Protective Effects of Chestnut (Castanea crenata) Inner Shell Extract in Macrophage-Driven Emphysematous Lesion Induced by Cigarette Smoke Condensate
Abstract
1. Introduction
2. Materials and Methods
2.1. Chestnut Inner Shell Extract Preparation
2.1.1. Chestnut Inner Shell Extraction
2.1.2. Chestnut Inner Shell Extract Analysis
2.2. In Vivo Experiments
2.2.1. Experimental Animal Model
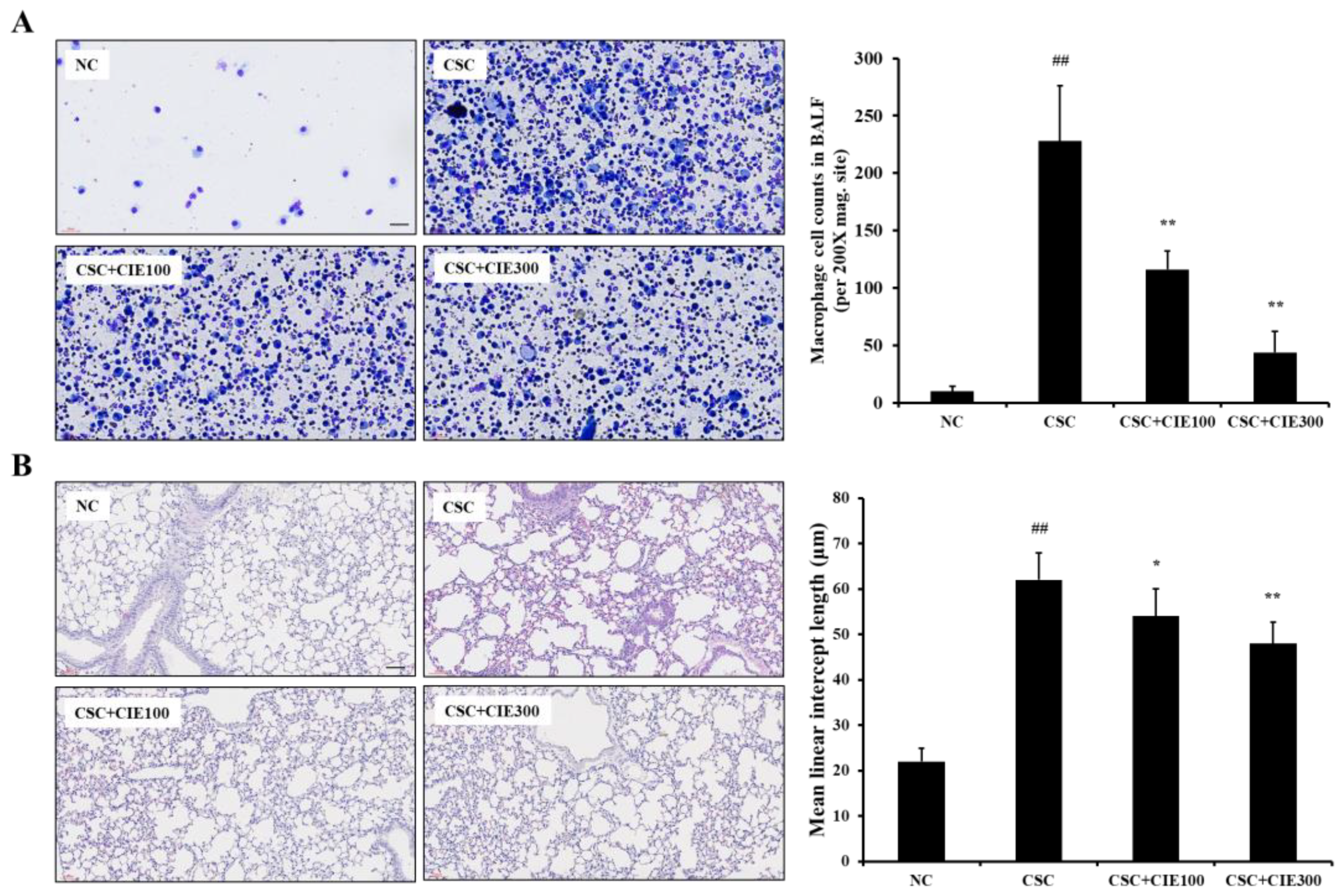
2.2.2. Analysis of Macrophage Count in Bronchoalveolar Lavage Fluid (BALF)
2.2.3. Histological Analysis
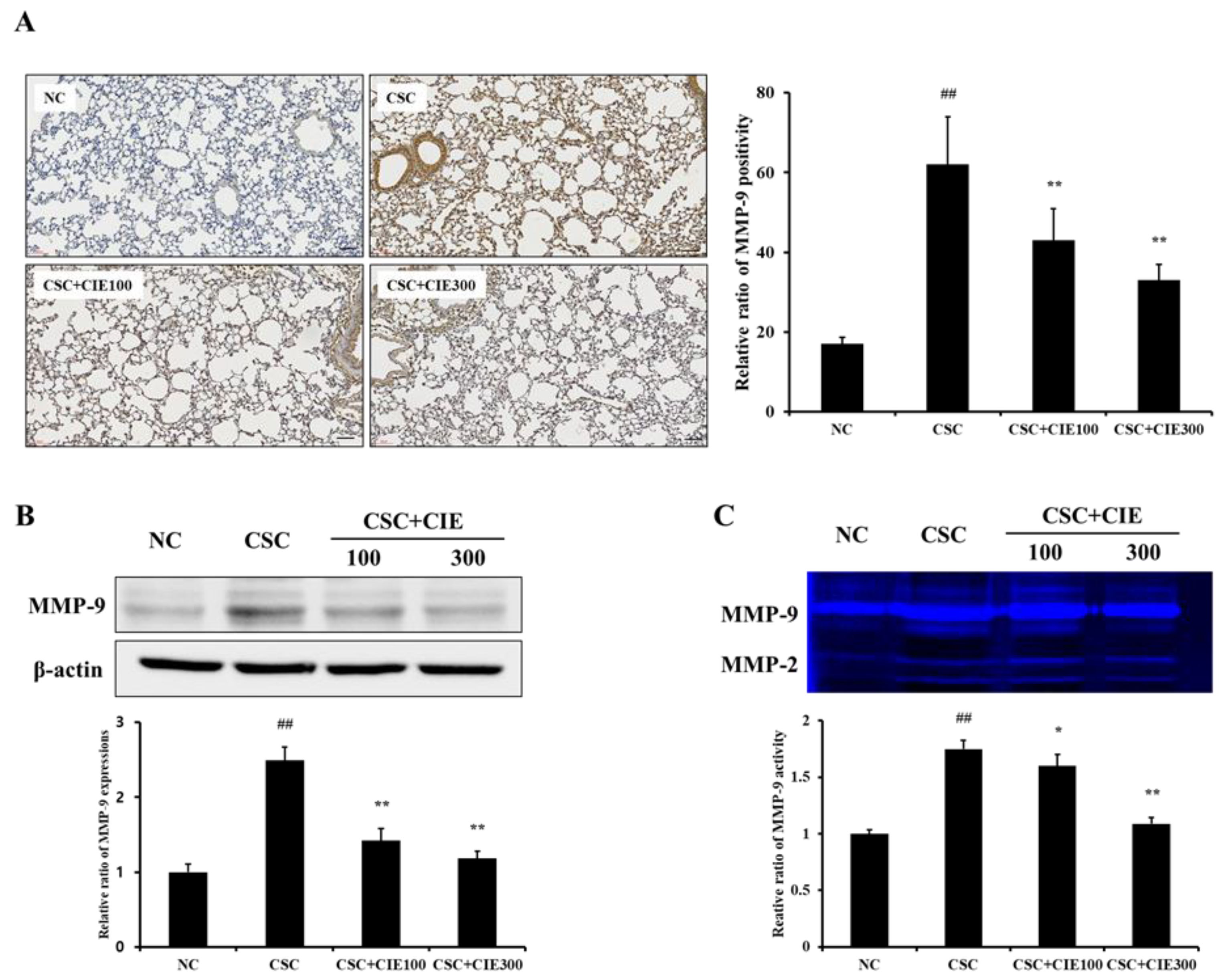
2.2.4. Immunohistochemistry (IHC) and Gelatin Zymography
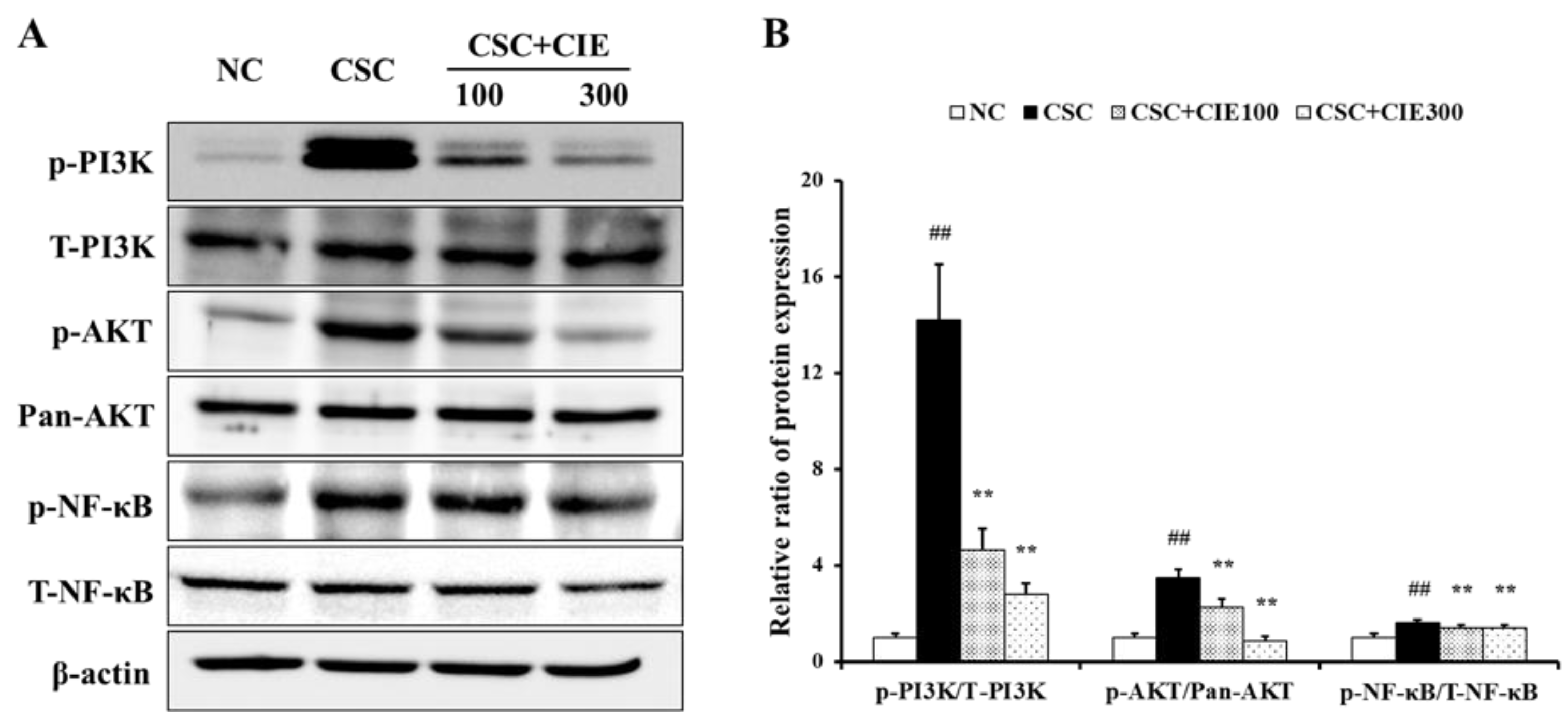
2.2.5. Immunoblotting
2.3. In Vitro Experiments
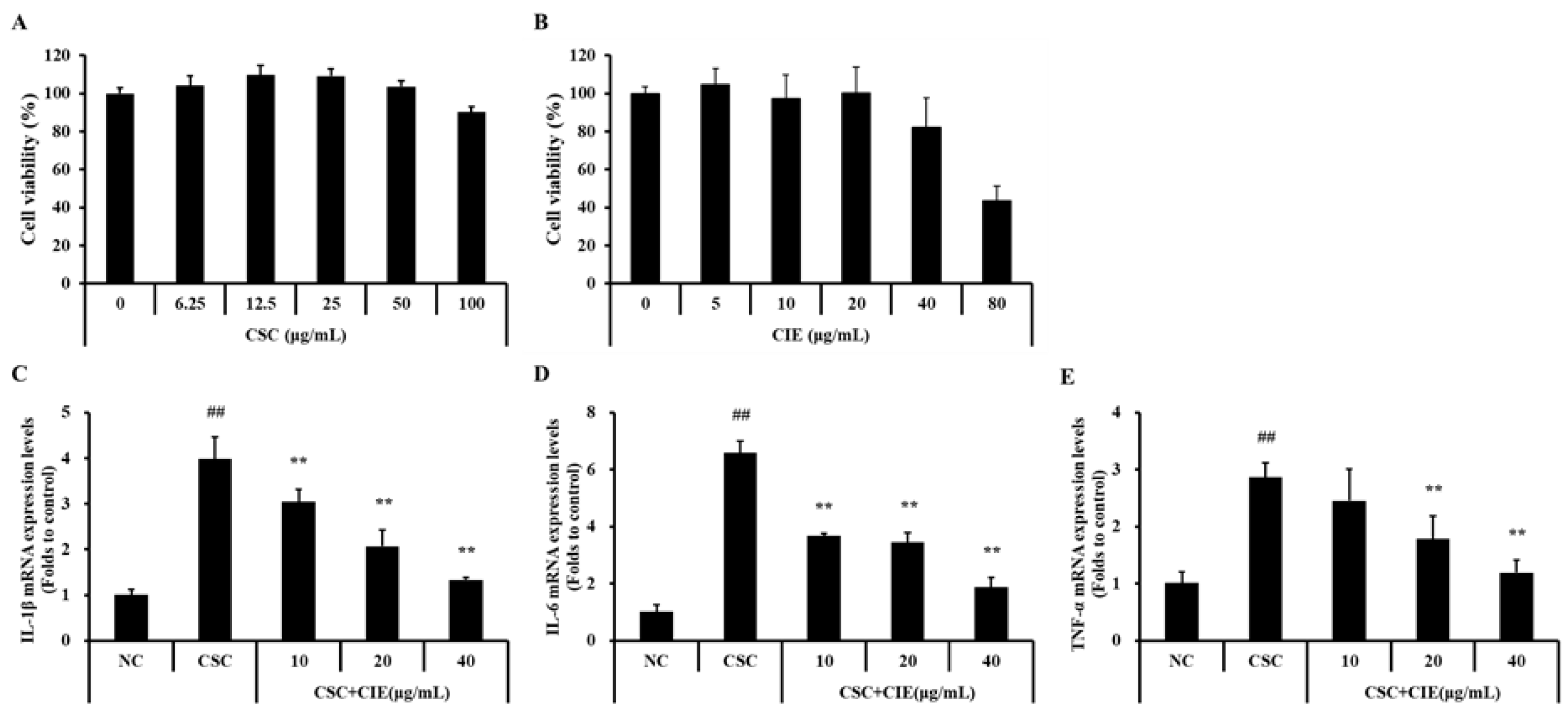
2.3.1. Cell Culture and Cell Viability
2.3.2. Analysis of mRNA Expression Levels
2.3.3. Immunoblotting
2.4. Statistical Analysis
3. Results
3.1. CIE Contents
3.2. In Vivo Experiments
3.2.1. Effects of CIE on Macrophage Counts in BALF from CSC-Instilled Mice
3.2.2. Effects of CIE on Emphysematous Lesions in CSC-Instilled Mice
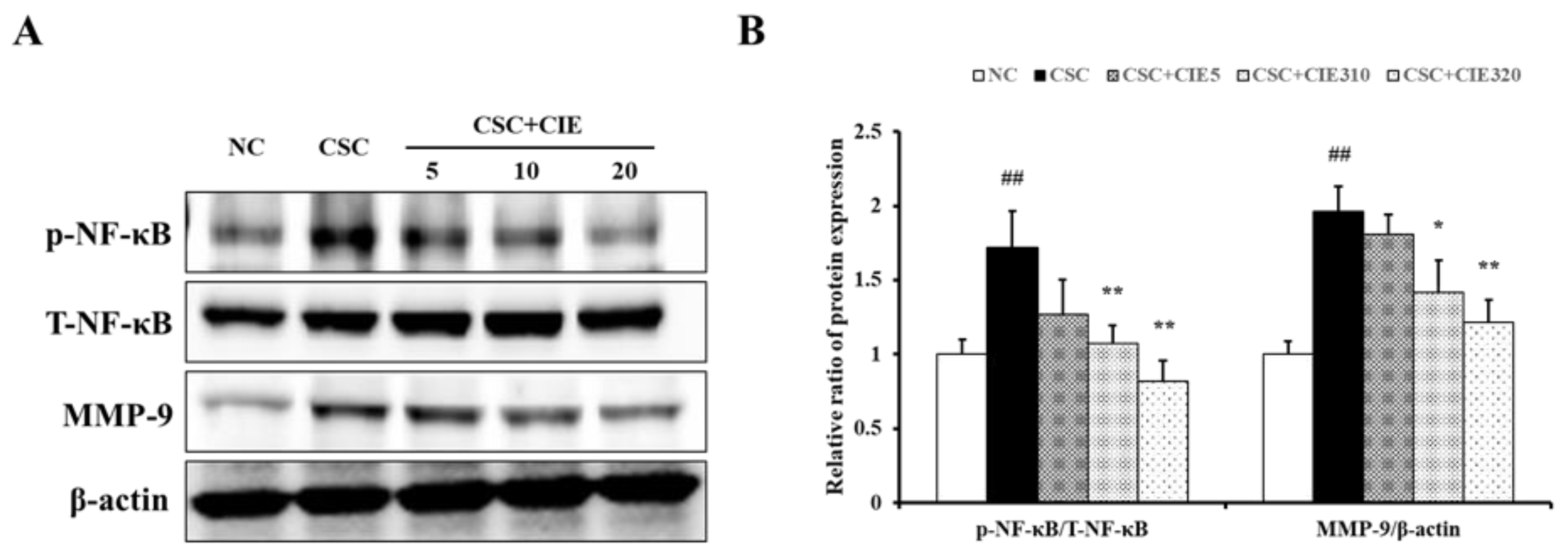
3.2.3. Effects of CIE on MMP-9 Expression in Lung Tissues of CSC-Instilled Mice
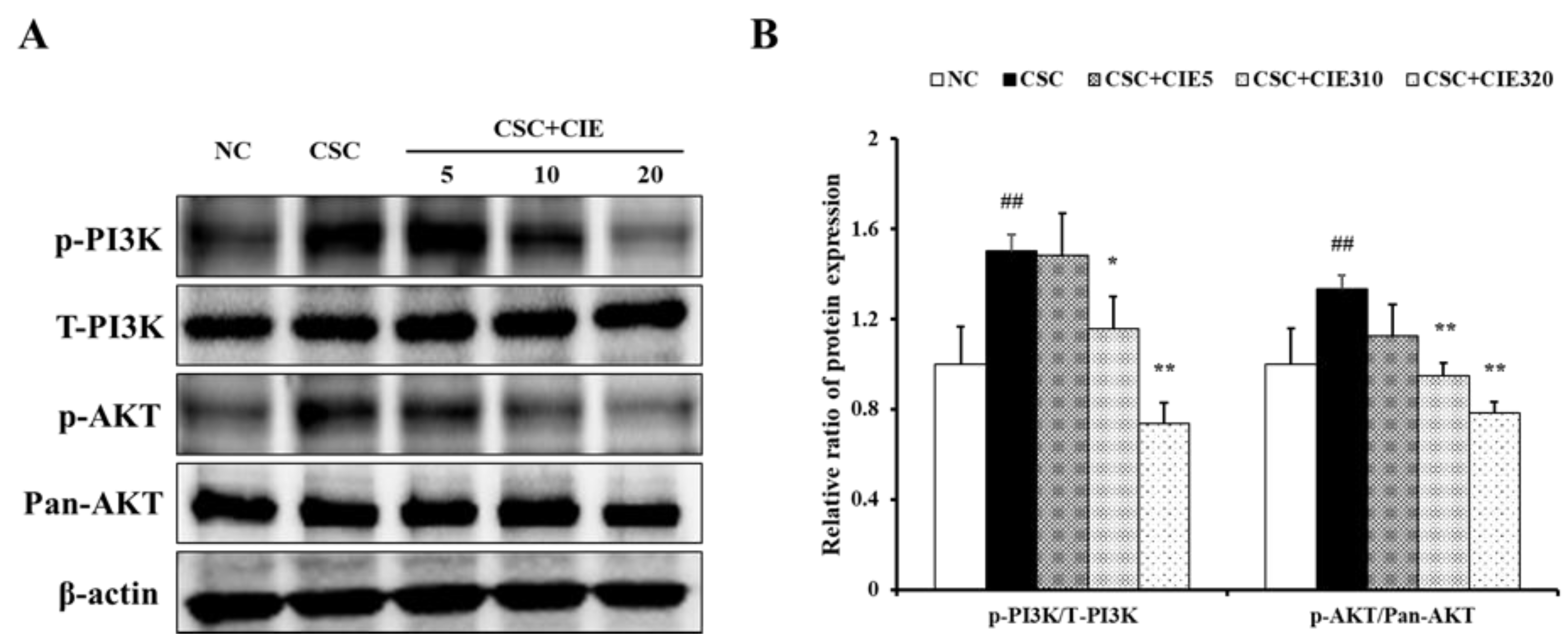
3.2.4. Effects of CIE on PI3K/AKT/NF-κB Pathway in Lung Tissues of CSC-Instilled Mice
3.3. In Vitro Experiments
3.3.1. Effects of CIE on Cell Viability and mRNA Expression of Cytokines in NCI-H292 Cells Stimulated with CSC
3.3.2. Effects of CIE on Expression of PI3K/AKT NF-κB Pathway in NCI-H292 Cells Stimulated with CSC
3.3.3. Effects of CIE on MMP-9 Expression in NCI-H292 Cells Stimulated with CSC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halpin, D.M.; Criner, G.; Papi, A.; Singh, D.; Anzueto, A.; Martinez, F.J.; Agusti, A.A.; Vogelmeier, C.J. Global Initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2021, 203, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, R. Global initiative for chronic obstructive lung diseases (GOLD): Time to act. Eur. Respir. J. 2021, 18, 901–902. [Google Scholar] [CrossRef]
