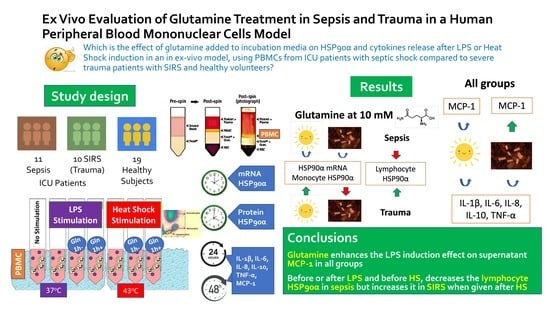
Ex Vivo Evaluation of Glutamine Treatment in Sepsis and Trauma in a Human Peripheral Blood Mononuclear Cells Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
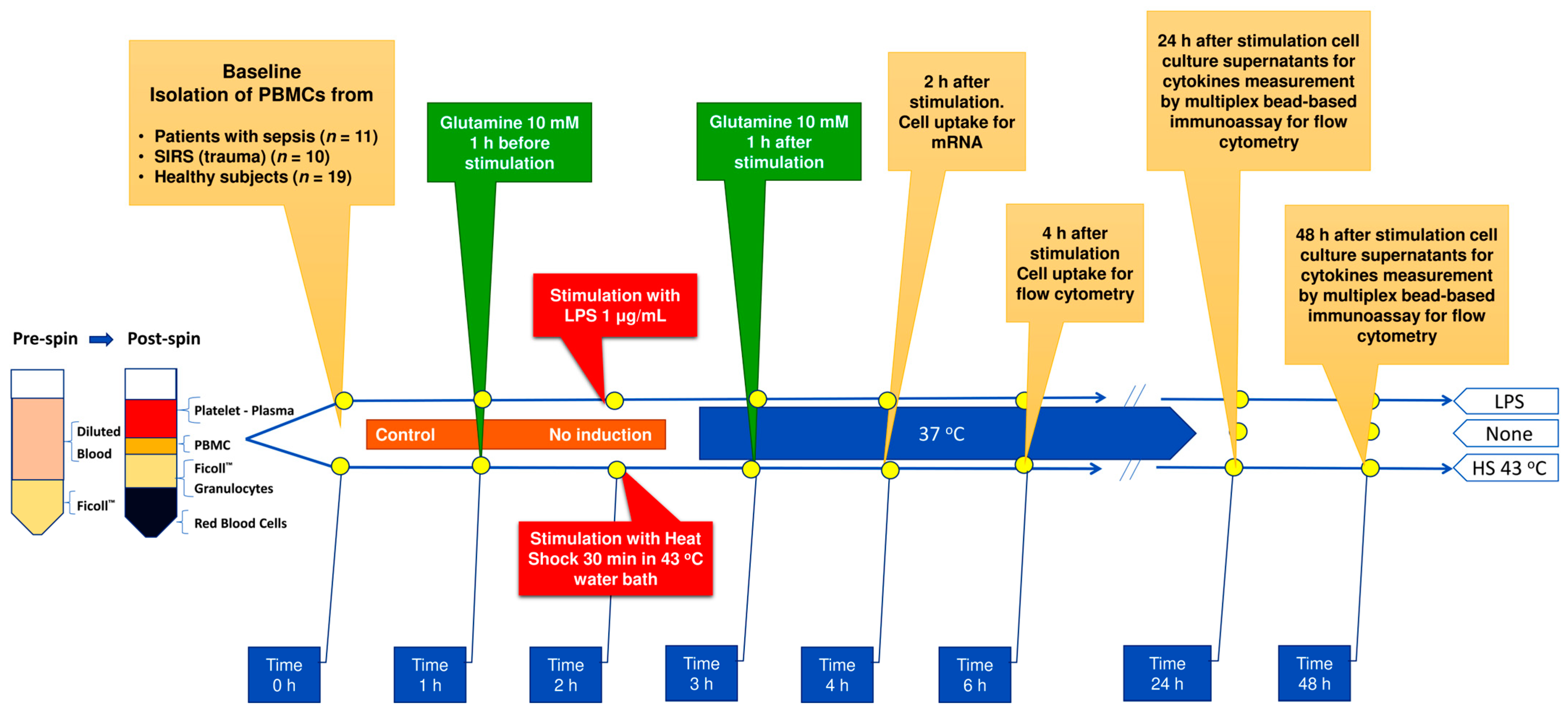
2.2. Sample Collection
2.3. Cell Culture Protocol
2.4. HSP90α Flow Cytometry
2.5. Gene Expression Assays
2.5.1. The Gene Expression Assays Have Been Previously Described in Detail [32]
2.5.2. RNA Isolation from PBMC
2.5.3. Reverse Transcription and Quantitative PCR
2.5.4. Complementary DNA (cDNA)
2.6. Supernatant Cytokines
2.7. Serum Cytokines
2.8. Statistical Analysis
3. Results
3.1. Study Population
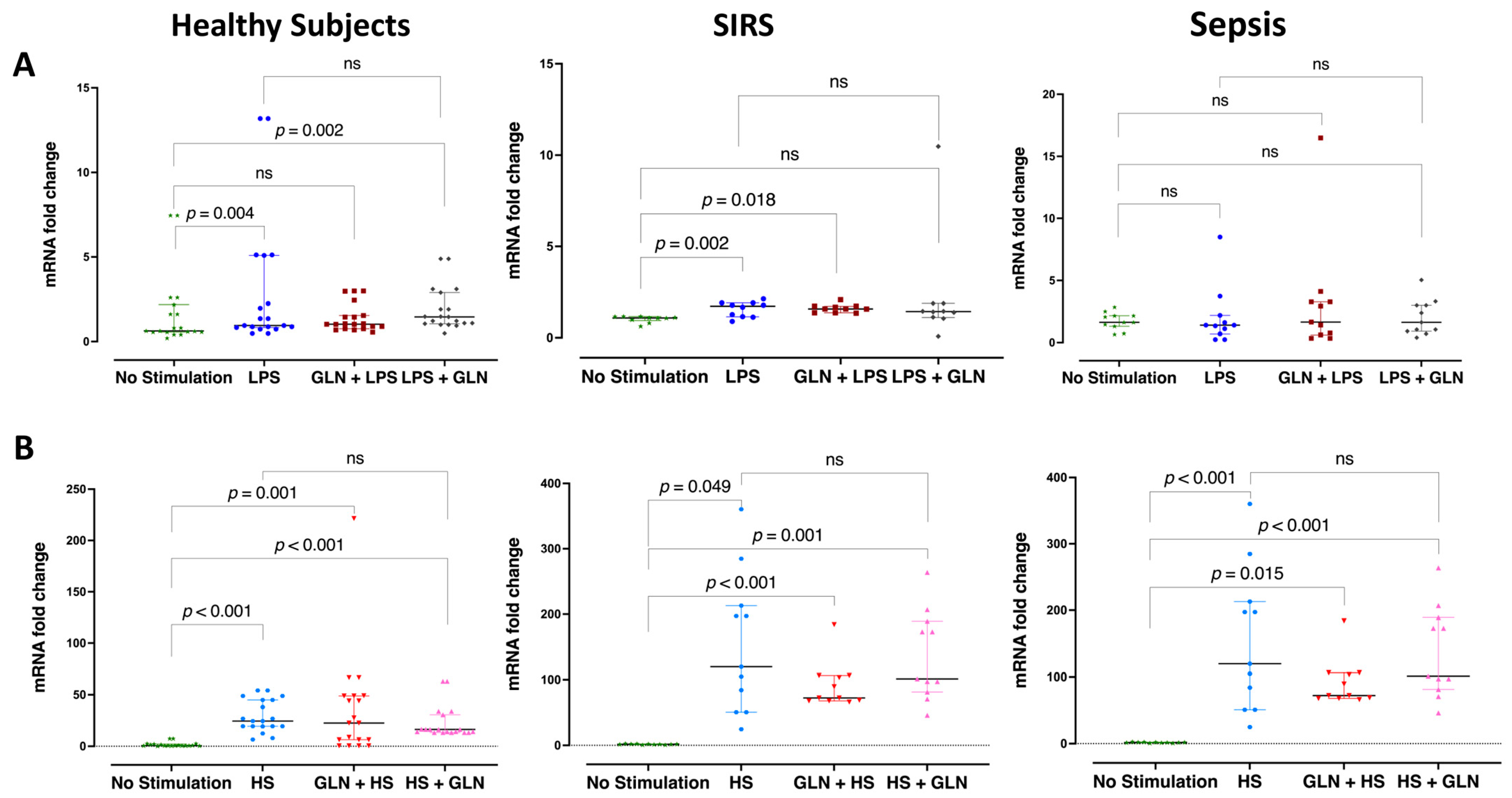
3.2. Differences and Glutamine Effect in Relative HSP90α Gene Expression
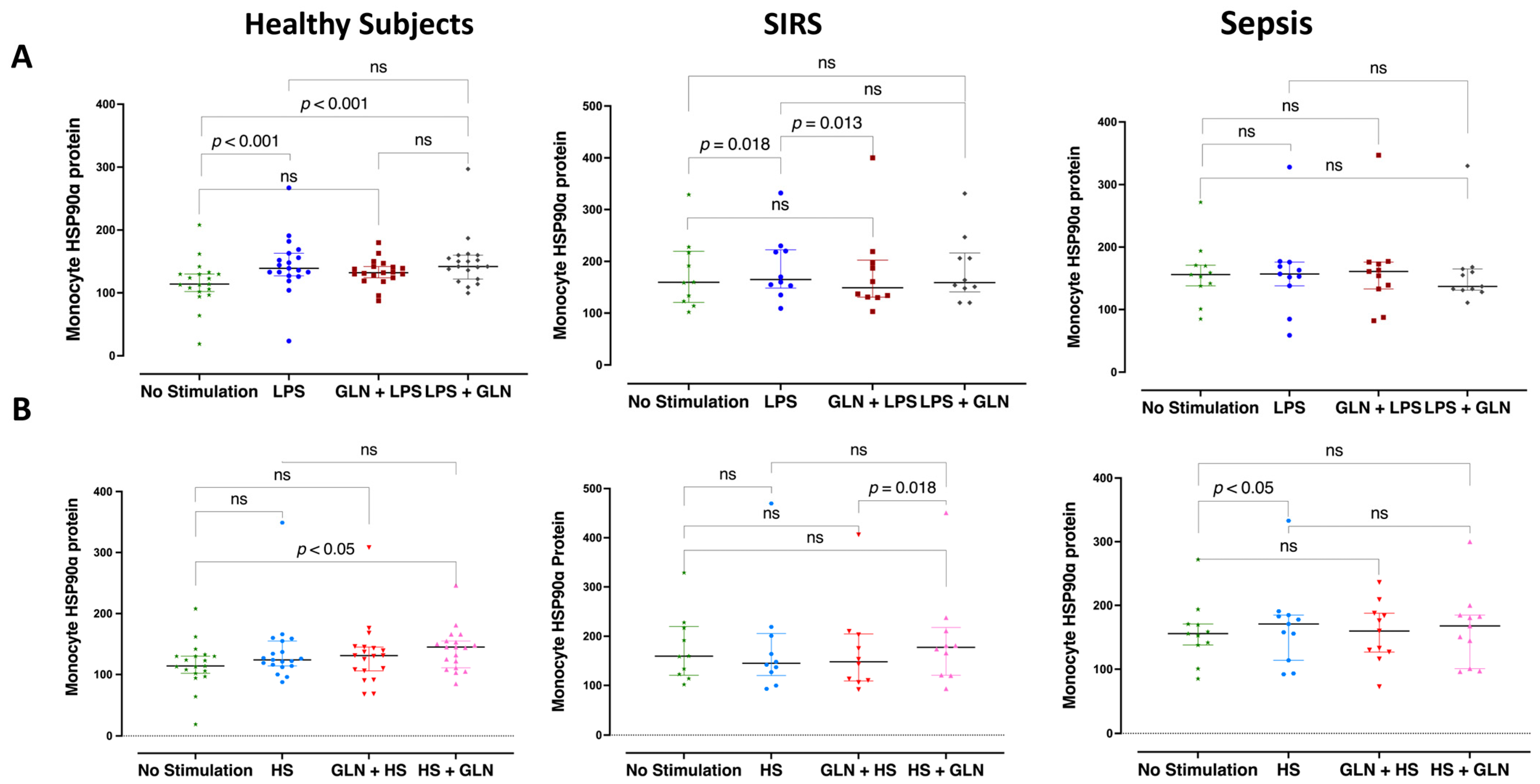
3.3. Differences and Glutamine Effect in Intracellular Monocyte HSP90α Protein Expression
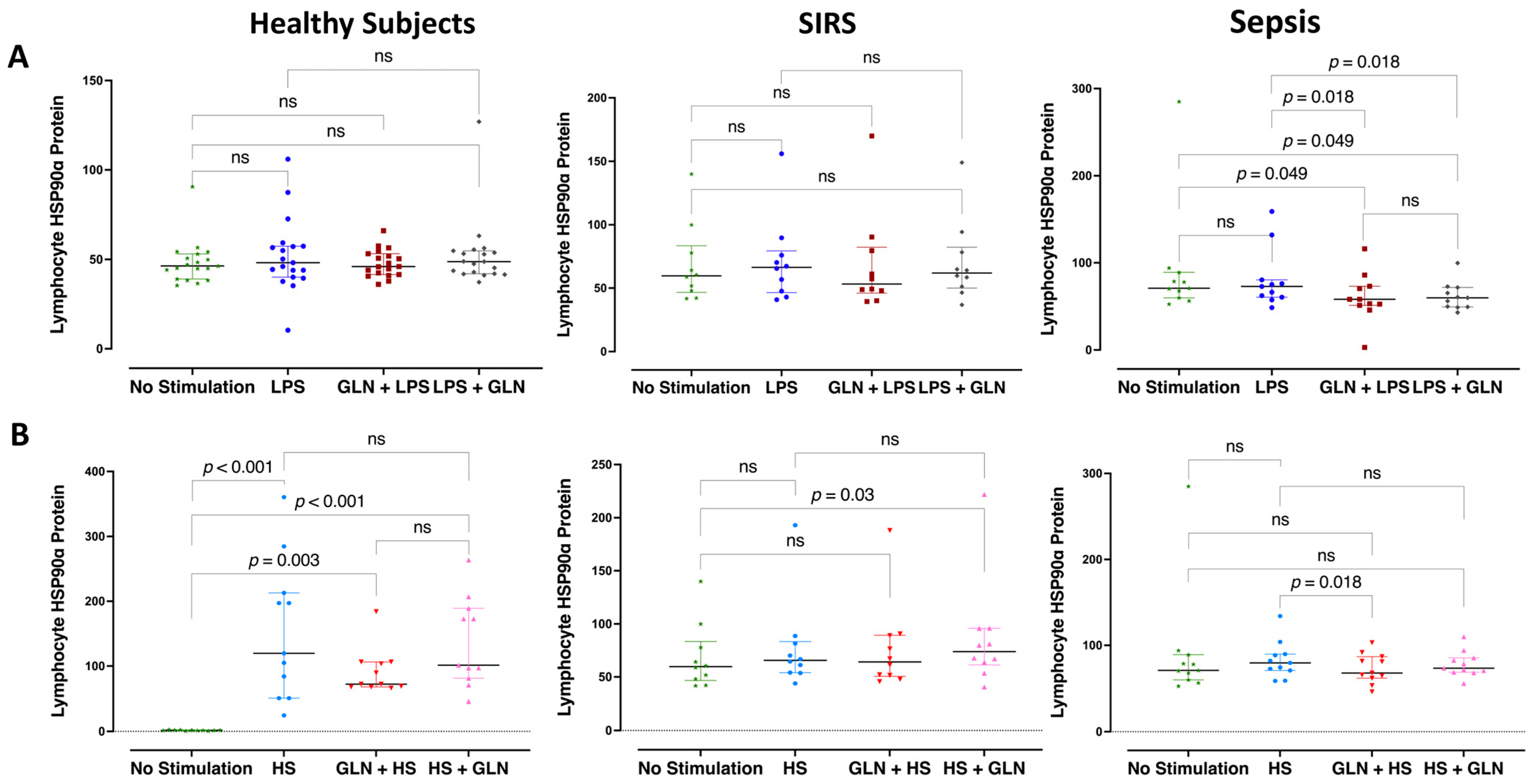
3.4. Differences and Glutamine Effect in Intracellular Lymphocyte HSP90α Protein Expression
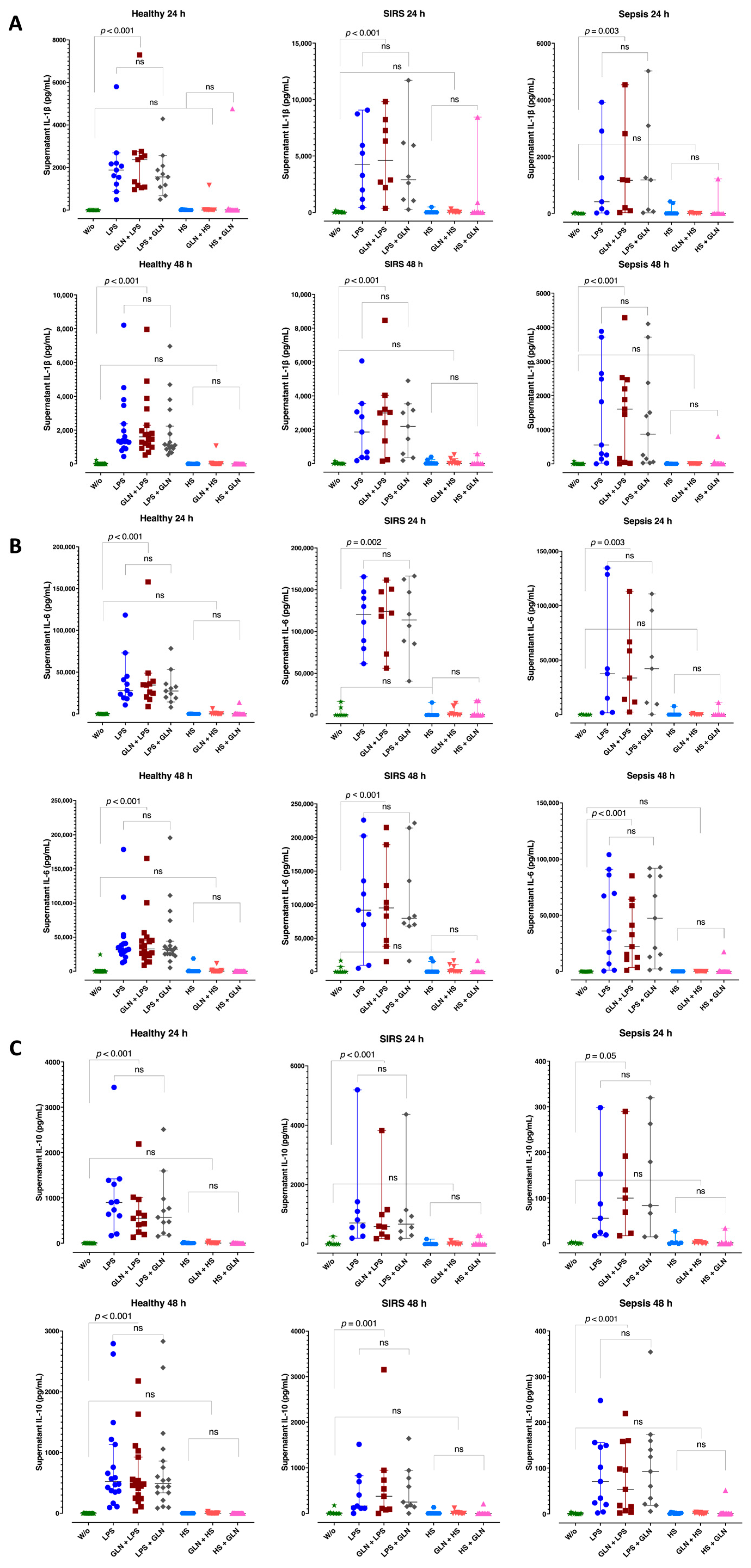
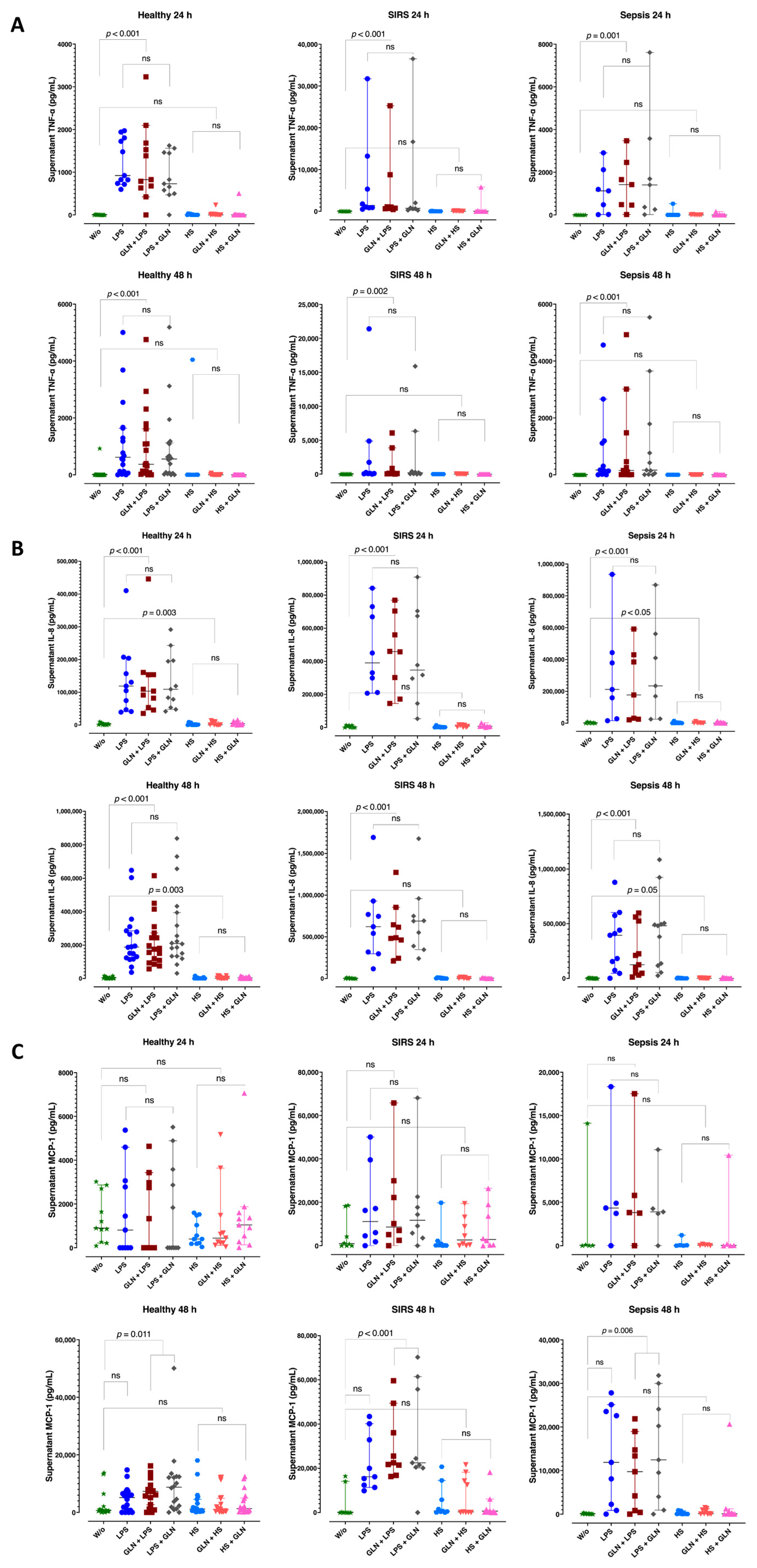
3.5. Group Differences in Supernatant Cytokine Level
3.6. Induction Differences and Glutamine Effect in Supernatant Cytokine Levels
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, P. Preserving the Quality of Life: Nutrition in the ICU. Crit. Care 2019, 23, 139. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Jones, G.; David, S.; Olariu, E.; Cadwell, K.K. Frequency and Mortality of Septic Shock in Europe and North America: A Systematic Review and Meta-Analysis. Crit. Care 2019, 23, 196. [Google Scholar] [CrossRef] [PubMed]
- Janus, P.; Kuś, P.; Vydra, N.; Toma-Jonik, A.; Stokowy, T.; Mrowiec, K.; Wojtaś, B.; Gielniewski, B.; Widłak, W. HSF1 Can Prevent Inflammation Following Heat Shock by Inhibiting the Excessive Activation of the ATF3 and JUN&FOS Genes. Cells 2022, 11, 2510. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.J.; Hanna-Jumma, S.; Carraretto, M.; Forni, L. The Pathophysiological Basis and Consequences of Fever. Crit. Care 2016, 20, 200. [Google Scholar] [CrossRef] [PubMed]
- Drewry, A.; Mohr, N.M. Temperature Management in the ICU. Crit. Care Med. 2022, 50, 1138–1147. [Google Scholar] [CrossRef]
- Shen, Y.; Lou, Y.; Zhu, S. Hyperthermia Is a Predictor of High Mortality in Patients with Sepsis. Crit. Care 2020, 24, 543. [Google Scholar] [CrossRef]
- Drewry, A.M.; Mohr, N.M.; Ablordeppey, E.A.; Dalton, C.M.; Doctor, R.J.; Fuller, B.M.; Kollef, M.H.; Hotchkiss, R.S. Therapeutic Hyperthermia Is Associated with Improved Survival in Afebrile Critically Ill Patients with Sepsis: A Pilot Randomized Trial. Crit. Care Med. 2022, 50, 924–934. [Google Scholar] [CrossRef]
- Moura, C.S.; Lollo, P.C.B.; Morato, P.N.; Risso, E.M.; Amaya-Farfan, J. Modulatory Effects of Arginine, Glutamine and Branched-Chain Amino Acids on Heat Shock Proteins, Immunity and Antioxidant Response in Exercised Rats. Food Funct. 2017, 8, 3228–3238. [Google Scholar] [CrossRef]
- Fitrolaki, D.-M.; Dimitriou, H.; Kalmanti, M.; Briassoulis, G. CD64-Neutrophil Expression and Stress Metabolic Patterns in Early Sepsis and Severe Traumatic Brain Injury in Children. BMC Pediatr. 2013, 13, 31. [Google Scholar] [CrossRef]
- Fitrolaki, M.-D.; Dimitriou, H.; Venihaki, M.; Katrinaki, M.; Ilia, S.; Briassoulis, G. Increased Extracellular Heat Shock Protein 90α in Severe Sepsis and SIRS Associated with Multiple Organ Failure and Related to Acute Inflammatory-Metabolic Stress Response in Children. Medicine 2016, 95, e4651. [Google Scholar] [CrossRef]
