Resting Energy Expenditure in the Critically Ill and Healthy Elderly—A Retrospective Matched Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.2.1. Critically Ill Patient Cohort
2.2.2. Matched Healthy Cohort
2.3. Statistical Analyses
3. Results
3.1. Study Flow and Patient Characteristics
3.2. Primary Outcome
3.3. Secondary Outcomes
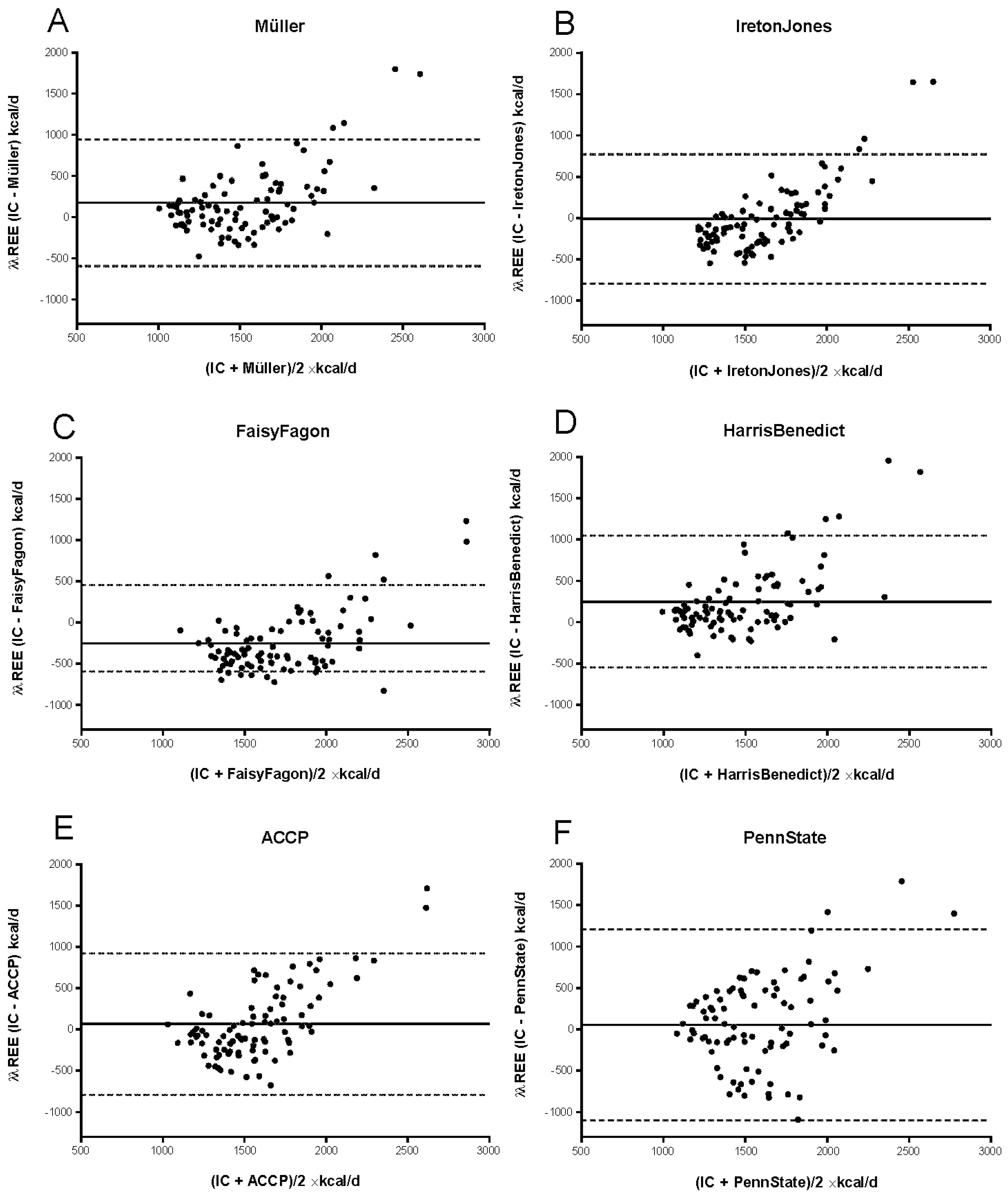
3.3.1. mREE and cREE in the Critically Ill Patient Cohort
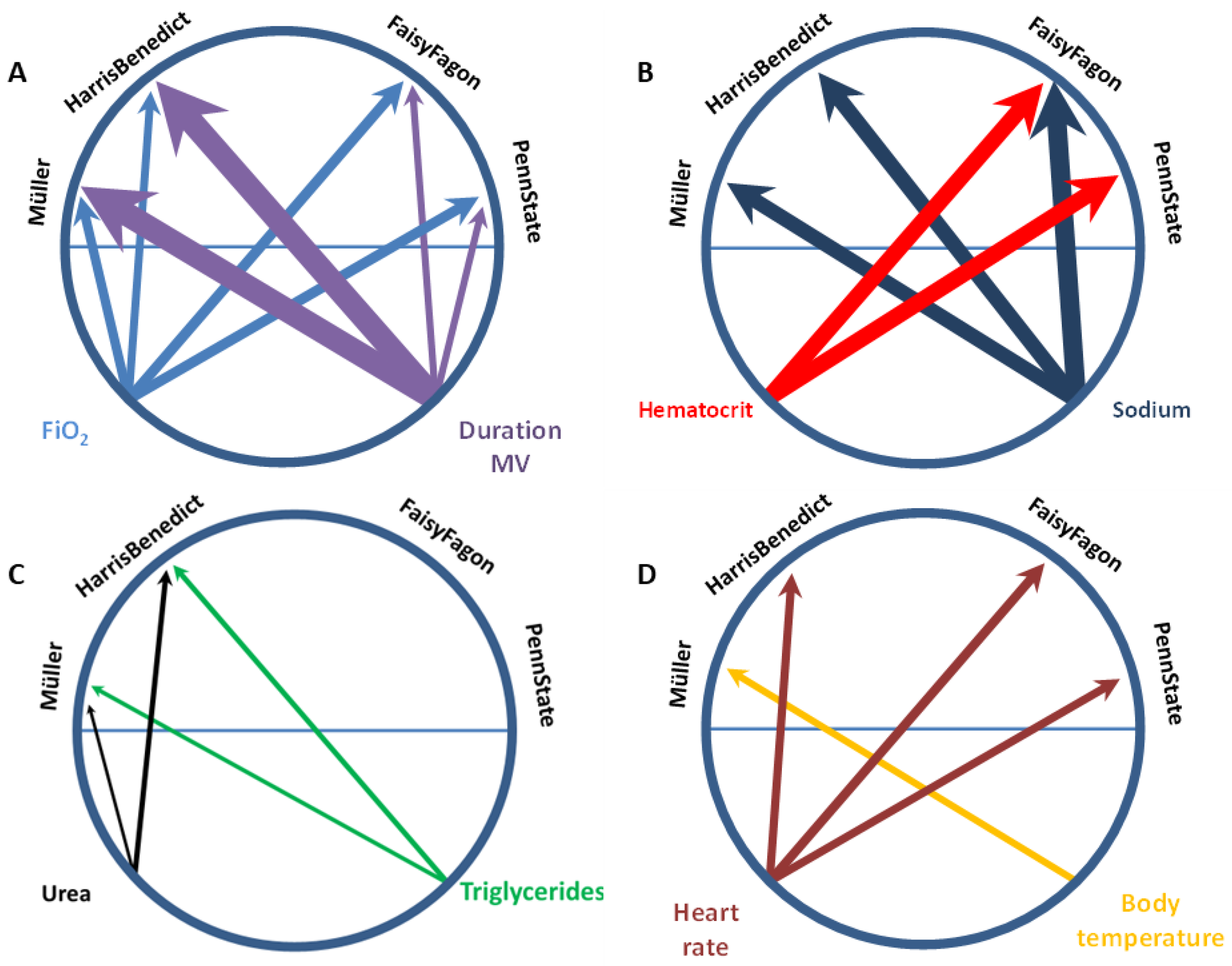
3.3.2. Predictors of Differences between mREE and cREE in the Critically Ill Patient Cohort
3.3.3. Predictors for Differences in mREE between the Critically Ill Patient and Healthy Control Cohort
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orimo, H. Reviewing the definition of elderly. Nihon Ronen Igakkai Zasshi 2006, 43, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Sjoding, M.W.; Prescott, H.C.; Wunsch, H.; Iwashyna, T.J.; Cooke, C.R. Longitudinal Changes in ICU Admissions Among Elderly Patients in the United States. Crit. Care Med. 2016, 44, 1353–1360. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Webb, S.A.; Delaney, A.; George, C.; Pilcher, D.; Hart, G.K.; Bellomo, R. Very old patients admitted to intensive care in Australia and New Zealand: A multi-centre cohort analysis. Crit. Care 2009, 13, R45. [Google Scholar] [CrossRef]
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.M.; Divatia, J.V.; Shrestha, B.R.; Arabi, Y.M.; Ng, J.; Gomersall, C.D.; et al. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir. Med. 2020, 8, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Lew, C.C.H.; Yandell, R.; Fraser, R.J.L.; Chua, A.P.; Chong, M.F.F.; Miller, M. Association Between Malnutrition and Clinical Outcomes in the Intensive Care Unit: A Systematic Review [Formula: See text]. JPEN J. Parenter. Enteral Nutr. 2017, 41, 744–758. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cereda, E.; Cruz-Jentoft, A.; Goisser, S.; de Groot, L.; Grosshauser, F.; Kiesswetter, E.; Norman, K.; et al. Management of Malnutrition in Older Patients-Current Approaches, Evidence and Open Questions. J. Clin. Med. 2019, 8, 974. [Google Scholar] [CrossRef] [PubMed]