- Vijayan, V.K. Chronic obstructive pulmonary disease. Indian J. Med. Res. 2013, 137, 251–269. [Google Scholar] [PubMed]
- Rahman, I. Pharmacological antioxidant strategies as therapeutic interventions for COPD. Biochim. Biophys. Acta 2012, 1822, 714–728. [Google Scholar] [CrossRef] [PubMed]
- Repine, J.E.; Bast, A.; Lankhorst, I. Oxidative stress study group oxidative stress in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1997, 156, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Goldklang, M.; Stockley, R. Pathophysiology of emphysema and implications. Chronic Obstr. Pulm. Dis. 2016, 3, 454–458. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gwinn, M.R.; Vallyathan, V. Respiratory burst: Role in signal transduction in alveolar macrophages. J. Toxicol. Environ. Health B Crit. Rev. 2006, 9, 27–39. [Google Scholar] [CrossRef]
- Sharafkhaneh, A.; Hanania, N.A.; Kim, V. Pathogenesis of emphysema: From the bench to the bedside. Proc. Am. Thorac. Soc. 2008, 5, 475–477. [Google Scholar] [CrossRef]
- Hiemstra, P.S. Macrophage function in chronic obstructive pulmonary disease: The many faces of notch signaling. EBioMedicine 2019, 43, 22–23. [Google Scholar] [CrossRef]
- Abboud, R.T.; Vimalanathan, S. Pathogenesis of COPD. Part I. The role of protease-antiprotease imbalance in emphysema. Int. J. Tuberc. Lung Dis. 2008, 12, 361–367. [Google Scholar]
- Ko, J.W.; Seo, C.S.; Shin, N.R.; Kim, J.S.; Lee, S.I.; Kim, J.C.; Kim, S.H.; Shin, I.S. Modificated Mahuang-Tang, a traditional herbal medicine suppresses inflammatory responses induced by cigarette smoke in human airway epithelial cell and mice. Phytomedicine 2019, 59, 152777. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.D. Elastolytic metalloproteinases produced by human mononuclear phagocytes: Potential roles in destructive lung disease. Am. J. Respir. Crit. Care Med. 1994, 150, S160–S164. