Synergistic Effect of Diet and Physical Activity on a NAFLD Cohort: Metabolomics Profile and Clinical Variable Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Groups
2.2. Dietary Intervention and Data Collection
2.3. Fecal Metabolome Analysis
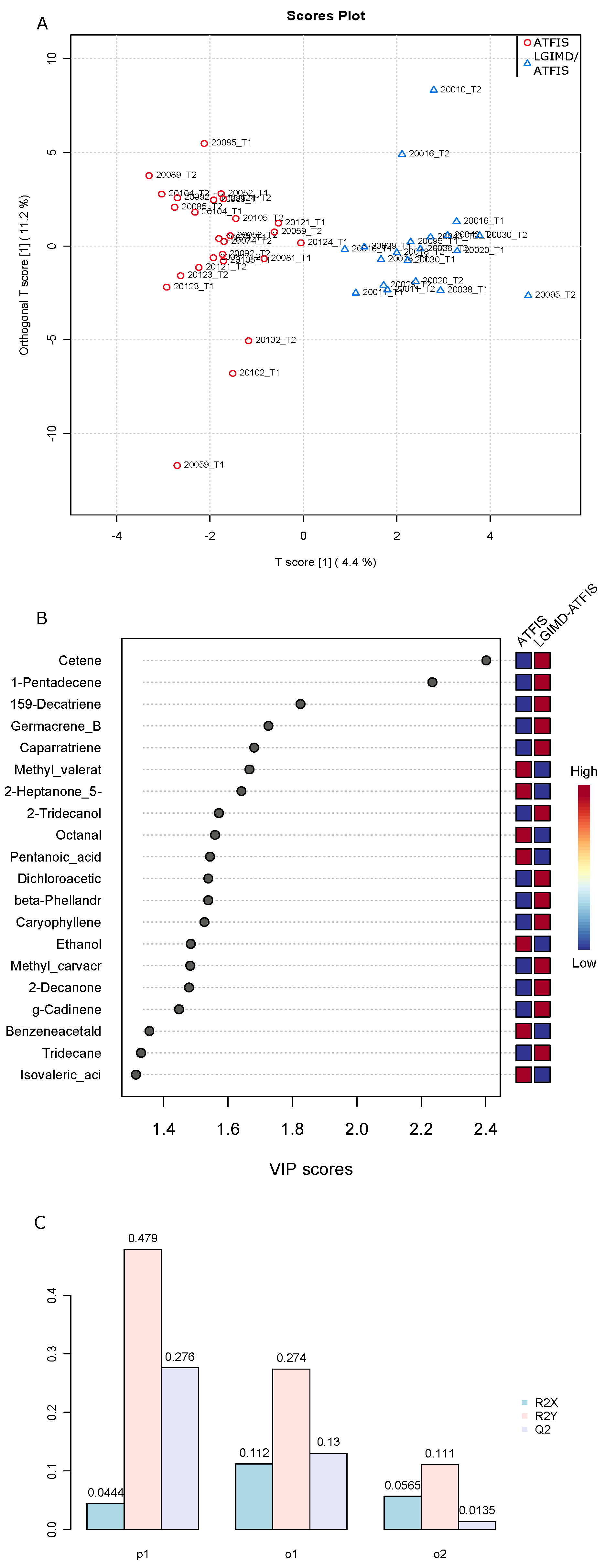
2.4. Differential Abundance Analysis, Principal Component Analysis, and Orthogonal Projections for Latent Structure Discriminant Analysis and Clustering Analysis
2.5. Multivariable Association via the MaAslin2 R Package
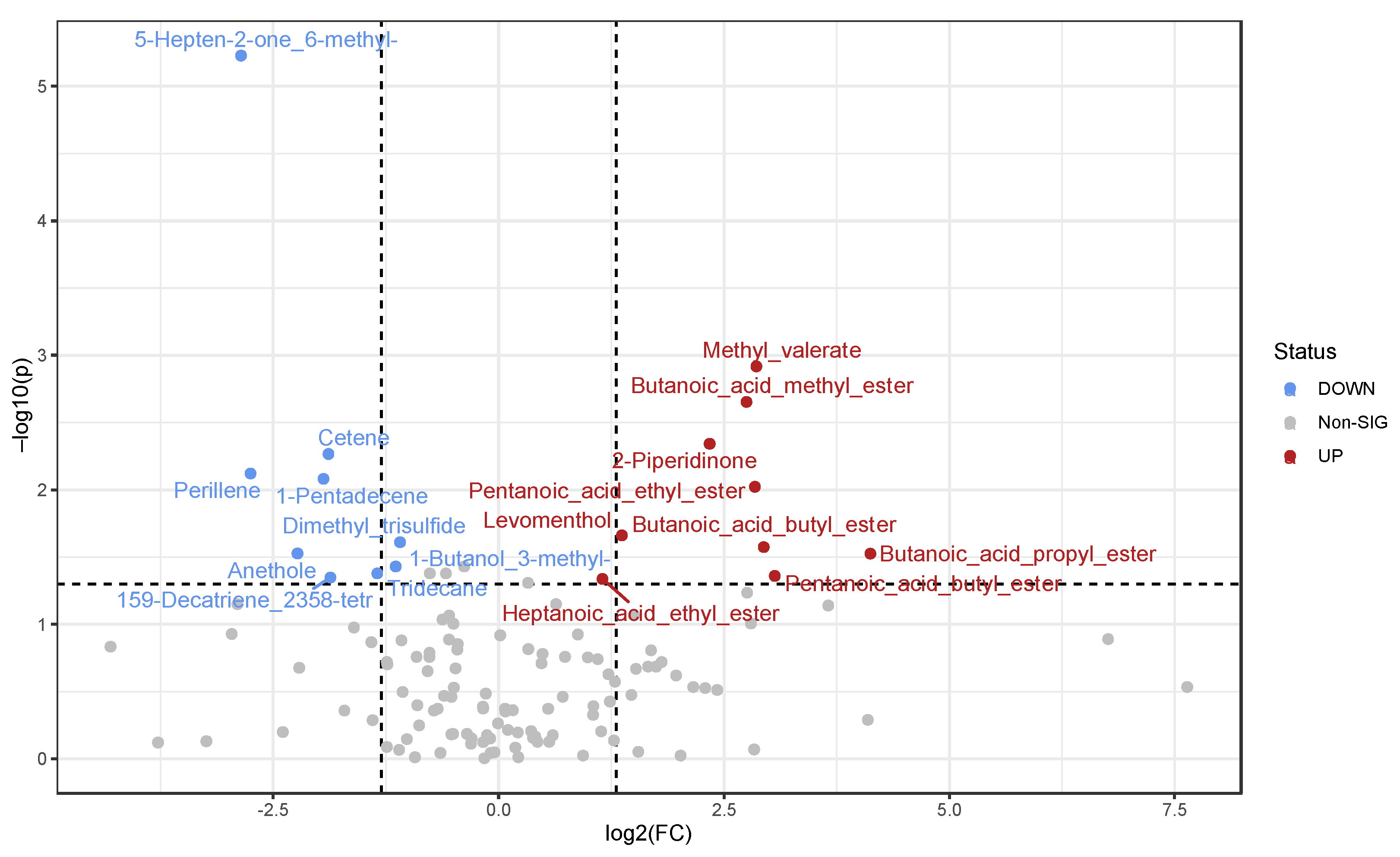
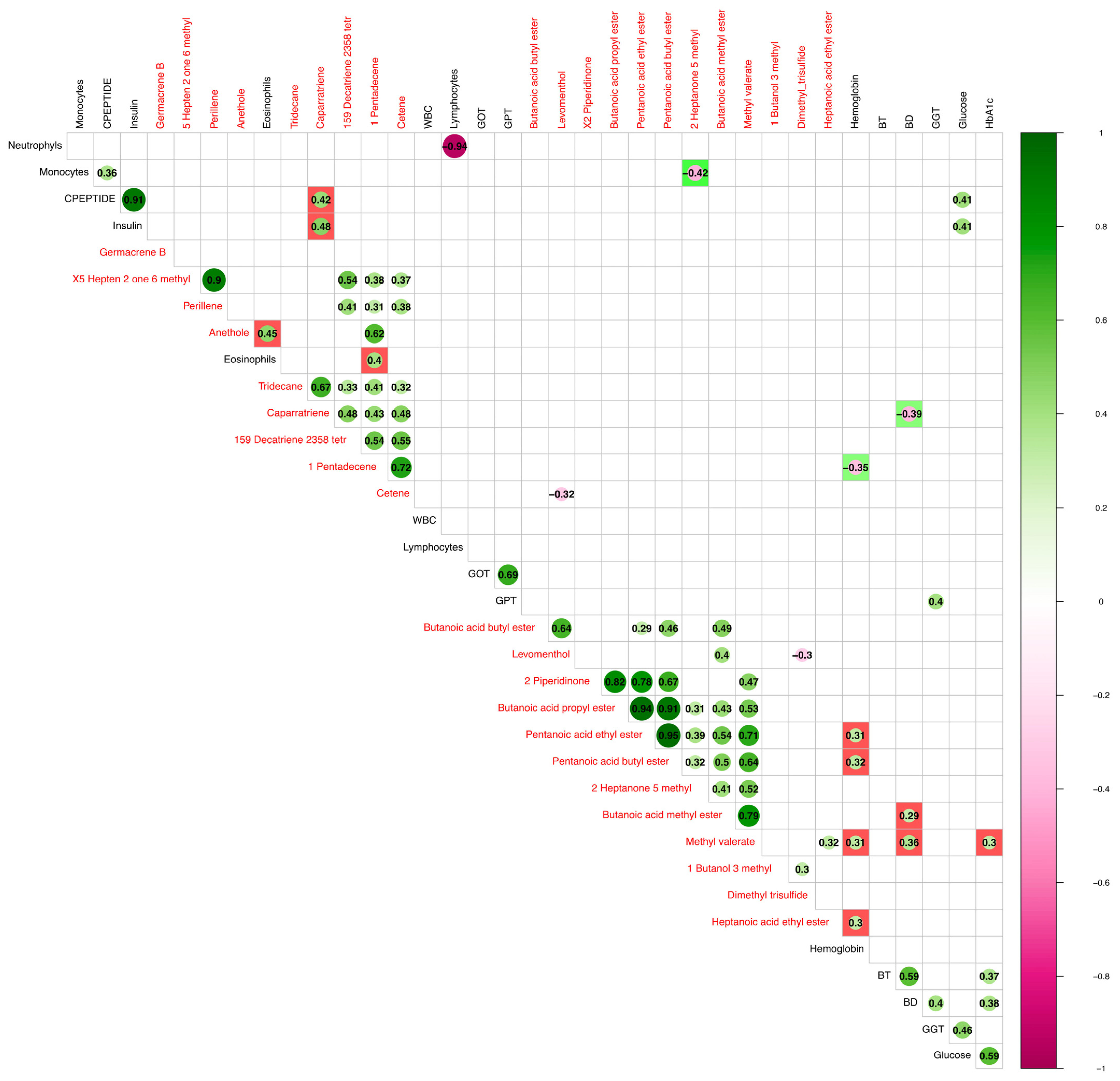
3. Results
3.1. Rotated Factor Loadings (Pattern Matrix) and Unique Variances of Biochemical Clinical Detected Variables
3.2. Detected Volatile Organic Compounds (VOCs)
3.3. Correlation between VOCs and Clinical Parameters
3.4. Comparison between Statistically Significant VOCs and Taxa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chrysavgis, L.; Giannakodimos, I.; Diamantopoulou, P.; Cholongitas, E. Non-Alcoholic Fatty Liver Disease and Hepatocellular Carcinoma: Clinical Challenges of an Intriguing Link. World J. Gastroenterol. 2022, 28, 310–331. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.G.; Roelstraete, B.; Hagström, H.; Sundström, J.; Ludvigsson, J.F. Non-Alcoholic Fatty Liver Disease and Incident Major Adverse Cardiovascular Events: Results from a Nationwide Histology Cohort. Gut 2022, 71, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Arranz, I.; Bruzzone, C.; Noureddin, M.; Gil-Redondo, R.; Mincholé, I.; Bizkarguenaga, M.; Arretxe, E.; Iruarrizaga-Lejarreta, M.; Fernández-Ramos, D.; Lopitz-Otsoa, F.; et al. Metabolic Subtypes of Patients with NAFLD Exhibit Distinctive Cardiovascular Risk Profiles. Hepatology 2022, 76, 1121–1134. [Google Scholar] [CrossRef]
- Visschers, R.G.J.; Luyer, M.D.; Schaap, F.G.; Olde Damink, S.W.M.; Soeters, P.B. The Gut-Liver Axis. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 576–581. [Google Scholar] [CrossRef]