- Vardas, K.; Apostolou, K.; Briassouli, E.; Goukos, D.; Psarra, K.; Botoula, E.; Tsagarakis, S.; Magira, E.; Routsi, C.; Nanas, S.; et al. Early Response Roles for Prolactin Cortisol and Circulating and Cellular Levels of Heat Shock Proteins 72 and 90α in Severe Sepsis and SIRS. Biomed Res. Int. 2014, 2014, 803561. [Google Scholar] [CrossRef]
- Wischmeyer, P.E.; Kahana, M.; Wolfson, R.; Ren, H.; Musch, M.M.; Chang, E.B. Glutamine Induces Heat Shock Protein and Protects against Endotoxin Shock in the Rat. J. Appl. Physiol. 2001, 90, 2403–2410. [Google Scholar] [CrossRef]
- Briassoulis, G.; Briassouli, E.; Fitrolaki, D.-M.; Plati, I.; Apostolou, K.; Tavladaki, T.; Spanaki, A.-M. Heat Shock Protein 72 Expressing Stress in Sepsis: Unbridgeable Gap between Animal and Human Studies--a Hypothetical “Comparative” Study. Biomed Res. Int. 2014, 2014, 101023. [Google Scholar] [CrossRef]
- Roth, E. Nonnutritive Effects of Glutamine. J. Nutr. 2008, 138, 2025S–2031S. [Google Scholar] [CrossRef]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, E1564. [Google Scholar] [CrossRef]
- Newsholme, P. Why Is L-Glutamine Metabolism Important to Cells of the Immune System in Health, Postinjury, Surgery or Infection? J. Nutr. 2001, 131, 2515S–2522S. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.F.; Pantaleão, L.C.; Donato, J.; de Bittencourt, P.I.H.; Tirapegui, J. Oral Supplementations with Free and Dipeptide Forms of L-Glutamine in Endotoxemic Mice: Effects on Muscle Glutamine-Glutathione Axis and Heat Shock Proteins. J. Nutr. Biochem 2014, 25, 345–352. [Google Scholar] [CrossRef]
- Kao, C.; Hsu, J.; Bandi, V.; Jahoor, F. Alterations in Glutamine Metabolism and Its Conversion to Citrulline in Sepsis. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1359–E1364. [Google Scholar] [CrossRef] [PubMed]
- Rodas, P.C.; Rooyackers, O.; Hebert, C.; Norberg, Å.; Wernerman, J. Glutamine and Glutathione at ICU Admission in Relation to Outcome. Clin. Sci. 2012, 122, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wu, J.; Wu, L.-D.; Li, G.-P.; Xiong, M.; Chen, X.; Meng, Q.-Y. Effect of Parenteral Glutamine Supplementation Combined with Enteral Nutrition on Hsp90 Expression and Lymphoid Organ Apoptosis in Severely Burned Rats. Burns 2016, 42, 1494–1506. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tao, S.; Wu, Q.; Wu, T.; Tao, R.; Fan, J. Glutamine Reduces Myocardial Cell Apoptosis in a Rat Model of Sepsis by Promoting Expression of Heat Shock Protein 90. J. Surg. Res. 2017, 220, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.; Muscedere, J.; Wischmeyer, P.E.; Cook, D.; Jones, G.; Albert, M.; Elke, G.; Berger, M.M.; Day, A.G. Canadian Critical Care Trials Group a Randomized Trial of Glutamine and Antioxidants in Critically Ill Patients. N. Engl. J. Med. 2013, 368, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- van Zanten, A.R.H.; Sztark, F.; Kaisers, U.X.; Zielmann, S.; Felbinger, T.W.; Sablotzki, A.R.; De Waele, J.J.; Timsit, J.-F.; Honing, M.L.H.; Keh, D.; et al. High-Protein Enteral Nutrition Enriched with Immune-Modulating Nutrients vs Standard High-Protein Enteral Nutrition and Nosocomial Infections in the ICU: A Randomized Clinical Trial. JAMA 2014, 312, 514–524. [Google Scholar] [CrossRef] [PubMed]
- van Zanten, A.R.H.; Dhaliwal, R.; Garrel, D.; Heyland, D.K. Enteral Glutamine Supplementation in Critically Ill Patients: A Systematic Review and Meta-Analysis. Crit. Care 2015, 19, 294. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulou, A.; Haidich, A.-B.; Kofina, K.; Manzanares, W.; Bouras, E.; Tsaousi, G.; Stoppe, C.; Dardavessis, T.I.; Chourdakis, M. Effects of Glutamine Supplementation on Critically Ill Patients: Focus on Efficacy and Safety. An Overview of Systematic Reviews. Nutrition 2020, 78, 110960. [Google Scholar] [CrossRef] [PubMed]
