultural and pre-industrial diets can have positive effects on health [14]. These findings are consistent with the evolutionary discordance theory, which suggests that the human body is adapted to certain dietary patterns and that modern diets, which differ significantly from those of our ancestors, may contribute to chronic diseases. Thus, by adopting healthier eating habits that are in line with our evolutionary past, we can potentially reduce the risk of chronic diseases and improve overall health.
However, we have been able to mitigate some of the effects of this discordance through advancements in medicine and technology, which has led to some new challenges given the natural disruption of viral and bacterial processes in modern medicine, such as antibiotic-resistant bacteria [15]. In addition, the impact of human activities on the environment is creating new selective pressures that could lead to further evolutionary changes in the future. As such, understanding the interplay between genetics and the environment is crucial for developing effective strategies to promote human health and well-being in the face of these challenges.
In light of the need for a greater understanding of this nutritional behavior, its effect on an individual’s metabolism and health, and its impact on national health systems, this review was conducted to describe the effect of the Western pattern diet on the metabolism, inflammation, and antioxidant status; the impact on gut microbiota and mitochondrial fitness; the effect cardiovascular health, mental health, and cancer; and the sanitary costs.
2. Methods
In this investigation, we performed a comprehensive examination of primary and secondary sources that incorporated scientific articles, bibliographic indexes, and databases such as PubMed, Scopus, Embase, Science Direct, Sports Discuss, ResearchGate, and the Web of Science. We applied MeSH-compliant keywords such as Western diet, inflammation, metabolic health, metabolic fitness, cardiovascular disease, cancer, oxidative stress, mental health, and metabolism to explore articles that were published between 1 January 2003 and 1 March 2023. The following exclusion criteria were used: (i) studies with inappropriate or irrelevant topics not pertinent to the main focus of the review, (ii) Ph.D. dissertations, conference proceedings, and unpublished studies. A team of five review authors meticulously evaluated the titles and abstracts of all collected manuscripts to ascertain their suitability. Studies that utilized outdated data, had irrelevant topics that did not align with the research objectives, or were not in English were eliminated. The same team of five review authors who undertook the study selection independently extracted pertinent data from the selected studies. Subsequently, the outcomes were discussed to create the current comprehensive review. It is worth mentioning that this study’s approach guarantees that the included data are current, relevant, and of high quality, thereby making the findings of this review reliable and useful for future research.
3. Nutritional Characteristic of Western Diet
The Western diet has been the subject of much discussion in the field of nutrition. It is a modern dietary pattern that is characterized by high intakes of processed and refined foods, red and processed meats, added sugars, and saturated and trans fats and low intakes of fruits, vegetables, whole grains, and nuts (Figure 1). The Western diet has been linked to a range of chronic diseases, including obesity, type 2 diabetes, cardiovascular disease, and certain cancers. In this paper, we will discuss the nutritional characteristics of the Western diet, its impact on health, and the potential solutions to improve dietary patterns in Western societies [12].
3.1. Western Diet Concept
The Western diet is characterized by a high intake of energy-dense, nutrient-poor foods such as fast foods, soft drinks, and highly processed foods, which are high in added sugars, salt, and saturated fats. In contrast, the traditional diets of non-Western countries, such as the Mediterranean diet, are characterized by high intakes of fruits, vegetables, whole grains, legumes, and healthy fats such as olive oil and nuts [16]. The consumption of processed and refined foods is a major characteristic of the Western diet. Processed foods are foods that have been altered from their natural state, typically to increase their shelf life or improve their taste. Examples include fast foods, packaged snacks, and sugary drinks. Refined foods are foods that have had their natural fiber and nutrients removed during processing, such as white flour, white rice, and added sugars [17].
The Western diet is also characterized by high consumption of red and processed meats. Red meat consumption has been associated with an increased risk of colorectal cancer, while processed meat consumption has been linked to an increased risk of colorectal cancer, cardiovascular disease, and type 2 diabetes [18]. Another major characteristic of the Western diet is the high intake of added sugars. Added sugars are sugars and syrups that are added to foods during processing, such as high-fructose corn syrup in soft drinks and table sugar in baked goods. High intake of added sugars has been linked to an increased risk of obesity, type 2 diabetes, and cardiovascular disease [19]. The Western diet is also characterized by high intakes of saturated and trans fats. Saturated fats are found in animal products such as meat, butter, and cheese, while trans fats are found in processed foods such as baked goods and fried foods. High intake of saturated and trans fats has been linked to an increased risk of cardiovascular disease [20].
In order to clarify which countries are exposed to this diet concept, we provide official FAO data. FAO-provided food balance sheets are adjusted for consumer waste and used to calculate a Western dietary similarity index (WSI) for each nation. Based on this information, a ratio of calories from animal foods, oils, lipids, and sweeteners to total calories per capita can be calculated [21,22,23]. A plausible value is WSI = 70, which corresponds to the US WSI in 2013, indicating that the global steady-state WSI will correspond to a dietary pattern in which 70% of calories originate from animal foods, oils and fats, and sweeteners. Iceland (72), Switzerland (72), the United States (70), Australia (69), Sweden (67), Hungary (66), France (66), Austria (66), Germany (66), Denmark (66), the Czech Republic (65), the Netherlands (65), Spain (65), Belgium (65), Finland (64), and New Zealand comprise the “Western diet countries” group (64).
3.2. Western Diet-Related Diseases
The Western diet includes low intakes of fruits, vegetables, whole grains, and nuts. These foods are important sources of vitamins, minerals, fiber, and antioxidants, which are essential for optimal health. Low intakes of these foods have been linked to an increased risk of chronic diseases such as obesity, type 2 diabetes, and cardiovascular disease [24]. The Western diet has been linked to a range of chronic diseases and inflammation processes (Figure 1), including obesity, type 2 diabetes, cardiovascular disease, and certain cancers. In the United States, the data reveal alarming trends between 1999 and 2018, with male obesity increasing from 27.5% to 43.0% and severe obesity from 3.1% to 6.0%. In women, the prevalence of obesity rose from 33.4% to 41.9%, and the prevalence of severe obesity rose from 6.2% to 11.5% [25]. Obesity is a major risk factor for a range of chronic diseases, including type 2 diabetes, cardiovascular disease, and certain cancers. The consumption of a Western-style diet has been shown to increase the risk of obesity and related diseases [17]. Type 2 diabetes is another major health concern associated with the Western diet. A high intake of processed and refined foods, added sugars, and saturated and trans fats has been linked to an increased risk of type 2 diabetes [19]. In addition, low intakes of fruits, vegetables, whole grains, and nuts have been associated with an increased risk of type 2 diabetes [24].
Cardiovascular disease is the leading cause of death worldwide, and the Western diet is a major risk factor for this disease. High intakes of saturated and trans fats, added sugars, and salt have been linked to an increased risk of cardiovascular disease [26]. In contrast, diets high in fruits, vegetables, whole grains, and healthy fats such as those found in the Mediterranean diet have been shown to reduce the risk of cardiovascular disease [16]. Certain cancers, particularly colorectal cancer, have also been associated with the Western diet. High intakes of red and processed meats, as well as low intakes of fruits, vegetables, and whole grains, have been linked to an increased risk of colorectal cancer [27].
The availability of processed and ultra-processed foods including sugar, industrial seed oils, and poultry has increased over the past two centuries, while butter/lard/shortening, dairy (primarily whole-fat), fresh fruits, fresh vegetables, and red meat (beef/pork) have decreased. Before 1900, ultra-processed foods were uncommon, but they now make up more than half of the American diet [28]. The gut microbiome is the collection of microorganisms that reside in the digestive tract, including bacteria, viruses, fungi, and other microbes. This complex ecosystem plays an important role in the health of the human body, contributing to various functions such as digestion, immune system regulation, and the synthesis of certain vitamins and other essential nutrients [29]. In the scientific literature, it has been previously stated that one of the main factors, together with physical exercise, that modulates the intestinal microbiota is nutrition [30]. However, due to this, diet impacts the gut microbiome and immune system. Concretely, the Western diet can disrupt the balance and diversity of the gut microbiome leading to dysbiosis, which is a condition characterized by an overgrowth of harmful bacteria and a reduction in beneficial bacteria. Dysbiosis can impair intestinal barrier function, increase intestinal permeability, promote bacterial translocation, and trigger systemic inflammation. Dysbiosis can also affect immune system function, modulate immune cell differentiation, alter immune cell activation, influence cytokine production, regulate immunoglobulin secretion, and modulate immune tolerance [31].
3.3. Western Diet’s Impact on Genetics
The Western diet also can influence gene expression and epigenetics. Gene expression refers to the process by which genetic information is translated into functional molecules such as proteins or RNA. Epigenetics refers to the study of heritable changes in gene expression that do not involve changes in the DNA sequence but rather modifications in DNA methylation, histone modification, or microRNA regulation. The Western diet can affect gene expression and epigenetics by altering nutrient availability, cofactor supply, hormonal levels, environmental cues, cellular signaling pathways, transcription factors, chromatin structure, DNA methylation, histone modification, microRNA regulation, feedback loops, genetic variants, gene–environment interactions, intergenerational effects, and transgenerational effects (Figure 1) [32].
The Western diet shifts the co-expression of 445 gene pairs in mice, including small RNAs and transcription factors associated with metabolism and adiposity in humans, and dramatically alters behavior. For example, Western-fed individuals were more anxious and less socially integrated [33]. Similarly, the Western diet limits microbial short-chain fatty acid (SCFA) production in humans, which prevents many of the microbiota-dependent events from occurring, such as histone deacetylation and DNA methylation, and leads to alterations in hepatic gene expression [34]. The Western diet evokes structural and behavioral changes in the resident microbiome, which alters the representation of metabolic pathways in the microbiome and alters microbiome gene expression [35], as well as inducing epigenetic changes in monocytes that affect their inflammatory response and cytokine production [33], altering the expression of genes involved in lipid metabolism and oxidative stress in liver tissue [36], and modulating the expression of genes related to the circadian rhythm and melatonin synthesis in the pineal gland [37]. Similarly, it can also increase gene expression alterations related to a fatty liver (non-alcoholic fatty liver disease).
4. Western Diet and Lifestyle
The Western diet has become a global phenomenon and has had significant impacts on human health. Social characteristics such as income, education, and cultural background play a crucial role in shaping dietary habits and preferences. There are some social characteristics of the Western diet, which are related to income and education, since income and education are essential social factors that influence dietary choices. People with higher incomes and more education are more likely to have healthier diets that include more fruits, vegetables, whole grains, and lean proteins. A study by Burrows et al. (2017) found that higher levels of education were associated with a higher intake of fruits and vegetables [38]. Income and education levels are associated with health outcomes related to the Western diet. Lower-income individuals and those with less education are more likely to have poor dietary habits and be at higher risk for chronic diseases such as obesity, type 2 diabetes, and cardiovascular disease (Figure 1). For example, it was found that low-income individuals were more likely to have a higher intake of processed foods and a lower intake of fruits and vegetables [39].
In addition to the Western diet, sedentary lifestyles and low levels of physical activity are also associated with an increased risk of chronic diseases. In a paper that studied 3316 Finnish participants between 25 and 74 years old, it was found that higher levels of physical activity were associated with a reduced risk of type 2 diabetes [40]. Similarly, a study with 88 140 participants aged 40–85 years old found that higher levels of physical activity were associated with a reduced risk of cardiovascular disease [41].
Similarly, social norms can also influence dietary choices. People are often influenced by the behavior of those around them, including family, friends, and peers. One study [42] found that social norms related to healthy eating were positively associated with fruit and vegetable intake among adolescents. Social norms related to healthy eating are associated with better health outcomes. Individuals who perceive social norms to support healthy eating behaviors are more likely to have healthier diets and lower risks for chronic diseases, and adolescents who perceived social norms to support healthy eating had higher intakes of fruits and vegetables and lower intakes of sugar-sweetened beverages [43].
The food environment, including the availability and accessibility of healthy food options, also plays a role in shaping dietary choices. People living in areas with limited access to healthy food options are more likely to have poor dietary habits. A study by Stowers et al. (2020) [44] found that individuals living in food deserts had lower intakes of fruits and vegetables and higher intakes of sugar-sweetened beverages compared to those living in areas with better food access. The food environment is also associated with health outcomes related to the Western diet. People living in food deserts are more likely to have poorer health outcomes due to limited access to healthy food options. A study by Caspi et al. (2012) [45] found that individuals living in food deserts had higher risks for obesity and type 2 diabetes compared to those living in areas with better food access. Understanding these social characteristics is essential for developing effective interventions aimed at promoting healthier dietary habits and reducing the risk of chronic diseases.
5. Western Diet and Antioxidant Status
The Western dietary pattern has been associated with an increased risk of chronic diseases, and one of the mechanisms by which the Western diet may contribute to the development of these diseases is through oxidative stress [46]. Oxidative stress occurs when there is an imbalance between the production of reactive oxygen species (ROS) and the body’s antioxidant defense system (Figure 2). In this process, a free radical can be defined as any molecular species capable of independent existence that contains an unpaired electron in an atomic orbital. The presence of an unpaired electron gives rise to certain common properties that most radicals share. Many radicals are unstable and highly reactive. They can either donate or accept an electron from other molecules, so they behave as oxidizers or reductants [47]. As a consequence of an imbalance between free radical production and antioxidant defenses, oxidative stress is associated with injury to a broad spectrum of molecular species, including lipids, proteins, and nucleic acids [48]. Due to important macromolecules being attacked by free radicals, cell injury and a disruption of homeostasis develop [49]. An antioxidant is a molecule that is stable enough to donate an electron to a free radical and neutralize it, thereby reducing its destructive potential [50]. These antioxidants delay or prevent cellular injury primarily due to their ability to neutralize free radicals. These low-molecular-weight antioxidants can interact with free radicals and terminate the chain reaction without causing injury to vital molecules. Some of these antioxidants, such as glutathione, ubiquinol, and uric acid, are produced by the body’s normal metabolism [51]. There are additional milder antioxidants in the diet. Although there are numerous enzyme systems in the body that neutralize free radicals, the most important micronutrient (vitamin) antioxidants are vitamin E (tocopherol), vitamin C (ascorbic acid), and beta-carotene. Because the body cannot produce these micronutrients, they must be consumed [52]. In summary, antioxidants can neutralize ROS and prevent oxidative damage. However, the Western diet is generally low in these antioxidant-rich foods and high in pro-oxidant substances such as omega-6 fatty acids and advanced glycation end products (AGEs) [53].
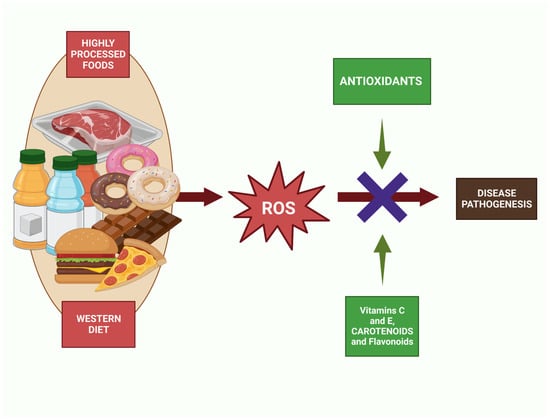
Figure 2.
Antioxidant action against the increase in ROS caused by the intake of foods that follow Western diet patterns such as sugary drinks, pastries, or fast food.
Since the diet type analyzed in this paper is deficient in antioxidants, the problem is exacerbated [54,55].The main sources of dietary antioxidants are fruits, vegetables, whole grains, and nuts. However, the Western diet is deficient in these foods, which means that individuals following this dietary pattern are more likely to have low levels of antioxidants [56]. Studies have shown that individuals following the Western diet have lower levels of the previously mentioned vitamin C, vitamin E, and beta-carotene than those consuming a healthy diet [57,58]. For example, a study conducted on a sample of adults in the United States found that those following the Western diet had lower serum levels of vitamin C and vitamin E than those following a healthy diet [59]. Similarly, a study conducted on Australian adults found that those consuming a Western diet had lower serum levels of beta-carotene than those consuming a healthy diet [60].
Several studies have highlighted the importance of a diet rich in antioxidants for maintaining optimal health (Figure 2) [61]. A high intake of fruits and vegetables, which are rich sources of antioxidants, has been associated with a reduced risk of chronic diseases, [62] including cardiovascular disease, cancer, and neurodegenerative disorders [63,64]. In contrast, a Western diet that is low in fruits, vegetables, and whole grains and high in saturated fats, sugar, and processed foods has been linked to a higher risk of chronic diseases. A study conducted in the United States found that individuals consuming a Western diet had a higher risk of heart disease, diabetes, and stroke than those consuming a healthy diet [65]. Furthermore, the Western diet not only lacks essential antioxidants but also contains pro-oxidant compounds that increase the production of free radicals and oxidative stress in the body [66]. A study conducted on a sample of healthy adults found that consuming a high-fat meal induced oxidative stress, increased inflammation, and decreased antioxidant capacity in the body [67]. The study also found that consuming a meal rich in fruits and vegetables before the high-fat meal reduced oxidative stress and inflammation and improved antioxidant capacity.
Studies have found that the Western diet is associated with lower antioxidant status in the body, as measured by levels of antioxidant enzymes and biomarkers [68]. This may contribute to the development of chronic diseases, as low antioxidant status has been linked to increased oxidative stress and inflammation [69]. Therefore, improving antioxidant status through dietary changes may have potential health benefits, particularly in individuals with a high intake of the Western diet. One study found that increasing the consumption of fruits and vegetables, which are high in antioxidants, was associated with a lower risk of mortality from cardiovascular disease and cancer [70]. Another study found that supplementation with antioxidant vitamins reduced the risk of cardiovascular events in high-risk individuals [71].
Moreover, the Western diet may exacerbate oxidative stress in the body by reducing the activity of endogenous antioxidants such as glutathione and superoxide dismutase (Figure 2) [72]. Oxidative stress occurs when the body’s antioxidant defenses are overwhelmed by the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS). These free radicals can damage DNA, proteins, and lipids, leading to cell dysfunction and death [73,74,75]. Oxidative stress has been implicated in the pathogenesis of numerous diseases, including cancer, neurodegenerative disorders, and cardiovascular disease [76]. The Western diet is a major contributor to oxidative stress [73,74,75]. High intake of processed foods and sugar-sweetened beverages has been linked to increased production of ROS and RNS (Figure 2) [10]. These foods are often high in refined carbohydrates and saturated and trans fats, which can promote inflammation and increase the production of ROS and RNS [77]. Red and processed meat, another hallmark of the Western diet, are also associated with oxidative stress. The heme iron in red meat can catalyze the formation of ROS, leading to oxidative damage [78]. Additionally, processed meats contain nitrates and nitrites, which can react with other compounds in the body to form RNS [79]. Refined grains, another staple of the Western diet, are low in fiber and other important nutrients, which can increase inflammation and oxidative stress [80]. High intake of refined grains has been associated with increased levels of markers of oxidative stress in the blood [81].
In a study conducted on overweight and obese adults, the consumption of a Western diet for eight weeks was found to reduce the activity of superoxide dismutase and increase oxidative stress markers in the body [82]. These findings suggest that a Western diet may increase the risk of chronic diseases by promoting oxidative stress and reducing the body’s ability to defend against it. Low levels of antioxidants can have significant implications for health. Oxidative stress, which results from an imbalance between free radicals and antioxidants in the body, can damage cells, proteins, and DNA. This damage can lead to chronic diseases such as cancer, cardiovascular disease, and neurodegenerative diseases [83]. In addition, low levels of antioxidants have been associated with an increased risk of inflammation and oxidative damage to the body’s tissues [76].
In addition to consuming a diet rich in antioxidants, other lifestyle factors can impact antioxidant status. Physical activity has been shown to increase antioxidant capacity in the body, reduce oxidative stress, and improve overall health [84]. It was found that individuals who engaged in regular physical activity had higher levels of antioxidant enzymes, such as superoxide dismutase and glutathione peroxidase, compared to sedentary individuals [85]. Additionally, smoking and alcohol consumption have been associated with decreased antioxidant levels in the body and increased risk of chronic diseases [86,87].
In conclusion, the Western diet is associated with a lower antioxidant status compared to healthy dietary patterns, and this may increase the risk of chronic diseases. A diet rich in fruits, vegetables, whole grains, nuts, and legumes can provide essential nutrients, including antioxidants, that are critical for optimal health. Therefore, it is recommended that individuals adopt a healthy dietary pattern that emphasizes plant-based foods and limits the intake of processed and high-fat foods to promote good health and reduce the risk of chronic diseases.
6. Western Diet and Inflammation
High consumption of saturated fats, processed foods, and refined sugars is characteristic of the Western diet and has been associated with persistent low-grade inflammation. Critical in the body’s defense against infections and injuries, inflammation is a complicated process involving the immune system [88]. Specifically, chronic inflammation can lead to the development of several chronic diseases, such as cardiovascular disease, type 2 diabetes, and cancer (Figure 1) [89,90]. In this regard, research has demonstrated that Western diet patterns, which are also associated with a high intake of red and processed meats, refined cereals, and caffeinated beverages, promote elevated levels of C-reactive protein (CRP), a well-established marker of inflammation according to previous research [91]. Additionally, authors have also reported that the Western diet was associated with increased levels of CRP and other inflammatory markers, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) [92]. In this case, the WD results in a persistent metabolic inflammatory state [66].