- Ji, J.; Wu, L.; Wei, J.; Wu, J.; Guo, C. The Gut Microbiome and Ferroptosis in MAFLD. J. Clin. Transl. Hepatol. 2023, 11, 174–187. [Google Scholar] [CrossRef]
- Higarza, S.G.; Arboleya, S.; Arias, J.L.; Gueimonde, M.; Arias, N. The Gut-Microbiota-Brain Changes across the Liver Disease Spectrum. Front. Cell. Neurosci. 2022, 16, 994404. [Google Scholar] [CrossRef]
- Taipale, T.; Seppälä, I.; Raitoharju, E.; Mononen, N.; Lyytikäinen, L.-P.; Illig, T.; Waldenberger, M.; Juonala, M.; Hutri-Kähönen, N.; Oksala, N.; et al. Fatty Liver Is Associated with Blood Pathways of Inflammatory Response, Immune System Activation and Prothrombotic State in Young Finns Study. Sci. Rep. 2018, 8, 10358. [Google Scholar] [CrossRef]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-Mediated Dysbiosis Regulates Progression of NAFLD and Obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef]
- Heymann, F.; Tacke, F. Immunology in the Liver--From Homeostasis to Disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 88–110. [Google Scholar] [CrossRef]
- Wu, L.; Gao, X.; Guo, Q.; Li, J.; Yao, J.; Yan, K.; Xu, Y.; Jiang, X.; Ye, D.; Guo, J. The Role of Neutrophils in Innate Immunity-Driven Nonalcoholic Steatohepatitis: Lessons Learned and Future Promise. Hepatol. Int. 2020, 14, 652–666. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Z.; Fan, J.-G. Peripheral Immune Cells in NAFLD Patients: A Spyhole to Disease Progression. EBioMedicine 2022, 75, 103768. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Khac, E.; Thiele, M.; Voican, C.; Nahon, P.; Moreno, C.; Boursier, J.; Mueller, S.; de Ledinghen, V.; Stärkel, P.; Gyune Kim, S.; et al. Non-Invasive Diagnosis of Liver Fibrosis in Patients with Alcohol-Related Liver Disease by Transient Elastography: An Individual Patient Data Meta-Analysis. Lancet Gastroenterol. Hepatol. 