- Briassoulis, G.; Filippou, O.; Hatzi, E.; Papassotiriou, I.; Hatzis, T. Early Enteral Administration of Immunonutrition in Critically Ill Children: Results of a Blinded Randomized Controlled Clinical Trial. Nutrition 2005, 21, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Briassoulis, G.; Filippou, O.; Kanariou, M.; Hatzis, T. Comparative Effects of Early Randomized Immune or Non-Immune-Enhancing Enteral Nutrition on Cytokine Production in Children with Septic Shock. Intensive Care Med. 2005, 31, 851–858. [Google Scholar] [CrossRef]
- Briassoulis, G.; Filippou, O.; Kanariou, M.; Papassotiriou, I.; Hatzis, T. Temporal Nutritional and Inflammatory Changes in Children with Severe Head Injury Fed a Regular or an Immune-Enhancing Diet: A Randomized, Controlled Trial. Pediatr. Crit. Care Med. 2006, 7, 56–62. [Google Scholar] [CrossRef]
- Jennaro, T.S.; Viglianti, E.M.; Ingraham, N.E.; Jones, A.E.; Stringer, K.A.; Puskarich, M.A. Serum Levels of Acylcarnitines and Amino Acids Are Associated with Liberation from Organ Support in Patients with Septic Shock. J. Clin. Med. 2022, 11, 627. [Google Scholar] [CrossRef]
- Briassoulis, P.; Ilia, S.; Briassouli, E.; Miliaraki, M.; Briassoulis, G. The Lonely Glutamine Tree in the Middle of the Infinite Critically Ill Forest. Crit. Care 2021, 25, 342. [Google Scholar] [CrossRef]
- Briassouli, E.; Briassoulis, G. Glutamine Randomized Studies in Early Life: The Unsolved Riddle of Experimental and Clinical Studies. Clin. Dev. Immunol. 2012, 2012, 749189. [Google Scholar] [CrossRef] [PubMed]
- Briassouli, E.; Tzanoudaki, M.; Goukos, D.; Routsi, C.; Nanas, S.; Vardas, K.; Apostolou, K.; Kanariou, M.; Daikos, G.; Briassoulis, G. Glutamine May Repress the Weak LPS and Enhance the Strong Heat Shock Induction of Monocyte and Lymphocyte HSP72 Proteins but May Not Modulate the HSP72 MRNA in Patients with Sepsis or Trauma. Biomed Res. Int. 2015, 2015, 806042. [Google Scholar] [CrossRef] [PubMed]
- Garib, R.; Garla, P.; Torrinhas, R.S.; Moretti, A.I.S.; Machado, M.C.C.; Waitzberg, D.L. Effect of Previous High Glutamine Infusion on Inflammatory Mediators and Mortality in an Acute Pancreatitis Model. Mediat. Inflamm. 2016, 2016, 4261419. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wu, J.; Wu, L.-D.; Cheng, B.; Tao, S.-Y.; Wang, W.; Chen, X.; Zeng, P.; Wang, Y.-B.; Meng, Q.-Y. Effect of Parenteral Glutamine Supplementation Combined with Enteral Nutrition on Hsp90 Expression and Peyer’s Patch Apoptosis in Severely Burned Rats. Nutrition 2018, 47, 97–103. [Google Scholar] [CrossRef]
- Cruzat, V.F.; Keane, K.N.; Scheinpflug, A.L.; Cordeiro, R.; Soares, M.J.; Newsholme, P. Alanyl-Glutamine Improves Pancreatic β-Cell Function Following Ex Vivo Inflammatory Challenge. J. Endocrinol. 2015, 224, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Briassouli, E.; Goukos, D.; Daikos, G.; Apostolou, K.; Routsi, C.; Nanas, S.; Briassoulis, G. Glutamine Suppresses Hsp72 Not Hsp90α and Is Not Inducing Th1, Th2, or Th17 Cytokine Responses in Human Septic PBMCs. Nutrition 2014, 30, 1185–1194. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M. Sepsis Definitions Task Force Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef]
- Kaukonen, K.-M.; Bailey, M.; Pilcher, D.; Cooper, D.J.; Bellomo, R. Systemic Inflammatory Response Syndrome Criteria in Defining Severe Sepsis. N. Engl. J. Med. 2015, 372, 1629–1638. [Google Scholar] [CrossRef]
- Basi, D.L.; Ross, K.F.; Hodges, J.S.; Herzberg, M.C. The Modulation of Tissue Factor by Endothelial Cells during Heat Shock. J. Biol. Chem. 2003, 278, 11065–11071. [Google Scholar] [CrossRef]