Multiple mechanisms, including oxidative stress, intestinal microbiota dysbiosis, and immune dysregulation, have been also shown to contribute to the pro-inflammatory effects of a Western diet (Figure 1). When there is an imbalance between the production of reactive oxygen species (ROS) and the body’s ability to detoxify, oxidative stress occurs [74,93]. Thus, saturated and trans fats, which can increase ROS production and impair antioxidant defenses, are the main promoters of oxidative stress creation [73]. This can result in damage to cells, tissues, and organs, and the release of pro-inflammatory cytokines, such as IL-6, and TNF-α [94]. ROS are highly reactive molecules that are formed as a byproduct of cellular metabolism, and their production is tightly regulated in the body. However, oxidative stress conditions injure cells and tissues [95]. This damage can contribute to the development of chronic diseases such as cardiovascular diseases, diabetes, and cancer [95].
In addition to providing evidence along these lines, rodent studies may be pertinent to dietary changes in humans. Concretely, recent studies found that the consumption of a high-fat diet led to increased production of ROS in rats [96]. It was also shown that antioxidants lose their efficacy in an inflammatory state, making this a progressive, hard-to-control rise in oxidative stress [97]. These findings suggest that the consumption of a Western diet may contribute to the development of chronic diseases through increased ROS production. Similarly, processed and fried foods, baked goods, and high-fat dairy products have been shown to increase LDL cholesterol levels and contribute to the development of cardiovascular diseases [98]. Thus, high-fat diets are particularly harmful, as they not only increase LDL cholesterol levels but also decrease HDL cholesterol levels, leading to an unfavorable lipid profile [98].
Given the preceding, all of the evidence indicates that high-fat diets result in immune dysregulation. Specifically, low-grade inflammation is promoted by an imbalance in the actions of the immune system. In the Western diet, sugar and fatty acids can either be pro-inflammatory—such as omega-6—and present in high numbers, or they can be anti-inflammatory—such as omega-3 or antioxidants—and present in lesser proportions [99]. This can promote the activation of immune cells, such as macrophages and T cells, and the release of pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α [100]. The consumption of high levels of saturated and trans fats found in the Western diet can activate Toll-like receptors (TLRs) on immune cells, leading to the production of pro-inflammatory cytokines and chemokines [101]. TLRs are important mediators of inflammatory pathways that detect pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) and initiate an immune response [102]. In response to a Western diet, the TLR pathway is activated by the high levels of endotoxins and other pro-inflammatory molecules present in the diet. This activation leads to the production of pro-inflammatory cytokines, such as TNF-α, IL-6, and interleukin-1 beta (IL-1β), which are involved in the pathogenesis of chronic diseases [101].
To continue, it is relevant to note that the method of cooking is also thought to alter or may affect metabolic processes. In relation to this, advanced glycation end products (AGEs)—compounds created when food is cooked at very high temperatures—are abundant in the Western diet. When AGEs bind to the RAGE receptor, pro-inflammatory cytokines are released [103]. AGEs are a group of complex molecules formed by the non-enzymatic reaction between reducing sugars and amino acids or proteins. This reaction is known as the Maillard reaction and occurs naturally in the body as part of normal metabolism, but also through external sources such as cooking methods, particularly high-temperature cooking, and the processing of food [104]. AGEs are known to accumulate in tissues over time and contribute to the development of various diseases [105,106]. AGEs are formed through the non-enzymatic reaction between reducing sugars and amino acids and are present in high levels in processed foods, particularly those that are high in fat and sugar [103]. The Western diet is high in AGEs due to the consumption of processed and refined foods, which are often cooked at high temperatures, leading to the formation of AGEs [107]. These AGEs can be found in various foods, including meat, dairy, and baked goods, which are staple items in the Western diet [103]. It was found that participants who consumed a high-AGE diet for four weeks had increased markers of inflammation and oxidative stress, which are known to contribute to the development of chronic diseases such as diabetes and cardiovascular diseases [108]. AGEs can activate the receptor for advanced glycation end products (RAGE), which is expressed on the surface of many cell types, including endothelial cells, macrophages, and adipocytes. RAGE activation leads to the production of pro-inflammatory cytokines and oxidative stress, which are involved in the pathogenesis of chronic diseases [103].
Regarding gut diseases, dysbiosis, or an imbalance of the intestinal microbiota, has been linked to the Western diet and may contribute to inflammation. Dysbiosis of the gut microbiota describes a state of discord between the microbes normally found in the digestive tract and their host. The Western diet lacks the fiber and prebiotics necessary to foster the development and variety of good gut flora [109]. Instead, the diet is high in fat, sugar, and salt, which can promote the growth of harmful bacteria and fungi. This can result in gut inflammation, increased gut permeability, and the release of bacterial endotoxins, such as lipopolysaccharides (LPS), into the bloodstream. These endotoxins can activate the immune system and promote the release of pro-inflammatory cytokines, such as IL-1β and IL-18 [110]. Previous authors found that the Western diet was associated with decreased gut microbial diversity and increased levels of pro-inflammatory bacteria, such as Proteobacteria [111]. The dysbiosis induced by the Western diet can lead to increased intestinal permeability, allowing bacterial endotoxins to enter the bloodstream and trigger inflammation [112].
7. The Effect of Nutrition and the Western Diet on the Intestinal Microbiota
Based on the above, our knowledge reveals that eating a lot of processed, high-calorie meals is not only associated with an increased risk of developing conditions including diabetes, obesity, heart disease, but also gut dysbiosis. Concretely, the high meat consumption related to the Western diet increases Bacteroides, Alistipes, and Bilophila (associated with pathological processes such as atherogenesis) and decreases Bifidobacterium, Roseburia, Eubacterium, and Ruminococcus [113]. Additionally, the Firmicutes/Bacteroidetes ratio has been frequently considered a plausible marker for obesity over the past decade and a parallel of Western diet patterns [114]. In this regard, the abundance of Firmicutes in the gastrointestinal microbiota of healthy individuals ranges from 11% to 95%, while that of Bacteroidetes ranges from 0.6% to 86.5%. However, Kasai et al. reported more accurately that Firmicutes comprised 37.0 ± 9.1% of non-obese and 40.8 ± 15.0% of obese individuals, whereas Bacteroidetes comprised 44.0 ± 9.8% of non-obese and 37.0 ± 14.0% of obese individuals [115]. These alterations in bacterial composition and/or diversity are typically associated with alterations in the microbiota’s metabolic profile, which also impact host health [116]. However, the relative abundance of the phyla Firmicutes and Bacteroidetes is highly variable among members of the same population [117]. This is likely due to the influence of numerous harmful lifestyle factors, such as the Western diet [118], low physical activity, dietary additives and contaminants, or antibiotic consumption. This may explain the contradictory results observed when comparing the microbiota of normal-weight and obese subjects, making it challenging to correlate the ratio of Firmicutes to Bacteroidetes with a particular health status [117]. Although the intestinal microbiota may contribute to the development of obesity, there is insufficient evidence to support a link between obesity and changes in the Firmicutes/Bacteroidetes ratio [119].
In addition, the combination of a high intake of fat and sugar with a low intake of fiber, fruits, and vegetables is associated with chronic inflammation. Consequently, inflammation is an important underlying mechanism connecting the Western diet to chronic diseases [120]. Regarding this, Bolte et al. demonstrated through long-term diet protocol research that higher abundances of Firmicutes, Ruminococcus species of the Blautia genus, and endotoxin synthesis pathways were consistently associated with processed and animal-derived diets. In contrast, plant and fish diets were found to be positively associated with short-chain fatty acid-producing commensals and nutrient metabolism pathways [121].
Similarly, authors have suggested that the Western diet promotes inflammation in the body by increasing the levels of pro-inflammatory molecules such as cytokines and C-reactive protein [122]. Furthermore, the intake of saturated and trans fats, as well as refined carbohydrates, typical in this diet may trigger the release of inflammatory mediators [123]. In contrast, a Mediterranean diet, rich in fruits and vegetables, whole grains, and lean protein sources such as fish, can help reduce inflammation and protect against chronic diseases [124]. Illescas et al. found in a meta-analysis that diets and gastrointestinal maladies are linked to inflammation and cancer, highlighting the distinctive characteristics of the bacterial population associated with the Mediterranean diet. In particular, the microbiota of subjects following a MD was enriched with pro-anti-inflammatory bacteria. For the first time, studies revealed an increase in Akkermansia and a decrease in Fusobacterium with a MD, even below the levels observed in healthy individuals with no defined diet without a diet plan. Fusobacterium is a known pathogenic bacterium associated with malignancy, whereas Akkermansia is indicative of a healthy gastrointestinal tract [125]. One of the main reasons for this is the anti-inflammatory capacity provided by the antioxidants in this diet. These foods are high in antioxidants, which can neutralize free radicals and prevent oxidative damage to cells. Additionally, they contain anti-inflammatory compounds, such as polyphenols and omega-3 fatty acids, which can help reduce inflammation in the body [126].
Dysbiosis and the process of chronic inflammation, as well as the profile and nutritional quality of these typical Western foods, occur simultaneously. Intestine dysbiosis is a condition that occurs when the natural equilibrium of microorganisms in the intestine is disturbed, resulting in an overgrowth of harmful bacteria or a reduction in beneficial bacteria [127]. The foods we consume provide not only the essential nutrients required for our health but also create an environment for the growth and sustenance of gut bacteria. The composition of our diet plays a significant role in determining the type of bacteria that flourish in our gut. As the importance of gut microbiota in maintaining good health has been recognized, there has been growing interest in studying the impact of diet on gut microbiota [111,128,129,130]. Specifically, the Western diet model is hypothesized to increase proinflammatory cytokines, disrupt the epithelial barrier, and alter the intestinal microbiota (with an increase in Bacteroides and Enterobacteriaceae and a decrease in Bifidobacterium and Lactobacillus), thereby promoting low-grade chronic inflammation in the gut [131].
One of the groups of foods that seem to have a greater impact on the intestinal microbiota and that receive the most attention in the scientific literature are ultra-processed foods [132]. These comprise food products made from substances extracted from whole foods that are then combined with various additives, such as preservatives, flavorings, and sweeteners [133]. Ultra-processed foods, such as soft drinks and savory snacks, are highly appealing due to their long shelf-life, affordability, and convenience for consumption anytime and anywhere [134]. Their consumption is alarmingly high in many high-income countries. For instance, in France [135], ultra-processed food contributes to 29.1% of total energy intake, while in Australia and the United States, it accounts for 42% and 57.9% [136], respectively. The negative effects on the gut microbiota [6] have been studied by authors such as Zinöcker MK and Lindseth IA. Authors suggest that ultra-processed foods that contain high levels of added sugars, unhealthy fats, and sodium alter the gut microbiota, leading to a reduction in beneficial bacteria and an increase in harmful bacteria. Moreover, these foods are low in fiber, which is a critical nutrient that promotes the growth of beneficial gut bacteria. In this regard, a recent meta-analysis concluded that vegetable consumption was associated with a lower risk of ulcerative colitis alone, whereas fruit consumption was associated with a lower risk of both Crohn’s disease (CD) and ulcerative colitis [137]. However, authors also suggest that the emulsifiers and additives used in the production of ultra-processed food may have harmful effects on gut microbiota, including inflammation and increased permeability of the gut lining. Additionally, some studies suggest that the consumption of ultra-processed foods may lead to changes in brain activity and behavior, including addiction-like behaviors, which can contribute to overconsumption and further health problems [138]. Analysis of the feces of adults consuming aspartame and acesulfame K in a recent cross-sectional clinical study with 31 participants did not reveal an increase in bacterial abundance, but rather a decrease in bacterial diversity compared to non-consumers. As mentioned, the data may be inconsistent; the median Bacteroidetes:Firmicutes ratio did not change in seven participants who consumed 1.7–33.2 mg/day of acesulfame K for four days, but bacterial diversity statistically differed from that of non-consumers [139,140].
In contrast, vegetarian or plant-based diets may be beneficial to the gut microbiome [141]. Dietary polyphenols are plant-based natural chemicals found in vegetables, grains, fruits, coffee, and tea [142]. These organic molecules are a complex and heterogeneous class of substances characterized by hydroxylated phenyl groups, which are present in very small concentrations in the Western diet [143]. They are frequently divided into flavonoids and non-flavonoids according to their chemical structure and complexity. The influence of dietary polyphenols on gut ecology and the mechanisms behind the effects on intestinal and extraintestinal illnesses has recently been explored. Various research works have discovered antioxidant, antidiabetic, anticarcinogenic, neuroprotective, anti-inflammatory, cardioprotective, antibacterial, antiadipogenic, and other properties in various dietary polyphenols [144].Concretely, epidemiological studies have found links between the consumption of polyphenol-rich foods such as vegetables and drinks and illness prevention. These results have been seen in certain types of cancer, cardiovascular disease, type 2 diabetes, osteoporosis, pancreatitis, gastrointestinal problems, lung damage and neurodegenerative diseases [145].
Although the gut microbiota can be widely utilized as a tool for food safety assessment, more research is needed to better understand the relationship between the gut microbiota and food consumption. While there is mounting evidence for the potential link between gut microbiota and health outcomes, further studies are necessary to establish a causal relationship.
8. Western Diet and Mitochondrial Fitness
Individuals’ diets consist of mixtures of foods and beverages (referred to as foods for simplicity). However, the precise combination of foods that constitutes a healthy diet is context-dependent and influenced by several cultural, economic, and other variables [146]. In recent decades, global food habits have shifted towards increasingly Westernized and less healthy diets. Based on the World Health Organization’s (WHO) worldwide nutrition report, while a portion of the world’s population suffers from starvation, the other portion suffers from obesity and its accompanying problems [147]. With the right proportion, content, quantity, and presence of macronutrients, micronutrients, and bioactive chemicals, a balanced diet counteracts these severe situations. Unfortunately, little is understood about how these components affect human health. These nutrients destined to nourish our bodies, tissues, and cells must first reach the mitochondria, where they are broken down into CO2 and H2O to produce energy. Mitochondria are the cell’s powerhouse and are primarily responsible for nutrition metabolism [148]. Nevertheless, they are also the primary cause of oxidative stress and apoptosis-mediated cell death [149]. However, mitochondrial performance might be “improved” by physical activity, which improves their integrity, efficiency, and dynamic adaptability to stressors, thus, “mitochondrial fitness” [150]. Mitochondria are extremely dynamic organelles, and their bioenergetics are directly linked to the fusion/fission equilibrium. Fusion activities relate to the enhancement of mitochondrial function, whereas fission processes are associated with the elimination of dysfunctional mitochondria. These processes are influenced by the consumption of a variety of foods, which gives cells several stimuli. Concretely, they are influenced by the energy substrate availability caused by food quality and diet timing. In this regard, it is well acknowledged that different dietary fat sources have distinct effects on the dynamics and bioenergetics of the mitochondria, including mitophagy (Figure 3) [6]. For instance, obesity-related disorders such as insulin resistance and non-alcoholic fatty liver disease are greatly influenced by mitochondrial dysfunction [151].
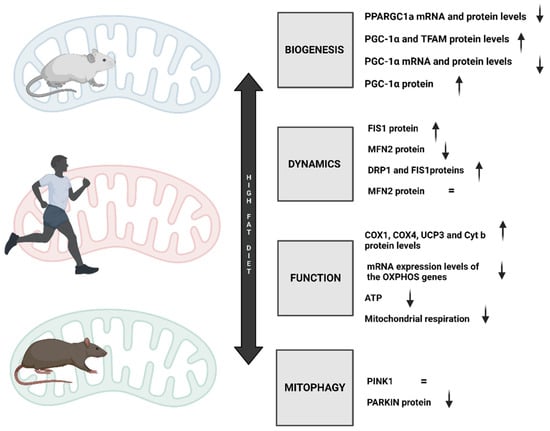
Figure 3.
Changes in the most essential mitochondrial processes, such as mitochondrial functioning, dynamics, biogenesis, and mitophagy, as a result of consuming a diet rich in typical Western foods.
The traditional Western diet (WD) pattern’s dietary traits are specifically a high intake of saturated fats and omega-6 fatty acids, a decreased intake of omega-3 fats, an abundance of processed carbohydrates, and an overuse of salt [8]. Drinks account for 47% of the added sugars in this surplus, which amounts to more than 13% of the daily calorie intake [7]. Thus, considering the WD as a high-fat diet (HFD), one of the most important pathophysiological symptoms of HFD is insulin resistance, which causes obesity, among other diseases [7]. One of the well-explained pathophysiological changes that an HFD promotes is impaired mitochondrial biogenesis (Figure 3). Concretely, excessive calorie consumption causes tissue hypertrophy and adipocyte hyperplasia in white adipose tissue [152]. The subsequent manifestations increase lipolysis in fat cells and ultimately result in raised amounts of free fatty acids (FA) in circulation. Enhanced lipid catabolism and energy production via the Krebs cycle have already been established as outcomes of increased FA oxidation in the mitochondria [152]. The adipocyte-secreted hormones adiponectin, leptin, acylation-stimulating protein, and resistin play a crucial role in regulating mitochondrial biogenesis and insulin sensitivity [153,154]. In this regard, adiponectin physically binds to its receptors during HFD intake, activating AMP-activated protein kinase (AMPK), which ultimately stimulates glucose uptake and FA oxidation. Moreover, AMPK has been linked to the control of PGC-1α, the principal regulator of mitochondrial biogenesis [155]. According to studies on animals, PGC1 α mRNA and protein levels in skeletal muscle were likewise decreased in C57Bl/6 mice fed an HFD for three weeks [156]. Diastolic dysfunction and poor mitochondrial function were associated in a different investigation. Nevertheless, PGC-1 and mitochondrial transcription factor A (TFAM) protein levels were elevated in the gastrocnemius muscle of male and female Wistar rats that were 2 months old and fed an HFD for 26 weeks. The same study hypothesized that as male rats showed a larger rise in PGC-1 α and TFAM levels than female rats, the effects of an HFD on mitochondrial biogenesis may be sex-dependent [154]. As previously mentioned, a prolonged HFD affects the availability of substrates that mitochondria can use; as a result, mitochondria need to adapt their molecular machinery to generate ATP effectively and sufficiently, especially in high-energy consuming tissues, such as the brain and myocardium. In HFD-fed (60% kcal) rats, Chen et al. reported that reduced complex I-III and citrate synthase activities have been found, which are significant findings that support compromised cardiac function. Similarly, rats showed decreased mitochondrial respiration activities [157], which also supports compromised cardiac function. Likewise, it has been demonstrated that feeding male Wistar rats an HFD for 4 weeks increases their levels of OXPHOS-related proteins such as cytochrome c oxidase (COX)I, COXIV, uncoupling protein (UCP) 3, and cytochrome b [158]. Despite the fact that some studies have shown an increase in the OXPHOS complex, perhaps as a compensatory mechanism to enable mitochondria to produce more energy, an HFD appears to impair mitochondrial function, affecting cellular energetics and ATP turnover [152].
Although an HFD has been shown to lead to insulin resistance, impaired mitochondrial function, and increased production of reactive oxygen species (ROS), much more needs to be known about the dysregulated expression profile of proteins involved in mitochondrial dynamics. Regarding the proteins involved in mitochondrial dynamics, an HFD diet caused mitochondrial fusion protein 2 (MFN2) to drop and proteins involved in fission processes to rise (Drp1 and FIS1) [159]. In this regard, Chen et al. also confirmed that male Sprague Dawley rats fed an HFD (60 percent kcal) showed increased mitochondrial FIS1 levels [16]. Consistent with this, it was also found that healthy male C57BL/6 mice that were 4 weeks old and fed an HFD for 16 weeks showed reduced relative expression of MFN2, but higher levels of mitochondrial DRP1 expression [160]. Lionetti et al., according to electron microscopy and immune activity analysis, suggested that this type of diet also caused a shift towards fission in skeletal muscle. These results are consistent with earlier reports that type 2 diabetic patients and obese Zucker rats have decreased MFN2 expression in their skeletal muscles [159]. Furthermore, in the work of Jheng et al., differentiated C2C12 skeletal muscle cells exposed to saturated fatty acids have been shown to trigger fission processes in vitro that are linked to mitochondrial dysfunction [161]. In addition, high-glucose (HG) treatment has been linked to mitochondrial fragmentation and an increase in fission processes in myoblasts and a rat liver cell line. It has been hypothesized that this fragmentation would increase the total surface area of metabolically active organelles, increasing the accessibility of metabolic substrate to carrier proteins. As a result, it is possible to hypothesize that HFD-induced mitochondrial fragmentation is a cellular adaptation to increase mitochondrial intake and oxidize excess dietary fatty acids, which would lead to increased ROS production [159,162].
Regarding mitophagy during HFD, it has been found that in healthy, non-obese, sedentary males and 10 endurance-trained male runners, the protein content of total PTEN-induced kinase 1 (PINK1) in skeletal muscle was found to be similar between groups before and after a high-fat meal, indicating that mitophagy is not required for metabolic flexibility in the healthy population [163]. However, it has been demonstrated that mice fed an HFD (60 kcal% fat) for two months display mitophagy, which is followed by decreased mitochondrial abundance (mtDNA/nuDNA) [164]. Additionally, a decrease in PARKIN in mitochondria that were supposed to be recruited by PINK1 has been demonstrated [165]. Although mitochondria can readily adapt to exogenous parameters and maintain their proper functionality to generate sufficient energy for cellular demands, these organelles are sensitized to variations in substrate availability due to different dietary habits, followed by molecular rewiring. This orchestrates alterations in mitochondrial function, regulation of biogenesis and mitophagy, and ‘self-recycling’ via fusion and fission in mitochondria. In conclusion, a high-fat diet impairs mitochondrial function and fusion while inducing fission.
