Abstract
Background: Muscle quality index (MQI) is an emerging health indicator obtained by dividing handgrip strength by body mass index (BMI) that needs to be studied in morbidly obese patients (defined by BMI ≥ 35 kg/m2). Objective: To determine the association between MQI, metabolic syndrome (MetS) markers, and cardiorespiratory fitness (CRF), and as a second objective to determine the potential mediation role of MQI in the relationship between abdominal obesity and systolic blood pressure (SBP) in this sample. Methods: This cross-sectional study included 86 severely/morbidly obese patients (age = 41.1 ± 11.9 y, nine men). MQI, metabolic syndrome markers, CRF, and anthropometric parameters were measured. Two groups were developed according to MQI; High-MQI (n = 41) and Low-MQI (n = 45). Results: The Low-MQI group reported higher abdominal obesity (High-MQI: 0.7 ± 0.1 vs. Low-MQI: 0.8 ± 0.1 WC/height; p = 0.011), SBP (High-MQI: 133.0 ± 17.5 vs. Low-MQI: 140.1 ± 15.1 mmHg; p = 0.048), and lower CRF (High-MQI; 26.3 ± 5.9 vs. Low-MQI; 22.4 ± 6.1 mL/kg/min, p = 0.003) than the High-MQI group. Waist-to-height ratio (β: −0.07, p = 0.011), SBP (β: −18.47, p = 0.001), and CRF (β: 5.21, p = 0.011) were linked to MQI. In a mediation model, the indirect effect confirms that MQI is a partial mediator of the association between abdominal obesity with SBP. Conclusions: MQI in morbidly obesity patients reported an inverse association with MetS markers and a positive association with CRF (VO2max). It mediates the relationship between abdominal obesity and SBP.
1. Introduction
Obesity is a multifactorial disease and has become a worldwide public health problem [1]. Close to a third of the global population is considered overweight or obese [2]—it has almost tripled since 1975 [3]—whereas malnutrition by excess in Latin America is growing quickly compared with other continents [4], which increases the health and economic impact in the countries [5,6]. There is a consensus that obesity may lead to premature disability and death (i.e., cardiovascular diseases (CDV), depression, dementia, and various types of cancers), and therefore, impact life expectancy [7]. Consequently, severe obesity will be the most common body mass index (BMI) classification among women and low-income adults by 2030 in the USA [8]. In this sense, one study reported that the increase in severe obesity was related to the decline of disease-free years in adulthood [9]. This increase in severe obesity could be attributed to the lack of success of public health in managing obesity in its first phases [7].
Similarly, morbidly obesity (defined by the body mass index (BMI) ≥ 40 kg/m2 or 35 kg/m2 with obesity-related health conditions) [10] has been related to the prevalence of CVD, poor subjective well-being, and bad functional capacity [11]. Moreover, metabolic syndrome (MetS) is a cluster of cardiovascular risk factors including abdominal obesity, high blood pressure, high blood glucose, and blood lipid abnormalities [12]. The MetS prevalence differed from 12.5% to 31.4% in the global population, whereas America and Eastern Mediterranean presented the highest prevalence [13]. Obesity and abdominal obesity have been related to MetS [14,15].
Additionally, the evidence has reported that abdominal obesity is considered a health problem [16,17]. An epidemiological study reported the prevalence of an abdominal obesity increase in adult subjects [18]. Moreover, a systematic review and meta-analysis of 13.3 million participants, indicated a sustained increase in abdominal obesity prevalence since 1990 [19]. Abdominal obesity is a good marker for metabolic disease [20] and may be defined as excess visceral fat in the abdominal region; this condition has reported the association with the development of arterial hypertension [21] and serious implications that promote non-communicable diseases such as heart diseases, non-alcoholic fatty liver diseases, kidney disorders, cancer, and other health problems [22,23].
In a complementary way, abdominal obesity can play a fundamental role in CDV prevention [19]. A recent cross-sectional study found that abdominal fat content was linked with higher blood pressure and arterial stiffness [24]. In this sense, it has been indicated that abdominal obesity was associated with systolic blood pressure and other cardiovascular risk factors [24]. A systematic review of prospective cohort studies reported that the risk of hypertension increased with the elevation of abdominal obesity (i.e., the relative risk of hypertension 1.49 for 10 cm increments in abdominal obesity) [25]. In this sense, a study conducted in adults from urban and rural areas indicated that subjects who had obesity and abdominal obesity obtained a higher risk of hypertension compared to subjects with normal anthropometric measures [26].
Similarly, subjects with abdominal obesity have a significantly higher prevalence of MetS factors involving high systolic blood pressure (SBP), high fasting blood glucose, impaired HDL-cholesterol, and high triglyceride levels [27]. Moreover, the present higher risk of acute myocardial infarction [28] substantially increases the total mortality rates, with most of the excess deaths due to heart disease, and reduces life expectancy when compared with normal weight [29].
On the other hand, handgrip strength (HGS) is an important health indicator and could be easily and universally applied, reporting association with all causes of mortality in different types of populations [30]. HGS is a powerful predictor of future disability, morbidity, and mortality [31]. The evidence suggests that in addition to obesity, a decrease in HGS is associated with an increase in all-cause mortality risk [32]. Moreover, both HGS and obesity evaluated by BMI are predictors of mortality [33]. For this reason, the muscle quality index (MQI) that is obtained by dividing HGS by BMI (MQI = HGS/BMI) is an emerging health and physical function indicator [34,35], which represents relative strength (units; kg/BMI). The evidence suggests that Low-MQI has been associated with all causes of mortality and MetS markers, increasing the risk of CVD, insulin resistance, sarcopenia, and even, death [36,37].
It has been indicated that muscle quality was related to more insulin-sensitive subjects with obesity [38]. Similarly, a recent study found that Low-MQI was associated with a higher risk of diabetes [39]. In this sense, recent evidence indicated that MetS markers such as blood pressure and fasting blood glucose were linked with muscle strength and physical fitness [12]. Yamada et al. [40] found a longitudinal link between low skeletal muscle and the development of MetS. In a complementary way, it has been shown that obese subjects had a high prevalence of poor MQI [41], and poor muscle quality can lead to the development of MetS [42].