- Wolters, M.; Volkert, D.; Streicher, M.; Kiesswetter, E.; Torbahn, G.; O’Connor, E.M.; O’Keeffe, M.; Kelly, M.; O’Herlihy, E.; O’Toole, P.W.; et al. Prevalence of malnutrition using harmonized definitions in older adults from different settings—A MaNuEL study. Clin. Nutr. 2019, 38, 2389–2398. [Google Scholar] [CrossRef]
- Alix, E.; Berrut, G.; Bore, M.; Bouthier-Quintard, F.; Buia, J.M.; Chlala, A.; Cledat, Y.; d’Orsay, G.; Lavigne, C.; Levasseur, R.; et al. Energy requirements in hospitalized elderly people. J. Am. Geriatr. Soc. 2007, 55, 1085–1089. [Google Scholar] [CrossRef]
- Conley, K.E.; Jubrias, S.A.; Esselman, P.C. Oxidative capacity and ageing in human muscle. J. Physiol. 2000, 526 Pt 1, 203–210. [Google Scholar] [CrossRef]
- Fraipont, V.; Preiser, J.C. Energy estimation and measurement in critically ill patients. JPEN J. Parenter. Enteral Nutr. 2013, 37, 705–713. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter. Enteral Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Anderegg, B.A.; Worrall, C.; Barbour, E.; Simpson, K.N.; Delegge, M. Comparison of resting energy expenditure prediction methods with measured resting energy expenditure in obese, hospitalized adults. JPEN J. Parenter. Enteral Nutr. 2009, 33, 168–175. [Google Scholar] [CrossRef]
- De Waele, E.; Opsomer, T.; Honore, P.M.; Diltoer, M.; Mattens, S.; Huyghens, L.; Spapen, H. Measured versus calculated resting energy expenditure in critically ill adult patients. Do mathematics match the gold standard? Minerva Anestesiol. 2015, 81, 272–282. [Google Scholar]
- Kross, E.K.; Sena, M.; Schmidt, K.; Stapleton, R.D. A comparison of predictive equations of energy expenditure and measured energy expenditure in critically ill patients. J. Crit. Care 2012, 27, 312.e5-12. [Google Scholar] [CrossRef]