2018, 3, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Okanoue, T.; Yamaguchi, K.; Shima, T.; Mitsumoto, Y.; Mizuno, M.; Katayama, T.; Seko, Y.; Moriguchi, M.; Umemura, A.; Itoh, Y.; et al. Serum Levels of Immunoglobulin M-Free Inhibitors of Macrophage/CD5L as a Predictive and Early Diagnostic Marker for Nonalcoholic Steatohepatitis-Associated Hepatocellular Carcinoma. Hepatol. Res. 2022, 52, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Hrncir, T.; Hrncirova, L.; Kverka, M.; Hromadka, R.; Machova, V.; Trckova, E.; Kostovcikova, K.; Kralickova, P.; Krejsek, J.; Tlaskalova-Hogenova, H. Gut Microbiota and NAFLD: Pathogenetic Mechanisms, Microbiota Signatures, and Therapeutic Interventions. Microorganisms 2021, 9, 957. [Google Scholar] [CrossRef]
- Semmler, G.; Datz, C.; Trauner, M. Eating, Diet, and Nutrition for the Treatment of NAFLD. Clin. Mol. Hepatol. 2022, 29, S244–S260. [Google Scholar] [CrossRef]
- Calabrese, F.M.; Disciglio, V.; Franco, I.; Sorino, P.; Bonfiglio, C.; Bianco, A.; Campanella, A.; Lippolis, T.; Pesole, P.L.; Polignano, M.; et al. A Low Glycemic Index Mediterranean Diet Combined with Aerobic Physical Activity Rearranges the Gut Microbiota Signature in NAFLD Patients. Nutrients 2022, 14, 1773. [Google Scholar] [CrossRef]
- Tutino, V.; De Nunzio, V.; Caruso, M.G.; Bonfiglio, C.; Franco, I.; Mirizzi, A.; De Leonardis, G.; Cozzolongo, R.; Giannuzzi, V.; Giannelli, G.; et al. Aerobic Physical Activity and a Low Glycemic Diet Reduce the AA/EPA Ratio in Red Blood Cell Membranes of Patients with NAFLD. Nutrients 2018, 10, 1299. [Google Scholar] [CrossRef]
- Honsek, C.; Kabisch, S.; Kemper, M.; Gerbracht, C.; Arafat, A.M.; Birkenfeld, A.L.; Dambeck, U.; Osterhoff, M.A.; Weickert, M.O.; Pfeiffer, A.F.H. Fibre Supplementation for the Prevention of Type 2 Diabetes and Improvement of Glucose Metabolism: The Randomised Controlled Optimal Fibre Trial (OptiFiT). Diabetologia 2018, 61, 1295–1305. [Google Scholar] [CrossRef]