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.S.; Lee, N.K.; Na, D.S.; Yu, H.H.; Paik, H.D. Comparative analysis of the antioxidant and anticancer activities of chestnut inner shell extracts prepared with various solvents. J. Sci. Food Agric. 2016, 96, 2097–2102. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.Y.; Chen, S.Y.; Shi, L.L.; Liu, Y.J.; Ma, C. Antioxidant potential of polyphenols and tannins fromburs of Castanea mollissima Blume. Molecules 2011, 16, 8590–8600. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Hwang, I.K.; Park, J.B. Analysis of physicochemical factors related to the automatic pellicle removal in Korean chestnut (Castanea crenata). J. Agric. Food Chem. 2001, 49, 6045–6049. [Google Scholar] [CrossRef]
- Kim, C.Y.; Kim, J.W.; Kim, J.H.; Jeong, J.S.; Lim, J.O.; Ko, J.W.; Kim, T.W. Inner shell of the chestnut (Castanea crenatta) suppresses inflammatory responses in ovalbumin-induced allergic asthma mouse model. Nutrients 2022, 14, 2067. [Google Scholar] [CrossRef]
- Chang, W.C.; Yu, Y.M.; Chiang, S.Y.; Tseng, C.Y. Ellagic acid suppresses oxidised low-density lipoprotein-induced aortic smooth muscle cell proliferation: Studies on the activation of extracellular signal-regulated kinase 1/2 and proliferating cell nuclear antigen expression. Br. J. Nutr. 2008, 99, 709–714. [Google Scholar] [CrossRef]
- Cornélio Favarin, D.; Martins Teixeira, M.; Lemos de Andrade, E.; De Freitas Alves, C.; Lazo Chica, J.E.; Artério Sorgi, C.; Faccioli, L.H.; Paula Rogerio, A. Anti-inflammatory effects of ellagic acid on acute lung injury induced by acid in mice. Mediators Inflamm. 2013, 2013, 164202. [Google Scholar] [CrossRef]
- Han, D.H.; Lee, M.J.; Kim, J.H. Antioxidant and apoptosis-inducing activities of ellagic acid. Anticancer Res. 2006, 26, 3601–3606. [Google Scholar]