In addition, cardiorespiratory fitness (CRF) is a term related to maximal capacity for oxygen consumption and reflected the individual capacity of the circulatory and respiratory system to supply oxygen to the skeletal muscle during physical exercise [43,44,45] and the evidence has reported that it is a strong and independent predictor of CVD [46] and all cause of mortality [44,47]. Moreover, the evidence has shown that CRF is an important health indicator [45]; hence, to present better physical fitness (i.e., CRF and muscular strength), lower MetS markers are reported [48].
A recent longitudinal study indicated that the combination of abdominal obesity with low skeletal mass could be increased the risk of diabetes [49]. Data from severely obese women reported that abdominal obesity was linked with hypertension and other MetS markers [50]. However, the potential mediator role of MQI in the relationship between abdominal obesity and MetS markers has not been studied in deep. Therefore, considering that morbidly obesity patients usually report a major cardiometabolic risk such as elevated MetS markers, the evaluation of the relationship between MQI and different health markers, such as anthropometric, plasmatic, and cardiovascular parameters, is interesting. Therefore, the objective of the present study was to determine the association between MQI with MetS markers and CRF, and as a second objective, to determine the potential mediation role of MQI in the relationship between abdominal obesity and SBP in morbidly obesity patients.
2. Materials and Methods
2.1. Participants
This cross-sectional study included 86 morbidly obesity patients (age = 41.1 ± 11.9 y, men = 9, BMI; 43.4 ± 7.2). The patients were invited to participate by an open invitation directly from the Morbid Obesity Association (Temuco, Chile) and open information on social networks. After all information and feedback about the risks/benefits were provided, all participants signed an informed consent form. The study was carried out in accordance with the Declaration of Helsinki (2013) and was approved by the Ethical Committee of the Universidad de La Frontera, Temuco, Chile (Act 080-21 and Act 071-18), the database corresponding to project DI21-0030 and DI18-0043.
The inclusion criteria were (i) 18–60 years of age, (ii) to present authorisation medical for physical testing, (iii) BMI ≥ 40 kg/m2 (i.e., ≥obesity class III) or BMI ≥ 35 kg/m2 with any comorbidity. The exclusion criteria were (i) physical limitations such as restrictive injuries of the musculoskeletal system, (ii) exercise-related dyspnoea or respiratory alterations, and (iii) chronic heart disease with any worsening in the last month. Of the total of participants, eight used drugs for insulin resistance or diabetes, fifteen for hypertension, and three for cholesterol treatment.
2.2. Measurements
2.2.1. Muscle Quality Index
The muscle quality index was estimated by HGS divided by BMI. A hydraulic hand dynamometer (BASELINE® Hydraulic Hand Dynamometers, NY, USA) was used to determine HGS, which has been used previously [11]. The measure of each dominant and non-dominant arm was made in two attempts, and the best result from each was selected. The average scores achieved by the left and right hands were registered for data analysis. MQI was categorized as follows: Low-MQI ≤ 50th and High-MQI > 50th (50th MQI = 0.67), according to previous studies [51].
2.2.2. Health Outcomes
To evaluate MetS markers all participants were instructed to arrive at the health centre following overnight fasting >8 h and measured between 08:00 and 09:00 in the morning. The MetS markers considered the following values: fasting plasma glucose (FPG ≥ 100 mg/dL), high-density lipoprotein cholesterol (cHDL < 50 women and <40 mg/dL men), and triglycerides (TG ≥ 150 mg/dL). The additional markers taken were total cholesterol (Tc) and low-density lipoprotein cholesterol (cLDL).
SBP and diastolic blood pressure (DBP) measurements (hypertension = SBP ≥ 140 mmHg, DBP ≥ 90 mmHg) were evaluated by the standard criteria [52]. Blood pressure was measured after 5 min of rest in the sitting position. Two evaluations were made using an OMRONTM digital electronic BP monitor (model HEM 7114, Chicago, IL, USA), and the mean of these measurements was used for data analysis. The participants were informed that they must not drink caffeine or smoke for at least 2 h prior to measurement.
2.2.3. Abdominal Obesity
The waist circumference (WC) of participants was assessed with a non-elastic measuring tape in centimetres (Adult SECATM, USA) at the upper hipbone and top of the right iliac crest, in a horizontal at the level of the iliac crest plane around the abdomen. The tape was snug but did not compress the skin and was parallel to the floor, at the end of a normal expiration the measurement was made. Waist-to-height ratio (WtHR = WC/height) was estimated to determine the central obesity (WtHR ≥ 0.5).
2.2.4. Anthropometric Parameters
The anthropometric variables of participants were made after fasting (>6 h). Body mass (kg) was measured using a digital bio-impedance BIA scale (TANITATM, model 331, Tokyo, Japan), and height (m) was measured using a SECATM stadiometer (model 214, Hamburg, Germany), with subjects in light clothing and without shoes. The BMI was calculated by dividing body mass in kg by the square of the height in m (kg/m2). The BMI was determined to estimate the degree of obesity (kg/m2) using the standard criteria for the obesity and severe/morbid-obesity classifications.
2.2.5. Fitness
CRF was measured through the six-minute walking test (6 Mwt). Participants were instructed to walk as far as they could for a 6 min period. An exercise physiologist assisted the participants with instructions. Anthropometric variables and the six-minute walk test results were used to estimate the maximal oxygen uptake (VO2max) (mL/kg/min)VO2max from the equation derived by Burr et al. [53]; VO2max = 70.161 + (0.023 × 6 MWT [m]) − (0.276 × weight [kg]) − (6.79 × sex, where m = 0, f = 1) − (0.193 × resting HR [beats per minute]) − (0.191 × age [y]).
2.3. Statistical Analysis
The Kolmogorov–Smirnov test was used to determine normal distribution. For continuous variables, values are presented as the mean and standard deviation (SD). Differences between mean values according to MQI were determined using the analysis of variance (ANOVA) and chi-square test (Chi2), respectively. A simple linear regression was used to analyse the association between MetS markers and MQI. The alpha level was set at p < 0.05 for statistical significance.