9. The Effect of Western Diet on Cardiovascular Health
In Western societies, cardiovascular disease (CVD) is the leading cause of death, with coronary heart disease (CHD) accounting for more than 50% of cases. Numerous risk factors, including family history, diabetes, hypertension, obesity, smoking, and inactivity, contribute significantly to the overall cardiovascular risk (Figure 4). Recent data indicate an interesting gradient in the incidence, morbidity, and mortality of cardiovascular disease across the socioeconomic status spectrum, as defined by educational level, occupation, or income [166]. Personal characteristics, such as physical inactivity, smoking, stress, and alcohol consumption, only account for 13.6% of the SE differences in CVD morbidity and 19.5% of the SE differences in CVD mortality (Figure 4) [167]. Despite the above-mentioned established facts, dietary interventions are much less frequently used in the management of cardiovascular disease than pharmacological and procedural interventions. Nutritional guidelines recommend eating a diet high in fish [168], whole grains [169], vegetables and fruits [170], legumes [171], and nuts to reduce the risk of developing atherosclerotic cardiovascular disease (ASCVD). To lower the risk of ASCVD, dietary monounsaturated and polyunsaturated fats should be substituted for saturated fat, along with sodium, cholesterol, processed meats, refined carbohydrates, and sweetened beverages [172].the consumption of Western-type diets (WDs), which exclude fiber, vitamins, and minerals, has increased over the past few decades in Westernized societies. WDs include processed foods, “fast food”, convenience foods, snacks, and sugary soft drinks (Figure 4). With the spread of these food items and their consumption from high- to low-income countries, there has also been a parallel rise in diseases, as mentioned for CVD, linked to the Western diet [77,173].
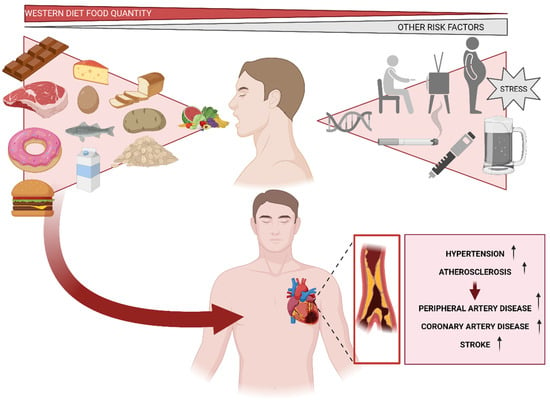
Figure 4.
Cardiovascular risks associated with a high-fat diet and other relevant factors that may influence their development, such as genetics, obesity, sedentary lifestyle, diabetes, and alcohol or tobacco consumption.
A variety of conditions affecting the heart and blood vessels are referred to as CVD, including hypertension, strokes, atherosclerosis, peripheral artery disease, and vein diseases [174]. The combination of unhealthy nutritional habits and habits typical of Western societies creates an environment that is highly prone to cardiovascular system failure (Figure 4) [175,176]. Concretely, ASCVD is an inflammatory disease that contributes significantly to the incidence and mortality of CVD. Libby, on the one side [177], and Mozzaffarian et al. [178], on the other, pointed out in a study based on atherosclerosis therapies that oxidative stress and systemic inflammation are modifiable by nutrition; however, excessive energy intake and physical inactivity contribute to the secretion of pro-inflammatory cytokines [179]. Inflammatory processes involve the subendothelial region of the arterial wall, where lipids and macrophages loaded with lipids accumulate [180]. Concretely, Virmani et al. specified that early stages of atherosclerosis are characterized by the internalization of lipids in the intima, primarily low-density lipoproteins (LDL), which results in endothelial dysfunction. Endothelial dysfunction disruption promotes inflammatory process, emboli, and multiple pathological outcomes, along with calcifications, stenosis, burst, and hemorrhage. Advanced atherosclerosis manifests clinically as coronary heart disease, ischemic stroke, peripheral artery disease, heart failure, or sudden death [181,182]. However, Yubero-Serrano’s findings demonstrate that the Mediterranean diet, especially in CHD patients with severe endothelial dysfunction, controls endothelial function better than a low-fat diet and is linked with a better balance of vascular homeostasis [183].
Current scientific evidence demonstrates that chronic inflammation plays an important role in the pathogenesis of coronary artery disease (CAD), including the initiation and progression of atheroma plaque and rupture, as well as post-angioplasty and restenosis. C-reactive protein (CRP), interleukin (IL)-1, IL-6, IL-8, IL-1, IL-18, monocyte chemoattractant protein (MCP)-1, and tumor necrosis factor (TNF α), among others, are the primary mediators of CAD development. Additionally, the results of Usui et al.´s study show that IL-17 is crucial for the emergence of moderate atherosclerosis and shed new light on IL-17’s function in the pathogenesis of atherosclerosis [184]. Moreover, these mediators are considered potential biomarkers of inflammation, and their expression may be correlated with the severity of coronary artery disease [185,186]. Oikonomou et al. provided further evidence assessing 118 stable symptomatic patients and concluded that the Western diet (increased intake of fat, red meat, and carbohydrates and low consumption of fruits and green leafy vegetables) was predictive of severe CAD [187].
Well-established knowledge suggests that Western dietary patterns, in comparison to healthier dietary patterns such as the “Mediterranean diet” (MeDiet), are associated with an increase in the production of proinflammatory cytokines and a decrease in the production of anti-inflammatory cytokines [188,189,190]. Therefore, greater adherence to healthier eating habits, which include consuming more fruits, vegetables, legumes, nuts, and whole grains, may lessen low-grade inflammation and thereby prevent cardiovascular disease [191,192]. A cohort study carried out by Gao et al. identified food-based dietary patterns that operate through excess energy intake and explained high variability in energy density, free sugars, saturated fat, and fiber intakes to investigate their association with total and fatal CVD and all-cause mortality [193]. High intakes of chocolate and sweets [194,195], butter, and low-fiber bread and low intakes of fresh fruits and vegetables comprised the predominant dietary pattern and were positively associated with total CVD [193]. In contrast, current evidence demonstrates that healthy dietary patterns are characterized by a high intake of fiber, antioxidants, vitamins, minerals, polyphenols, and monounsaturated and polyunsaturated fatty acids and a low intake of salt, refined sugar, saturated and trans fats, and carbohydrates with a low glycemic load [99,196]. This corresponds to a high intake of fruits, vegetables, legumes, fish and seafood, nuts, seeds, whole grains, vegetable oils (primarily extra virgin olive oil), and dairy foods, and a low intake of sweets, soft drinks, and red and processed meat [197,198]. Concretely, in a recent meta-analysis, which included 86 cross-sectional and 10 cohort prospective studies for a total population of more than 130,000 vegetarians and 15,000 vegans (plant-based diet, complete abstention from meat and meat products, poultry, seafood, and consumption of any other product from an animal), it was found that vegetarians and vegans had significantly lower levels of BMI, total cholesterol, LDL-cholesterol, and glucose than omnivores. Additionally, an analysis of prospective studies showed a 25% pooled significant risk reduction in ischemic heart disease incidence and/or mortality [199,200].
Conversely, the published literature generally does not support statistically significant associations between dietary cholesterol and CVD risk. The heterogeneity in the adjustment for total energy, other dietary components, and serum cholesterol concentrations shows that studies of dietary cholesterol have been conducted over a long time span during which nutritional epidemiology methods have significantly changed. Nonetheless, it has been demonstrated by Zhong et al. that there is a positive correlation between dietary cholesterol and egg consumption and the risk of CVD. According to the authors’ calculations, the risk of CVD increased significantly with every additional half egg consumed each day. However, when dietary cholesterol was taken into account, the link between egg consumption and CVD was eliminated [201]. Table 1 provides further references for all of the above and specifically for the benefits of plant-based or vegetarian diets.

Table 1.
Contributions from references pointing to cardiovascular improvements in individuals following a diet without the Western diet patterns.
Implementing various strategies that resulted in a marked decrease in inflammation diseases and consequently CVD, with an enormous increase in life expectancy as a result, was one of public health’s greatest achievements. However, over the past few decades, more and more populations have been exposed to a Western lifestyle, which is associated with a high consumption of processed foods high in calories, which has resulted in an increase in diseases that are largely preventable and linked to a chronic inflammatory state [7]. In fact, we face a pandemic of diseases linked to lifestyle because more than one third of adults worldwide are considered overweight or obese. As society becomes increasingly health-conscious, healthy dietary patterns may increase. In the upcoming years, formal dietary recommendations by major health organizations will ultimately be guided by newly emerging data highlighting new definitions of a healthy diet and which foods make up this diet. In the coming decades, the current trend towards healthier choices may extend life expectancy and improve cardiovascular outcomes [172,209].
10. The Effect of Western Diet on Mental Health
The bidirectional communication that maintains the microbiota–gut–brain axis is carried out through three main axes, and nervous, immune, and endocrine signaling pathways are involved [210]. This communication is carried out through the transmission of information from neurotransmitters and through the bloodstream, including metabolites, cytokines, and hormones [211]. The nervous system (NS) participates in this essential interaction, as well as the neuroimmune and neuroendocrine systems. The enteric nervous system (ENS) is a structure that is part of the autonomic nervous system (ANS), especially qualified for the regulation of gastrointestinal functions [212]. It is the most complex system of the ANS, and among its most important functions we find the regulation of the esophagus and stomach, and colorectal functions. The latter are intimately associated with digestion and nutrient absorption. In addition, it protects these cellular structures from inflammation of the organs that are part of the digestive system [213]. The ENS is called the second brain, as there are about 100 million neurons in this area, making it the largest presence of these specialized cells outside the brain [214]. Communication with the CNS is through the sensory neurons that provide information about the intestine and are connected by the vagus nerve and the motor neurons that send information to the intestine [215].
In this axis, the microbiota performs regulation of the concentration of bacterial families along the intestine as a protective action, trophic functions, metabolic functions, and nutritional functions. Due to this involvement, the microbiota is associated with the regulation of mood and cognitive functions, so it is possible to affirm its impact on the presence of mental disorders [216]. When there is an alteration in the microbiota, various physiological effects appear, such as systemic inflammation or chronic low-grade inflammation. Systemic inflammation favors neuroinflammation through different very complex mechanisms that maintain a constant interaction [217]. This inflammation is characterized by an increased presence of macrophages in peripheral cellular tissues, and an increased level of inflammatory cytokines in the blood [218]. Similarly, although low-grade systemic inflammation does not in itself cause loss or damage to the functions of the infiltrated tissues, it does facilitate vulnerability to psychopathologies directly associated with CNS functioning [219]. When this inflammation exists, microglia, which are cells that are part of the immune system, are activated, and this activation causes neuroinflammation [220].
In relation to the study of mental disorders associated with alterations in the communication of the microbiota–gut–brain axis, it has been determined that in the presence of stress disorders, patients present elevated levels of proinflammatory cytokines [221]. In this sense, mast cells, important effector cells, translate stress signals and release neurotransmitters and cytokines, and these actions have a negative impact on the patient’s anxiogenic symptomatology [222]. In addition, these patients present microinflammation of the gut mucosa and mast cell hyperplasia, which explains the maintenance of symptoms [223]. This alteration in the intestinal microbiota, called dysbiosis, is associated with the consumption of the Western diet due to its proinflammatory action [224].
Similarly, it is known that patients with mental pathologies present a higher risk of cardiometabolic diseases [225]. The comorbidity of mental disorders with metabolic syndrome (MS) is already a reality, with an incidence of 60% in these patients. Although it was initially thought that MS could appear because of the pharmacology associated with psychopathology, we now know that patients with depressive disorder, anxiety disorder, psychotic disorder, and bipolar disorder who do not take drugs to treat these also have a higher risk of suffering from MS [226,227].
It is precisely for this reason that it is essential to understand that there are pathophysiological elements that function as connecting structures between these pathologies. In psychiatric patients, MS can appear due to the acquisition of unhealthy nutritional habits, as well as the consumption of alcohol, tobacco, irregular sleep behaviors, and sedentary lifestyles [228]. At the molecular level, psychiatric patients seem to present definite characteristics that may make them more vulnerable to MS, for example, a modification in the sensitivity to glucocorticoids, which implies a dysregulation of the hypothalamic–pituitary–adrenal (HHA) axis and is caused by high levels of stress in the organism [229]. This high adrenocortical stimulation favors dyslipidemia, an alteration in the circulation of lipids in the blood [230].
Moreover, the presence of certain pleiotropic genes that seem to be involved in the connection between mental pathologies and cardiac and metabolic conditions has been studied with great interest in recent years [231,232]. These studies have shown that psychiatric patients have an increased risk of MS. This can be explained by the fact that serotonin 2C receptor genes and obesity-associated genes such as leptin are involved in the pathogenesis of both diseases [233,234]. The role of the microbiota has also been studied in terms of its involvement in modulating the production of signals associated with neuronal plasticity [235].
In the case of metabolic and endocrine disturbances and depression, we can see that recent studies have shown that the effects of stress act on the response of the hypothalamus by releasing corticotropin and vasopressin, which causes corticotropin-bearing neurons to project to noradrenergic centers and stimulate the spinal cord [236]. This promotes the action of sympathetic branch neurons through the stimulation of α1-adrenergic receptors and, in turn, this stimulates corticotropin release in the hypothalamus, creating a positive bidirectional feedback loop [237]. In recent years, research on intestinal microorganisms that can cause neurological problems and psychopathologies has been increasing, thanks to the knowledge we now have about the functioning of the bidirectional microbiota–intestine–brain axis [238]. Previous studies have been able to show that in patients with depression, there are two types of bacteria whose levels are low or even non-existent, Dialister and Corproccus. In this sense, it is known that certain nutrients have an antidepressant action; among the most important, we find the long-chain omega-3 fatty acids (EPA and DHA), magnesium, potassium, iron, and vitamins B6, B12, A, and C [239,240].
Currently, the incorporation of prebiotic and probiotic products in the diet is presented as a beneficial strategy for people at risk of suffering a mental disorder or people who have been diagnosed with a psychopathology [241,242,243]. In fact, these foods are now considered to be psychobiotics. Prebiotics are products that nourish the growth of some beneficial bacterial species such as lactobacilli and bifidobacteria. Starch, which promotes insulin sensitivity and mobilizes fats in the process of obtaining energy, is also present in this group [244,245].
Similarly, inulin and galacto-oligosaccharides (GOS), in addition to improving the cholesterol profile presented in animal [246] studies, increase the attentional level as well as improve cognitive processing. In addition, they decrease cortisol levels, which has a positive impact on the presence of mood disorders. On the other hand, the Lactobacillus Casei Shirota family of bacteria can inhibit the proliferation of harmful bacteria, in addition to increasing the immune response [247]. In its interaction in the gut–brain axis, it reduces anxiety levels, decreases chronic fatigue, and improves mood [248,249].
With regard to probiotics, the formula Lactobacillus helveticus and Bifidobacterium longum results in a significant reduction in psychological anxiety, improved emotional well-being, and a reduction in the vagus activation of the sympathetic branch [250,251]. Another formulation that has beneficial effects on mental health are species-specific formulations of lactobacilli and bifidobacteria, called friendly bacteria, a group of saccharolytic bacteria that produce short-chain fatty acids such as lactate and acetate [252,253]. This composition has been shown to reduce the symptoms associated with depression and negative and aggressive emotions [254].
After reviewing the Western diet, it is possible to affirm that its effects on general health, and more specifically on mental health, are not beneficial. The food composition of this diet, rich in processed foods, red meat, and saturated fats among others, as well as the lack of fiber and vitamins, makes the body vulnerable to the presence of mental pathologies. Among the most important are mood disorders in the face of chronic low levels of inflammation, which appear as a defense and repair mechanism in the face of intestinal dysfunction. Systemic inflammation increases the circulation of inflammatory cytokines and the passage of macrophages in cellular tissues, which facilitates the activation of other dendritic cellular inflammation processes that can be found in neurodegenerative diseases, although it remains to be confirmed whether this inflammation is a cause or effect of the pathology.
11. The Effect of Western Diet on Metabolism
Diabetes, including hyperinsulinemia and insulin resistance, obesity, hyperlipidemia, and cardiovascular disease have been extensively linked to the Western diet [10,77].Recent literature has highlighted how the Western diet may contribute to systemic inflammation enhancement, due to the large amount of fats present in the Western diet, as well as it effects triggering oxidative stress [255].
Considering both the quantity and quality of macronutrients, it is intriguing to note how the Western diet may affect general health in this passage. In terms of quantity, the Western diet has been associated with increased intakes of ultra-processed foods, such as sweets, soft beverages, processed meats, refined potato and maize products, and high-fat dairy products, as well as increased intakes of animal fats. In addition, it has been noted that the Western diet was associated with a lower ingestion of unprocessed fruits, vegetables, seeds, and whole cereals, as well as fish [12,197,256,257]. With regard to quality aspects, the Western diet has been related to elevated saturated and omega-6 polyunsaturated fatty acid consumption as well as to a decreased omega-3 polyunsaturated fatty acid intake, which may compromise cardiovascular condition [258,259]. However, Nicholls et al. note that not all evidence points in the same direction; in patients with a high cardiovascular risk, the addition of omega-3 to standard background regimens did not result in a significant difference in the composite outcome of severe adverse cardiovascular events when compared to maize oil. These results do not support the use of this omega-3 fatty acid formulation to prevent severe cardiovascular adverse events in patients at high risk [260].
Moreover, regarding a lower fruit and vegetable intake, the latest literature proposed that this could be related to a decreased fiber and antioxidant exposition, which is present in this type of food, avoiding their benefits for the general health status [261]. Similarly, the recent literature proposed that reduced fruit and vegetable consumption has been inversely associated with metabolic syndrome, supporting the protective role of these foods in overall health management [262]. In a study by Li et al. combining a fruit and vegetable diet with physical activity(PA), metabolic syndrome (MS) was prevalent in 28.7% of participants, with prevalence rates of 24.7% in men and 32.9% in women. Compared to participants with inadequate PA and inadequate FV intake, those with adequate PA and adequate FV intake had the lowest risk of MS [263].
In order to improve the comprehension of the Western diet in health, it is necessary to understand the key role of inflammation, which may contribute enhancing the pathogenesis of metabolic syndrome [264]. MS, also known as insulin resistance syndrome or syndrome X, is a complex pathology due to the phenotypic profile that presents with wide variations and is associated with the acquisition of unhealthy nutritional habits, sedentary lifestyles, and obesity [265].Thus, pro-inflammatory substances increase oxidative stress, triggering different pathologies associated with metabolic syndrome, such as type 2 diabetes, dyslipidemia, obesity, atherosclerosis, cardiomyopathy, hypertension, and heart failure [266,267,268]. Hence, previous authors proposed that these findings may be explained by the fact that elevated saturated fatty acid consumption may be related to postprandial inflammation [269]. The postprandial state is defined as the moment that occurs after food ingestion, lasting between 6 and 12 h, in which an increase in carbohydrates and fatty acids occurs [270]. Furthermore, the postprandial state may be considered as a source of pro-inflammatory substances, since previous authors proposed that saturated fatty acids may activate the immune response [271,272]. These studies proposed that saturated fatty acids may present analogous molecular effects to lipopolysaccharide, a molecule which is responsible for Toll-like receptor 4 stimulation, which consequently promotes pro-inflammatory cytokine liberation and disturbs cellular metabolism. Additionally, previous authors pointed out that polyunsaturated fatty acids may also have a great impact on metabolic diseases, since omega-3 fatty acids were related to postprandial inflammation suppression, whereas omega-6 fatty acids were linked to inflammatory processes [273,274,275,276,277,278]. These outcomes were also supported by the recent and previous literature, in which positive associations were found between type 2 diabetes, atherosclerosis, non-alcoholic fatty liver disease, and postprandial state [279,280,281], suggesting the importance of postprandial inflammation and its relationship between different diseases that compound metabolic syndrome. In relation to this, a review of the use of citrus fruits notes that hesperidin, hesperetin, naringenin, naringin, and narirutin have anti-inflammatory effects in model systems, and human trials with hesperidin report a decrease in inflammatory markers. Orange juice reduced inflammation caused by a high-fat, high-carbohydrate meal in humans [282].
Regarding fiber, the previous literature suggested that patients who followed the Western diet were more likely to suffer obesogenic surroundings, increasing visceral obesity growth and insulin resistance [283]. According to these findings, diets that involved a raised fiber intake were related to a decreased cardiovascular disease, metabolic syndrome, and gastrointestinal disease prevalence [284]. The beneficious role of fiber may be explained through its capability to modulate inflammatory and proliferation processes as a result of its effect in the gastrointestinal tract. Thus, when fibers are introduced into an organism, they are not digested in the gastrointestinal tube, but rather fermented by gut microbiota, generating short-chain fatty acids, which play an important role as anti-inflammatory molecules [285,286]. For instance, after conducting a randomized controlled trial with a 3-week intensive diet-exercise intervention, Tremblay et al. concluded that adequate dietary fiber intake should be a primary factor in diet-based weight loss programs [287].