- Stucky, C.C.; Moncure, M.; Hise, M.; Gossage, C.M.; Northrop, D. How accurate are resting energy expenditure prediction equations in obese trauma and burn patients? JPEN J. Parenter. Enteral Nutr. 2008, 32, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Tah, P.C.; Lee, Z.Y.; Poh, B.K.; Abdul Majid, H.; Hakumat-Rai, V.R.; Mat Nor, M.B.; Kee, C.C.; Kamarul Zaman, M.; Hasan, M.S. A Single-Center Prospective Observational Study Comparing Resting Energy Expenditure in Different Phases of Critical Illness: Indirect Calorimetry Versus Predictive Equations. Crit. Care Med. 2020, 48, e380–e390. [Google Scholar] [CrossRef] [PubMed]
- Segadilha, N.; Rocha, E.E.M.; Tanaka, L.M.S.; Gomes, K.L.P.; Espinoza, R.E.A.; Peres, W.A.F. Energy Expenditure in Critically Ill Elderly Patients: Indirect Calorimetry vs Predictive Equations. JPEN J. Parenter. Enteral Nutr. 2017, 41, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Sessler, C.N.; Gosnell, M.S.; Grap, M.J.; Brophy, G.M.; O’Neal, P.V.; Keane, K.A.; Tesoro, E.P.; Elswick, R.K. The Richmond Agitation-Sedation Scale: Validity and reliability in adult intensive care unit patients. Am. J. Respir. Crit. Care Med. 2002, 166, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonca, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Heyland, D.K.; Dhaliwal, R.; Jiang, X.; Day, A.G. Identifying critically ill patients who benefit the most from nutrition therapy: The development and initial validation of a novel risk assessment tool. Crit. Care 2011, 15, R268. [Google Scholar] [CrossRef] [PubMed]
- Cerra, F.B.; Benitez, M.R.; Blackburn, G.L.; Irwin, R.S.; Jeejeebhoy, K.; Katz, D.P.; Pingleton, S.K.; Pomposelli, J.; Rombeau, J.L.; Shronts, E.; et al. Applied nutrition in ICU patients. A consensus statement of the American College of Chest Physicians. Chest 1997, 111, 769–778. [Google Scholar] [CrossRef]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Ireton-Jones, C.; Jones, J.D. Improved equations for predicting energy expenditure in patients: The Ireton-Jones Equations. Nutr. Clin. Pract. 2002, 17, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Faisy, C.; Guerot, E.; Diehl, J.L.; Labrousse, J.; Fagon, J.Y. Assessment of resting energy expenditure in mechanically ventilated patients. Am. J. Clin. Nutr. 2003, 78, 241–249. [Google Scholar] [CrossRef]