- Franco, I.; Bianco, A.; Mirizzi, A.; Campanella, A.; Bonfiglio, C.; Sorino, P.; Notarnicola, M.; Tutino, V.; Cozzolongo, R.; Giannuzzi, V.; et al. Physical Activity and Low Glycemic Index Mediterranean Diet: Main and Modification Effects on NAFLD Score. Results from a Randomized Clinical Trial. Nutrients 2020, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, H.F. An Index of Factorial Simplicity. Psychometrika 1974, 39, 31–36. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Dudek, M.; Knolle, P. Non-Alcoholic Fatty Liver Disease: The Interplay between Metabolism, Microbes and Immunity. Nat. Metab. 2021, 3, 1596–1607. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Wang, X.; Liu, P.; Wei, R.; Chen, W.; Rajani, C.; Hernandez, B.Y.; Alegado, R.; Dong, B.; Li, D.; et al. Distinctly Altered Gut Microbiota in the Progression of Liver Disease. Oncotarget 2016, 7, 19355–19366. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, C.; Zhang, G.; Yuan, Z.; Liu, Y.; Ding, L.; Sun, X.; Jia, H.; Xue, F. Association between White Blood Cell Count and Non-Alcoholic Fatty Liver Disease in Urban Han Chinese: A Prospective Cohort Study. BMJ Open 2016, 6, e010342. [Google Scholar] [CrossRef]
- Diniz, A.B.; Antunes, M.M.; de Souza Lacerda, V.A.; Nakagaki, B.N.; Lopes, M.A.F.; de Castro-Oliveira, H.M.; Mattos, M.S.; Mafra, K.; de Miranda, C.D.M.; de Oliveira Costa, K.M.; et al. Imaging and Immunometabolic Phenotyping Uncover Changes in the Hepatic Immune Response in the Early Phases of NAFLD. JHEP Rep. 2020, 2, 100117. [Google Scholar] [CrossRef]
- Schleich, F.N.; Zanella, D.; Stefanuto, P.-H.; Bessonov, K.; Smolinska, A.; Dallinga, J.W.; Henket, M.; Paulus, V.; Guissard, F.; Graff, S.; et al. Exhaled Volatile Organic Compounds Are Able to Discriminate between Neutrophilic and Eosinophilic Asthma. Am. J. Respir. Crit. Care Med. 2019, 200, 444–453. [Google Scholar] [CrossRef]
- Byun, J.-S.; Yi, H.-S. Hepatic Immune Microenvironment in Alcoholic and Nonalcoholic Liver Disease. Biomed. Res. Int. 2017, 2017, 6862439. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, B.; Chen, A.; Yin, Q.; Wang, H. Trans-Anethole Attenuates Diet-Induced Nonalcoholic Steatohepatitis through Suppressing TGF-β-Mediated Fibrosis. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101833. [Google Scholar] [CrossRef]