- Singleton, K.D.; Beckey, V.E.; Wischmeyer, P.E. Glutamine prevents activation of NF-kB and Stress Kinase pathways, attenuates inflammatory cytokine release, and prevents Acute Respiratory Distress Syndrome (ARDS) following sepsis. Shock 2005, 24, 583–589. [Google Scholar] [CrossRef]
- Wischmeyer, P.E.; Riehm, J.; Singleton, K.D.; Ren, H.; Musch, M.W.; Kahana, M.; Chang, E.B. Glutamine Attenuates Tumor Necrosis Factor-Alpha Release and Enhances Heat Shock Protein 72 in Human Peripheral Blood Mononuclear Cells. Nutrition 2003, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Diniz, V.L.S.; Dodd, G.T.; Cruzat, V. Glutamine Metabolism and Optimal Immune and CNS Function. Proc. Nutr. Soc. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- van Zanten, A.R.H.; Hofman, Z.; Heyland, D.K. Consequences of the REDOXS and METAPLUS Trials: The End of an Era of Glutamine and Antioxidant Supplementation for Critically Ill Patients? JPEN J Parenter Enter. Nutr 2015, 39, 890–892. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN Guideline on Clinical Nutrition in the Intensive Care Unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Tume, L.N.; Valla, F.V.; Joosten, K.; Jotterand Chaparro, C.; Latten, L.; Marino, L.V.; Macleod, I.; Moullet, C.; Pathan, N.; Rooze, S.; et al. Nutritional Support for Children during Critical Illness: European Society of Pediatric and Neonatal Intensive Care (ESPNIC) Metabolism, Endocrine and Nutrition Section Position Statement and Clinical Recommendations. Intensive Care Med. 2020, 46, 411–425. [Google Scholar] [CrossRef]
- Compher, C.; Bingham, A.L.; McCall, M.; Patel, J.; Rice, T.W.; Braunschweig, C.; McKeever, L. Guidelines for the Provision of Nutrition Support Therapy in the Adult Critically Ill Patient: The American Society for Parenteral and Enteral Nutrition. JPEN J. Parenter. Enter. Nutr. 2022, 46, 12–41. [Google Scholar] [CrossRef]
- Miliaraki, M.; Briassoulis, P.; Ilia, S.; Polonifi, A.; Mantzourani, M.; Briassouli, E.; Vardas, K.; Nanas, S.; Pistiki, A.; Theodorakopoulou, M.; et al. Survivin and Caspases Serum Protein Levels and Survivin Variants MRNA Expression in Sepsis. Sci. Rep. 2021, 11, 1049. [Google Scholar] [CrossRef]
- Gupta, S.; Lee, C.-M.; Wang, J.-F.; Parodo, J.; Jia, S.-H.; Hu, J.; Marshall, J.C. Heat-Shock Protein-90 Prolongs Septic Neutrophil Survival by Protecting c-Src Kinase and Caspase-8 from Proteasomal Degradation. J. Leukoc. Biol. 2018, 103, 933–944. [Google Scholar] [CrossRef]
- Yan, G.; Huang, J.; Jarbadan, N.R.; Jiang, Y.; Cheng, H. Sequestration of NF-KappaB Signaling Complexes in Lipid Rafts Contributes to Repression of NF-KappaB in T Lymphocytes under Hyperthermia Stress. J. Biol. Chem. 2008, 283, 12489–12500. [Google Scholar] [CrossRef]
- Janus, P.; Stokowy, T.; Jaksik, R.; Szoltysek, K.; Handschuh, L.; Podkowinski, J.; Widlak, W.; Kimmel, M.; Widlak, P. Cross Talk between Cytokine and Hyperthermia-Induced Pathways: Identification of Different Subsets of NF-ΚB-Dependent Genes Regulated by TNFα and Heat Shock. Mol. Genet. Genom. 2015, 290, 1979–1990. [Google Scholar] [CrossRef]
- Paszek, A.; Kardyńska, M.; Bagnall, J.; Śmieja, J.; Spiller, D.G.; Widłak, P.; Kimmel, M.; Widlak, W.; Paszek, P. Heat Shock Response Regulates Stimulus-Specificity and Sensitivity of the pro-Inflammatory NF-ΚB Signalling. Cell Commun. Signal. 2020, 18, 77. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, P.; Pistiki, A.; Theodorakopoulou, M.; Christodoulopoulou, T.; Damoraki, G.; Goukos, D.; Briassouli, E.; Dimopoulou, I.; Armaganidis, A.; Nanas, S.; et al. Immunoparalysis: Clinical and Immunological Associations in SIRS and Severe Sepsis Patients. Cytokine 2017, 92, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, M.; Jeong, H.; Chae, J.S.; Kim, Y.S.; Lee, J.G.; Cho, Y.; Lee, J.H. Hyporesponsiveness of Natural Killer Cells and Impaired Inflammatory Responses in Critically Ill Patients. BMC Immunol. 2017, 18, 48. [Google Scholar] [CrossRef] [PubMed]