Moreover, to verify the effect of the mediating variables MQI (M), considering abdominal obesity (WtHR) as the independent variable (X) and SBP as the dependent variable (Y) regression analyses were performed. Within the analysis, for the samples, the total effect as (c), direct effect as (c′), and indirect effect as (a*b; IE) were calculated, as well as the 95% confidence interval (95%CI), using the macro/interface process v. 3.3 for SPSS v. 23 and the bootstrapping method with a resampling rate of 5000 [54]. The statistical analyses were performed using SPSS statistical software (SPSSTM Inc., Chicago, IL, USA) version 23.0.
3. Results
Table 1 shows the comparison according to MQI. In the total sample, the mean age (years), BMI (kg/m2), and WC (cm) were 41.1 ± 11.9 years, 43.4 ± 7.2 kg/m2 and 121.0 ± 15.2 cm, respectively. The SBP and DBP were 137.7 ± 16.6 mmHg and 86.3 ± 10.3 mmHg, respectively. The mean of mg/dL was 102.4 ± 18.7 for fasting glucose, 185.2 ± 37.4 for total cholesterol, 132.0 ± 64.9 for TG, 115.3 ± 30.6 for cLDL and 48.3 ± 10.9 for cHDL. In fitness, the mean of VO2max (mL/kg/min), HGS (kg), and MQI (ratio) were 24.2 ± 6.3, 31.4 ± 12.3 and 0.7 ± 0.3, respectively. According to MetS factors, the Low-MQI group reported higher abdominal obesity (WtHR, High-MQI: 0.7 ± 0.1 vs. Low-MQI: 0.8 ± 0.1; p = 0.011) and SBP (High-MQI: 133.0 ± 17.5 vs. Low-MQI: 140.1 ± 15.1, p = 0.048) than the High-MQI group. According to fitness, the Low-MQI reported lower CRF than the High-MQI group (High-MQI; 26.3 ± 5.9 vs. Low-MQI; 22.4 ±6.1 mL/kg/min, p = 0.003).

Table 1.
Characteristics of sample study according to muscle quality index.
According to MetS markers, there were 47.7% of participants with hypertension, 46.9% reported high fasting glucose, 72% high TG, 39.5% of participants reported low cHDL, and 100% presented abdominal obesity. There were no differences between groups according to MQI (Table 2).

Table 2.
Frequency of impaired metabolic syndrome markers according to muscle quality index.
The simple linear regression reported that WtHR (β = 0.07, 95%CI: −0.13, −0.02; p = 0.011), SBP (β = −18.47, 95%CI: −28.69, −8.25; p = 0.001), and CRF (β = 5.21, 95%CI: 1.21, 9.20; p = 0.011) were significantly linked to MQI. When the variables were adjusted by sex and age, the significance was maintained in all (Table 3).

Table 3.
Association between muscle quality index with MetS markers and physical status.
The results of the mediation analysis are shown in Figure 1 for the total sample (n = 86). In the relationship between WtHR and SBP, MQI appears as a mediate variable. WtHR was inversely related to MQI (p = 0.003) (a) in the first regression step. Second (c), the regression coefficient of WtHR in SBP was also significant (p = 0.029). In the third step (b), the potential mediator MQI presented a positive relationship with the dependent variable (Y) (p = 0.004), but when both variables in the model WtHR and MQI were included (c′), the regression coefficient remained statistically significant (p = 0.029). Finally, the IE confirms that MQI is a partial mediator of SBP (Figure 1).
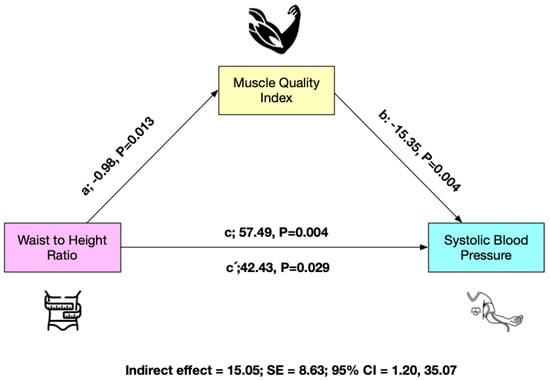
Figure 1.
Mediation model testing whether the association between waist-to-height ratio and systolic blood pressure was mediated by muscle quality index.
4. Discussion
The objective of the present study was to determine the association between MQI and MetS markers and CRF, and as a second objective, to determine the potential mediation role of MQI in the relationship between abdominal obesity and SBP in morbidly obesity patients. The main results were: (i) MQI was linked inversely to MetS markers (i.e., SBP and abdominal obesity), (ii) the association between abdominal obesity and SBP was mediated by MQI, and (iii) CRF was positively linked to MQI.
MQI was linked inversely to MetS markers (i.e., abdominal obesity) in morbidly obesity participants. In accordance with our results, it has been reported that Low-MQI is associated with a cluster of MetS markers that included SBP and elevated WC [55]. In addition, another study reported that low relative skeletal muscle was associated with a higher risk of MetS [40]. Another retrospective cohort study indicated that low muscle quality and quantity incremented the incidence of MetS [56]. In this sense, it has been shown that low muscle quality, together with visceral fat accumulation, were linked with more prevalence of CVD [57]. Moreover, a study conducted on subjects with overweight or obesity condition indicated that abdominal obesity was related to low muscle quality [58].
In this study, abdominal obesity was linked to SBP, and this relationship was mediated by MQI in morbidly obese patients. In this sense, subjects with abdominal obesity have a significantly higher prevalence of high SBP, high FPG, high Tc, and high TG levels [27]. Additionally, a previous study showed that abdominal obesity was associated with cardiovascular risk factors, low-grade inflammation, SBP, and TG [59]. Another study found that subjects with abdominal obesity presented significantly higher hypertension compared with their counterparts; it has been indicated that abdominal obesity was significantly related to hypertension in a cohort of severely obese women [50] and it has been related to physical fitness impairments such as low CRF and HGS, both limiting the capacity to perform activities of daily living and increasing prevalence of MetS [58,60].