- Palomino, E.; Maldonado, C.; Kempff, M.B.; Ksebati, M.B. Caparratriene, an Active Sesquiterpene Hydrocarbon from Ocotea Caparrapi. J. Nat. Prod. 1996, 59, 77–79. [Google Scholar] [CrossRef]
- Han, X.; Xu, P.; Zhou, J.; Liu, Y.; Xu, H. Fasting C-Peptide Is a Significant Indicator of Nonalcoholic Fatty Liver Disease in Obese Children. Diabetes Res. Clin. Pract. 2020, 160, 108027. [Google Scholar] [CrossRef] [PubMed]
- Aleryani, S.L.; Cluette-Brown, J.E.; Khan, Z.A.; Hasaba, H.; Lopez de Heredia, L.; Laposata, M. Fatty Acid Methyl Esters Are Detectable in the Plasma and Their Presence Correlates with Liver Dysfunction. Clin. Chim. Acta 2005, 359, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Akyuz, U.; Yesil, A.; Yilmaz, Y. Characterization of Lean Patients with Nonalcoholic Fatty Liver Disease: Potential Role of High Hemoglobin Levels. Scand. J. Gastroenterol. 2015, 50, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Hernández, E.; Chávez-Tapia, N.C.; Brizuela-Alcántara, D.C.; Uribe, M.; Ramos-Ostos, M.H.; Nuño-Lámbarri, N. Association Between Serum Hemoglobin Levels and Non Alcoholic Fatty Liver Disease in a Mexican Population. Ann. Hepatol. 2018, 17, 577–584. [Google Scholar] [CrossRef]
- Gerber, Y.; VanWagner, L.B.; Yaffe, K.; Terry, J.G.; Rana, J.S.; Reis, J.P.; Sidney, S. Non-Alcoholic Fatty Liver Disease and Cognitive Function in Middle-Aged Adults: The CARDIA Study. BMC Gastroenterol. 2021, 21, 96. [Google Scholar] [CrossRef]
- De Chiara, F.; Heebøll, S.; Marrone, G.; Montoliu, C.; Hamilton-Dutoit, S.; Ferrandez, A.; Andreola, F.; Rombouts, K.; Grønbæk, H.; Felipo, V.; et al. Urea Cycle Dysregulation in Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2018, 69, 905–915. [Google Scholar] [CrossRef]