- West, M.A.; Koons, A. Endotoxin Tolerance in Sepsis: Concentration-Dependent Augmentation or Inhibition of LPS-Stimulated Macrophage TNF Secretion by LPS Pretreatment. J. Trauma 2008, 65, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Jordan, I.; Balaguer, M.; Esteban, M.E.; Cambra, F.J.; Felipe, A.; Hernández, L.; Alsina, L.; Molero, M.; Villaronga, M.; Esteban, E. Glutamine Effects on Heat Shock Protein 70 and Interleukines 6 and 10: Randomized Trial of Glutamine Supplementation versus Standard Parenteral Nutrition in Critically Ill Children. Clin. Nutr. 2016, 35, 34–40. [Google Scholar] [CrossRef]
- Marino, L.V.; Pathan, N.; Meyer, R.; Wright, V.J.; Habibi, P. The Effect of 2 MMol Glutamine Supplementation on HSP70 and TNF-α Release by LPS Stimulated Blood from Healthy Children. Clin. Nutr. 2014, 34, 1195–1201. [Google Scholar] [CrossRef]
- Marino, L.V.; Pathan, N.; Meyer, R.W.; Wright, V.J.; Habibi, P. An in Vitro Model to Consider the Effect of 2 MM Glutamine and KNK437 on Endotoxin-Stimulated Release of Heat Shock Protein 70 and Inflammatory Mediators. Nutrition 2015, 32, 375–383. [Google Scholar] [CrossRef]
- Vardas, K.; Ilia, S.; Sertedaki, A.; Charmandari, E.; Briassouli, E.; Goukos, D.; Apostolou, K.; Psarra, K.; Botoula, E.; Tsagarakis, S.; et al. Increased Glucocorticoid Receptor Expression in Sepsis Is Related to Heat Shock Proteins, Cytokines, and Cortisol and Is Associated with Increased Mortality. Intensive Care Med. Exp. 2017, 5, 10. [Google Scholar] [CrossRef]
- Spanaki, A.M.; Tavladaki, T.; Dimitriou, H.; Kozlov, A.V.; Duvigneau, J.C.; Meleti, E.; Weidinger, A.; Papakonstantinou, E.; Briassoulis, G. Longitudinal Profiles of Metabolism and Bioenergetics Associated with Innate Immune Hormonal Inflammatory Responses and Amino-Acid Kinetics in Severe Sepsis and Systemic Inflammatory Response Syndrome in Children. JPEN J. Parenter. Enter. Nutr. 2018, 42, 1061–1074. [Google Scholar] [CrossRef]
- Tavladaki, T.; Spanaki, A.M.; Dimitriou, H.; Kondili, E.; Choulaki, C.; Georgopoulos, D.; Briassoulis, G. Similar Metabolic, Innate Immunity, and Adipokine Profiles in Adult and Pediatric Sepsis Versus Systemic Inflammatory Response Syndrome-A Pilot Study. Pediatr. Crit. Care Med. 2017, 18, e494–e505. [Google Scholar] [CrossRef]
- Huang, J.; Liu, J.; Chang, G.; Wang, Y.; Ma, N.; Roy, A.C.; Shen, X. Glutamine Supplementation Attenuates the Inflammation Caused by LPS-Induced Acute Lung Injury in Mice by Regulating the TLR4/MAPK Signaling Pathway. Inflammation 2021, 44, 2180–2192. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dong, Y.; Cai, Y. Alanyl-glutamine Prophylactically Protects against Lipopolysaccharide-induced Acute Lung Injury by Enhancing the Expression of HSP70. Mol. Med. Rep. 2017, 16, 2807–2813. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.; Nowell, M.; Chima, R.; Zingarelli, B. Pioglitazone Reduces Inflammation through Inhibition of NF-ΚB in Polymicrobial Sepsis. Innate Immun. 2014, 20, 519–528. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhou, X.; Wu, Z.; Feng, Y.; Liu, D.; Li, T.; Yin, Y. Glutamine in Suppression of Lipopolysaccharide-Induced Piglet Intestinal Inflammation: The Crosstalk between AMPK Activation and Mitochondrial Function. Anim. Nutr. 2022, 10, 137–147. [Google Scholar] [CrossRef]