Moreover, it has been indicated that the excess of visceral fat leads to the increased production of adipokines which could have serious implications in non-communicable diseases such as hypertension [22]. Furthermore, a study conducted in 500,000 adult Chinese men and women reported that general adiposity was strongly linked with SBP [61]. According to the mediation role of MQI, the evidence highlights the positive role of muscle fitness in MetS markers [62], where a high muscle fitness could reduce MetS markers (i.e., WC, Tc, cLDL, TG, cHDL, and median blood pressure) [63]; hence, MQI could be a sensitive measure of overall health [64]. MQI is also a great predictor of mortality in both men and women [64]. In addition, low muscle quality has been related to increased CVD risks and all-cause mortality [65] and hypertension [66]. Fortunately, this is something that can be avoided by improving MQI, which significantly changes in response to resistance training [35]. A recent study has indicated that resistance training with both low and moderate loads is just as effective in improving muscular strength and muscle growth as it is in improving MQI [67].
Similar to the above, abdominal obesity was related to MQI and the results of this study agree with those indicated by Palacio-Agüero et al. [68] who reported that subjects with high abdominal obesity presented significantly lower MQI levels using HGS. In this sense, another study conducted in Chilean adults showed that the MQI was negatively linked with abdominal adiposity [69]. A previous study indicated that abdominal obesity had a negative direct effect on HGS in older adults [70]. Similarly, it has been reported that a high body fat percentage is linked to lower muscle quality and predicts a quicker decrease in lean mass [71]. Additionally, overweight and obese women tend to have a lower MQI [72], and a higher adipose tissue is associated with a lower MQI [73]. Moreover, data from 6.455 subjects indicated that HGS was associated with body composition [74]. In addition, evidence has shown that low MQI was associated with CVD in all participants [57]. In an adult population, low values of HGS were linked with high abdominal obesity and the presence of cardiovascular risk factors [75]. In this sense, the increase in the MQI was inversely associated with the risk of MetS in Korean adults [76].
Moreover, MQI was related to CRF (i.e., VO2max in mL/kg/min). An experimental study conducted in diabetics participants reported that changes in muscle quality were linked with improvement in CRF [77]. In addition, a cross-sectional study showed that CRF was positively linked with the skeletal muscle mass index and negatively related to visceral obesity in Korean adults [78]. Similarly, it has been reported that the skeletal muscle mass index was linked to VO2max in older adults [79]. Furthermore, another study conducted in obese and lean sedentary women showed that muscle strength and power were linked with CRF; moreover, the BMI predicted the VO2max [80]. Likewise, another study indicated that better muscle quality was related to higher results in physical function scores in adults [81]. Moreover, it has been shown that there is a link between CRF and muscle quality, either directly or indirectly [81], which agrees with our findings. This is because in the obese population, a higher BMI, which is associated with a lower MQI, leads to a higher percentage of fat mass [82], which in turn leads to low CRF [83]. Excessive fat mass imposes an unfavourable burden on cardiac function and oxygen uptake by the working muscles. This indicates that reduced oxygen utilisation by adipose tissue during exercise reduces the overall CRF [84]. Similarly, the evidence has shown that the population with obesity have severely reduced CRF [85], and muscle mass could be closely linked with CRF [79].
Strengths and Limitations
The main limitations of the present study are the cross-sectional design. Understanding the mentioned relationships could be better served by the development of a longitudinal study. However, the main strength is novel information on a simple indicator that presents powerful information and relationships with MetS markers. In addition, the sample is small, which prevents the extrapolation of data. For future studies, the role of the MQI should be analysed with more complex methods, such as gold standards (DEXA).
5. Conclusions
In conclusion, MQI in morbidly obesity patients reported an inverse association with MetS markers such as abdominal obesity and SBP and a positive association with CRF (VO2max). Moreover, it mediates the relationship between abdominal obesity and SBP. Thereby, MQI could be used as a health marker in this population.
Author Contributions
Conceptualization, P.D.-F., C.A. and F.C.-N.; methodology, P.D.-F. and D.J.-M.; software, P.D.-F. and F.C.-N.; validation, F.C.-N., D.J.-M., I.d.-C. and M.C.-B.; formal analysis, investigation, P.D.-F.; resources, P.D.-F.; data curation, P.D.-F.; writing—original draft preparation, P.D.-F.; writing—review and editing, F.C.-N., D.J.-M., I.d.-C., C.A. and M.C.-B.; visualization, D.J.-M.; supervision, P.D.-F.; project administration, P.D.-F.; funding acquisition, P.D.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was carried out in accordance with the Declaration of Helsinki (2013) and was approved by the Ethical Committee of the Universidad de La Frontera, Temuco, Chile (Act 080-21 and act 071-18), the database corresponding to project DI21-0030 and DI18-0043.