- Li, X.; Huang, K.; Liu, X.; Ruan, H.; Ma, L.; Liang, J.; Cui, Y.; Wang, Y.; Wu, S.; Li, H.; et al. Ellagic acid attenuates BLM-induced pulmonary fibrosis via inhibiting Wnt signaling pathway. Front. Pharmacol. 2021, 12, 639574. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, Z.; Dianat, M.; Radan, M.; Badavi, M.; Mansouri, Z. Ellagic acid ameliorates lung inflammation and heart oxidative stress in elastase-induced emphysema model in rat. Inflammation 2020, 43, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Hsia, C.C.; Hyde, D.M.; Ochs, M.; Weibel, E.R. An Official Research Policy Statement of the American Thoracic Society/European Respiratory Society: Standards for Quantitative Assessment of Lung Structure. Am. J. Respir. Care Med. 2010, 181, 394–418. [Google Scholar] [CrossRef] [PubMed]
- Nikota, J.K.; Stämpfli, M.R. Cigarette smoke-induced inflammation and respiratory host defense: Insights from animal models. Pulm. Pharmacol. Ther. 2012, 25, 257–262. [Google Scholar] [CrossRef]
- Chung, K.F.; Adcock, I.M. Multifaceted mechanisms in COPD: Inflammation, immunity, and tissue repair and destruction. Eur. Respir. J. 2008, 31, 1334–1356. [Google Scholar] [CrossRef]
- Akata, K.; van Eeden, S.F. Lung Macrophage Functional Properties in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2020, 21, 853. [Google Scholar] [CrossRef]
- Hogg, J.C.; Chu, F.; Utokaparch, S.; Woods, R.; Elliott, W.M.; Buzatu, L.; Cherniack, R.M.; Rogers, R.M.; Sciurba, F.C.; Coxson, H.O.; et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 2645–2653. [Google Scholar] [CrossRef]
- Peleman, R.A.; Rytila, P.H.; Kips, J.C.; Joos, G.F.; Pauwels, R.A. The cellular composition of induced sputum in chronic obstructive pulmonary disease. Eur. Respir. J. 1999, 13, 839–843. [Google Scholar] [CrossRef]
- Di Stefano, A.; Capelli, A.; Lusuardi, M.; Balbo, P.; Vecchio, C.; Maestrelli, P.; Mapp, C.E.; Fabbri, L.M.; Donner, C.F.; Saetta, M. Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am. J. Respir. Crit. Care Med. 1998, 158, 1277–1285. [Google Scholar] [CrossRef]