- Scharte, M.; Baba, H.A.; Van Aken, H.; Schulzki, C.; Meyer, J.; Goeters, C.; Bone, H.G. Alanyl-Glutamine Dipeptide Does Not Affect Hemodynamics despite a Greater Increase in Myocardial Heat Shock Protein 72 Immunoreactivity in Endotoxemic Sheep. J. Nutr. 2001, 131, 1433–1437. [Google Scholar] [CrossRef][Green Version]
- Ramnath, R.D.; Ng, S.W.; Guglielmotti, A.; Bhatia, M. Role of MCP-1 in Endotoxemia and Sepsis. Int. Immunopharmacol. 2008, 8, 810–818. [Google Scholar] [CrossRef]
- Akhter, M.S.; Uddin, M.A.; Kubra, K.-T.; Barabutis, N. Elucidation of the Molecular Pathways Involved in the Protective Effects of AUY-922 in LPS-Induced Inflammation in Mouse Lungs. Pharmaceuticals 2021, 14, 522. [Google Scholar] [CrossRef]
| Variables | Healthy Individuals | Patients with Trauma (SIRS) | Patients with Sepsis | #p Value |
|---|---|---|---|---|
| n = 40 | n = 19 | n = 10 | n = 11 | |
| Age (years) | 39.3 ± 13 | 39.5 ± 15 | 49.2 ± 16 | 0.214 |
| Sex (male/female) | 7/12 (36.8/63.2) | 5/6 (45.5/54.5) | 4/7 (36.7/63.6) | 0.877 |
| APACHE II | - | 10.6 ± 4.8 | 15.9 ± 4.8 | 0.040 |
| SOFA | - | 7.75 ± 3.2 | 9.3± 2.6 | 0.274 |
| Temperature (°C) | - | 36.4 ± 1.2 | 37.8 ± 1.1 | 0.021 |
| mHSP90α (MFI) | 80.5 (8; 90.5) | 67.7 (59.9; 80.9) | 55.5 (40.6; 58.5) | 0.975 |
| nHSP90α (MFl) | 24.3 (5.0; 30.0) * | 33.7 (21.0; 35.7) | 40.2 (31.4; 41.9) * | 0.009 |
| IL-6 (pg/mL) | 1.4 (1.0; 2.1) * | 67.0 (45.4; 130.5) ** | 499.5 (82.3; 645.0) *** | <0.001 |
| IL-10 (pg/mL) | 7.7 (3.4; 13.7) * | 19.4 (10.6; 26.6) | 32.9 (15.7; 61.9) * | <0.001 |
| IL-17 (pg/mL) | 0.01 (0.01; 0.01) | 0.86 (0.01; 5.96) | 0.01 (0.01; 0.01) | 0.557 |
| IFN-γ (pg/mL) | 4.85 (2.88; 7.44) | 5.0 (4.3; 6.7) | 45.0 (10.5; 144.5) | <0.001 |
| Mortality | - | 0 (0) | 2 (5.1) | 0.047 |
| PBMCs from | Healthy Individuals | Patients with Trauma (SIRS) | Patients with Sepsis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n = 40 | n = 19 | n = 10 | n = 11 | ||||||
| Compared Conditions | Time (h) | HSP90α mRNA Fold Change | |||||||
| 0 | 1 | 2 | 3 | 4 | Median (IQR) | Median (IQR) | Median (IQR) | #p Value | |
| Stimulation | None | mRNA | 0.63 (0.56; 1.90) | 1.08 (0.90; 1.14) | 1.84 (1.31; 2.15) | 0.052 | |||
| LPS | mRNA | 0.95 (0.86; 3.67) ^^ | 1.67 (1.21; 1.85) ^^ | 1.37 (0.70; 2.18) | 0.795 | ||||
| GLN | LPS | mRNA | 1.03 (0.83; 1.50) | 1.58 (1.47; 1.70) | 2.29 (0.61; 3.28) | 0.155 | |||
| LPS | GLN | mRNA | 1.46 (1.08; 2.40) | 1.42 (1.13; 1.67) | 1.72 (0.91; 3.00) | 0.956 | |||
| ^p < 0.001 | ^p = 0.002 | ^p = 0.819 | |||||||
| Stimulation | None | mRNA | 0.63 (0.56; 1.90) | 1.08 (0.90; 1.14) | 1.84 (1.31; 2.15) | 0.052 | |||
| HS | mRNA | 24.59 (19.56; 44.98) ^^ * | 19.03 (16.05; 44.98) ^^ ** | 112.32 (50.68; 197.88) ^^ * ** | <0.001 | ||||
| GLN | HS | mRNA | 22.63 (6.36; 48.84) * | 34.54 (31.02; 44.94) ** | 72.17 (67.81; 106.40) * ** | <0.001 | |||
| HS | GLN | mRNA | 16.45 (13.84; 23.68) * | 23.92 (16.58; 32.34) ** | 137.10 (97.23; 189.14) * ** | <0.001 | |||
| ^p < 0.001 | ^p < 0.001 | ^p < 0.001 | |||||||
| PBMCs from | Healthy Individuals | Patients with Trauma (SIRS) | Patients with Sepsis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n = 40 | n = 19 | n = 10 | n = 11 | ||||||
| Compared Conditions | Time (h) | Monocyte HSP90α Protein Expression | |||||||
| 0 | 1 | 2 | 3 | 6 | Median (IQR) | Median (IQR) | Median (IQR) | #p Value | |
| Stimulation | None | mHSP90α | 114.0 (104.0; 130.0) * *** | 159.0 (128.5; 204.5) *** | 156.0 (140.0; 170.5) * | 0.008 | |||
| LPS | mHSP90α | 139.0 (130.0; 159.5) ^^ * *** | 170.0 (154.0; 219.0) ^^ *** | 163.0 (155.0; 176.5) * | 0.021 | ||||