- Muller, M.J.; Bosy-Westphal, A.; Klaus, S.; Kreymann, G.; Luhrmann, P.M.; Neuhauser-Berthold, M.; Noack, R.; Pirke, K.M.; Platte, P.; Selberg, O.; et al. World Health Organization equations have shortcomings for predicting resting energy expenditure in persons from a modern, affluent population: Generation of a new reference standard from a retrospective analysis of a German database of resting energy expenditure. Am. J. Clin. Nutr. 2004, 80, 1379–1390. [Google Scholar] [CrossRef]
- Frankenfield, D. Validation of an equation for resting metabolic rate in older obese, critically ill patients. JPEN J. Parenter. Enteral Nutr. 2011, 35, 264–269. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Danielzik, S.; Geisler, C.; Onur, S.; Korth, O.; Selberg, O.; Pfeuffer, M.; Schrezenmeir, J.; Muller, M.J. Use of height3: Waist circumference3 as an index for metabolic risk assessment? Br. J. Nutr. 2006, 95, 1212–1220. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Geisler, C.; Onur, S.; Korth, O.; Selberg, O.; Schrezenmeir, J.; Muller, M.J. Value of body fat mass vs anthropometric obesity indices in the assessment of metabolic risk factors. Int. J. Obes. 2006, 30, 475–483. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Onur, S.; Geisler, C.; Wolf, A.; Korth, O.; Pfeuffer, M.; Schrezenmeir, J.; Krawczak, M.; Muller, M.J. Common familial influences on clustering of metabolic syndrome traits with central obesity and insulin resistance: The Kiel obesity prevention study. Int. J. Obes. 2007, 31, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Neelemaat, F.; van Bokhorst-de van der Schueren, M.A.; Thijs, A.; Seidell, J.C.; Weijs, P.J. Resting energy expenditure in malnourished older patients at hospital admission and three months after discharge: Predictive equations versus measurements. Clin. Nutr. 2012, 31, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Boullata, J.; Williams, J.; Cottrell, F.; Hudson, L.; Compher, C. Accurate determination of energy needs in hospitalized patients. J. Am. Diet. Assoc. 2007, 107, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Melzer, K.; Laurie Karsegard, V.; Genton, L.; Kossovsky, M.P.; Kayser, B.; Pichard, C. Comparison of equations for estimating resting metabolic rate in healthy subjects over 70 years of age. Clin. Nutr. 2007, 26, 498–505. [Google Scholar] [CrossRef]