- Hu, G.; Dong, T.; Wang, S.; Jing, H.; Chen, J. Vitamin D3-vitamin D receptor axis suppresses pulmonary emphysema by maintaining alveolar macrophage homeostasis and function. EBioMedicne 2019, 45, 563–577. [Google Scholar] [CrossRef]
- Keatings, V.M.; Collins, P.D.; Barnes, P.J. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am. J. Respir. Crit. Care Med. 1996, 153, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Pesci, A.; Balbi, B.; Majori, M.; Cacciani, G.; Bertacco, S.; Alciato, P.; Donner, C.F. Inflammatory cells and mediators in bronchial lavage of patients with chronic obstructive pulmonary disease. Eur. Respir. J. 1998, 12, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R.; Fraser, R.S.; Ghezzo, H.; Cosio, M.G. Alveolar inflammation and its relation to emphysema in smokers. Am. J. Respir. Crit. Care Med. 1995, 152, 1666–1672. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, J.; Wang, J.; Li, S.; Fukunaga, A.; Yodoi, J.; Tian, H. Progress in the mechanism and targeted drug therapy for COPD. Signal Transduct. Target. Ther. 2020, 5, 248. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Hammond, M.; Dungwa, J.; Shah, R.; Montero-Fernandez, A.; Higham, A.; Lea, S.; Singh, D. Red blood cell-derived iron alters macrophage function in COPD. Biomedicines 2021, 9, 1939. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, D.; Higham, A.; Wolosianka, S.; Gai, X.; Zhou, L.; Petersen, H.; Pinto-Plata, V.; Divo, M.; Silverman, E.K.; et al. ADAM15 expression is increased in lung CD8+ T cells, macrophages, and bronchial epithelial cells in patients with COPD and is inversely related to airflow obstruction. Respir. Res. 2020, 21, 188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ramsey, C.; Berical, A.; Yu, L.; Leng, L.; McGinnis, K.A.; Song, Y.; Michael, H.; McCormack, M.C.; Allore, H.; et al. A functional macrophage migration inhibitory factor promoter polymorphism is associated with reduced diffusing capacity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L400–L405. [Google Scholar] [CrossRef]