These findings suggest that fiber may be an effective weapon against metabolic disorders by virtue of its ability to lower oxidative stress. Antioxidant chemicals are molecules that have the capacity to reduce oxidative stress and, as a result, may have a beneficial influence on health. Polyphenols, which have been shown to have anti-oxidative and anti-inflammatory properties, have been suggested as a potential tool in the prevention and control of diabetes [288]. Additionally, the previous literature proposed that the use of flavonoids, present in citrus products, may have antioxidant properties since the citrus flavonoid narangin may reduce proinflammatory substances such as TNF-a and cyclooxygenase-2, as well as inducible NO synthase activity [289]. Considering these findings, citrus flavonoids seem to be beneficious for metabolic syndrome and obesity treatment [290], as they perform an important role as an antioxidant, reducing inflammatory processes. With regard to fatty acids and their effect on oxidative stress, the latest literature proposed that a higher ratio between omega-6 and omega-3fatty acids was strongly related to greater mortality from all causes of death, cancer, and cardiovascular disease [291]. These results may be explained by the fact that omega-6 fatty acids have strong pro-inflammatory activity while omega-3 polyunsaturated fatty acids present antioxidant effects. Furthermore, it has been largely proposed that the Mediterranean diet, in contrast to the Western diet, is composed of several antioxidant substances, such as oleic acid, a mono-unsaturated fatty acid that can be found in olive oil, as well as alpha- linolenic acid, an omega-3-polyunsaturated fatty acid present in nuts and fish such as salmon, tuna, or sardines [292,293,294]. Finally, it has been reported that this kind of diet also involves a raised amount of polyphenols such as flavonoids, present in fruits and vegetables, as well as a substantial quantity of fiber, with all these being antioxidant components able to enhance health status by reducing oxidative damage [295]. Thus, considering these outcomes, it could be proposed that the Western diet may have a negative impact on metabolic syndrome.
12. The Effect of Western Diet on Cancer
The impact of the Western diet on noncommunicable diseases, including its negative effects on cancer, has been extensively studied. However, there has been conflicting evidence from research looking at the link between specific foods and diseases, and even fewer studies have been carried out on diet as a whole, with conflicting results [296]. Nevertheless, the Western diet could be considered a dietary choice that may compromise health status by promoting inflammation and increasing the risk of developing these types of pathologies, as well as increasing their mortality and morbidity [12,297,298].
With regard to cancer, several studies pointed out that it may be modulated by different molecular processes, including oxidative stress and inflammation [299]. Similarly, previous authors related the raised production of reactive oxygen species (ROS) to numerous different types of cancer, due to the raised oxidative stress suffered by cells [299]. For instance, the utilization of endogenously produced reactive oxygen species (ROS) through the activation of signaling pathways including PI3K/AKT and MAPK can also contribute to the development of ovarian cancer [300]. This fact may be explained through ROS’ capability of promoting the initiation of oncogenes as well as by their ability to reduce tumor suppressor activity. Moreover, ROS play a crucial role in inflammatory response, since they have been associated with a decrease in p53 levels, with p53 being one of the most important tumor suppressors that can slow cancer progression. Additionally, increased ROS levels were related to a significant increase in cytokine levels, which may compromise the inflammatory response [301]. Subsequently, the previous literature has related the elevation of cytokine levels to an increased tumor proliferation as well as to cancer progression [302,303], compromising cancer management and survival.
The transition from whole foods to processed ones in the Western diet triggers a reduction in the micronutrient quality of this kind of diet [304]. However, fruits and vegetables with significant antioxidant content may help prevent malignancies of the mouth, pharynx, larynx, esophagus, stomach, and lungs, according to the available research on malignancies of the lungs, esophagus, stomach, and larynx [305].
Thus, the absence of these foods prevents individuals from obtaining nutrients that can be beneficial, whereas they consume saturated fats and refined sugar, which may negatively affect their overall health due to their pro-inflammatory effects. Regarding lipid consumption, the recent literature proposed that individuals with lower levels of omega-3 polyunsaturated fatty acids presented a 30% greater risk of cancer mortality [291,306], suggesting the protective effect that omega-3 polyunsaturated fatty acids may have in cancer development due to their capability modulating oxidative stress and inflammation response. With regard to specific types of cancer, a recent metanalysis noted that lower risk of developing colorectal cancer has been associated with raised docosahexanoic acid (DHA) and eicosapentaenoic acid (EPA) intake, both omega-3 polyunsaturated fatty acids, as well as with decreased linoleic acid intake, an omega-6 polyunsaturated fatty acid [307]. Furthermore, colorectal cancer also has been associated with an elevated trans fatty acid intake [308], a special kind of fatty acid that suffers a partial hydrogenation in the industrial process in order to deodorize and heat vegetable oils at great temperatures [309]. Thus, trans fatty acids may have a negative effect on health due to their potentially pro-inflammatory activity, enhancing cytokine release from monocytes and macrophages, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and monocyte chemoattractant protein (MCP) [310,311]. Not only has it been found that industrial trans fatty acids are linked to an increased risk of colon cancer, industrial TFAs were also shown to have a stronger correlation with colorectal cancer [312].
Concerning the current refined sugar intake in the Western diet, it is important to consider carbohydrates’ effects on tumoral events, since the latest studies proposed the high glucose levels could be related to an improvement in vascular endothelium growth, as well as to the reduction in anti-angiogenic factors [313]. This could be explained by the fact that cancer cells might enhance their growth and proliferation when they are in a nutrient-rich environment, as was largely described several years ago by Otto Warburg, who considered that cancer cells may obtain energy through aerobic glycolysis [314]. According to these findings, it may be considered that an increased refined sugar intake may be associated with a worse efficacy in tumoral events, since several studies proposed that higher simple carbohydrate intake may be related to a worse cancer prognosis [315,316,317,318,319]. Therefore, these outcomes support the idea that the Western diet, which involves a raised simple carbohydrate intake, may have a negative impact on cancer disease. For instance, breast cancer risks were shown to rise by more than 10% for every 10% increase in the amount of ultra-processed foods in the diet in a large prospective investigation [320].
Regarding fruit and vegetable consumption, several authors suggested their intake may have a beneficious outcome in cancer, due to their higher fiber and complex carbohydrate content. More specifically, reduced fruit and vegetable ingestion has been inversely correlated with a raised risk of developing different types of cancer, including lung cancer [321]. This may be explained by the ability of complex carbohydrates to modify insulin-like growth factor binding protein 3 (IGFBP-3) activity. This protein has been shown to be responsible for insulin/IGF-1 axis disturbance, blocking cell proliferation and tumoral growth, which may control cancer development. Additionally, both complex carbohydrates and fiber could modify the gut microbiota, and as mentioned above, they might modulate short-chain fatty acid production from the gut microbiota, decreasing inflammation and promoting tumoral control. Lastly, the potential effect of complex carbohydrates and fiber on excretion processes has been highlighted, since they might be liable for raising carcinogen fecal excretion, enhancing their elimination and reducing their time inside the organism, and, consequently, their associated negative effects [322]. Finally, considering the antioxidants present in fruit and vegetables, several studies pointed out their benefits as cancer-modulating molecules, due to their ability to reduce cellular stress and, consequently, modulate tumorigenesis through modifications in gene expression [323,324,325]. Interestingly, the highest values for antioxidant substances were found in fruits, such as berries, as well as in nuts, including walnuts, sunflower seeds, and pecans [326,327]. Regarding nuts, it has been widely described that they may constitute an advantageous tool in order to modulate cancer development. Thus, their polyphenols, including ellagic acid, anacardic acid, resveratrol, and inositol, among others, may be related to apoptosis modulation, cell growth, migration and invasion inhibition, and angiogenesis and metastasis control [328,329,330,331,332].
Therefore, taking into account that the Western diet avoids this type of nutrients, it would be considered that individuals who follow this type of diet are less likely to benefit from these phytochemicals and their intake.
13. Sanitary Costs of Western Diet
There are several pathologies associated with the different risk factors associated with the consumption of the Western diet, such as obesity and type 2 diabetes [333,334,335]. In this regard, obesity has tripled over the last 5 years. According to the Organization for Economic Cooperation and Development (OECD), in a report made in 2019 [336], it is predicted that within 30 years, obesity or overweight is going to be triggering the onset of 70% of cases of patients diagnosed with diabetes, around 20% of heart disease, and 9% of cancer cases. This means that the treatment of obesity and associated diseases is not only a public health problem but also a very high economic cost for societies [336,337].
According to data collected in another study conducted by the World Obesity Federation (WOF) in 2022 [338], it is possible to predict that in 2030, one in five women and one in seven men will be obese. This projection is generalized for all countries in the world. In addition, approximately 14% of the child population will be obese. This means that around one billion people will suffer from obesity worldwide [338,339]. From the numbers collected in relation to the cost involved, we can see that in Australia, USD 24 billion is spent annually on the treatment of these pathologies. In Brazil, the annual expenditure is USD 39 billion. In India, this expenditure amounts to USD 23 billion a year. Although these figures are already alarming, a very significant increase is expected within a few decades [339].
Type 2 diabetes is a disease associated with a high rate of comorbidity and mortality. Both incidence and prevalence have increased in recent years, with a greater presence in developed countries and associated with sedentary lifestyles, obesity, and increased life expectancy. The organic degeneration it entails makes it serious because of its morbidity and mortality. It is, therefore, a major public health problem worldwide [340].
Sources consulted indicate that the prevalence worldwide is approximately 8.5%, and a growth in this range is expected by 2035, exceeding 590 million people with obesity [341,342] as a high-impact factor. In the US, about 15.5 million people have type 2 diabetes and at least 13 million people have impaired glucose tolerance. In Europe, chronic and acute complications appear in more than 10 million people. In Asian countries, there has been a rapid increase in the incidence, mainly due to the change in nutritional habits during the last few decades [342].
In relation to comorbidity, it is estimated that the chronic complications that occur in this pathology because of its vascular degenerative course are the most frequent [343]. Among the most important are heart failure, peripheral and cerebral vascular disease, retinopathy, and neuropathy [344,345]. Another of the highest costs is hospitalization because of the disease, hyperglycemia, hypoglycemia, infections, exacerbations, and/or ketoacidosis, among others. Hypoglycemia is frequently a reason for consultation that is also associated with a reduction in or loss of work [346]. Its incidence is around 75 cases per 1000 patients per year after 5 years of diagnosis. Associated diseases such as intestinal complications and/or nasal and pharyngeal infections are also a reason for consultation and hospitalization, accounting in some countries for up to 20% of the costs associated with the disease [347].
The healthcare costs incurred in 2013 worldwide indicate that approximately USD 550 million was spent on the treatment and follow-up of these patients [348]. This includes direct healthcare costs, non-direct healthcare costs related to aspects such as the number of healthcare personnel needed, transportation costs, and care services, among others, and costs due to the loss of productivity, which refer to work incapacity, absenteeism, and low productivity, among others [349]. Finally, intangible costs are included, which refer to the reduction in or even loss of well-being, both of patients and their immediate environment, associated with suffering and pain [350,351].
Metabolic syndrome, also known as insulin resistance syndrome or syndrome X, is a complex pathology due to the phenotypic profile that presents with wide variations and is associated with the acquisition of unhealthy nutritional habits, sedentary lifestyles, and obesity [352]. It is a pathology in which different metabolic and inflammatory alterations are present that favor the appearance of cardiovascular pathologies [353]. Statistics reveal that, when stratified by diabetes, the average yearly total expenses of participants with and without metabolic syndrome varied by an overall magnitude of 1.6 (USD 5732 vs. USD 3581) and an overall magnitude of 1.3 (USD 7896 vs. USD 6038; USD 4476 vs. USD 3422), respectively. Per extra risk factor, overall expenses rose by an average of 24% (p = 0.001). Costs for patients with diabetes who also had weight risk, dyslipidemia, and hypertension were nearly twice as expensive (USD 8067 vs. USD 4638) as those for patients with prediabetes who also had these risk factors [354].
The prevalence in adults in the US stands at almost 23% for both men and women. In Europe, this prevalence is somewhat lower, being 10% in women and 15% in men. In recent years, the incidence has increased significantly and in relation to obesity [355]. With regard to the health costs associated with metabolic syndrome, there are also those derived from treatments for obesity, type 2 diabetes, hypertension, and hypercholesterolemia. All this means that this cluster of pathologies represents a very high annual cost. For this reason, in 2004, the WHO published a document called Global Strategy on Diet and Physical Activity for Health, with the aim of implementing strategies and actions to reduce the impact of these diseases on society. In 2011, the United Nations General Assembly scheduled a meeting in which it adopted a resolution called “Political declaration of the high-level meeting of the General Assembly on the prevention and control of non-communicable disease”, with the same objective of prevention [356].
Cardiovascular disease is the leading cause of death in the world. It affects all countries equally and is associated with the presence of risk factors such as unhealthy eating habits, obesity, and hypertension, among others [357]. These factors are present in a large part of society due to the general lack of physical activity, as well as the habit of hypercaloric diets with low consumption of vitamins and fiber, so its consequences worldwide are very serious. Among the most important are coronary heart disease, cerebrovascular disease, rheumatic heart disease, deep-vein thrombosis, and pulmonary embolism [358]. Approximately 18 million people worldwide died from this cause in 2015, accounting for more than 30% of all recorded deaths, being above deaths from cancer, HIV, or respiratory system infections. The recorded data indicate that the costs of these pathologies are the highest in the world. In the United States, more than USD 450 billion is spent annually on treatment and associated indirect costs [358]. In Europe, more than 20% of people admitted to hospitals are admitted for these diseases, and the associated cost exceeds EUR 170 billion per year. In countries such as France, Germany, and the United Kingdom, the direct cost of these pathologies amounted to more than EUR 100 billion in 2014, a figure comparable to the GDP of some small or medium-sized countries [359]. In addition, the indirect costs involved must be added to this, the psychological and other costs associated with the triggers and diagnosed pathologies [360,361].
Cancer is another pathology associated with the Western diet. Related to metabolic syndrome and obesity, it is known that the presence of these characteristics causes changes in an organism that facilitate the appearance of certain cancers [362,363]. As a result of these changes, chronic inflammation may appear, increasing insulin levels. Studies conducted in 2014 indicated that the increase in cancers in the US related to obesity and overweight was 7%, recording in contrast that cancers associated with other factors decreased by 13% [364,365]. The figures indicate that nearly 700,000 people a year are diagnosed with obesity-related cancer [366].
In addition, the occurrence of diseases associated with the inflammation and infection of the intestinal system should be considered [367]. In this regard, inflammatory bowel disease has an unknown prevalence, although it appears more frequently in developed societies [368]. Among the most important of this group of pathologies, we find Crohn’s disease and ulcerative colitis. With regard to Western countries, there is a higher incidence of diagnosis of Crohn’s disease in the US, with figures of 20.2 per 100,000 inhabitants [369]. In Europe, however, there is a higher incidence of ulcerative colitis, standing at 24.3 per 100,000 people [370]. It is interesting to see how in Asia, Africa, and the Middle East, a few years ago, very low prevalence figures were recorded, although an increase in the diagnosis of inflammatory bowel disease seems to be detected, mainly associated with the social and economic development of some specific countries [371].
These diseases currently have no known cure, so treatment is a matter of indefinite follow-up, with the intention of keeping the patient in remission [372,373]. This means that once the diagnosis is made, we will have these people in follow-up consultations, check-ups, and pharmacology throughout life, which is associated with high healthcare costs during this process [374,375,376,377,378,379,380]. In addition, in these diseases, we must consider that there will be moments of remission, but also episodes in which the symptomatology worsens significantly during outbreaks [381,382,383].
The annual health costs in all developed countries are currently very high if we consider the pathologies associated with the consumption of the Western diet. This diet, which includes an excess of calories and the intake of foods with pro-inflammatory action such as polyunsaturated fats and sugar, favors the presence of various diseases by itself or as a cause of other associated pathologies. These facts allow us to understand the social and economic repercussions that are involved with the habit of this style of nutrition. This should be enough for institutions to introduce actions and measures aimed at a correct understanding of the characteristics of the Western diet and its risks for the population, as well as actions aimed at preventing the appearance of organic pathologies. Special mention should also be made of the current situation of overcoming the COVID-19 pandemic, where these factors only increase the risk for this type of pathology and other related ones [384,385,386].
14. Practical Applications
Improving the Western diet and lifestyle can have a significant impact on health outcomes, reducing the risk of chronic diseases such as heart disease, diabetes, and certain types of cancer. We can propose some practical applications that can help improve the Western diet and lifestyle:
- Increase intake of fruits and vegetables: A diet rich in fruits and vegetables provides a range of vitamins, minerals, and antioxidants that can promote health and reduce the risk of chronic diseases. Encouraging people to eat a variety of colorful fruits and vegetables and including them in meals and snacks can help increase their intake.
- Reduce intake of processed and fast food: Processed and fast foods tend to be high in calories, unhealthy fats, sugar, and sodium and low in nutrients. Encouraging people to cook meals at home, emphasizing the importance of reading food labels, and promoting healthier fast food options can help reduce intake.
- Encourage physical activity: Regular physical activity is essential for maintaining a healthy weight and reducing the risk of chronic diseases. Encouraging people to find enjoyable ways to be active, such as walking, biking, or dancing, and emphasizing the importance of incorporating physical activity into daily routines can help increase physical activity levels.
- Limit sedentary behavior: Sedentary behavior, such as sitting for long periods of time, can increase the risk of chronic diseases. Encouraging people to take breaks from sitting, such as standing or walking, and promoting workplace wellness programs can help reduce sedentary behavior.
- Promote mindful eating: Mindful eating involves paying attention to the present moment and being aware of food choices, hunger, and fullness cues. Encouraging people to eat slowly, savor their food, and listen to their body’s cues can help promote mindful eating.
- Emphasize the importance of sleep: Getting enough sleep is important for overall health and well-being. Encouraging people to prioritize sleep and establish healthy sleep habits, such as going to bed and waking up at consistent times, can help improve sleep quality.
- By incorporating and enhancing psychometric evaluation questionnaires specifically designed for assessing one’s lifestyle choices, health professionals will be empowered to provide more personalized and targeted interventions.
Overall, these practical applications can help improve the Western diet and lifestyle, promoting better health outcomes and reducing the risk of chronic diseases.
15. Conclusions
This review aimed to describe the effects of the Western diet on the metabolism, inflammation, and antioxidant status, as well as on the gut microbiota and mitochondrial fitness, cardiovascular and mental health, and cancer, in addition to the Western diet’s hygienic costs. As presented in Figure 5, the most important highlights of this review are, firstly, that fruits, vegetables, fiber, and complex carbohydrates can combat not only metabolic disorders that an individual may be afflicted with, but also cancer and its development in relation to the consumption of typical Western diet foods. Secondly, in this regard, consuming a healthier diet rich in micronutrients and antioxidants such as magnesium, potassium, iron, vitamins B6, B12, A, and C, carotenoids, and flavonoids as well as engaging in physical activity reduce inflammatory processes, which has consequences such as improving mental health, increasing beneficial bacteria in the gut, enhancing mitochondrial function, and boosting our immune system.
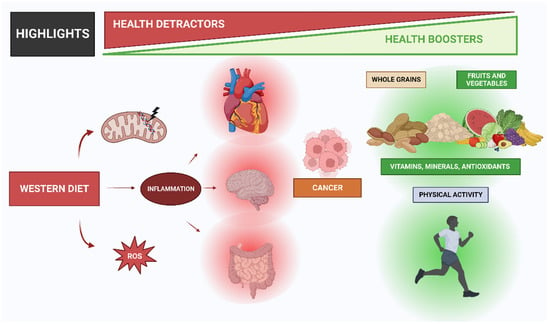
Figure 5.
Key highlights from this analysis of the Western diet that emphasize its negative effects on health and suggestions for improving these effects on your body.