- Lazzer, S.; Agosti, F.; Resnik, M.; Marazzi, N.; Mornati, D.; Sartorio, A. Prediction of resting energy expenditure in severely obese Italian males. J. Endocrinol. Investig. 2007, 30, 754–761. [Google Scholar] [CrossRef]
- De Waele, E.; van Zanten, A.R.H. Routine use of indirect calorimetry in critically ill patients: Pros and cons. Crit. Care 2022, 26, 123. [Google Scholar] [CrossRef]
- Moonen, H.; Beckers, K.J.H.; van Zanten, A.R.H. Energy expenditure and indirect calorimetry in critical illness and convalescence: Current evidence and practical considerations. J. Intensive Care 2021, 9, 8. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Eichhorn, C.; Kutzner, D.; Illner, K.; Heller, M.; Muller, M.J. The age-related decline in resting energy expenditure in humans is due to the loss of fat-free mass and to alterations in its metabolically active components. J. Nutr. 2003, 133, 2356–2362. [Google Scholar] [CrossRef]
- Mtaweh, H.; Soto Aguero, M.J.; Campbell, M.; Allard, J.P.; Pencharz, P.; Pullenayegum, E.; Parshuram, C.S. Systematic review of factors associated with energy expenditure in the critically ill. Clin. Nutr. ESPEN 2019, 33, 111–124. [Google Scholar] [CrossRef]
- Dollberg, S.; Marom, R.; Mimouni, F.B.; Littner, Y. Increased energy expenditure after dilutional exchange transfusion for neonatal polycythemia. J. Am. Coll. Nutr. 2007, 26, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Harmatz, P.; Heyman, M.B.; Cunningham, J.; Lee, P.D.; Styles, L.; Quirolo, K.; Kopp-Hoolihan, L.; Ghiron, J.; Hintz, R.L.; Vichinsky, E. Effects of red blood cell transfusion on resting energy expenditure in adolescents with sickle cell anemia. J. Pediatr. Gastroenterol. Nutr. 1999, 29, 127–131. [Google Scholar] [CrossRef]
- Holzel, C.; Weidhase, L.; Petros, S. The effect of age and body mass index on energy expenditure of critically ill medical patients. Eur. J. Clin. Nutr. 2021, 75, 464–472. [Google Scholar] [CrossRef]