| GLN | LPS | mHSP90α | 132.0 (126.0; 141.5) | 161.0 (132.5; 192.0) ^^^ | 161.0 (136.0; 174.5) | 0.066 | |||
| LPS | GLN | mHSP90α | 142.0 (129.0; 157.5) ^^ | 164.0 (149.5; 206.0) | 137.0 (132.0; 162.5) | 0.177 | |||
| ^p < 0.001 | ^p = 0.004 | ^p = 0.383 | |||||||
| Stimulation | None | mHSP90α | 114.0 (104.0; 130.0) * *** | 159.0 (128.5; 204.5) *** | 156.0 (140.0; 170.5) * | 0.008 | |||
| HS | mHSP90α | 124.0 (115.0; 146.0) * | 148.0 (132.0; 182.5) | 183.0 (164.5; 191.5) ^^ * | 0.005 | ||||
| GLN | HS | mHSP90α | 131.0 (107.0; 144.0) | 153.0 (111.5; 189.0) | 160.0 (128.5; 186.0) | 0.161 | |||
| HS | GLN | mHSP90α | 145.0 (116.5; 154.0) ^^ | 174.0 (143.5; 196.0) ^^^^ | 168.0 (123.0; 184.0) | 0.123 | |||
| ^p = 0.031 | ^p = 0.031 | ^p = 0.047 | |||||||
| PBMCs from | Healthy Individuals | Patients with Trauma (SIRS) | Patients with Sepsis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n = 40 | n = 19 | n = 10 | n = 11 | ||||||
| Compared Conditions | Time (h) | Lymphocyte HSP90α Expression | |||||||
| 0 | 1 | 2 | 3 | 6 | Median (IQR) | Median (IQR) | Median (IQR) | #p Value | |
| Stimulation | None | lHSP90α | 46.4 (41.65; 51.9) * *** | 60.5 (50.0; 71.15) *** | 70.8 (63.8; 83.85) * | <0.001 | |||
| LPS | lHSP90α | 48.2 (42.0; 57.3) * | 67.3 (52.2; 73.1) | 80.4 (73.9; 152.85) ^^^ * | <0.001 | ||||
| GLN | LPS | lHSP90α | 46.0 (42.0; 52.55) * | 57.3 (48.4; 70.3) | 58.0 (51.8; 71.75) ^^ * | 0.033 | |||
| LPS | GLN | lHSP90α | 48.8 (42.45; 54.3) * *** | 64.1 (54.85; 71.6) *** | 59.7 (49.5; 68.45) ^^ * | 0.013 | |||
| ^ p < 0.422 | ^ p = 0.210 | ^ p = 0.001 | |||||||
| Stimulation | None | lHSP90α | 46.4 (41.65; 51.9) * *** | 60.5 (50.0; 71.15) *** | 70.8 (63.8; 83.85) * | <0.001 | |||
| HS | lHSP90α | 63.9 (54.75; 73.35) ^^ * | 66.8 (57.65; 81.6) ** | 88.4 (76.85; 119.0) * ** | 0.002 | ||||
| GLN | HS | lHSP90α | 57.2 (45.54; 64.15) ^^ | 67.1 (51.75; 82.65) | 63.1 (67.7; 84.15) ^^^ | 0.206 | |||
| HS | GLN | lHSP90α | 60.2 (55.2; 66.3) ^^ * | 79.9 (65.55; 88.5) ^^ | 73.4 (67.65; 83.95) * | 0.007 | |||
| ^ p < 0.001 | ^ p = 0.039 | ^ p = 0.024 | |||||||
| PBMCs from | Healthy Individuals | Patients with Trauma (SIRS) | Patients with Sepsis | |||||
|---|---|---|---|---|---|---|---|---|
| n = 40 | n = 19 (15 at 24 h) | n = 10 (8 at 24 h) | n = 11 (9 at 24 h) | |||||
| Compared Conditions | Time (h) | Cytokine Supernatant (pg/mL) | ||||||
| 0 | 2 | 24 | 48 | Median (IQR) | Median (IQR) | Median (IQR) | #p Value | |
| Stimulation | None | IL-1β | 2.00 (0.01; 6.76) | 4.87 (0.01; 38.12) | 2.00 (1.05; 3.55) | 0.664 | ||
| LPS | IL-1β | 1881 (1382; 2189) | 4261 (1576; 7334) | 414 (95.75; 2080) | 0.058 | |||
| HS | IL-1β | 0.01 (0.01; 14.46) | 5.46 (0.01; 10.33) | 2.00 (0.89; 185) | 0.775 | |||
| None | IL-1β | 1.62 (0.01; 6.51) | 0.01 (0.01; 8.93) | 0.01 (0.01; 2.91) | 0.758 | |||
| LPS | IL-1β | 1338 (1277; 2371) | 1863 (363; 3055) | 552 (201; 2565) | 0.395 | |||
| HS | IL-1β | 0.01 (0.01; 5.66) | 1.96 (0.01; 17.51) | 2.00 (0.01; 17.51) | 0.734 | |||
| Stimulation | None | IL-6 | 2.09 (0.01; 7.32) | 6.24 (0.01; 4607) | 2.09 (0.01; 7.32) | 0.625 | ||
| LPS | IL-6 | 28,154 (21,366; 42,992) *** | 120,559 (84,310; 143,653) *** | 37549 (8563; 85446) | 0.005 | |||
| HS | IL-6 | 2.16 (0.01; 25.15) | 30.31 (2.92; 164) | 2.00 (1.05; 25.30) | 0.597 | |||
| None | IL-6 | 6.01 (1.51; 86.79) | 0.10 (0.01; 6.24) | 3.81 (1.48; 12.23) | 0.237 | |||
| LPS | IL-6 | 31,538 (27,992; 40,739) | 917,669 (70,412; 135,394) | 36,045 (11,817; 77,740) | 0.100 | |||
| HS | IL-6 | 4.48 (0.01; 16.82) | 119.42 (0.01; 218) | 6.62 (3.32; 10.02) | 0.525 | |||