- Lustgarten, M.S.; Price, L.L.; Chale, A.; Phillips, E.M.; Fielding, R.A. Branched Chain Amino Acids Are Associated with Muscle Mass in Functionally Limited Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 717–724. [Google Scholar] [CrossRef]
- Shin, M.-J.; Park, E.; Lee, J.H.; Chung, N. Relationship between Insulin Resistance and Lipid Peroxidation and Antioxidant Vitamins in Hypercholesterolemic Patients. Ann. Nutr. Metab. 2006, 50, 115–120. [Google Scholar] [CrossRef]
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut Microbiome-Based Metagenomic Signature for Non-Invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell. Metab. 2017, 25, 1054–1062.e5. [Google Scholar] [CrossRef]
- Ren, Z.; Li, A.; Jiang, J.; Zhou, L.; Yu, Z.; Lu, H.; Xie, H.; Chen, X.; Shao, L.; Zhang, R.; et al. Gut Microbiome Analysis as a Tool towards Targeted Non-Invasive Biomarkers for Early Hepatocellular Carcinoma. Gut 2019, 68, 1014–1023. [Google Scholar] [CrossRef]
- Zhang, Q.; Xing, W.; Wang, Q.; Tang, Z.; Wang, Y.; Gao, W. Gut Microbiota-Mitochondrial Inter-Talk in Non-Alcoholic Fatty Liver Disease. Front. Nutr. 2022, 9, 934113. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Hamaya, J.; Hoshi, T.; Yokoyama, C. Self-Aldol Condensation of Unmodified Aldehyde in Supercritical Carbon Dioxide Catalyzed by Amine Grafted on Silica. Tetrahedron Lett. 2005, 46, 393–395. [Google Scholar] [CrossRef]
- Hasegawa, T.; Iino, C.; Endo, T.; Mikami, K.; Kimura, M.; Sawada, N.; Nakaji, S.; Fukuda, S. Changed Amino Acids in NAFLD and Liver Fibrosis: A Large Cross-Sectional Study without Influence of Insulin Resistance. Nutrients 2020, 12, 1450. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-C.; Yu, H.-F.; Chang, C.-H.; Liu, Y.-T.; Yao, H.-T. Effects of Lemongrass Oil and Citral on Hepatic Drug-Metabolizing Enzymes, Oxidative Stress, and Acetaminophen Toxicity in Rats. J. Food Drug. Anal. 2018, 26, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-S.; Lee, W.-C.; Lin, Y.-E.; Ho, C.-T.; Lu, K.-H.; Lin, S.-H.; Panyod, S.; Chu, Y.-L.; Sheen, L.-Y. Ginger Essential Oil Ameliorates Hepatic Injury and Lipid Accumulation in High Fat Diet-Induced Nonalcoholic Fatty Liver Disease. J. Agric. Food Chem. 2016, 64, 2062–2071. [Google Scholar] [CrossRef]