- Bruder, N.; Raynal, M.; Pellissier, D.; Courtinat, C.; Francois, G. Influence of body temperature, with or without sedation, on energy expenditure in severe head-injured patients. Crit. Care Med. 1998, 26, 568–572. [Google Scholar] [CrossRef]
- Chiolero, R.; Revelly, J.P.; Tappy, L. Energy metabolism in sepsis and injury. Nutrition 1997, 13, 45S–51S. [Google Scholar] [CrossRef] [PubMed]
- Kreymann, G.; Grosser, S.; Buggisch, P.; Gottschall, C.; Matthaei, S.; Greten, H. Oxygen consumption and resting metabolic rate in sepsis, sepsis syndrome, and septic shock. Crit. Care Med. 1993, 21, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Botran, M.; Lopez-Herce, J.; Mencia, S.; Urbano, J.; Solana, M.J.; Garcia, A.; Carrillo, A. Relationship between energy expenditure, nutritional status and clinical severity before starting enteral nutrition in critically ill children. Br. J. Nutr. 2011, 105, 731–737. [Google Scholar] [CrossRef]
- McLellan, S.; Walsh, T.; Burdess, A.; Lee, A. Comparison between the Datex-Ohmeda M-COVX metabolic monitor and the Deltatrac II in mechanically ventilated patients. Intensive Care Med. 2002, 28, 870–876. [Google Scholar] [CrossRef]
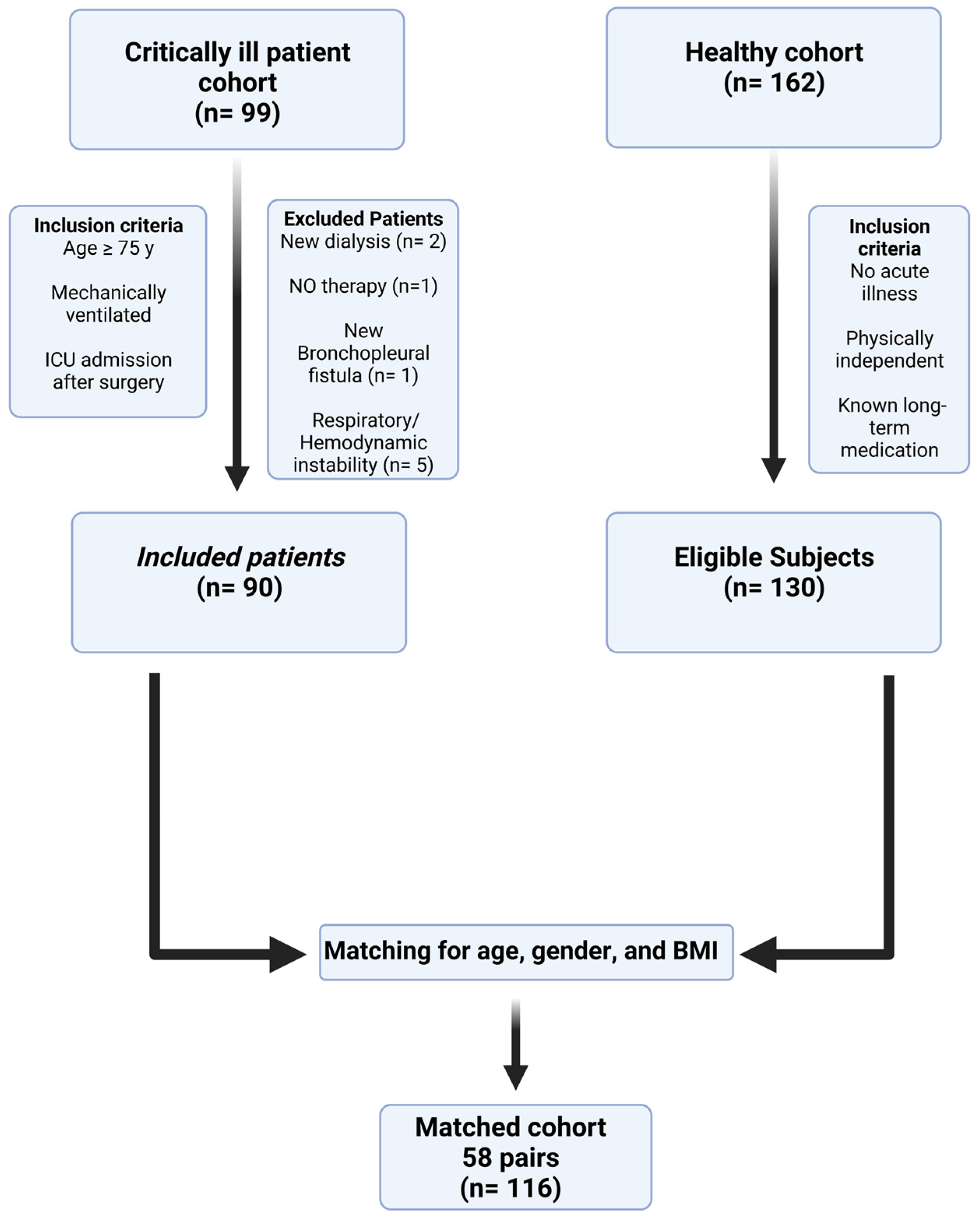
| Critically Ill Patients Cohort | Matched Cohort | p-Value | ||
|---|---|---|---|---|
| Anthropometrics | (N = 90) | Healthy Control (N = 58) | Critically Ill Patients (N = 58) | |
| Age, years | 80 (77–84) | 79 (76–81) | 79 (76–82) | 0.777 |
| Gender, male, n (%) | 44 (49). 46 w (51%) | 25 m (43%). 33 f (57%) | 25 m (43%). 33 f (57%) | 0.139 |
| Weight, kg | 72 (60–88) | 69 (60–80) | 70 (60–81) | 0.677 |
| Height, days | 168 (160–172) | 165 (157–171) | 165 (160–170) | 0.206 |
| BMI, kg/m2 | 25 (23–30) | 25 (23–29) | 25 (23–28) | 0.738 |
| Admission diagnosis | ||||
| Postoperative | 25 | - | 18 | n.a. |
| Perforation of a hollow organ | ||||
| (n) | 14 | 9 | ||
| Pneumonia (n) | 10 | 5 | ||
| Ischaemia of limbs or organ | ||||
| (n) | 9 | 7 | ||
| Sepsis (n) | 7 | 4 | ||
| Ileus (n) | 6 | 3 | ||