Informed Consent Statement
Informed consent was obtained from all the participants involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
Pedro Delgado Floody has a contract through the programme “Recualificación del Profesorado Universitario. Modalidad María Zambrano”, Universidad de Granada/Ministerio de Universidades y Fondos Next Generation de la Unión Europea Institutional. Daniel Jerez-Mayorga has a contract through the programme “Recualificación del Profesorado Universitario Modalidad Margarita Salas”, Universidad de Granada/Ministerio de Universidades y Fondos Next Generation de la Unión Europea.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Cole, T.J.; Lobstein, T. Exploring an algorithm to harmonize International Obesity Task Force and World Health Organization child overweight and obesity prevalence rates. Pediatr. Obes. 2022, 17, e12905. [Google Scholar] [CrossRef] [PubMed]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef] [PubMed]
- Halpern, B.; Louzada, M.L.d.C.; Aschner, P.; Gerchman, F.; Brajkovich, I.; Faria-Neto, J.R.; Polanco, F.E.; Montero, J.; Juliá, S.M.M.; Lotufo, P.A. Obesity and COVID-19 in Latin America: A tragedy of two pandemics—Official document of the Latin American Federation of Obesity Societies. Obes. Rev. 2021, 22, e13165. [Google Scholar] [CrossRef]
- Okunogbe, A.; Nugent, R.; Spencer, G.; Ralston, J.; Wilding, J. Economic impacts of overweight and obesity: Current and future estimates for eight countries. BMJ Glob. Health 2021, 6, e006351. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Kraak, V.I.; Allender, S.; Atkins, V.J.; Baker, P.I.; Bogard, J.R.; Brinsden, H.; Calvillo, A.; De Schutter, O.; Devarajan, R. The global syndemic of obesity, undernutrition, and climate change: The Lancet Commission report. Lancet 2019, 393, 791–846. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected US state-level prevalence of adult obesity and severe obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef]
- Nyberg, S.T.; Batty, G.D.; Pentti, J.; Virtanen, M.; Alfredsson, L.; Fransson, E.I.; Goldberg, M.; Heikkilä, K.; Jokela, M.; Knutsson, A.; et al. Obesity and loss of disease-free years owing to major non-communicable diseases: A multicohort study. Lancet Public Health 2018, 3, e490–e497. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Vargas, C.A.; Guzmán-Guzmán, I.P.; Caamaño-Navarrete, F.; Jerez-Mayorga, D.; Chirosa-Ríos, L.J.; Delgado-Floody, P. Syndrome metabolic markers, fitness and body fat is associated with sleep quality in women with severe/morbid obesity. Int. J. Environ. Res. Public Health 2021, 18, 9294. [Google Scholar] [CrossRef]
- Tong, Q.; Wang, X.; Sheng, Y.; Chen, S.; Lai, B.; Lv, R.; Yu, J. Metabolic syndrome and its association with components of sarcopenia in older community-dwelling Chinese. J. Biomed. Res. 2022, 36, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.-L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res. Clin. Pract. 2022, 188, 109924. [Google Scholar] [CrossRef] [PubMed]
- Ntougou Assoumou, H.-G.; Pichot, V.; Barthelemy, J.-C.; Celle, S.; Garcin, A.; Thomas, T.; Roche, F. Obesity related to metabolic syndrome: Comparison of obesity indicators in an older french population. Diabetol. Metab. Syndr. 2023, 15, 98. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.-H.; Huang, Y.-C.; Chiu, H.; Wu, P.-Y.; Chiou, H.-Y.C.; Huang, J.-C.; Chen, S.-C. Comparison of Various Obesity-Related Indices for Identification of Metabolic Syndrome: A Population-Based Study from Taiwan Biobank. Diagnostics 2020, 10, 1081. [Google Scholar] [CrossRef]
- Hu, L.; Huang, X.; You, C.; Li, J.; Hong, K.; Li, P.; Wu, Y.; Wu, Q.; Wang, Z.; Gao, R. Prevalence of overweight, obesity, abdominal obesity and obesity-related risk factors in southern China. PLoS ONE 2017, 12, e0183934. [Google Scholar] [CrossRef]
- Ford, E.S.; Li, C.; Zhao, G.; Tsai, J. Trends in obesity and abdominal obesity among adults in the United States from 1999–2008. Int. J. Obes. 2011, 35, 736–743. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Hou, W.; Arcan, C. Obesity Trends and Associations with Types of Physical Activity and Sedentary Behavior in US Adults: National Health and Nutrition Examination Survey, 2007–2016. Obesity 2021, 29, 240–250. [Google Scholar] [CrossRef]
- Wong, M.C.; Huang, J.; Wang, J.; Chan, P.S.; Lok, V.; Chen, X.; Leung, C.; Wang, H.H.; Lao, X.Q.; Zheng, Z.-J. Global, regional and time-trend prevalence of central obesity: A systematic review and meta-analysis of 13.2 million subjects. Eur. J. Epidemiol. 2020, 35, 673–683. [Google Scholar] [CrossRef]
- Caspard, H.; Jabbour, S.; Hammar, N.; Fenici, P.; Sheehan, J.J.; Kosiborod, M. Recent trends in the prevalence of type 2 diabetes and the association with abdominal obesity lead to growing health disparities in the USA: An analysis of the NHANES surveys from 1999 to 2014. Diabetes Obes. Metab. 2018, 20, 667–671. [Google Scholar] [CrossRef]
- Rhee, E.J.; Cho, J.H.; Kwon, H.; Park, S.E.; Jung, J.H.; Han, K.D.; Park, Y.G.; Park, H.S.; Kim, Y.H.; Yoo, S.J. Association between abdominal obesity and increased risk for the development of hypertension regardless of physical activity: A nationwide population-based study. J. Clin. Hypertens. 2018, 20, 1417–1426. [Google Scholar] [CrossRef]
- Dhawan, D.; Sharma, S. Abdominal Obesity, Adipokines and Non-communicable Diseases. J. Steroid Biochem. Mol. Biol. 2020, 203, 105737. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, J.K.; Shin, G.G.; Han, J.A.; Kim, J.W. Association between abdominal obesity and cardiovascular risk factors in adults with normal body mass index: Based on the sixth Korea National Health and Nutrition Examination Survey. J. Obes. Metab. Syndr. 2019, 28, 262. [Google Scholar] [CrossRef]