Author Contributions
Conceptualization, V.J.C.-S.; methodology, J.F.T.-A.; investigation, all authors; writing—original draft preparation, all authors; writing—review and editing, all authors; visualization, all authors; supervision, V.J.C.-S.; project administration, V.J.C.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Figures were created with BioRender.com (accessed on 25 March 2023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- González Olmo, B.M.; Butler, M.J.; Barrientos, R.M. Evolution of the Human Diet and Its Impact on Gut Microbiota, Immune Responses, and Brain Health. Nutrients 2021, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Shively, C.A.; Appt, S.E.; Vitolins, M.Z.; Uberseder, B.; Michalson, K.T.; Silverstein-Metzler, M.G.; Register, T.C. Mediterranean versus Western Diet Effects on Caloric Intake, Obesity, Metabolism, and Hepatosteatosis in Nonhuman Primates. Obesity (Silver Spring Md.) 2019, 27, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, G.; Nantel, G.; Shetty, P.; Food and Agriculture Organization of the United Nations. Globalization of food systems in developing countries: Impact on food security and nutrition. FAO Food Nutr. Pap. 2004, 83, 1–300. [Google Scholar]
- Bustamante-Sanchez, A.; Villegas-Mora, B.E.; Martínez-Guardado, I.; Tornero-Aguilera, J.F.; Ardigò, L.P.; Nobari, H.; Clemente-Suárez, V.J. Physical Activity and Nutritional Pattern Related to Maturation and Development. Sustainability 2022, 14, 16958. [Google Scholar] [CrossRef]
- Kopp, W. How Western Diet and Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2221–2236. [Google Scholar] [CrossRef]
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet-Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef]
- Rakhra, V.; Galappaththy, S.L.; Bulchandani, S.; Cabandugama, P.K. Obesity and the Western Diet: How We Got Here. Mo. Med. 2020, 117, 536–538. [Google Scholar]
- Rice Bradley, B.H. Dietary Fat and Risk for Type 2 Diabetes: A Review of Recent Research. Curr. Nutr. Rep. 2018, 7, 214–226. [Google Scholar] [CrossRef]
- Al Ghorani, H.; Götzinger, F.; Böhm, M.; Mahfoud, F. Arterial hypertension—Clinical trials update 2021. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 21–31. [Google Scholar] [CrossRef]
- Carrera-Bastos, P.; Fontes-Villalba, M.; O’Keefe, J.H.; Lindeberg, S.; Cordain, L. The western diet and lifestyle and diseases of civilization. Res. Rep. Clin. Cardiol. 2011, 2, 15–35. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Naude, C.E.; Schoonees, A.; A Nguyen, K.; Senekal, M.; Young, T.; Garner, P.; Chaplin, M.; Volmink, J.; Richardson, M. Low-carbohydrate versus balanced-carbohydrate diets for reducing weight and cardiovascular risk. Cochrane Database Syst. Rev. 2022, 1, CD013334. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, D.; Vellanki, K.; Kramer, H. The Western Diet and Chronic Kidney Disease. Curr. Hypertens. Rep. 2015, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Arsene, M.M.; Jorelle, A.B.; Sarra, S.; Viktorovna, P.I.; Davares, A.K.; Ingrid, N.K.; Steve, A.A.; Andreevna, S.L.; Vyacheslavovna, Y.N.; Carime, B.Z. Short review on the potential alternatives to antibiotics in the era of antibiotic resistance. J. Appl. Pharm. Sci. 2021, 12, 29–40. [Google Scholar]
- Salas-Salvadó, J.; Becerra-Tomás, N.; García-Gavilán, J.F.; Bulló, M.; Barrubés, L. Mediterranean Diet and Cardiovascular Disease Prevention: What Do We Know? Prog. Cardiovasc. Dis. 2018, 61, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, F. Animal and Plant Protein Sources and Cardiometabolic Health. Adv. Nutr. 2019, 10, S351–S366. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.J.; Sohn, S.-K.; Song, H.K.; Lee, S.M.; Youn, Y.H.; Lee, S.; Park, H. Associations of colorectal cancer incidence with nutrient and food group intakes in korean adults: A case-control study. Clin. Nutr. Res. 2015, 4, 110–123. [Google Scholar] [CrossRef]
- Malik, V.S.; Li, Y.; Tobias, D.K.; Pan, A.; Hu, F.B. Dietary Protein Intake and Risk of Type 2 Diabetes in US Men and Women. Am. J. Epidemiol. 2016, 183, 715–728. [Google Scholar] [CrossRef]
- Hunter, J.E.; Zhang, J.; Kris-Etherton, P.M. Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated, and unsaturated fatty acids: A systematic review. Am. J. Clin. Nutr. 2010, 91, 46–63. [Google Scholar] [CrossRef]
- Wolever, T. Carbohydrates and health—The FAO/WHO consultation. Aust. J. Nutr. Diet. 2001, 58, S3. [Google Scholar]
- Mann, J.; Cummings, J.H.; Englyst, H.N.; Key, T.; Liu, S.; Riccardi, G.; Summerbell, C.; Uauy, R.; van Dam, R.M.; Venn, B.; et al. FAO/WHO Scientific Update on carbohydrates in human nutrition: Conclusions. Eur. J. Clin. Nutr. 2007, 61, S132–S137. [Google Scholar] [CrossRef] [PubMed]
- The State of Food Security and Nutrition in the World 2018|Agrifood Economics|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/agrifood-economics/publications/detail/en/c/1153252/ (accessed on 20 April 2023).
- Wu, H.; Flint, A.J.; Qi, Q.; Van Dam, R.M.; Sampson, L.A.; Rimm, E.B.; Holmes, M.D.; Willett, W.C.; Hu, F.B.; Sun, Q. Association between dietary whole grain intake and risk of mortality: Two large prospective studies in US men and women. JAMA Intern. Med. 2015, 175, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Fryar, C.D.; Martin, C.B.; Freedman, D.S.; Carroll, M.D.; Gu, Q.; Hales, C.M. Trends in Obesity Prevalence by Race and Hispanic Origin-1999-2000 to 2017-2018. JAMA 2020, 324, 1208–1210. [Google Scholar] [CrossRef]
- de Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef]
- Park, Y.; Lee, J.; Oh, J.H.; Shin, A.; Kim, J. Dietary patterns and colorectal cancer risk in a Korean population: A case-control study. Medicine 2016, 95, e3759. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Duster, M.; Roberts, T.; Devinsky, O. United States Dietary Trends Since 1800: Lack of Association Between Saturated Fatty Acid Consumption and Non-communicable Diseases. Front. Nutr. 2022, 8, 1267. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, B.; Pérez, M.; Pérez-Santiago, J.D.; Tornero-Aguilera, J.F.; González-Soltero, R.; Larrosa, M. Gut Microbiota Modification: Another Piece in the Puzzle of the Benefits of Physical Exercise in Health? Front. Physiol. 2016, 7, 51. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Mielgo-Ayuso, J.; Martín-Rodríguez, A.; Ramos-Campo, D.J.; Redondo-Flórez, L.; Tornero-Aguilera, J.F. The Burden of Carbohydrates in Health and Disease. Nutrients 2022, 14, 3809. [Google Scholar] [CrossRef]
- López-Taboada, I.; González-Pardo, H.; Conejo, N.M. Western Diet: Implications for Brain Function and Behavior. Front. Psychol. 2020, 11, 564413. [Google Scholar] [CrossRef]
- Cinquina, V.; Calvigioni, D.; Farlik, M.; Halbritter, F.; Fife-Gernedl, V.; Shirran, S.L.; Fuszard, M.A.; Botting, C.H.; Poullet, P.; Piscitelli, F.; et al. Life-long epigenetic programming of cortical architecture by maternal ‘Western’ diet during pregnancy. Mol. Psychiatry 2020, 25, 22–36. [Google Scholar] [CrossRef]
- Johnson, C.S.; Shively, C.A.; Michalson, K.T.; Lea, A.J.; DeBo, R.J.; Howard, T.D.; Hawkins, G.A.; Appt, S.E.; Liu, Y.; McCall, C.E.; et al. Contrasting effects of Western vs Mediterranean diets on monocyte inflammatory gene expression and social behavior in a primate model. eLife 2021, 10, e68293. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; De Miguel, M.; Moreno Fernández, A.M.; Carmona López, I.M.; Garrido Maraver, J.; Cotán, D.; Gómez Izquierdo, L.; Bonal, P.; Campa, F.; Bullon, P.; et al. Mitochondrial dysfunction and mitophagy activation in blood mononuclear cells of fibromyalgia patients: Implications in the pathogenesis of the disease. Arthritis Res. Ther. 2010, 12, R17. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef]
- Roberts, M.D.; Mobley, C.B.; Toedebush, R.G.; Heese, A.J.; Zhu, C.; Krieger, A.E.; Cruthirds, C.L.; Lockwood, C.M.; Hofheins, J.C.; Wiedmeyer, C.E.; et al. Western diet-induced hepatic steatosis and alterations in the liver transcriptome in adult Brown-Norway rats. BMC Gastroenterol. 2015, 15, 151. [Google Scholar] [CrossRef]
- Arendt, J.; Aulinas, A. Physiology of the Pineal Gland and Melatonin. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Burrows, T.L.; Whatnall, M.C.; Patterson, A.J.; Hutchesson, M.J. Associations between Dietary Intake and Academic Achievement in College Students: A Systematic Review. Healthcare 2017, 5, 60. [Google Scholar] [CrossRef]
- Yuan, M.; Chen, W.; Teng, B.; Fang, Y. Occupational Disparities in the Association between Self-Reported Salt-Eating Habit and Hypertension in Older Adults in Xiamen, China. Int. J. Environ. Res. Public Health 2016, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Eriksson, J.; Barengo, N.C.; Lakka, T.A.; Valle, T.T.; Nissinen, A.; Jousilahti, P.; Tuomilehto, J. Occupational, commuting, and leisure-time physical activity in relation to total and cardiovascular mortality among Finnish subjects with type 2 diabetes. Circulation 2004, 110, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Veeranki, S.P.; Li, S.; Steffen, L.M.; Xi, B. Beneficial associations of low and large doses of leisure time physical activity with all-cause, cardiovascular disease and cancer mortality: A national cohort study of 88,140 US adults. Br. J. Sport. Med. 2019, 53, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Salam, N.; Jiao, J.-Y.; Zhang, X.-T.; Li, W.-J. Update on the classification of higher ranks in the phylum Actinobacteria. Int. J. Syst. Evol. Microbiol. 2020, 70, 1331–1355. [Google Scholar] [CrossRef]
- Lally, P.; Bartle, N.; Wardle, J. Social norms and diet in adolescents. Appetite 2011, 57, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Cooksey Stowers, K.; Jiang, Q.; Atoloye, A.; Lucan, S.; Gans, K. Racial Differences in Perceived Food Swamp and Food Desert Exposure and Disparities in Self-Reported Dietary Habits. Int. J. Environ. Res. Public Health 2020, 17, 7143. [Google Scholar] [CrossRef]
- Caspi, C.E.; Sorensen, G.; Subramanian, S.V.; Kawachi, I. The local food environment and diet: A systematic review. Health Place 2012, 18, 1172–1187. [Google Scholar] [CrossRef]
- Ballal, K.; Wilson, C.R.; Harmancey, R.; Taegtmeyer, H. Obesogenic high fat western diet induces oxidative stress and apoptosis in rat heart. Mol. Cell. Biochem. 2010, 344, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, K.H.; Slater, T.F. An introduction to free radical biochemistry. Br. Med. Bull. 1993, 49, 481–493. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Halliwell, B. How to characterize an antioxidant: An update. Biochem. Soc. Symp. 1995, 61, 73–101. [Google Scholar]
- Shi, H.; Noguchi, N.; Niki, E. Comparative study on dynamics of antioxidative action of alpha-tocopheryl hydroquinone, ubiquinol, and alpha-tocopherol against lipid peroxidation. Free Radic. Biol. Med. 1999, 27, 334–346. [Google Scholar] [CrossRef]
- Kunwar, A.; Priyadarsini, K.I. Free radicals, oxidative stress and importance of antioxidants in human health. J. Med. Allied Sci. 2011, 1, 53–60. [Google Scholar]
- Galli, C.; Calder, P.C. Effects of fat and fatty acid intake on inflammatory and immune responses: A critical review. Ann. Nutr. Metab. 2009, 55, 123–139. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.-Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant Activity and Healthy Benefits of Natural Pigments in Fruits: A Review. Int. J. Mol. Sci. 2021, 22, 4945. [Google Scholar] [CrossRef]
- Manzel, A.; Muller, D.N.; Hafler, D.A.; Erdman, S.E.; Linker, R.A.; Kleinewietfeld, M. Role of ‘Western diet’ in inflammatory autoimmune diseases. Curr. Allergy Asthma. Rep. 2014, 14, 404. [Google Scholar] [CrossRef]
- Blaner, W.S.; Shmarakov, I.O.; Traber, M.G. Vitamin A and Vitamin E: Will the Real Antioxidant Please Stand Up? Annu. Rev. Nutr. 2021, 41, 105–131. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.M.; Simpson, J.A.; Gibson, R.A.; Sinclair, A.J.; Makrides, M.; O’Dea, K.; English, D.R.; Giles, G.G. Plasma phospholipid fatty acid composition as a biomarker of habitual dietary fat intake in an ethnically diverse cohort. Nutr. Metab. Cardiovasc. Dis. NMCD 2007, 17, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Packer, J.E.; Slater, T.F.; Willson, R.L. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature 1979, 278, 737–738. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Appel, L.J.; Van Horn, L. Components of a cardioprotective diet: New insights. Circulation 2011, 123, 2870–2891. [Google Scholar] [CrossRef] [PubMed]
- Charles-Messance, H.; Mitchelson, K.A.; Castro, E.D.M.; Sheedy, F.J.; Roche, H.M. Regulating metabolic inflammation by nutritional modulation. J. Allergy Clin. Immunol. 2020, 146, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Gunnerud, U.J.; Heinzle, C.; Holst, J.J.; Östman, E.M.; Björck IM, E. Effects of pre-meal drinks with protein and amino acids on glycemic and metabolic responses at a subsequent composite meal. PLoS ONE 2012, 7, e44731. [Google Scholar] [CrossRef]
- De la Fuente, M. Effects of antioxidants on immune system ageing. Eur. J. Clin. Nutr. 2002, 56 (Suppl. 3), S5–S8. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Bertoia, M.L.; Mukamal, K.J.; Cahill, L.E.; Hou, T.; Ludwig, D.S.; Mozaffarian, D.; Willett, W.C.; Hu, F.B.; Rimm, E.B. Changes in Intake of Fruits and Vegetables and Weight Change in United States Men and Women Followed for Up to 24 Years: Analysis from Three Prospective Cohort Studies. PLoS Med. 2015, 12, e1001878. [Google Scholar] [CrossRef]
- Sesso, H.D.; Buring, J.E.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Glynn, R.J.; Gaziano, J.M. Vitamins E and C in the prevention of cardiovascular disease in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2008, 300, 2123–2133. [Google Scholar] [CrossRef]
- Aguilar TA, F.; Navarro BC, H.; Pérez, J.A.M. Endogenous antioxidants: A review of their role in oxidative stress. In A Master Regulator of Oxidative Stress-the Transcription Factor nrf2; BoD–Books on Demand: Norderstedt, Germany, 2016; pp. 3–20. [Google Scholar]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Dato, S.; Crocco, P.; D’Aquila, P.; De Rango, F.; Bellizzi, D.; Rose, G.; Passarino, G. Exploring the role of genetic variability and lifestyle in oxidative stress response for healthy aging and longevity. Int. J. Mol. Sci. 2013, 14, 16443–16472. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem.-Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Macho-González, A.; Garcimartín, A.; López-Oliva, M.E.; Bastida, S.; Benedí, J.; Ros, G.; Nieto, G.; Sánchez-Muniz, F.J. Can Meat and Meat-Products Induce Oxidative Stress? Antioxidants 2020, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Ursin, G.; Veierød, M.B. Meat consumption and the risk of type 2 diabetes: A systematic review and meta-analysis of cohort studies. Diabetologia 2009, 52, 2277–2287. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarczyk, M.M.; Miller, M.J.; Freund, G.G. The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metab. Clin. Exp. 2012, 61, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Why whole grains are protective: Biological mechanisms. Proc. Nutr. Soc. 2003, 62, 129–134. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Khabour, O.F.; Salah, H.A.; Abu Rashid, B.E. The combined effect of sleep deprivation and Western diet on spatial learning and memory: Role of BDNF and oxidative stress. J. Mol. Neurosci. MN 2013, 50, 124–133. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals and other reactive species in disease. e LS 2001. [Google Scholar] [CrossRef]
- Radak, Z.; Zhao, Z.; Koltai, E.; Ohno, H.; Atalay, M. Oxygen consumption and usage during physical exercise: The balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid. Redox Signal. 2013, 18, 1208–1246. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Vina, J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [CrossRef]
- Hecht, S.S. Cigarette smoking and lung cancer: Chemical mechanisms and approaches to prevention. Lancet Oncol. 2002, 3, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Ravn-Haren, G.; Olsen, A.; Tjønneland, A.; Dragsted, L.O.; Nexø, B.A.; Wallin, H.; Overvad, K.; Raaschou-Nielsen, O.; Vogel, U. Associations between GPX1 Pro198Leu polymorphism, erythrocyte GPX activity, alcohol consumption and breast cancer risk in a prospective cohort study. Carcinogenesis 2006, 27, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Barton, G.M. A calculated response: Control of inflammation by the innate immune system. J. Clin. Investig. 2008, 118, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.S.; Wu, T.L.; Chou, S.C.; Ho, C.; Chang, P.Y.; Tsao, K.C.; Huang, J.Y.; Sun, C.F.; Wu, J.T. Development of multiple complications in type 2 diabetes is associated with the increase of multiple markers of chronic inflammation. J. Clin. Lab. Anal. 2008, 22, 6–13. [Google Scholar] [CrossRef]
- Multhoff, G.; Molls, M.; Radons, J. Chronic inflammation in cancer development. Front. Immunol. 2011, 2, 98. [Google Scholar] [CrossRef]
- Esmaillzadeh, A.; Kimiagar, M.; Mehrabi, Y.; Azadbakht, L.; Hu, F.B.; Willett, W.C. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am. J. Clin. Nutr. 2007, 85, 910–918. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106 (Suppl. 3), S5–S78. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Chen, J.; Ding, X.; Wu, R.; Tong, B.; Zhao, L.; Lv, H.; Meng, X.; Liu, Y.; Ren, B.; Li, J.; et al. Novel Sesquiterpene Glycoside from Loquat Leaf Alleviates Type 2 Diabetes Mellitus Combined with Nonalcoholic Fatty Liver Disease by Improving Insulin Resistance, Oxidative Stress, Inflammation, and Gut Microbiota Composition. J. Agric. Food Chem. 2021, 69, 14176–14191. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Kim, M.-S.; Chun, S.-S.; Choi, J.-H. Effects of turmeric (Curcuma longa L.) on antioxidative systems and oxidative damage in rats fed a high fat and cholesterol diet. J. Korean Soc. Food Sci. Nutr. 2013, 42, 570–576. [Google Scholar] [CrossRef]
- Niki, E.; Yoshida, Y.; Saito, Y.; Noguchi, N. Lipid peroxidation: Mechanisms, inhibition, and biological effects. Biochem. Biophys. Res. Commun. 2005, 338, 668–676. [Google Scholar] [CrossRef]
- Micha, R.; Mozaffarian, D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: A fresh look at the evidence. Lipids 2010, 45, 893–905. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Nutrition, immunity and COVID-19. BMJ Nutr. Prev. Health 2020, 3, 74–92. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Abuaysheh, S.; Sia, C.L.; Korzeniewski, K.; Chaudhuri, A.; Fernandez-Real, J.M.; Dandona, P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: Implications for insulin resistance. Diabetes Care 2009, 32, 2281–2287. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. TLR signaling pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Uribarri, J. Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr. Diabetes Rep. 2014, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Birlouez-Aragon, I.; Saavedra, G.; Tessier, F.J.; Galinier, A.; Ait-Ameur, L.; Lacoste, F.; Niamba, C.N.; Alt, N.; Somoza, V.; Lecerf, J.M. A diet based on high-heat-treated foods promotes risk factors for diabetes mellitus and cardiovascular diseases. Am. J. Clin. Nutr. 2010, 91, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxid. Med. Cell Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Stadler, R.H.; Theurillat, V. Heat-generated toxicants in foods (acrylamide, MCPD esters, glycidyl esters, furan, and related compounds). In Chemical Contaminants and Residues in Food; Elsevier: Amsterdam, The Netherlands, 2017; pp. 171–195. [Google Scholar]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916.e12. [Google Scholar] [CrossRef]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Cani, P.D.; Knauf, C. How gut microbes talk to organs: The role of endocrine and nervous routes. Mol. Metab. 2016, 5, 743–752. [Google Scholar] [CrossRef]