- Zeybel, M.; Arif, M.; Li, X.; Altay, O.; Yang, H.; Shi, M.; Akyildiz, M.; Saglam, B.; Gonenli, M.G.; Yigit, B.; et al. Multiomics Analysis Reveals the Impact of Microbiota on Host Metabolism in Hepatic Steatosis. Adv. Sci. 2022, 9, e2104373. [Google Scholar] [CrossRef]
- Kimura, M.; Ito, M. Bioconversion of Essential Oil Components of Perilla Frutescens by Saccharomyces Cerevisiae. J. Nat. Med. 2020, 74, 189–199. [Google Scholar] [CrossRef]
- Mou, Y.; Meng, J.; Fu, X.; Wang, X.; Tian, J.; Wang, M.; Peng, Y.; Zhou, L. Antimicrobial and Antioxidant Activities and Effect of 1-Hexadecene Addition on Palmarumycin C2 and C3 Yields in Liquid Culture of Endophytic Fungus Berkleasmium Sp. Dzf12. Molecules 2013, 18, 15587–15599. [Google Scholar] [CrossRef]
| Variable | Factor1 | Factor2 | Factor3 | Factor4 | Uniqueness |
|---|---|---|---|---|---|
| RBC | 0.4276 | 0.0287 | 0.1607 | 0.2875 | 0.7078 |
| hemoglobin | 0.2019 | −0.0004 | 0.2233 | 0.5525 | 0.6041 |
| Platelets | 0.2915 | −0.0773 | 0.2949 | −0.6362 | 0.4173 |
| WBC | 0.8994 | 0.0361 | −0.0157 | −0.1788 | 0.1575 |
| neutrophils | 0.7489 | 0.0059 | −0.1133 | −0.0779 | 0.4202 |
| lymphocytes | 0.5892 | 0.1021 | 0.1975 | −0.3466 | 0.4832 |
| monocytes | 0.6879 | 0.1248 | −0.0648 | 0.1637 | 0.4803 |
| eosinophils | 0.6177 | −0.205 | 0.1259 | −0.1964 | 0.522 |
| basophils | 0.5387 | −0.026 | 0.1105 | 0.1217 | 0.6821 |
| glucose | −0.0541 | 0.7698 | −0.1217 | 0.0499 | 0.3871 |
| HbA1c | −0.2133 | 0.7414 | 0.0225 | −0.1435 | 0.3837 |
| UREA | −0.0032 | 0.1894 | −0.2616 | 0.2071 | 0.8528 |
| Creatinine | 0.0352 | 0.1035 | 0.0187 | 0.5968 | 0.6315 |
| eGFR | −0.1503 | −0.1806 | −0.1691 | −0.1227 | 0.9012 |
| BT | −0.0903 | −0.0983 | 0.2386 | 0.6759 | 0.4684 |
| BD | −0.2108 | 0.0297 | 0.1298 | 0.7783 | 0.3321 |
| GOT | −0.0714 | 0.0742 | 0.8491 | 0.013 | 0.2682 |