- Taurio, J.; Hautaniemi, E.J.; Koskela, J.K.; Eräranta, A.; Hämäläinen, M.; Tikkakoski, A.; Kettunen, J.A.; Kähönen, M.; Niemelä, O.; Moilanen, E.; et al. The characteristics of elevated blood pressure in abdominal obesity correspond to primary hypertension: A cross-sectional study. BMC Cardiovasc. Disord. 2023, 23, 161. [Google Scholar] [CrossRef]
- Jayedi, A.; Rashidy-Pour, A.; Khorshidi, M.; Shab-Bidar, S. Body mass index, abdominal adiposity, weight gain and risk of developing hypertension: A systematic review and dose–response meta-analysis of more than 2.3 million participants. Obes. Rev. 2018, 19, 654–667. [Google Scholar] [CrossRef]
- Shen, C.; Zhou, Z.; Lai, S.; Tao, X.; Zhao, D.; Dong, W.; Li, D.; Lan, X.; Gao, J. Urban-rural-specific trend in prevalence of general and central obesity, and association with hypertension in Chinese adults, aged 18–65 years. BMC Public Health 2019, 19, 661. [Google Scholar] [CrossRef]
- Lukács, A.; Horváth, E.; Máté, Z.; Szabó, A.; Virág, K.; Papp, M.; Sándor, J.; Ádány, R.; Paulik, E. Abdominal obesity increases metabolic risk factors in non-obese adults: A Hungarian cross-sectional study. BMC Public Health 2019, 19, 1533. [Google Scholar] [CrossRef]
- Von Eyben, F.; Mouritsen, E.; Holm, J.; Montvilas, P.; Dimcevski, G.; Suciu, G.; Helleberg, I.; Kristensen, L.; Von Eyben, R. Intra-abdominal obesity and metabolic risk factors: A study of young adults. Int. J. Obes. 2003, 27, 941–949. [Google Scholar] [CrossRef]
- Kitahara, C.; Flint, A.; de Gonzalez, A.; Bernstein, L.; Brotzman, M.; MacInnis, R.; Moore, S.; Robien, K.; Rosenberg, P.; Singh, P. Association between class III obesity (BMI of 40–59 kg/m2) and mortality: A pooled analysis of 20 prospective studies. PLoS Med. 2014, 11, e1001673. [Google Scholar] [CrossRef]
- Kawamoto, R.; Ninomiya, D.; Kasai, Y.; Kusunoki, T.; Ohtsuka, N.; Kumagi, T.; Abe, M. Handgrip strength is associated with metabolic syndrome among middle-aged and elderly community-dwelling persons. Clin. Exp. Hypertens. 2016, 38, 245–251. [Google Scholar] [CrossRef]
- Sayer, A.A.; Kirkwood, T.B. Grip strength and mortality: A biomarker of ageing? Lancet 2015, 386, 226–227. [Google Scholar] [CrossRef]
- García-Hermoso, A.; Cavero-Redondo, I.; Ramírez-Vélez, R.; Ruiz, J.R.; Ortega, F.B.; Lee, D.-C.; Martínez-Vizcaíno, V. Muscular strength as a predictor of all-cause mortality in an apparently healthy population: A systematic review and meta-analysis of data from approximately 2 million men and women. Arch. Phys. Med. Rehabil. 2018, 99, 2100–2113.e2105. [Google Scholar] [CrossRef]
- Rantanen, T.; Harris, T.; Leveille, S.G.; Visser, M.; Foley, D.; Masaki, K.; Guralnik, J.M. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2000, 55, M168–M173. [Google Scholar] [CrossRef]
- Jerez-Mayorga, D.; Ríos, L.J.C.; Reyes, A.; Delgado-Floody, P.; Payer, R.M.; Requena, I.M.G. Muscle quality index and isometric strength in older adults with hip osteoarthritis. PeerJ 2019, 7, e7471. [Google Scholar] [CrossRef]
- Fragala, M.S.; Fukuda, D.H.; Stout, J.R.; Townsend, J.R.; Emerson, N.S.; Boone, C.H.; Beyer, K.S.; Oliveira, L.P.; Hoffman, J.R. Muscle quality index improves with resistance exercise training in older adults. Exp. Gerontol. 2014, 53, 1–6. [Google Scholar] [CrossRef]
- Cooper, R.; Kuh, D.; Hardy, R. Objectively measured physical capability levels and mortality: Systematic review and meta-analysis. BMJ 2010, 341, c4467. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Lubrano, C.; Gnessi, L.; Mariani, S.; Di Martino, M.; Catalano, C.; Lenzi, A.; Donini, L.M. The decline in muscle strength and muscle quality in relation to metabolic derangements in adult women with obesity. Clin. Nutr. 2019, 38, 2430–2435. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.; White, D.A.; Kuk, J.L.; Arslanian, S. Relationships between insulin sensitivity, skeletal muscle mass and muscle quality in obese adolescent boys. Eur. J. Clin. Nutr. 2012, 66, 1366–1368. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, H.K.; Lee, M.J.; Bae, S.J.; Kim, K.W.; Choe, J. Association between type 2 diabetes and skeletal muscle quality assessed by abdominal computed tomography scan. Diabetes/Metab. Res. Rev. 2022, 38, e3513. [Google Scholar] [CrossRef]
- Yamada, Y.; Murakami, H.; Kawakami, R.; Gando, Y.; Nanri, H.; Nakagata, T.; Watanabe, D.; Yoshida, T.; Hatamoto, Y.; Yoshimura, E. Impact of low relative skeletal muscle mass and power on the development of metabolic syndrome in Japanese women: A 7-year prospective study. medRxiv 2022. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Maffiuletti, N.A.; Tringali, G.; De Col, A.; Sartorio, A. Obesity-associated poor muscle quality: Prevalence and association with age, sex, and body mass index. BMC Musculoskelet. Disord. 2020, 21, 200. [Google Scholar] [CrossRef]
- Sayer, A.A.; Dennison, E.M.; Syddall, H.E.; Gilbody, H.J.; Phillips, D.I.; Cooper, C. Type 2 diabetes, muscle strength, and impaired physical function: The tip of the iceberg? Diabetes Care 2005, 28, 2541–2542. [Google Scholar] [CrossRef]
- Steele, R.M.; Brage, S.; Corder, K.; Wareham, N.J.; Ekelund, U. Physical activity, cardiorespiratory fitness, and the metabolic syndrome in youth. J. Appl. Physiol. 2008, 105, 342–351. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, H.; Qie, R.; Han, M.; Zhang, M.; Shi, X.; Yang, Y.; Lu, J.; Hu, D.; Sun, L. Association between cardiorespiratory fitness and risk of all-cause and cause-specific mortality. Eur. J. Clin. Investig. 2022, 52, e13770. [Google Scholar] [CrossRef]