- Świątecka, D.; Narbad, A.; Ridgway, K.P.; Kostyra, H. The study on the impact of glycated pea proteins on human intestinal bacteria. Int. J. Food Microbiol. 2011, 145, 267–272. [Google Scholar]
- De Bandt, J.-P.; Waligora-Dupriet, A.-J.; Butel, M.-J. Intestinal microbiota in inflammation and insulin resistance: Relevance to humans. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 334–340. [Google Scholar] [CrossRef]
- Kasai, C.; Sugimoto, K.; Moritani, I.; Tanaka, J.; Oya, Y.; Inoue, H.; Tameda, M.; Shiraki, K.; Ito, M.; Takei, Y.; et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Finucane, M.M.; Sharpton, T.J.; Laurent, T.J.; Pollard, K.S. A taxonomic signature of obesity in the microbiome? Getting to the guts of the matter. PLoS ONE 2014, 9, e84689. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Beam, A.; Clinger, E.; Hao, L. Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef]
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef]
- Astudillo-López, C.C.; Castro-Alarcón, N.; Ariza, A.C.; Muñoz-Valle, J.F.; de la Cruz-Mosso, U.; Flores-Alfaro, E.; del Moral-Hernández, O.; Moreno-Godínez, M.E.; Ramírez-Vargas, M.A.; Matia-Garcia, I.; et al. Influence of Diet and Levels of Zonulin, Lipopolysaccharide and C-Reactive Protein on Cardiometabolic Risk Factors in Young Subjects. Nutrients 2021, 13, 4472. [Google Scholar] [CrossRef]
- Celiberto, L.; Graef, F.; Healey, G.; Bosman, E.S.; Jacobson, K.; Sly, L.M.; Vallance, B. Inflammatory bowel disease and immunonutrition: Novel therapeutic approaches through modulation of diet and the gut microbiome. Immunology 2018, 155, 36–52. [Google Scholar] [CrossRef]
- Tsigalou, C.; Konstantinidis, T.; Paraschaki, A.; Stavropoulou, E.; Voidarou, C.; Bezirtzoglou, E. Mediterranean Diet as a Tool to Combat Inflammation and Chronic Diseases. An Overview. Biomedicines 2020, 8, 201. [Google Scholar] [CrossRef]
- Illescas, O.; Rodríguez-Sosa, M.; Gariboldi, M. Mediterranean Diet to Prevent the Development of Colon Diseases: A Meta-Analysis of Gut Microbiota Studies. Nutrients 2021, 13, 2234. [Google Scholar] [CrossRef]
- Bottero, V.; Potashkin, J.A. A Comparison of Gene Expression Changes in the Blood of Individuals Consuming Diets Supplemented with Olives, Nuts or Long-Chain Omega-3 Fatty Acids. Nutrients 2020, 12, 3765. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Ramos-Campo, D.J.; Mielgo-Ayuso, J.; Dalamitros, A.A.; Nikolaidis, P.A.; Hormeño-Holgado, A.; Tornero-Aguilera, J.F. Nutrition in the Actual COVID-19 Pandemic. A Narrative Review. Nutrients 2021, 13, 1924. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Hullar MA, J.; Fu, B.C. Diet, the gut microbiome, and epigenetics. Cancer J. 2014, 20, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.S.; Koller, K.R.; Ramaboli, M.C.; Nesengani, L.T.; Ocvirk, S.; Chen, C.; Flanagan, C.A.; Sapp, F.R.; Merritt, Z.T.; Bhatti, F.; et al. Diet and the Human Gut Microbiome: An International Review. Dig. Dis. Sci. 2020, 65, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.Y.; Devkota, S.; Moscoso, D.; Chang, E.B.; Leone, V.A. The role of diet in triggering human inflammatory disorders in the modern age. Microbes Infect. 2013, 15, 765–774. [Google Scholar] [CrossRef]
- Shi, Z. Gut Microbiota: An Important Link between Western Diet and Chronic Diseases. Nutrients 2019, 11, 2287. [Google Scholar] [CrossRef]
- Srour, B.; Kordahi, M.C.; Bonazzi, E.; Deschasaux-Tanguy, M.; Touvier, M.; Chassaing, B. Ultra-processed foods and human health: From epidemiological evidence to mechanistic insights. Lancet Gastroenterol. Hepatol. 2022, 7, 1128–1140. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.-C.; Louzada, M.L.C.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Schnabel, L.; Kesse-Guyot, E.; Allès, B.; Touvier, M.; Srour, B.; Hercberg, S.; Buscail, C.; Julia, C. Association Between Ultraprocessed Food Consumption and Risk of Mortality Among Middle-aged Adults in France. JAMA Intern. Med. 2019, 179, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Machado, P.P.; Steele, E.M.; Levy, R.B.; Sui, Z.; Rangan, A.; Woods, J.; Gill, T.; Scrinis, G.; Monteiro, C.A. Ultra-processed foods and recommended intake levels of nutrients linked to non-communicable diseases in Australia: Evidence from a nationally representative cross-sectional study. BMJ Open 2019, 9, e029544. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, X.; Wang, W.; Zhang, D. Consumption of vegetables and fruit and the risk of inflammatory bowel disease: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2015, 27, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.M.; Gamage, E.; Travica, N.; Dissanayaka, T.; Ashtree, D.N.; Gauci, S.; Lotfaliany, M.; O’Neil, A.; Jacka, F.N.; Marx, W. Ultra-Processed Food Consumption and Mental Health: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2022, 14, 2568. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef]
- Frankenfeld, C.L.; Sikaroodi, M.; Lamb, E.; Shoemaker, S.; Gillevet, P.M. High-intensity sweetener consumption and gut microbiome content and predicted gene function in a cross-sectional study of adults in the United States. Ann. Epidemiol. 2015, 25, 736–742.e4. [Google Scholar] [CrossRef]
- Ketnawa, S.; Reginio, F.C., Jr.; Thuengtung, S.; Ogawa, Y. Changes in bioactive compounds and antioxidant activity of plant-based foods by gastrointestinal digestion: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 4684–4705. [Google Scholar] [CrossRef]
- Redondo-Useros, N.; Nova, E.; González-Zancada, N.; Díaz, L.E.; Gómez-Martínez, S.; Marcos, A. Microbiota and Lifestyle: A Special Focus on Diet. Nutrients 2020, 12, 1776. [Google Scholar] [CrossRef]
- Ray, S.K.; Mukherjee, S. Evolving Interplay Between Dietary Polyphenols and Gut Microbiota—An Emerging Importance in Healthcare. Front. Nutr. 2021, 8, 634944. [Google Scholar] [CrossRef]
- Wu, T.; Grootaert, C.; Pitart, J.; Vidovic, N.K.; Kamiloglu, S.; Possemiers, S.; Glibetic, M.; Smagghe, G.; Raes, K.; Van de Wiele, T.; et al. Aronia (Aronia melanocarpa) Polyphenols Modulate the Microbial Community in a Simulator of the Human Intestinal Microbial Ecosystem (SHIME) and Decrease Secretion of Proinflammatory Markers in a Caco-2/endothelial Cell Coculture Model. Mol. Nutr. Food Res. 2018, 62, e1800607. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front Nutr 2018, 5, 87. [Google Scholar] [CrossRef]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2011, 23, 255–264.e119. [Google Scholar] [CrossRef] [PubMed]
- Martín-Rodríguez, A.; Bustamante-Sánchez, Á; Martínez-Guardado, I.; Navarro-Jiménez, E.; Plata-SanJuan, E.; Tornero-Aguilera, J.F.; Clemente-Suárez, V.J. Infancy Dietary Patterns, Development, and Health: An Extensive Narrative Review. Children 2022, 9, 1072. [Google Scholar] [CrossRef] [PubMed]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for The Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- García-García, F.J.; Monistrol-Mula, A.; Cardellach, F.; Garrabou, G. Nutrition, Bioenergetics, and Metabolic Syndrome. Nutrients 2020, 12, 2785. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, J.; Burtscher, M.; Millet, G.P. The central role of mitochondrial fitness on antiviral defenses: An advocacy for physical activity during the COVID-19 pandemic. Redox Biol. 2021, 43, 101976. [Google Scholar] [CrossRef]
- Bach, D.; Pich, S.; Soriano, F.X.; Vega, N.; Baumgartner, B.; Oriola, J.; Daugaard, J.R.; Lloberas, J.; Camps, M.; Zierath, J.R.; et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J. Biol. Chem. 2003, 278, 17190–17197. [Google Scholar] [CrossRef]
- Yang, A.; Mottillo, E.P. Adipocyte lipolysis: From molecular mechanisms of regulation to disease and therapeutics. Biochem. J. 2020, 477, 985–1008. [Google Scholar] [CrossRef]
- Chen, Z.; Tao, S.; Li, X.; Yao, Q. Resistin destroys mitochondrial biogenesis by inhibiting the PGC-1α/NRF1/TFAM signaling pathway. Biochem. Biophys. Res. Commun. 2018, 504, 13–18. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kadowaki, T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int. J. Obes. 2008, 32 (Suppl. 7), S13–S18. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nature reviews. Immunology 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Sparks, L.M.; Xie, H.; Koza, R.A.; Mynatt, R.; Hulver, M.W.; Bray, G.A.; Smith, S.R. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 2005, 54, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, X.; Zhang, L.; Zhu, M.; Gao, L. A high-fat diet impairs mitochondrial biogenesis, mitochondrial dynamics, and the respiratory chain complex in rat myocardial tissues. J. Cell. Biochem. 2018, 119, 9602. [Google Scholar] [CrossRef]
- Hancock, C.R.; Han, D.H.; Chen, M.; Terada, S.; Yasuda, T.; Wright, D.C.; Holloszy, J.O. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc. Natl. Acad. Sci. USA 2008, 105, 7815–7820. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, L.; Mollica, M.P.; Donizzetti, I.; Gifuni, G.; Sica, R.; Pignalosa, A.; Cavaliere, G.; Gaita, M.; De Filippo, C.; Zorzano, A.; et al. High-lard and high-fish-oil diets differ in their effects on function and dynamic behaviour of rat hepatic mitochondria. PLoS ONE 2014, 9, e92753. [Google Scholar] [CrossRef]
- Li, N.; Li, H.P.; Zhang, B.Y.; Zhang, L.; Shen, J.M.; Li, Q.Y. Effect of high-fat diet on respiratory function and diaphragm fibers in mice and its mitochondrial mechanism. Zhonghua Yi Xue Za Zhi 2021, 101, 2893–2899. [Google Scholar] [PubMed]
- Jheng, H.-F.; Tsai, P.-J.; Guo, S.-M.; Kuo, L.-H.; Chang, C.-S.; Su, I.-J.; Chang, C.-R.; Tsai, Y.-S. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol. Cell. Biol. 2012, 32, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Robotham, J.L.; Yoon, Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl. Acad. Sci. USA 2006, 103, 2653–2658. [Google Scholar] [CrossRef]
- Tarpey, M.D.; Davy, K.P.; McMillan, R.P.; Bowser, S.M.; Halliday, T.M.; Boutagy, N.E.; Davy, B.M.; Frisard, M.I.; Hulver, M.W. Skeletal muscle autophagy and mitophagy in endurance-trained runners before and after a high-fat meal. Mol. Metab. 2017, 6, 1597–1609. [Google Scholar] [CrossRef]
- Tong, M.; Saito, T.; Zhai, P.; Oka, S.; Mizushima, W.; Nakamura, M.; Sadoshima, J. Mitophagy Is Essential for Maintaining Cardiac Function During High Fat Diet-Induced Diabetic Cardiomyopathy. Circ. Res. 2019, 124, 1360–1371. [Google Scholar] [CrossRef]
- Shao, D.; Kolwicz, S.C., Jr.; Wang, P.; Roe, N.D.; Villet, O.; Nishi, K.; Hsu, Y.-W.A.; Flint, G.V.; Caudal, A.; Wang, W.; et al. Increasing Fatty Acid Oxidation Prevents High-Fat Diet-Induced Cardiomyopathy through Regulating Parkin-Mediated Mitophagy. Circulation 2020, 142, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Psaltopoulou, T.; Hatzis, G.; Papageorgiou, N.; Androulakis, E.; Briasoulis, A.; Tousoulis, D. Socioeconomic status and risk factors for cardiovascular disease: Impact of dietary mediators. Hell. J. Cardiol. HJC Hell. Kardiol. Ep. 2017, 58, 32–42. [Google Scholar] [CrossRef]
- Méjean, C.; Droomers, M.; van der Schouw, Y.T.; Sluijs, I.; Czernichow, S.; Grobbee, D.E.; Bueno-De-Mesquita, H.B.; Beulens, J.W. The contribution of diet and lifestyle to socioeconomic inequalities in cardiovascular morbidity and mortality. Int. J. Cardiol. 2013, 168, 5190–5195. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; AlAbdulghafoor, F.K.; Summerbell, C.D.; Worthington, H.V.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 7, CD003177. [Google Scholar] [PubMed]
- Cho, S.S.; Qi, L.; Fahey, G.C.; Klurfeld, D.M. Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. Am. J. Clin. Nutr. 2013, 98, 594–619. [Google Scholar] [CrossRef] [PubMed]
- Hartley, L.; Igbinedion, E.; Holmes, J.; Flowers, N.; Thorogood, M.; Clarke, A.; Stranges, S.; Hooper, L.; Rees, K. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst. Rev. 2013, 2013, CD009874. [Google Scholar] [PubMed]
- Wallace, T.C.; Murray, R.; Zelman, K.M. The Nutritional Value and Health Benefits of Chickpeas and Hummus. Nutrients 2016, 8, 766. [Google Scholar] [CrossRef]
- Salehin, S.; Rasmussen, P.; Mai, S.; Mushtaq, M.; Agarwal, M.; Hasan, S.M.; Salehin, S.; Raja, M.; Gilani, S.; Khalife, W.I. Plant Based Diet and Its Effect on Cardiovascular Disease. Int. J. Environ. Res. Public Health 2023, 20, 3337. [Google Scholar] [CrossRef]
- Ghosh, J.C.; Perego, M.; Agarwal, E.; Bertolini, I.; Wang, Y.; Goldman, A.R.; Tang, H.-Y.; Kossenkov, A.V.; Landis, C.J.; Languino, L.R.; et al. Ghost mitochondria drive metastasis through adaptive GCN2/Akt therapeutic vulnerability. Proc. Natl. Acad. Sci. USA 2022, 119, e2115624119. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Artinian, N.T.; Fletcher, G.F.; Mozaffarian, D.; Kris-Etherton, P.; Van Horn, L.; Lichtenstein, A.H.; Kumanyika, S.; Kraus, W.E.; Fleg, J.L.; Nancy, S.; et al. Redeker Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: A scientific statement from the American Heart Association. Circulation 2010, 122, 406–441. [Google Scholar] [CrossRef]
- Anand, S.S.; Hawkes, C.; De Souza, R.J.; Mente, A.; Dehghan, M.; Nugent, R.; Zulyniak, M.A.; Weis, T.; Bernstein, A.M.; Krauss, R.M.; et al. Food Consumption and its Impact on Cardiovascular Disease: Importance of Solutions Focused on the Globalized Food System: A Report From the Workshop Convened by the World Heart Federation. J. Am. Coll. Cardiol. 2015, 66, 1590–1614. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Ludwig, D.S. Dietary guidelines in the 21st century--a time for food. JAMA 2010, 304, 681–682. [Google Scholar] [CrossRef] [PubMed]
- Bowen, K.J.; Sullivan, V.K.; Kris-Etherton, P.M.; Petersen, K.S. Nutrition and cardiovascular disease—an update. Curr. Atheroscler. Rep. 2018, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Interleukin-1 Beta as a Target for Atherosclerosis Therapy: Biological Basis of CANTOS and Beyond. J. Am. Coll. Cardiol. 2017, 70, 2278–2289. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Gerdes, N.; Winkels, H. ATVB Distinguished Scientist Award: How Costimulatory and Coinhibitory Pathways Shape Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Joner, M.; Sakakura, K. Recent highlights of ATVB: Calcification. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Low Wang, C.C.; Hess, C.N.; Hiatt, W.R.; Goldfine, A.B. Clinical Update: Cardiovascular Disease in Diabetes Mellitus: Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus—Mechanisms, Management, and Clinical Considerations. Circulation 2016, 133, 2459–2502. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Fernandez-Gandara, C.; Garcia-Rios, A.; Rangel-Zuñiga, O.A.; Gutierrez-Mariscal, F.M.; Torres-Peña, J.D.; Marin, C.; Lopez-Moreno, J.; Castaño, J.P.; Delgado-Lista, J.; et al. Mediterranean diet and endothelial function in patients with coronary heart disease: An analysis of the CORDIOPREV randomized controlled trial. PLoS Med. 2020, 17, e1003282. [Google Scholar] [CrossRef]
- Usui, F.; Kimura, H.; Ohshiro, T.; Tatsumi, K.; Kawashima, A.; Nishiyama, A.; Iwakura, Y.-I.; Ishibashi, S.; Takahashi, M. Interleukin-17 deficiency reduced vascular inflammation and development of atherosclerosis in Western diet-induced apoE-deficient mice. Biochem. Biophys. Res. Commun. 2012, 420, 72–77. [Google Scholar] [CrossRef]
- Denova-Gutiérrez, E.; Tucker, K.L.; Flores, M.; Barquera, S.; Salmerón, J. Dietary Patterns Are Associated with Predicted Cardiovascular Disease Risk in an Urban Mexican Adult Population. J. Nutr. 2016, 146, 90–97. [Google Scholar] [CrossRef]
- Atkins, J.L.; Whincup, P.H.; Morris, R.W.; Lennon, L.T.; Papacosta, O.; Wannamethee, S.G. Dietary patterns and the risk of CVD and all-cause mortality in older British men. Br. J. Nutr. 2016, 116, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.; Psaltopoulou, T.; Georgiopoulos, G.; Siasos, G.; Kokkou, E.; Antonopoulos, A.; Vogiatzi, G.; Tsalamandris, S.; Gennimata, V.; Papanikolaou, A.; et al. Western Dietary Pattern Is Associated with Severe Coronary Artery Disease. Angiology 2018, 69, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Rees, K.; Takeda, A.; Martin, N.; Ellis, L.; Wijesekara, D.; Vepa, A.; Das, A.; Hartley, L.; Stranges, S. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2019, 3, CD009825. [Google Scholar] [CrossRef] [PubMed]
- Viscogliosi, G.; Cipriani, E.; Liguori, M.L.; Marigliano, B.; Saliola, M.; Ettorre, E.; Andreozzi, P.; Georgousopoulou, E.N.; D’Cunha, N.M.; Mellor, D.D.; et al. Mediterranean dietary pattern adherence: Associations with prediabetes, metabolic syndrome, and related microinflammation. Metab. Syndr. Relat. Disord. 2013, 11, 210–216. [Google Scholar] [CrossRef]
- Martín-Peláez, S.; Fito, M.; Castaner, O. Mediterranean Diet Effects on Type 2 Diabetes Prevention, Disease Progression, and Related Mechanisms. A Review. Nutrients 2020, 12, 2236. [Google Scholar] [CrossRef]
- Badimon, L.; Chagas, P.; Chiva-Blanch, G. Diet and Cardiovascular Disease: Effects of Foods and Nutrients in Classical and Emerging Cardiovascular Risk Factors. Curr. Med. Chem. 2019, 26, 3639–3651. [Google Scholar] [CrossRef]
- Casas, R.; Castro-Barquero, S.; Estruch, R.; Sacanella, E. Nutrition and Cardiovascular Health. Int. J. Mol. Sci. 2018, 19, 3988. [Google Scholar] [CrossRef]
- Gao, M.; Jebb, S.A.; Aveyard, P.; Ambrosini, G.L.; Perez-Cornago, A.; Carter, J.; Sun, X.; Piernas, C. Associations between dietary patterns and the incidence of total and fatal cardiovascular disease and all-cause mortality in 116,806 individuals from the UK Biobank: A prospective cohort study. BMC Med. 2021, 19, 83. [Google Scholar] [CrossRef]
- Paglia, L. The sweet danger of added sugars. Eur. J. Paediatr. Dent. 2019, 20, 89. [Google Scholar]
- Khan, T.A.; Tayyiba, M.; Agarwal, A.; Mejia, S.B.; de Souza, R.J.; Wolever, T.M.; Leiter, L.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Sievenpiper, J.L. Relation of Total Sugars, Sucrose, Fructose, and Added Sugars with the Risk of Cardiovascular Disease: A Systematic Review and Dose-Response Meta-analysis of Prospective Cohort Studies. Mayo Clin. Proc. 2019, 94, 2399–2414. [Google Scholar] [CrossRef]
- Batista, E.S.; da Silva Rios, T.; Muñoz, V.R.; Jesus, J.S.; Vasconcelos, M.M.; da Cunha, D.T.; Marques-Rocha, J.L.; Nakandakari, S.C.B.R.; Lara, R.; da Silva, A.S.R.; et al. Omega-3 mechanism of action in inflammation and endoplasmic reticulum stress in mononuclear cells from overweight non-alcoholic fatty liver disease participants: Study protocol for the ‘Brazilian Omega Study’ (BROS)-a randomized controlled trial. Trials 2021, 22, 927. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Silveira BK, S.; Oliveira TM, S.; Andrade, P.A.; Hermsdorff HH, M.; Rosa CD, O.B.; Franceschini SD, C.C. Dietary Pattern and Macronutrients Profile on the Variation of Inflammatory Biomarkers: Scientific Update. Cardiol. Res. Pract. 2018, 2018, 4762575. [Google Scholar] [PubMed]
- Dinu, M.; Abbate, R.; Gensini, G.F.; Casini, A.; Sofi, F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3640–3649. [Google Scholar] [CrossRef]
- Zampelas, A.; Magriplis, E. Dietary patterns and risk of cardiovascular diseases: A review of the evidence. Proc. Nutr. Soc. 2020, 79, 68–75. [Google Scholar] [CrossRef]