- Wells, J.M.; Parker, M.M.; Oster, R.A.; Bowler, R.P.; Dransfield, M.T.; Bhatt, S.P.; Cho, M.H.; Kim, V.; Curtis, J.L.; Martinez, F.J.; et al. SPIROMICS and COPDGene Investigators, Elevated circulating MMP-9 is linked to increased COPD exacerbation risk in SPIROMICS and COPDGene. JCI Insight 2018, 3, e123614. [Google Scholar] [CrossRef]
- Vu, T.H.; Werb, Z. Matrix metalloproteinases: Effectors of development and normal physiology. Genes Dev. 2000, 14, 2123–2133. [Google Scholar] [CrossRef]
- Zheng, L.; Lam, W.K.; Tipoe, G.L.; Shum, I.H.; Yan, C.; Leung, R.; Sun, J.; Ooi, G.C.; Tsang, K.W. Overexpression of matrix metalloproteinase-8 and -9 in bronchiectatic airways in vivo. Eur. Respir. J. 2002, 20, 170–176. [Google Scholar] [CrossRef]
- Finlay, G.A.; O’Driscoll, L.; Russell, K.J.; D’Arcy, E.M.; Masterson, J.B. Matrix metalloproteinase expression and production by alveolar macrophages in emphysema. Am. J. Res. Crit. Care Med. 1997, 156, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.E.; Culpitt, S.V.; DeMatos, C.; Donnelly, L.; Smith, M. Release and activity of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2002, 26, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Pompura, S.L.; Dominguez-Villar, M. The PI3K/AKT signaling pathway in regulatory T-cell development, stability, and function. J. Leukoc. Biol. 2018, 103, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Shi, X.; Sheng, K.; Han, G.; Li, W.; Zhao, Q.; Jiang, B.; Feng, J.; Li, J.; Gu, Y. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review). Mol. Med. Req. 2019, 19, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Mercado, N.; Ito, K.; Barnes, P.J. Accelerated ageing of the lung in COPD: New concepts. Thorax 2015, 70, 482–489. [Google Scholar] [CrossRef]
- Wang, F.; Wang, F.; Li, F.; Wang, D.; Li, H.; He, X.; Zhang, J. Methane attenuates lung ischemia-reperfusion injury via regulating PI3K-AKT-NFκB signaling pathway. J. Recept. Signal Transduct. Res. 2020, 40, 209–217. [Google Scholar] [CrossRef]