- Raghuveer, G.; Hartz, J.; Lubans, D.R.; Takken, T.; Wiltz, J.L.; Mietus-Snyder, M.; Perak, A.M.; Baker-Smith, C.; Pietris, N.; Edwards, N.M. Cardiorespiratory fitness in youth: An important marker of health: A scientific statement from the American Heart Association. Circulation 2020, 142, e101–e118. [Google Scholar] [CrossRef]
- Lee, D.-C.; Artero, E.G.; Sui, X.; Blair, S.N. Mortality trends in the general population: The importance of cardiorespiratory fitness. J. Psychopharmacol. 2010, 24, 27–35. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Church, T.S.; Janssen, I.; Ross, R.; Blair, S.N. Metabolic syndrome, obesity, and mortality: Impact of cardiorespiratory fitness. Diabetes Care 2005, 28, 391–397. [Google Scholar] [CrossRef]
- Weisstaub, G.; Bravo, M.A.G.; García-Hermoso, A.; Salazar, G.; López-Gil, J.F. Cross-sectional association between physical fitness and cardiometabolic risk in Chilean schoolchildren: The fat but fit paradox. Transl. Pediatr. 2022, 11, 1085. [Google Scholar] [CrossRef]
- Jun, J.E.; Lee, S.-E.; Lee, Y.-B.; Kim, G.; Jin, S.-M.; Jee, J.H.; Kim, J.H. Low skeletal muscle mass accompanied by abdominal obesity additively increases the risk of incident type 2 diabetes. J. Clin. Endocrinol. Metab. 2023, 108, 1173–1180. [Google Scholar] [CrossRef]
- Borel, A.-L.; Coumes, S.; Reche, F.; Ruckly, S.; Pépin, J.-L.; Tamisier, R.; Wion, N.; Arvieux, C. Waist, neck circumferences, waist-to-hip ratio: Which is the best cardiometabolic risk marker in women with severe obesity? The SOON cohort. PLoS ONE 2018, 13, e0206617. [Google Scholar] [CrossRef]
- Choi, S.; Nah, S.; Jang, H.; Moon, J.; Han, S. Association between Relative Handgrip Strength and Chronic Lower Back Pain: A Nationwide Cross-Sectional Analysis of the Korea National Health and Nutrition Examination Survey. Int. J. Environ. Res. Public Health 2021, 18, 10770. [Google Scholar] [CrossRef]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A. 2013 ESH/ESC practice guidelines for the management of arterial hypertension: ESH-ESC the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Blood Press. 2014, 23, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Burr, J.F.; Bredin, S.S.; Faktor, M.D.; Warburton, D.E. The 6-minute walk test as a predictor of objectively measured aerobic fitness in healthy working-aged adults. Physician Sportsmed. 2011, 39, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Preacher, k.; Hayes, A. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav. Res. Methods Instrum. Comput. 2004, 36, 717–731. [Google Scholar] [CrossRef]
- Steene-Johannessen, J.; Anderssen, S.A.; Kolle, E.; Andersen, L.B. Low muscle fitness is associated with metabolic risk in youth. Med. Sci. Sport. Exerc. 2009, 41, 1361–1367. [Google Scholar] [CrossRef]
- Tanaka, M.; Okada, H.; Hashimoto, Y.; Kumagai, M.; Nishimura, H.; Fukui, M. Trunk muscle quality and quantity predict the development of metabolic syndrome and the increase in the number of its components in individuals without metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1161–1168. [Google Scholar] [CrossRef]
- Murai, J.; Nishizawa, H.; Otsuka, A.; Fukuda, S.; Tanaka, Y.; Nagao, H.; Sakai, Y.; Suzuki, M.; Yokota, S.; Tada, H.; et al. Low muscle quality in Japanese type 2 diabetic patients with visceral fat accumulation. Cardiovasc. Diabetol. 2018, 17, 112. [Google Scholar] [CrossRef]
- Mesinovic, J.; McMillan, L.B.; Shore-Lorenti, C.; De Courten, B.; Ebeling, P.R.; Scott, D. Metabolic Syndrome and Its Associations with Components of Sarcopenia in Overweight and Obese Older Adults. J. Clin. Med. 2019, 8, 145. [Google Scholar] [CrossRef]
- Melin, E.O.; Thulesius, H.O.; Hillman, M.; Landin-Olsson, M.; Thunander, M. Abdominal obesity in type 1 diabetes associated with gender, cardiovascular risk factors and complications, and difficulties achieving treatment targets: A cross sectional study at a secondary care diabetes clinic. BMC Obes. 2018, 5, 15. [Google Scholar] [CrossRef]
- Ji, C.; Xia, Y.; Tong, S.; Wu, Q.; Zhao, Y. Association of handgrip strength with the prevalence of metabolic syndrome in US adults: The national health and nutrition examination survey. Aging (Albany N. Y.) 2020, 12, 7818. [Google Scholar] [CrossRef]
- Chen, Z.; Smith, M.; Du, H.; Guo, Y.; Clarke, R.; Bian, Z.; Collins, R.; Chen, J.; Qian, Y.; Wang, X. Blood pressure in relation to general and central adiposity among 500 000 adult Chinese men and women. Int. J. Epidemiol. 2015, 44, 1305–1319. [Google Scholar] [CrossRef] [PubMed]
- Agostinis-Sobrinho, C.; Ruiz, J.R.; Moreira, C.; Lopes, L.; Ramírez-Vélez, R.; García-Hermoso, A.; Mota, J.; Santos, R. Changes in muscular fitness and its association with blood pressure in adolescents. Eur. J. Pediatr. 2018, 177, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vélez, R.; Correa-Bautista, J.E.; Lobelo, F.; Izquierdo, M.; Alonso-Martínez, A.; Rodríguez-Rodríguez, F.; Cristi-Montero, C. High muscular fitness has a powerful protective cardiometabolic effect in adults: Influence of weight status. BMC Public Health 2016, 16, 1012. [Google Scholar] [CrossRef]
- Brown, J.C.; Harhay, M.O.; Harhay, M.N. The muscle quality index and mortality among males and females. Ann. Epidemiol. 2016, 26, 648–653. [Google Scholar] [CrossRef]