- Zhong, V.W.; Van Horn, L.; Cornelis, M.C.; Wilkins, J.T.; Ning, H.; Carnethon, M.R.; Greenland, P.; Mentz, R.J.; Tucker, K.L.; Zhao, L.; et al. Associations of Dietary Cholesterol or Egg Consumption with Incident Cardiovascular Disease and Mortality. JAMA 2019, 321, 1081–1095. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Newman, J.D.; Woolf, K. Anti-Inflammatory Effects of a Vegan Diet Versus the American Heart Association-Recommended Diet in Coronary Artery Disease Trial. J. Am. Heart Assoc. 2018, 7, e011367. [Google Scholar] [CrossRef]
- Djekic, D.; Shi, L.; Brolin, H.; Carlsson, F.; Särnqvist, C.; Savolainen, O.; Cao, Y.; Bäckhed, F.; Tremaroli, V.; Landberg, R.; et al. Effects of a Vegetarian Diet on Cardiometabolic Risk Factors, Gut Microbiota, and Plasma Metabolome in Subjects with Ischemic Heart Disease: A Randomized, Crossover Study. J. Am. Heart Assoc. 2020, 9, e016518. [Google Scholar] [CrossRef] [PubMed]
- Djekic, D.; Shi, L.; Calais, F.; Carlsson, F.; Landberg, R.; Hyötyläinen, T.; Frøbert, O. Effects of a Lacto-Ovo-Vegetarian Diet on the Plasma Lipidome and Its Association with Atherosclerotic Burden in Patients with Coronary Artery Disease-A Randomized, Open-Label, Cross-over Study. Nutrients 2020, 12, 3586. [Google Scholar] [CrossRef]
- Mishra, S.; Xu, J.; Agarwal, U.; Gonzales, J.; Levin, S.; Barnard, N.D. A multicenter randomized controlled trial of a plant-based nutrition program to reduce body weight and cardiovascular risk in the corporate setting: The GEICO study. Eur. J. Clin. Nutr. 2013, 67, 718–724. [Google Scholar] [CrossRef]
- Turner-McGrievy, G.M.; Wilcox, S.; Frongillo, E.A.; Murphy, E.A.; Hutto, B.; Wilson, M.; Davey, M.; Bernhart, J.A.; Okpara, N.; Bailey, S.; et al. Effect of a Plant-Based vs Omnivorous Soul Food Diet on Weight and Lipid Levels Among African American Adults. JAMA Netw. Open 2023, 6, e2250626. [Google Scholar] [CrossRef]
- Wright, N.; Wilson, L.; Smith, M.; Duncan, B.; McHugh, P. The BROAD study: A randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr. Diabetes 2017, 7, e256. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Wong, J.M.W.; Kendall, C.W.C.; Esfahani, A.; Ng, V.W.Y.; Leong, T.C.K.; A Faulkner, D.; Vidgen, E.; Paul, G.; Mukherjea, R.; et al. Effect of a 6-month vegan low-carbohydrate (‘Eco-Atkins’) diet on cardiovascular risk factors and body weight in hyperlipidaemic adults: A randomised controlled trial. BMJ Open 2014, 4, e003505. [Google Scholar] [CrossRef]
- Carson, J.A.S.; Lichtenstein, A.H.; Anderson, C.A.; Appel, L.J.; Kris-Etherton, P.M.; Meyer, K.A.; Petersen, K.; Polonsky, T.; Van Horn, L.; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; et al. Dietary cholesterol and cardiovascular risk: A science advisory from the American Heart Association. Circulation 2020, 141, e39–e53. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, A.; Matsuda, K.; Kuwahara, Y.; Asano, S.; Inui, T.; Marunaka, Y. Microbiota-gut-brain axis: Enteroendocrine cells and the enteric nervous system form an interface between the microbiota and the central nervous system. Biomed. Res. 2020, 41, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Bienenstock, J.; Kunze, W.; Forsythe, P. Microbiota and the gut-brain axis. Nutr. Rev. 2015, 73 (Suppl. 1), 28–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-X.; Wang, Y.-P. Gut Microbiota-brain Axis. Chin. Med. J. 2016, 129, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Dowling, L.R.; Strazzari, M.R.; Keely, S.; Kaiko, G.E. Enteric nervous system and intestinal epithelial regulation of the gut-brain axis. J. Allergy Clin. Immunol. 2022, 150, 513–522. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Cussotto, S.; Sandhu, K.V.; Dinan, T.G.; Cryan, J.F. The Neuroendocrinology of the Microbiota-Gut-Brain Axis: A Behavioural Perspective. Front. Neuroendocrinol. 2018, 51, 80–101. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Valsamakis, G.; Mastorakos, G.; Hanson, P.; Kyrou, I.; Randeva, H.S.; Weickert, M.O. Dietary Influences on the Microbiota–Gut–Brain Axis. Int. J. Mol. Sci. 2021, 22, 3502. [Google Scholar] [CrossRef] [PubMed]
- Durgan, D.J.; Lee, J.; McCullough, L.D.; Bryan RM, J. Examining the Role of the Microbiota-Gut-Brain Axis in Stroke. Stroke 2019, 50, 2270–2277. [Google Scholar] [CrossRef] [PubMed]
- Rönnbäck, C.; Hansson, E. The Importance and Control of Low-Grade Inflammation Due to Damage of Cellular Barrier Systems That May Lead to Systemic Inflammation. Front. Neurol. 2019, 10, 533. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 2014, 63, 116–124. [Google Scholar] [CrossRef]
- Rohleder, N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom. Med. 2014, 76, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.A. Dysbiosis. In The Microbiota in Gastrointestinal Pathophysiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 227–232. [Google Scholar]
- Noble, E.E.; Hsu, T.M.; Kanoski, S.E. Gut to Brain Dysbiosis: Mechanisms Linking Western Diet Consumption, the Microbiome, and Cognitive Impairment. Front. Behav. Neurosci. 2017, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Stopińska, K.; Radziwoń-Zaleska, M.; Domitrz, I. The Microbiota-Gut-Brain Axis as a Key to Neuropsychiatric Disorders: A Mini Review. J. Clin. Med. 2021, 10, 4640. [Google Scholar] [CrossRef]
- Shahda, M.; El-Sayed, A. Study of the prevalence of metabolic syndrome among psychiatric patients and its correlation with diagnosis and medications. Egypt. J. Psychiatry 2010, 31, n2. [Google Scholar]
- Mousa, F.A.; Dessoki, H.H.; El Kateb, S.M.; Ezzat, A.A.; Soltan, M.R. Metabolic syndrome in psychiatric patients (comparative study). Egypt. J. Psychiatry 2017, 38, 179. [Google Scholar]
- Penninx, B.W.J.H.; Lange, S.M.M. Metabolic syndrome in psychiatric patients: Overview, mechanisms, and implications. Dialogues Clin. Neurosci. 2018, 20, 63–73. [Google Scholar] [CrossRef]
- De Leon, J. Metabolic Syndrome and Psychiatric Illness: Interactions, Pathophysiology, Assessment, and Treatment. Am. J. Psychiatry 2008, 165, 1056–1057. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, K.; Kwak, M.H.; Kim, H.J.; Kim, H.-S.; Han, K.H. The contribution of abdominal obesity and dyslipidemia to metabolic syndrome in psychiatric patients. Korean J. Intern. Med. 2010, 25, 168–173. [Google Scholar] [CrossRef]
- Fabbri, C.; Corponi, F.; Albani, D.; Raimondi, I.; Forloni, G.; Schruers, K.; Kasper, S.; Kautzky, A.; Zohar, J.; Souery, D.; et al. Pleiotropic genes in psychiatry: Calcium channels and the stress-related FKBP5 gene in antidepressant resistance. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 81, 203–210. [Google Scholar] [CrossRef]
- Lee, P.H.; Feng, Y.-C.A.; Smoller, J.W. Pleiotropy and Cross-Disorder Genetics Among Psychiatric Disorders. Biol. Psychiatry 2021, 89, 20–31. [Google Scholar] [CrossRef]
- Gorwood, P. Generalized anxiety disorder and major depressive disorder comorbidity: An example of genetic pleiotropy? Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2004, 19, 27–33. [Google Scholar] [CrossRef]
- Zheutlin, A.B.; Dennis, J.; Linnér, R.K.; Moscati, A.; Restrepo, N.; Straub, P.; Ruderfer, D.; Castro, V.M.; Chen, C.-Y.; Ge, T.; et al. Penetrance and Pleiotropy of Polygenic Risk Scores for Schizophrenia in 106,160 Patients Across Four Health Care Systems. Am. J. Psychiatry 2019, 176, 846–855. [Google Scholar] [CrossRef]
- Torrico, B.; Shaw, A.D.; Mosca, R.; Vivó-Luque, N.; Hervás, A.; Fernàndez-Castillo, N.; Aloy, P.; Bayés, M.; Fullerton, J.M.; Cormand, B.; et al. Truncating variant burden in high-functioning autism and pleiotropic effects of LRP1 across psychiatric phenotypes. J. Psychiatry Neurosci. JPN 2019, 44, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Tu, H.; Chen, T. The Microbiota-Gut-Brain Axis in Depression: The Potential Pathophysiological Mechanisms and Microbiota Combined Antidepression Effect. Nutrients 2022, 14, 2081. [Google Scholar] [CrossRef] [PubMed]
- Hamamah, S.; Aghazarian, A.; Nazaryan, A.; Hajnal, A.; Covasa, M. Role of Microbiota-Gut-Brain Axis in Regulating Dopaminergic Signaling. Biomedicines 2022, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P.; Farzi, A. Neuropeptides and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014, 817, 195–219. [Google Scholar]
- Xie, Y.; Zhou, G.; Wang, C.; Xu, X.; Li, C. Specific Microbiota Dynamically Regulate the Bidirectional Gut-Brain Axis Communications in Mice Fed Meat Protein Diets. J. Agric. Food Chem. 2019, 67, 1003–1017. [Google Scholar] [CrossRef]
- Walker, W.A.; Duffy, L.C. Diet and bacterial colonization: Role of probiotics and prebiotics. J. Nutr. Biochem. 1998, 9, 668–675. [Google Scholar] [CrossRef]
- Douglas, L.C.; Sanders, M.E. Probiotics and prebiotics in dietetics practice. J. Am. Diet. Assoc. 2008, 108, 510–521. [Google Scholar] [CrossRef]
- Green, M.; Arora, K.; Prakash, S. Microbial Medicine: Prebiotic and Probiotic Functional Foods to Target Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 2890. [Google Scholar] [CrossRef]
- Muir, J.G.; Lu, Z.X.; Young, G.P.; Cameron-Smith, D.; Collier, G.R.; O’dea, K. Resistant starch in the diet increases breath hydrogen and serum acetate in human subjects. Am. J. Clin. Nutr. 1995, 61, 792–799. [Google Scholar] [CrossRef]
- Popova, M.; Martin, C.; Eugène, M.; Mialon, M.; Doreau, M.; Morgavi, D. Effect of fibre-and starch-rich finishing diets on methanogenic Archaea diversity and activity in the rumen of feedlot bulls. Anim. Feed. Sci. Technol. 2011, 166, 113–121. [Google Scholar] [CrossRef]
- Giacco, R.; Clemente, G.; Luongo, D.; Lasorella, G.; Fiume, I.; Brouns, F.; Bornet, F.; Patti, L.; Cipriano, P.; Rivellese, A.A.; et al. Effects of short-chain fructo-oligosaccharides on glucose and lipid metabolism in mild hypercholesterolaemic individuals. Clin. Nutr. 2004, 23, 331–340. [Google Scholar] [CrossRef]
- Nanno, M.; Matsumoto, S.; Shida, K. Lactobacillus casei strain Shirota: Benefits based on a long history of usage. In ECAB Health Impact of Probiotics: Vision & Opportunities-E-Book; Elsevier: Bengaluru, India, 2014; p. 85. [Google Scholar]
- Weichselbaum, E. Potential benefits of probiotics--main findings of an in-depth review. Br. J. Community Nurs. 2010, 15, 110–114. [Google Scholar] [CrossRef]
- Pimentel, T.C.; Brandão, L.R.; de Oliveira, M.P.; da Costa WK, A.; Magnani, M. Health benefits and technological effects of Lacticaseibacillus casei-01: An overview of the scientific literature. Trends Food Sci. Technol. 2021, 114, 722–737. [Google Scholar] [CrossRef]
- Foster, L.M.; Tompkins, T.A.; Dahl, W.J. A comprehensive post-market review of studies on a probiotic product containing Lactobacillus helveticus R0052 and Lactobacillus rhamnosus R0011. Benef. Microbes 2011, 2, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Taverniti, V.; Guglielmetti, S. Health-Promoting Properties of Lactobacillus helveticus. Front. Microbiol. 2012, 3, 392. [Google Scholar] [CrossRef]
- Benno, Y.; Mitsuoka, T. Impact of Bifidobacterium longum on human fecal microflora. Microbiol. Immunol. 1992, 36, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium longum subspecies infantis: Champion colonizer of the infant gut. Pediatr. Res. 2015, 77, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Abhari, K.; Hosseini, H. Psychobiotics: Next Generation Treatment for Mental Disorders. J. Clin. Nutr. Diet 2018, 4, 1–2. [Google Scholar] [CrossRef]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Halton, T.L.; Willett, W.C.; Liu, S.; E Manson, J.; Stampfer, M.J.; Hu, F.B. Potato and french fry consumption and risk of type 2 diabetes in women. Am. J. Clin. Nutr. 2006, 83, 284–290. [Google Scholar] [CrossRef]
- Shan, Z.; Rehm, C.D.; Rogers, G. Trends in Dietary Carbohydrate, Protein, and Fat Intake and Diet Quality Among US Adults, 1999–2016. JAMA 2019, 322, 1178–1187. [Google Scholar] [CrossRef]
- Jain, A.P.; Aggarwal, K.K.; Zhang, P.-Y. Omega-3 fatty acids and cardiovascular disease. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 441–445. [Google Scholar]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef]
- Dreher, M.L. Whole Fruits and Fruit Fiber Emerging Health Effects. Nutrients 2018, 10, 1833. [Google Scholar] [CrossRef] [PubMed]
- Lonnie, M.; Hooker, E.; Brunstrom, J.M.; Corfe, B.M.; Green, M.A.; Watson, A.W.; Williams, E.A.; Stevenson, E.J.; Penson, S.; Johnstone, A.M. Protein for Life: Review of Optimal Protein Intake, Sustainable Dietary Sources and the Effect on Appetite in Ageing Adults. Nutrients 2018, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-T.; Liao, W.; Yu, H.-J.; Liu, M.-W.; Yuan, S.; Tang, B.-W.; Yang, X.-H.; Song, Y.; Huang, Y.; Cheng, S.-L.; et al. Combined effects of fruit and vegetables intake and physical activity on the risk of metabolic syndrome among Chinese adults. PLoS ONE 2017, 12, e0188533. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Black, P.H. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav. Immun. 2003, 17, 350–364. [Google Scholar] [CrossRef]
- Drews, G.; Krippeit-Drews, P.; Düfer, M. Oxidative stress and beta-cell dysfunction. Pflug. Arch. Eur. J. Physiol. 2010, 460, 703–718. [Google Scholar] [CrossRef]
- Park, K.-H.; Park, W.J. Endothelial Dysfunction: Clinical Implications in Cardiovascular Disease and Therapeutic Approaches. J. Korean Med. Sci. 2015, 30, 1213–1225. [Google Scholar] [CrossRef]
- Cohen, D.H.; LeRoith, D. Obesity, type 2 diabetes, and cancer: The insulin and IGF connection. Endocr.-Relat. Cancer 2012, 19, F27–F45. [Google Scholar] [CrossRef]
- Margioris, A.N. Fatty acids and postprandial inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 129–137. [Google Scholar] [CrossRef]
- Soeters, M.R.; Soeters, P.B.; Schooneman, M.G.; Houten, S.M.; Romijn, J.A. Adaptive reciprocity of lipid and glucose metabolism in human short-term starvation. Am. J. Physiology. Endocrinol. Metab. 2012, 303, E1397–E1407. [Google Scholar] [CrossRef]
- Ohashi, K.; Shibata, R.; Murohara, T.; Ouchi, N. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol. Metab. TEM 2014, 25, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Luu, N.-T.; Madden, J.; Calder, P.C.; Grimble, R.F.; Shearman, C.P.; Chan, T.; Dastur, N.; Howell, W.M.; Rainger, G.E.; Nash, G.B. Dietary supplementation with fish oil modifies the ability of human monocytes to induce an inflammatory response. J. Nutr. 2007, 137, 2769–2774. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Biochem. Soc. Trans. 2005, 33, 423–427. [Google Scholar] [CrossRef]
- Bogani, P.; Galli, C.; Villa, M.; Visioli, F. Postprandial anti-inflammatory and antioxidant effects of extra virgin olive oil. Atherosclerosis 2007, 190, 181–186. [Google Scholar] [CrossRef]
- Pacheco, Y.M.; López, S.; Bermúdez, B.; Abia, R.; Villar, J.; Muriana, F.J.G. A meal rich in oleic acid beneficially modulates postprandial sICAM-1 and sVCAM-1 in normotensive and hypertensive hypertriglyceridemic subjects. J. Nutr. Biochem. 2008, 19, 200–205. [Google Scholar] [CrossRef]
- Calder, P.C. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 327–335. [Google Scholar] [CrossRef]
- Calder, P.C.; Deckelbaum, R.J. Omega-3 fatty acids: Time to get the messages right! Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Bozzetto, L.; Annuzzi, G.; Ragucci, M.; Di Donato, O.; Della Pepa, G.; Della Corte, G.; Griffo, E.; Anniballi, G.; Giacco, A.; Mancini, M.; et al. Insulin resistance, postprandial GLP-1 and adaptive immunity are the main predictors of NAFLD in a homogeneous population at high cardiovascular risk. Nutr. Metab. Cardiovasc. Dis. NMCD 2016, 26, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.W.; Koopman, F.A.; Visscher, J.P.; de Hair, M.J.; Gerlag, D.M.; Tak, P.P. Hormone, metabolic peptide, and nutrient levels in the earliest phases of rheumatoid arthritis-contribution of free fatty acids to an increased cardiovascular risk during very early disease. Clin. Rheumatol. 2017, 36, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Zilversmit, D.B. Atherogenesis: A postprandial phenomenon. Circulation 1979, 60, 473–485. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Effects of Citrus Fruit Juices and Their Bioactive Components on Inflammation and Immunity: A Narrative Review. Front. Immunol. 2021, 12, 712608. [Google Scholar] [CrossRef]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing. Nutrients 2021, 13, 1951. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- De Filippo, C.; Di Paola, M.; Ramazzotti, M.; Albanese, D.; Pieraccini, G.; Banci, E.; Miglietta, F.; Cavalieri, D.; Lionetti, P. Diet, Environments, and Gut Microbiota. A Preliminary Investigation in Children Living in Rural and Urban Burkina Faso and Italy. Front. Microbiol. 2017, 8, 1979. [Google Scholar] [CrossRef]
- Calabrese, C.M.; Valentini, A.; Calabrese, G. Gut Microbiota and Type 1 Diabetes Mellitus: The Effect of Mediterranean Diet. Front. Nutr. 2020, 7, 612773. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M. SBRN Terminology Consensus Project Participants. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef]
- Gopinath, K.; Sudhandiran, G. Naringin modulates oxidative stress and inflammation in 3-nitropropionic acid-induced neurodegeneration through the activation of nuclear factor-erythroid 2-related factor-2 signalling pathway. Neuroscience 2012, 227, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Y.; Brenna, J.T.; Shen, Y.; Ye, K. Higher ratio of plasma omega-6/omega-3 fatty acids is associated with greater risk of all-cause, cancer, and cardiovascular mortality: A population-based cohort study in UK Biobank. medRxiv Prepr. Serv. Health Sci. 2023; in press. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3: The good oil. Nutr. Bull. 2017, 42, 132–140. [Google Scholar] [CrossRef]
- Calder, P.C. Very long-chain n-3 fatty acids and human health: Fact, fiction and the future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef]
- Charbonneau, B.; O’Connor, H.M.; Wang, A.H.; Liebow, M.; Thompson, C.A.; Fredericksen, Z.S.; Macon, W.R.; Slager, S.L.; Call, T.G.; Habermann, T.M.; et al. Trans fatty acid intake is associated with increased risk and n3 fatty acid intake with reduced risk of non-hodgkin lymphoma. J. Nutr. 2013, 143, 672–681. [Google Scholar] [CrossRef]
- Kolb, H.; Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef]
- Minelli, A.; Bellezza, I.; Conte, C.; Culig, Z. Oxidative stress-related aging: A role for prostate cancer? Biochim. Biophys. Acta 2009, 1795, 83–91. [Google Scholar] [CrossRef]
- Stieg, D.C.; Wang, Y.; Liu, L.-Z.; Jiang, B.-H. ROS and miRNA Dysregulation in Ovarian Cancer Development, Angiogenesis and Therapeutic Resistance. Int. J. Mol. Sci. 2022, 23, 6702. [Google Scholar] [CrossRef]
- Poyton, R.O.; Ball, K.A.; Castello, P.R. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol. Metab. TEM 2009, 20, 332–340. [Google Scholar] [CrossRef]
- Walens, A.; DiMarco, A.V.; Lupo, R.; Kroger, B.R.; Damrauer, J.S.; Alvarez, J.V. CCL5 promotes breast cancer recurrence through macrophage recruitment in residual tumors. eLife 2019, 8, e43653. [Google Scholar] [CrossRef]
- Chao, T.; Furth, E.E.; Vonderheide, R.H. CXCR2-Dependent Accumulation of Tumor-Associated Neutrophils Regulates T-cell Immunity in Pancreatic Ductal Adenocarcinoma. Cancer Immunol. Res. 2016, 4, 968–982. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc. Natl. Acad. Sci. USA 2006, 103, 17589–17594. [Google Scholar] [CrossRef] [PubMed]
- Aune, D. Plant Foods, Antioxidant Biomarkers, and the Risk of Cardiovascular Disease, Cancer, and Mortality: A Review of the Evidence. Adv. Nutr. 2019, 10, S404–S421. [Google Scholar] [CrossRef]