- Stark, A.K.; Sriskantharajah, S.; Hessel, E.M.; Okkenhaug, K. PI3K inhibitors in inflammation, autoimmunity and cancer. Curr. Opin. Pharmacol. 2015, 23, 82–91. [Google Scholar] [CrossRef]
- Bozinovski, S.; Vlahos, R.; Hansen, M.; Liu, K.; Anderson, G.P. Akt in the Pathogenesis of COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2006, 1, 31–38. [Google Scholar] [CrossRef]
- Schuliga, M. NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules 2015, 5, 1266–1283. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Hsieh, H.L.; Hsiao, L.D.; Yang, C.M. PI3-K/Akt/JNK/NF-κB is essential for MMP-9 expression and outgrowth in human limbal epithelial cells on intact amniotic membrane. Stem Cell Res. 2012, 9, 9–23. [Google Scholar] [CrossRef]
- Liang, J.A.; Wu, S.L.; Lo, H.Y.; Hsiang, C.Y.; Ho, T.Y. Vanillin inhibits matrix metalloproteinase-9 expression through downregulation of nuclear factor-κB signaling pathway in human hepatocellular carcinoma cells. Mol. Pharmacol. 2009, 75, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Falcone, D.; Gallelli, L.; Di Virgilio, A.; Tucci, L.; Scaramuzzino, M.; Terracciano, R.; Pelaia, G.; Savino, R. Effects of simvastatin and rosuvastatin on RAS protein, matrix metalloproteinases and NF-κB in lung cancer and in normal pulmonary tissues. Cell Prolif. 2013, 46, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Sokar, S.S.; Afify, E.H.; Osman, E.Y. Dexamethasone and losartan combination treatment protected cigarette smoke-induced COPD in rats. Hum. Exp. Toxicol. 2021, 40, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Sun, K.; Xie, W.; Hu, G.; Kong, H.; Wang, Q.; Wang, H. Etanercept attenuates short-term cigarette-smoke-exposure-induced pulmonary arterial remodelling in rats by suppressing the activation of TNF-a/NF-kB signal and the activities of MMP-2 and MMP-9. Pulm. Pharmacol. Ther. 2012, 25, 208–215. [Google Scholar] [CrossRef]
- Murugan, V.; Mukherjee, K.; Maiti, K.; Mukherjee, P.K. Enhanced oral bioavailability and antioxidant profile of ellagic acid by phospholipids. J. Agric. Food Chem. 2009, 57, 4559–4565. [Google Scholar] [CrossRef]