| GPT | 0.0603 | 0.0501 | 0.8683 | 0.0991 | 0.23 |
| GGT | −0.0316 | 0.0312 | 0.6658 | 0.0636 | 0.5506 |
| Iron | −0.0772 | −0.1194 | 0.1474 | −0.103 | 0.9474 |
| Cholesterol | 0.1812 | −0.3587 | 0.1512 | −0.3082 | 0.7206 |
| HDL | −0.3543 | −0.4732 | −0.2486 | −0.0437 | 0.5868 |
| FT3 | 0.1707 | −0.0438 | 0.4859 | 0.0339 | 0.7317 |
| FT4 | 0.0086 | 0.0938 | −0.1718 | 0.0823 | 0.9549 |
| Cortisol | −0.2473 | −0.2376 | -0.1526 | 0.1537 | 0.8355 |
| C-PEPTIDE | 0.177 | 0.7786 | 0.0823 | 0.107 | 0.3443 |
| Insulin | 0.1228 | 0.7452 | 0.1835 | 0.0024 | 0.396 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, F.M.; Celano, G.; Bonfiglio, C.; Campanella, A.; Franco, I.; Annunziato, A.; Giannelli, G.; Osella, A.R.; De Angelis, M. Synergistic Effect of Diet and Physical Activity on a NAFLD Cohort: Metabolomics Profile and Clinical Variable Evaluation. Nutrients 2023, 15, 2457. https://doi.org/10.3390/nu15112457
Calabrese FM, Celano G, Bonfiglio C, Campanella A, Franco I, Annunziato A, Giannelli G, Osella AR, De Angelis M. Synergistic Effect of Diet and Physical Activity on a NAFLD Cohort: Metabolomics Profile and Clinical Variable Evaluation. Nutrients. 2023; 15(11):2457. https://doi.org/10.3390/nu15112457
Chicago/Turabian StyleCalabrese, Francesco Maria, Giuseppe Celano, Caterina Bonfiglio, Angelo Campanella, Isabella Franco, Alessandro Annunziato, Gianluigi Giannelli, Alberto Ruben Osella, and Maria De Angelis. 2023. "Synergistic Effect of Diet and Physical Activity on a NAFLD Cohort: Metabolomics Profile and Clinical Variable Evaluation" Nutrients 15, no. 11: 2457. https://doi.org/10.3390/nu15112457
APA StyleCalabrese, F. M., Celano, G., Bonfiglio, C., Campanella, A., Franco, I., Annunziato, A., Giannelli, G., Osella, A. R., & De Angelis, M. (2023). Synergistic Effect of Diet and Physical Activity on a NAFLD Cohort: Metabolomics Profile and Clinical Variable Evaluation. Nutrients, 15(11), 2457. https://doi.org/10.3390/nu15112457