- Harris, W.S.; Tintle, N.L.; Imamura, F.; Qian, F.; Korat, A.V.A.; Marklund, M.; Djoussé, L.; Bassett, J.K.; Carmichael, P.-H.; Chen, Y.-Y.; et al. Blood n-3 fatty acid levels and total and cause-specific mortality from 17 prospective studies. Nat. Commun. 2021, 12, 2329. [Google Scholar] [CrossRef]
- Nguyen, S.; Li, H.; Yu, D.; Cai, H.; Gao, J.; Gao, Y.; Luu, H.N.; Tran, H.; Xiang, Y.B.; Zheng, W.; et al. Dietary fatty acids and colorectal cancer risk in men: A report from the Shanghai Men’s Health Study and a meta-analysis. Int. J. Cancer 2021, 148, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Michels, N.; Specht, I.O.; Heitmann, B.L.; Chajès, V.; Huybrechts, I. Dietary trans-fatty acid intake in relation to cancer risk: A systematic review and meta-analysis. Nutr. Rev. 2021, 79, 758–776. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.M.; Loehr, S.; Wu, J.H.Y. Trans Fatty Acids: A Summary of the Evidence Relating Consumption to Cardiovascular Outcomes and the Efficacy of Prevention Policy to Reduce Levels in the Food Supply. In Preventive Nutrition: The Comprehensive Guide for Health Professionals; Springer: Cham, Switzerland, 2015; pp. 273–296. [Google Scholar]
- Han, S.N.; Leka, L.S.; Lichtenstein, A.H.; Ausman, L.M.; Schaefer, E.J.; Meydani, S.N. Effect of hydrogenated and saturated, relative to polyunsaturated, fat on immune and inflammatory responses of adults with moderate hypercholesterolemia. J. Lipid Res. 2002, 43, 445–452. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Pischon, T.; E Hankinson, S.; Rifai, N.; Joshipura, K.; Willett, W.C.; Rimm, E.B. Dietary intake of trans fatty acids and systemic inflammation in women. Am. J. Clin. Nutr. 2004, 79, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Seyyedsalehi, M.S.; Collatuzzo, G.; Rashidian, H.; Hadji, M.; Gholipour, M.; Mohebbi, E.; Kamangar, F.; Pukkala, E.; Huybrechts, I.; Gunter, M.J.; et al. Dietary Ruminant and Industrial Trans-Fatty Acids Intake and Colorectal Cancer Risk. Nutrients 2022, 14, 4912. [Google Scholar] [CrossRef] [PubMed]
- Supabphol, S.; Seubwai, W.; Wongkham, S.; Saengboonmee, C. High glucose: An emerging association between diabetes mellitus and cancer progression. J. Mol. Med. 2021, 99, 1175–1193. [Google Scholar] [CrossRef]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef]
- Czekajło, A.; Rozanska, D.; Mandecka, A.; Konikowska, K.; Madalińska, M.; Szuba, A.; Regulska-Ilow, B. Glycemic load and carbohydrates content in the diets of cancer patients. Rocz. Panstw. Zakl. Hig. 2017, 68, 261–268. [Google Scholar]
- Dang, C.V. Rethinking the Warburg effect with Myc micromanaging glutamine metabolism. Cancer Res. 2010, 70, 859–862. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef]
- de Groot, S.; Vreeswijk, M.P.; Welters, M.J.; Gravesteijn, G.; Boei, J.J.; Jochems, A.; Houtsma, D.; Putter, H.; van der Hoeven, J.J.; Nortier, J.W.; et al. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: A randomized pilot study. BMC Cancer 2015, 15, 652. [Google Scholar] [CrossRef]
- Derr, R.L.; Ye, X.; Islas, M.U.; Desideri, S.; Saudek, C.D.; Grossman, S.A. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Srour, B.; Sellem, L.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Deschasaux, M.; Fassier, P.; Latino-Martel, P.; Beslay, M.; et al. Consumption of ultra-processed foods and cancer risk: Results from NutriNet-Santé prospective cohort. BMJ 2018, 360, k322. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Su, L.; Wang, J.; Duan, X.; Jiang, X. Fruit and vegetable consumption and risk of the metabolic syndrome: A meta-analysis. Public Health Nutr. 2018, 21, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Maino Vieytes, C.A.; Taha, H.M.; Burton-Obanla, A.A.; Douglas, K.G.; Arthur, A.E. Carbohydrate Nutrition and the Risk of Cancer. Curr. Nutr. Rep. 2019, 8, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Böhm, V.; Lietz, G.; Olmedilla-Alonso, B.; Phelan, D.; Reboul, E.; Bánati, D.; Borel, P.; Corte-Real, J.; de Lera, A.R.; Desmarchelier, C.; et al. From carotenoid intake to carotenoid blood and tissue concentrations—Implications for dietary intake recommendations. Nutr. Rev. 2021, 79, 544–573. [Google Scholar] [CrossRef]
- Ornish, D.; Magbanua, M.J.M.; Weidner, G.; Weinberg, V.; Kemp, C.; Green, C.; Mattie, M.D.; Marlin, R.; Simko, J.; Shinohara, K.; et al. Changes in prostate gene expression in men undergoing an intensive nutrition and lifestyle intervention. Proc. Natl. Acad. Sci. USA 2008, 105, 8369–8374. [Google Scholar] [CrossRef]
- Slavin, J.L. Mechanisms for the impact of whole grain foods on cancer risk. J. Am. Coll. Nutr. 2000, 19, 300S–307S. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Holte, K.; Myhrstad, M.C.W.; Barikmo, I.; Hvattum, E.; Remberg, S.F.; Wold, A.-B.; Haffner, K.; Baugerød, H.; Andersen, L.F.; et al. A systematic screening of total antioxidants in dietary plants. J. Nutr. 2002, 132, 461–471. [Google Scholar] [CrossRef]
- Comba, A.; Maestri, D.M.; A Berra, M.; Garcia, C.P.; Das, U.N.; Eynard, A.R.; E Pasqualini, M. Effect of ω-3 and ω-9 fatty acid rich oils on lipoxygenases and cyclooxygenases enzymes and on the growth of a mammary adenocarcinoma model. Lipids Health Dis. 2010, 9, 112. [Google Scholar] [CrossRef]
- Labrecque, L.; Lamy, S.; Chapus, A.; Mihoubi, S.; Durocher, Y.; Cass, B.; Bojanowski, M.W.; Gingras, D.; Béliveau, R. Combined inhibition of PDGF and VEGF receptors by ellagic acid, a dietary-derived phenolic compound. Carcinogenesis 2005, 26, 821–826. [Google Scholar] [CrossRef]
- Sung, B.; Pandey, M.K.; Ahn, K.S.; Yi, T.; Chaturvedi, M.M.; Liu, M.; Aggarwal, B.B. Anacardic acid (6-nonadecyl salicylic acid), an inhibitor of histone acetyltransferase, suppresses expression of nuclear factor-kappaB-regulated gene products involved in cell survival, proliferation, invasion, and inflammation through inhibition of the inhibitory subunit of nuclear factor-kappaBalpha kinase, leading to potentiation of apoptosis. Blood 2008, 111, 4880–4891. [Google Scholar]
- Carvalho, A.L.; Annoni, R.; Torres, L.H.; Durão, A.C.; Shimada, A.L.; Almeida, F.M.; Hebeda, C.B.; Lopes, F.D.; Dolhnikoff, M.; Martins, M.A.; et al. Anacardic acids from cashew nuts ameliorate lung damage induced by exposure to diesel exhaust particles in mice. Evid. Based Complement. Altern. Med. eCAM 2013, 2013, 549879. [Google Scholar] [CrossRef] [PubMed]
- Tsoukas, M.A.; Ko, B.-J.; Witte, T.R.; Dincer, F.; Hardman, W.E.; Mantzoros, C.S. Dietary walnut suppression of colorectal cancer in mice: Mediation by miRNA patterns and fatty acid incorporation. J. Nutr. Biochem. 2015, 26, 776–783. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Davidson, T.L. Western diet consumption and cognitive impairment: Links to hippocampal dysfunction and obesity. Physiol. Behav. 2011, 103, 59–68. [Google Scholar] [CrossRef]
- Poutahidis, T.; Kleinewietfeld, M.; Smillie, C.; Levkovich, T.; Perrotta, A.; Bhela, S.; Varian, B.; Ibrahim, Y.; Lakritz, J.; Kearney, S.; et al. Microbial reprogramming inhibits Western diet-associated obesity. PLoS ONE 2013, 8, e68596. [Google Scholar] [CrossRef]
- Christ, A.; Latz, E. The Western lifestyle has lasting effects on metaflammation. Nat. Rev. Immunol. 2019, 19, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Vuik, S.; Lerouge, A.; Guillemette, Y.; Feigl, A.; Aldea, A. The Economic Burden of Obesity; OECD iLibrary: Paris, France, 2019. [Google Scholar]
- Cecchini, M.; Vuik, S. The Heavy Burden of Obesity; OECD iLibrary: Paris, France, 2019. [Google Scholar]
- Peng, W.; Zhang, J.; Zhou, H.; Zhang, A.; Wang, Y.; Tian, X.; Wen, D.; Wang, Y. Obesity intervention efforts in China and the 2022 World Obesity Day. Glob. Health J. 2022, 6, 118–121. [Google Scholar] [CrossRef]
- Gooey, M.; Bacus, C.A.; Ramachandran, D.; Piya, M.K.; Baur, L.A. Health service approaches to providing care for people who seek treatment for obesity: Identifying challenges and ways forward. Public Health Res. Pract. 2022, 32, e3232228. [Google Scholar] [CrossRef]
- Shaw, J.E.; Zimmet, P.Z.; McCarty, D.; de Courten, M. Type 2 diabetes worldwide according to the new classification and criteria. Diabetes Care 2000, 23 (Suppl. 2), B5–B10. [Google Scholar]
- Fazeli Farsani, S.; van der Aa, M.P.; van der Vorst MM, J.; Knibbe CA, J.; de Boer, A. Global trends in the incidence and prevalence of type 2 diabetes in children and adolescents: A systematic review and evaluation of methodological approaches. Diabetologia 2013, 56, 1471–1488. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Chowdhury, T.A. The Impact of Comorbidities on the Pharmacological Management of Type 2 Diabetes Mellitus. Drugs 2019, 79, 231–242. [Google Scholar] [CrossRef]
- Kim, H.S.; Shin, A.M.; Kim, M.K.; Kim, Y.N. Comorbidity study on type 2 diabetes mellitus using data mining. Korean J. Intern. Med. 2012, 27, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Pantalone, K.M.; Hobbs, T.M.; Wells, B.J.; Kong, S.X.; Kattan, M.W.; Bouchard, J.; Yu, C.; Sakurada, B.; Milinovich, A.; Weng, W.; et al. Clinical characteristics, complications, comorbidities and treatment patterns among patients with type 2 diabetes mellitus in a large integrated health system. BMJ Open Diabetes Res. Care 2015, 3, e000093. [Google Scholar] [CrossRef] [PubMed]
- Nittari, G.; Scuri, S.; Petrelli, F.; Pirillo, I.; Di Luca, N.M.; Grappasonni, I. Fighting obesity in children from European World Health Organization member states. Epidemiological data, medical-social aspects, and prevention programs. La Clin. Ter. 2019, 170, e223–e230. [Google Scholar]
- Afolabi, H.A.; bin Zakariya, Z.; Shokri, A.B.A.; Hasim, M.N.B.M.; Vinayak, R.; Afolabi-Owolabi, O.T.; Elesho, R.F. The relationship between obesity and other medical comorbidities. Obes. Med. 2020, 17, 100164. [Google Scholar] [CrossRef]
- Seuring, T.; Archangelidi, O.; Suhrcke, M. The Economic Costs of Type 2 Diabetes: A Global Systematic Review. PharmacoEconomics 2015, 33, 811–831. [Google Scholar] [CrossRef]
- Hernan, W.H.; Brandle, M.; Zhang, P.; Williamson, D.F.; Matulik, M.J.; Ratner, R.E.; Lachin, J.M.; Engelgau, M.M.; Diabetes Prevention Program Research Group. Costs associated with the primary prevention of type 2 diabetes mellitus in the diabetes prevention program. Diabetes Care 2003, 26, 36–47. [Google Scholar]
- Dall, T.M.; Mann, S.E.; Zhang, Y.; Quick, W.W.; Seifert, R.F.; Martin, J.; Huang, E.A.; Zhang, S. Distinguishing the economic costs associated with type 1 and type 2 diabetes. Popul. Health Manag. 2009, 12, 103–110. [Google Scholar] [CrossRef]
- Kennedy-Martin, T.; Boye, K.S.; Peng, X. Cost of medication adherence and persistence in type 2 diabetes mellitus: A literature review. Patient Prefer. Adherence 2017, 11, 1103–1117. [Google Scholar] [CrossRef]
- Sikter, A. Psychosomatic Molecular Mechanisms of Metabolic Syndrome and Type 2 Diabetes. Part 2. Psychosomatic Mechanism of Metabolic Syndrome (a Theory). Acta Sci. Med. Sci. 2020, 4, 98–107. [Google Scholar]
- Harada, M.D. The Role of Health and Diet in the Development of Metabolic Syndrome Stratified by Race, Sex, and Age. Ph.D. Thesis, Walden University, Minneapolis, MN, USA, 2022. [Google Scholar]
- Boudreau, D.; Malone, D.; Raebel, M.; Fishman, P.; Nichols, G.; Feldstein, A.; Boscoe, A.; Ben-Joseph, R.; Magid, D.; Okamoto, L. Health care utilization and costs by metabolic syndrome risk factors. Metab. Syndr. Relat. Disord. 2009, 7, 305–314. [Google Scholar] [CrossRef]
- Belete, R.; Ataro, Z.; Abdu, A.; Sheleme, M. Global prevalence of metabolic syndrome among patients with type I diabetes mellitus: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2021, 13, 25. [Google Scholar] [CrossRef]
- Al-Rubeaan, K.; Bawazeer, N.; Al Farsi, Y.; Youssef, A.M.; Al-Yahya, A.A.; AlQumaidi, H.; Al-Malki, B.M.; Naji, K.A.; Al-Shehri, K.; Al Rumaih, F.I. Prevalence of metabolic syndrome in Saudi Arabia—A cross sectional study. BMC Endocr. Disord. 2018, 18, 16. [Google Scholar] [CrossRef]
- Amini, M.; Zayeri, F.; Salehi, M. Trend analysis of cardiovascular disease mortality, incidence, and mortality-to-incidence ratio: Results from global burden of disease study 2017. BMC Public Health 2021, 21, 401. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.-H.; Kim, S.U.; Kim, H.C. Metabolic Dysfunction-Associated Fatty Liver Disease and Incident Cardiovascular Disease Risk: A Nationwide Cohort Study. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2021, 19, 2138–2147.e10. [Google Scholar] [CrossRef]
- Cainzos-Achirica, M.; Fedeli, U.; Sattar, N.; Agyemang, C.; Jenum, A.K.; McEvoy, J.W.; Murphy, J.D.; Brotons, C.; Elosua, R.; Bilal, U.; et al. Epidemiology, risk factors, and opportunities for prevention of cardiovascular disease in individuals of South Asian ethnicity living in Europe. Atherosclerosis 2019, 286, 105–113. [Google Scholar] [CrossRef]
- Vikulova, D.; Grubisic, M.; Zhao, Y.; Lynch, K.; Humphries, K.H.; Pimstone, S.N.; Brunham, L.R. Premature Atherosclerotic Cardiovascular Disease: Trends in Incidence, Risk Factors, and Sex-Related Differences, 2000 to 2016. J. Am. Heart Assoc. 2019, 8, e012178. [Google Scholar] [CrossRef] [PubMed]
- Townsend, N.; Kazakiewicz, D.; Wright, F.L.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Dermadi, D.; Valo, S.; Ollila, S.; Soliymani, R.; Sipari, N.; Pussila, M.; Sarantaus, L.; Linden, J.; Baumann, M.; Nyström, M. Western Diet Deregulates Bile Acid Homeostasis, Cell Proliferation, and Tumorigenesis in Colon. Cancer Res. 2017, 77, 3352–3363. [Google Scholar] [CrossRef] [PubMed]
- Hintze, K.J.; Benninghoff, A.D.; Cho, C.E.; Ward, R.E. Modeling the Western Diet for Preclinical Investigations. Adv. Nutr. 2018, 9, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.B.; Thomas, C.C.; Henley, S.J.; Massetti, G.M.; Galuska, D.A.; Agurs-Collins, T.; Puckett, M.; Richardson, L.C. Vital Signs: Trends in Incidence of Cancers Associated with Overweight and Obesity—United States, 2005–2014. MMWR. Morb. Mortal. Wkly. Rep. 2017, 66, 1052–1058. [Google Scholar] [CrossRef]
- Sung, H.; Siegel, R.L.; Torre, L.A.; Pearson-Stuttard, J.; Islami, F.; Fedewa, S.A.; Sauer, A.G.; Shuval, K.; Gapstur, S.M.; Jacobs, E.J.; et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J. Clin. 2019, 69, 88–112. [Google Scholar] [CrossRef] [PubMed]
- López-Suárez, A. Burden of cancer attributable to obesity, type 2 diabetes and associated risk factors. Metab. Clin. Exp. 2019, 92, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Mirsepasi-Lauridsen, H.C.; Vallance, B.A.; Krogfelt, K.A.; Petersen, A.M. Escherichia coli Pathobionts Associated with Inflammatory Bowel Disease. Clin. Microbiol. Rev. 2019, 32, e00060-18. [Google Scholar] [CrossRef]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef]
- Chan, S.S.M.; Chen, Y.; Casey, K.; Olen, O.; Ludvigsson, J.F.; Carbonnel, F.; Oldenburg, B.; Gunter, M.J.; Tjønneland, A.; Grip, O.; et al. Obesity is Associated with Increased Risk of Crohn’s disease, but not Ulcerative Colitis: A Pooled Analysis of Five Prospective Cohort Studies. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2022, 20, 1048–1058. [Google Scholar] [CrossRef]
- Szilagyi, A.; Smith, B.E.; Sebbag, N.; Leighton, H.; Xue, X. Changing Patterns of Relationships Between Geographic Markers and IBD: Possible Intrusion of Obesity. Crohn’s Colitis 360 2020, 2, otaa044. [Google Scholar] [CrossRef]
- Park, S.; Kang, B.; Kim, S.; Choi, S.; Suh, H.R.; Kim, E.S.; Park, J.H.; Kim, M.J.; Choe, Y.H.; Lee, Y.J.; et al. Comparison between Pediatric Crohn’s Disease and Ulcerative Colitis at Diagnosis in Korea: Results from a Multicenter, Registry-Based, Inception Cohort Study. Gut Liver 2022, 16, 921–929. [Google Scholar] [CrossRef]
- Szilagyi, A. Relationship(s) between obesity and inflammatory bowel diseases: Possible intertwined pathogenic mechanisms. Clin. J. Gastroenterol. 2020, 13, 139–152. [Google Scholar] [CrossRef]
- El-Dallal, M.; Stein, D.J.; Raita, Y.; Feuerstein, J.D. The impact of obesity on hospitalized patients with ulcerative colitis. Ann. Gastroenterol. 2021, 34, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Milajerdi, A.; Abbasi, F.; Esmaillzadeh, A. A systematic review and meta-analysis of prospective studies on obesity and risk of inflammatory bowel disease. Nutr. Rev. 2022, 80, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Gao, X.; Dai, C.; Huang, Y.; Wu, Y.; Zhou, W.; Cao, Q.; Jing, X.; Jiang, H.; et al. Validation of the GLIM criteria for diagnosis of malnutrition and quality of life in patients with inflammatory bowel disease: A multicenter, prospective, observational study. Clin. Nutr. 2022, 41, 1297–1306. [Google Scholar] [CrossRef]
- Carreira-Míguez, M.; Ramos-Campo, D.J.; Clemente-Suárez, V.J. Differences in Nutritional and Psychological Habits in Hypertension Patients. BioMed Res. Int. 2022, 2022, 1920996. [Google Scholar] [CrossRef] [PubMed]
- Carreira-Míguez, M.; Belinchón-deMiguel, P.P.; Clemente-Suárez, V.J. Behavioural, odontological and physical activity patterns of hypertense and control population. Physiol. Behav. 2022, 252, 113841. [Google Scholar] [CrossRef]
- Martín-Rodríguez, A.; Tornero-Aguilera, J.F.; López-Pérez, P.J.; Clemente-Suárez, V.J. Overweight and executive functions, psychological and behavioral profile of Spanish adolescents. Physiol. Behav. 2022, 254, 113901. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Redondo-Flórez, L.; Ruisoto, P.; Navarro-Jiménez, E.; Ramos-Campo, D.J.; Tornero-Aguilera, J.F. Metabolic Health, Mitochondrial Fitness, Physical Activity, and Cancer. Cancers 2023, 15, 814. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. Mitochondrial Transfer as a Novel Therapeutic Approach in Disease Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 8848. [Google Scholar] [CrossRef]
- Gu, P.; Luo, J.; Kim, J.; Paul, P.; Limketkai, B.; Sauk, J.S.; Park, S.; Parekh, N.; Zheng, K.; Rudrapatna, V.; et al. Effect of Obesity on Risk of Hospitalization, Surgery, and Serious Infection in Biologic-Treated Patients With Inflammatory Bowel Diseases: A CA-IBD Cohort Study. Am. J. Gastroenterol. 2022, 117, 1639–1647. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Navarro-Jiménez, E.; Jimenez, M.; Hormeño-Holgado, A.; Martinez-Gonzalez, M.B.; Benitez-Agudelo, J.C.; Perez-Palencia, N.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. Impact of COVID-19 Pandemic in Public Mental Health: An Extensive Narrative Review. Sustainability 2021, 13, 3221. [Google Scholar] [CrossRef]
- Tornero-Aguilera, J.F.; Sánchez-Molina, J.; Parraca, J.A.; Morais, A.; Clemente-Suárez, V.J. Are Crohn’s Disease Patients Limited in Sport Practise? An UltraEndurance Case–Control Study Response. Int. J. Environ. Res. Public Health 2022, 19, 10007. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Bustamante-Sanchez, Á.; Tornero-Aguilera, J.F.; Ruisoto, P.; Mielgo-Ayuso, J. Inflammation in COVID-19 and the Effects of Non-Pharmacological Interventions during the Pandemic: A Review. Int. J. Mol. Sci. 2022, 23, 15584. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Role of Adipokines in Health and Disease. Biomedicines 2023, 11, 1290. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Ramírez-Goerke, M.I.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Ramos-Campo, D.J.; Navarro-Jiménez, E.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. The Impact of Anorexia Nervosa and the Basis for Non-Pharmacological Interventions. Nutrients 2023, 15, 2594. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).