| Stimulation | None | IL-8 | 3017 (1130; 3256) | 3788 (263; 14328) | 212 (129; 4969) | 0.485 | ||
| LPS | IL-8 | 11,901 (60,841; 180,449) *** | 390,558 (255,516; 699,501) *** | 211,736 (93,579; 411,038) | 0.010 | |||
| HS | IL-8 | 1099 (258; 1783) | 2127 (812; 4113) | 159 (45.53; 3381) | 0.294 | |||
| None | IL-8 | 3772 (947; 7463) * | 912 (289; 6889) | 537 (110; 3690) * | 0.042 | |||
| LPS | IL-8 | 188,532 (123,770; 285,525) *** | 622,075 (318,646; 769,093) *** | 114,337 (11,817; 507,894) | 0.015 | |||
| HS | IL-8 | 512 (289; 2710) | 2685 (601; 8462) | 235 (66.95; 2736) | 0.068 | |||
| Stimulation | None | IL-10 | 1.85 (0.01; 4.29) | 3.55 (0.01; 30.58) | 1.50 (1.08; 2.37) | 0.615 | ||
| LPS | IL-10 | 898 (621; 1343) * | 713 (414; 1264) ** | 55.90 (22.06; 120) * ** | 0.001 | |||
| HS | IL-10 | 2.00 (0.01; 4.90) | 1.73 (0.01; 5.91) | 2.00 (0.66; 2.68) | 0.999 | |||
| None | IL-10 | 1.41 (0.01; 4.15) | 0.01 (0.01; 7.41) | 0.01 (0.01; 1.79) | 0.651 | |||
| LPS | IL-10 | 526 (366; 1132) * | 162 (115; 698) ** | 71 (22.14; 148) * ** | <0.001 | |||
| HS | IL-10 | 0.01 (0.01; 1.46) | 0.01 (0.01; 2.12) | 1.33 (0.01; 2.36) | 0.602 | |||
| Stimulation | None | TNF-α | 1.35 (0.01; 7.91) | 4.96 (0.01; 9.88) | 0.01 (0.01; 3.83) | 0.420 | ||
| LPS | TNF-α | 922 (781; 1763) | 1401 (867; 9263) | 1129 (250; 1658) | 0.416 | |||
| HS | TNF-α | 1.58 (0.01; 13.27) | 5.36 (0.01; 16.05) | 0.01 (0.01; 3.29) | 0.736 | |||
| None | TNF-α | 0.45 (0.01; 4.81) | 0.01 (0.01; 1.08) | 0.01 (0.01; 0.88) | 0.514 | |||
| LPS | TNF-α | 618 (116; 1636) | 115 (74.46; 1742) | 170 (76.90; 1154) | 0.796 | |||
| HS | TNF-α | 0.55 (0.01; 3.01) | 0.01 (0.01; 4.05) | 0.01 (0.01; 1.94) | 0.905 | |||
| Stimulation | None | MCP-1 | 901 (555; 2179) | 986 0.01; 11234) | 37.59 (22.52; 110) | 0.410 | ||
| LPS | MCP-1 | 811 (0.01; 2919) *** | 11,141 (3176; 28,345) *** | 4344 (3727; 4891) | 0.029 | |||
| HS | MCP-1 | 400 (200; 1259) | 203 (105; 1556) | 49.98 (6.05; 54.9) | 0.119 | |||
| None | MCP-1 | 655 (418; 1032) * *** | 15.59 (0.01; 253) *** | 66.75 (35.16; 194) * | 0.002 | |||
| LPS | MCP-1 | 5171 (729; 7385) * *** | 161,96 (12,400; 32,876) *** | 11,883 (2254; 23,562) * | <0.001 | |||
| HS | MCP-1 | 1187 (549; 4572) | 717 (351; 5784) | 53.72 (49.78; 239) | 0.199 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briassouli, E.; Tzanoudaki, M.; Goukos, D.; Vardas, K.; Briassoulis, P.; Ilia, S.; Kanariou, M.; Routsi, C.; Nanas, S.; Daikos, G.L.; et al. Ex Vivo Evaluation of Glutamine Treatment in Sepsis and Trauma in a Human Peripheral Blood Mononuclear Cells Model. Nutrients 2023, 15, 252. https://doi.org/10.3390/nu15010252
Briassouli E, Tzanoudaki M, Goukos D, Vardas K, Briassoulis P, Ilia S, Kanariou M, Routsi C, Nanas S, Daikos GL, et al. Ex Vivo Evaluation of Glutamine Treatment in Sepsis and Trauma in a Human Peripheral Blood Mononuclear Cells Model. Nutrients. 2023; 15(1):252. https://doi.org/10.3390/nu15010252
Chicago/Turabian StyleBriassouli, Efrossini, Marianna Tzanoudaki, Dimitris Goukos, Kostas Vardas, Panagiotis Briassoulis, Stavroula Ilia, Maria Kanariou, Christina Routsi, Serafim Nanas, George L. Daikos, and et al. 2023. "Ex Vivo Evaluation of Glutamine Treatment in Sepsis and Trauma in a Human Peripheral Blood Mononuclear Cells Model" Nutrients 15, no. 1: 252. https://doi.org/10.3390/nu15010252
APA StyleBriassouli, E., Tzanoudaki, M., Goukos, D., Vardas, K., Briassoulis, P., Ilia, S., Kanariou, M., Routsi, C., Nanas, S., Daikos, G. L., & Briassoulis, G. (2023). Ex Vivo Evaluation of Glutamine Treatment in Sepsis and Trauma in a Human Peripheral Blood Mononuclear Cells Model. Nutrients, 15(1), 252. https://doi.org/10.3390/nu15010252