- Rogerio, A.P.; Fontanari, C.; Borducchi, E. Anti-inflammatory effects of Lafoensia pacari and ellagic acid in a murine model of asthma. Eur. J. Pharmacol. 2008, 580, 262–270. [Google Scholar] [CrossRef]
- Guan, S.; Zheng, Y.; Yu, X.; Li, W.; Han, B.; Lu, J. Ellagic acid protects against LPS-induced acute lung injury through inhibition of nuclear factor kappa B, proinflammatory cytokines and enhancement of interleukin-10. Food Agric. Immunol. 2017, 28, 1347–1361. [Google Scholar] [CrossRef]
- Losso, J.N.; Bansode, R.P.; 2nd Trappey, A.; Bawadi, H.A.; Truax, R. In vitro anti-proliferative activities of ellagic acid. J. Nutr. Biochem. 2004, 15, 672–678. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.-S.; Kim, J.-W.; Kim, J.-H.; Kim, C.-Y.; Ko, J.-W.; Kim, T.-W. Protective Effects of Chestnut (Castanea crenata) Inner Shell Extract in Macrophage-Driven Emphysematous Lesion Induced by Cigarette Smoke Condensate. Nutrients 2023, 15, 253. https://doi.org/10.3390/nu15020253
Jeong J-S, Kim J-W, Kim J-H, Kim C-Y, Ko J-W, Kim T-W. Protective Effects of Chestnut (Castanea crenata) Inner Shell Extract in Macrophage-Driven Emphysematous Lesion Induced by Cigarette Smoke Condensate. Nutrients. 2023; 15(2):253. https://doi.org/10.3390/nu15020253
Chicago/Turabian StyleJeong, Ji-Soo, Jeong-Won Kim, Jin-Hwa Kim, Chang-Yeop Kim, Je-Won Ko, and Tae-Won Kim. 2023. "Protective Effects of Chestnut (Castanea crenata) Inner Shell Extract in Macrophage-Driven Emphysematous Lesion Induced by Cigarette Smoke Condensate" Nutrients 15, no. 2: 253. https://doi.org/10.3390/nu15020253
APA StyleJeong, J.-S., Kim, J.-W., Kim, J.-H., Kim, C.-Y., Ko, J.-W., & Kim, T.-W. (2023). Protective Effects of Chestnut (Castanea crenata) Inner Shell Extract in Macrophage-Driven Emphysematous Lesion Induced by Cigarette Smoke Condensate. Nutrients, 15(2), 253. https://doi.org/10.3390/nu15020253