- Farmer, R.E.; Mathur, R.; Schmidt, A.F.; Bhaskaran, K.; Fatemifar, G.; Eastwood, S.V.; Finan, C.; Denaxas, S.; Smeeth, L.; Chaturvedi, N. Associations between measures of sarcopenic obesity and risk of cardiovascular disease and mortality: A cohort study and Mendelian randomization analysis using the UK Biobank. J. Am. Heart Assoc. 2019, 8, e011638. [Google Scholar] [CrossRef]
- Mallah, M.A.; Liu, M.; Liu, Y.; Xu, H.-F.; Wu, X.-J.; Chen, X.-T.; Wang, H.; Liu, C.-L.; Tian, Y.-R.; Li, M.-X. Association of handgrip strength with the prevalence of hypertension in a Chinese Han population. Chronic Dis. Transl. Med. 2019, 5, 113–121. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Picoloto, A.; Nunes, J.P.; Bezerra, E.S.; Schoenfeld, B.J.; Cyrino, E.S. Effects of different resistance training loads on the muscle quality index in older women. J. Strength Cond. Res. 2022, 36, 1445–1449. [Google Scholar] [CrossRef]
- Palacio-Agüero, A.; Díaz-Torrente, X.; Quintiliano Scarpelli Dourado, D. Relative handgrip strength, nutritional status and abdominal obesity in Chilean adolescents. PLoS ONE 2020, 15, e0234316. [Google Scholar] [CrossRef]
- Palacio, A.C.; Díaz-Torrente, X.; Quintiliano-Scarpelli, D. Higher abdominal adiposity is associated with lower muscle strength in chilean adults. Front. Nutr. 2022, 9, 167. [Google Scholar] [CrossRef]
- Pérez-Sousa, M.Á.; del Pozo-Cruz, J.; Cano-Gutiérrez, C.A.; Ferrebuz, A.J.; Sandoval-Cuellar, C.; Izquierdo, M.; Hernández-Quiñonez, P.A.; Ramírez-Vélez, R. Glucose levels as a mediator of the detrimental effect of abdominal obesity on relative handgrip strength in older adults. J. Clin. Med. 2020, 9, 2323. [Google Scholar] [CrossRef]
- Koster, A.; Ding, J.; Stenholm, S.; Caserotti, P.; Houston, D.K.; Nicklas, B.J.; You, T.; Lee, J.S.; Visser, M.; Newman, A.B. Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2011, 66, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Brady, A.O.; Straight, C.; Schmidt, M.; Evans, E. Impact of body mass index on the relationship between muscle quality and physical function in older women. J. Nutr. Health Aging 2014, 18, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Wearing, J.; deBruin, E.D.; Stokes, M. Investigations on muscle composition and muscle power in older people. J. Bodyw. Mov. Ther. 2018, 22, 855. [Google Scholar] [CrossRef]
- Pasdar, Y.; Darbandi, M.; Mirtaher, E.; Rezaeian, S.; Najafi, F.; Hamzeh, B. Associations between muscle strength with different measures of obesity and lipid profiles in men and women: Results from RaNCD cohort study. Clin. Nutr. Res. 2019, 8, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Guzmán, I.P.; Delgado-Floody, P.; Gutiérrez-Pérez, I.A.; Caamaño-Navarrete, F.; Jerez-Mayorga, D.; Zaragoza-García, Ó.; Parra-Rojas, I. Asociación entre la fuerza prensil relativa de la mano y la obesidad abdominal, la diabetes de tipo 2 y la hipertensión en una población mexicana. Nutr. Hosp. 2022, 39, 82–92. [Google Scholar]
- Yi, D.; Khang, A.R.; Lee, H.W.; Son, S.M.; Kang, Y.H. Relative handgrip strength as a marker of metabolic syndrome: The Korea National Health and Nutrition Examination Survey (KNHANES) VI (2014–2015). Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 227–240. [Google Scholar] [CrossRef]
- Sénéchal, M.; Johannsen, N.M.; Swift, D.L.; Earnest, C.P.; Lavie, C.J.; Blair, S.N.; Church, T.S. Association between changes in muscle quality with exercise training and changes in cardiorespiratory fitness measures in individuals with type 2 diabetes mellitus: Results from the HART-D study. PLoS ONE 2015, 10, e0135057. [Google Scholar] [CrossRef]
- Kim, T.N.; Park, M.S.; Kim, Y.J.; Lee, E.J.; Kim, M.-K.; Kim, J.M.; Ko, K.S.; Rhee, B.D.; Won, J.C. Association of low muscle mass and combined low muscle mass and visceral obesity with low cardiorespiratory fitness. PLoS ONE 2014, 9, e100118. [Google Scholar] [CrossRef]
- Boo, S.-H.; Joo, M.C.; Lee, J.M.; Kim, S.C.; Yu, Y.M.; Kim, M.-S. Association between skeletal muscle mass and cardiorespiratory fitness in community-dwelling elderly men. Aging Clin. Exp. Res. 2019, 31, 49–57. [Google Scholar] [CrossRef]
- Carvalho, L.P.; Di Thommazo-Luporini, L.; Aubertin-Leheudre, M.; Bonjorno Junior, J.C.; de Oliveira, C.R.; Luporini, R.L.; Mendes, R.G.; Zangrando, K.T.L.; Trimer, R.; Arena, R. Prediction of cardiorespiratory fitness by the six-minute step test and its association with muscle strength and power in sedentary obese and lean young women: A cross-sectional study. PLoS ONE 2015, 10, e0145960. [Google Scholar] [CrossRef]
- Barbat-Artigas, S.; Pion, C.H.; Leduc-Gaudet, J.-P.; Rolland, Y.; Aubertin-Leheudre, M. Exploring the Role of Muscle Mass, Obesity, and Age in the Relationship Between Muscle Quality and Physical Function. J. Am. Med. Dir. Assoc. 2014, 15, 303.e313–303.e320. [Google Scholar] [CrossRef] [PubMed]
- Peltz, G.; Aguirre, M.T.; Sanderson, M.; Fadden, M.K. The role of fat mass index in determining obesity. Am. J. Hum. Biol. 2010, 22, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Dewi, M.; Kustiyah, L.; Kuswari, M. Percent fat mass and body mass index as cardiorespiratory fitness predictors in young adults. J. Gizi Dan Pangan 2015, 10, 179–184. [Google Scholar]
- Chatterjee, S.; Chatterjee, P.; Bandhopadhyay, A. Cardiorespiratory fitness of obese boys. Indian J. Physiol. Pharmacol. 2005, 49, 353. [Google Scholar]
- Gallagher, M.J.; Franklin, B.A.; Ehrman, J.K.; Keteyian, S.J.; Brawner, C.A.; dejong, A.T.; McCullough, P.A. Comparative impact of morbid obesity vs. heart failure on cardiorespiratory fitness. Chest 2005, 127, 2197–2203. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).