| Bleeding (n) | 6 | 5 | ||
| Trauma (n) | 6 | 3 | ||
| ACS (n) | 3 | 2 | ||
| Intracerebral bleeding (n) | 1 | 1 | ||
| Seizures (n) | 1 | 1 | ||
| Subdural hematoma (n) | 1 | 0 | ||
| Vital Signs | ||||
| Heart rate, min−1 | 88 (76–103) | 68 (60–72) | 86 (74–103) | <0.00001 |
| Systolic blood pressure, mmHg | 121 (104–136) | 150 (130–160) | 122 (103–136) | <0.00001 |
| Diastolic blood pressure, mmHg | 53 (47–60) | 90 (75–90) | 54 (49–65) | <0.00001 |
| Maximum body temperature, °C | 37 (37–38) | 36 (36–37) | 38 (37–38) | <0.00001 |
| Indirect Calorimetry | ||||
| Measured REE, kcal/d | 1475 (1251–1892) | 1351 (1187–1503) | 1457 (1247–1876) | 0.008 |
| Respiratory Quotient, VCO2/VO2 | 0.76 (0.71–0.81) | 0.82 (0.76–0.87) | 0.76 (0.71–0.85) | 0.016 |
| Ventilatory parameters | ||||
| Days since start of MV | 1 (1–2) | - | 1 (1–2) | - |
| Days on MV | 1 (1–2) | - | 1 (1–2) | - |
| FIO2, % | 40 (35–50) | - | 40 (35–50) | - |
| Minute volume ventilation, L/min | 8 (6–10) | - | 8 (7–10) | - |
| Highest Respiratory rate, min−1 | 17 (9–23) | - | 18 (9–27) | - |
| Partial pressure of O2, mmHg | 108 (92–133) | - | 104 (89–125) | - |
| Partial pressure of CO2, mmHg | 46 (39–52) | - | 44 (39–52) | - |
| Laboratory data | ||||
| Triglycerids, mg/dL | 91 (53.3–134.5) | 126.4 (87.7–166.0) | 92.0 (50.0–134.0) | 0.017 |
| Cholesterine, mg/dL | 85 (60.5–132.5) | 188.7 (2.6–234.3) | 86.0 (67.0–143.5) | 0.083 |
| Blood glucose, mg/dL | 145 (119–127) | 97 (84–106) | 143 (118–173) | <0.00001 |
| Thyrotropine, pg/mL | 2.2 (0.9–3.7) | 1.0 (0.8–1.4) | 2.2 (1.0–3.8) | 0.003 |
| Unbound Tri-jodthyronine, pg/ml | 1.7 (1.4–2.2) | 3.0 (2.6–3.8) | 1.7 (1.3–1.9) | 0 |
| Unbound Thyroxine, ng/dL | 1.1 (1.0–1.4) | 1.3 (1.1–1.6) | 1.1 (0.9–1.2) | 0.014 |
| Creatinine, µmol/L | 1.3 (0.9–1.8) | - | 1.3 (0.9–1.7) | - |
| Urea, mmol/L | 53.5 (33.0–81.5) | - | 48.0 (27.0–80.0) | - |
| Hematocrit, % | 30 (27.0–33.0) | - | 30.0 (25.5–33.0) | - |
| Albumin, g/dL | 2.1 (1.8–2.8) | - | 2.2 (1.8–2.7) | - |
| Arterial pH | 7.4 (7.3–7.4) | - | 7.4 (7.3–7.4) | - |
| Base excess | −1.4 (−3.3–1.6) | - | −0.3 (−2.5–2.5) | - |
| Na+, mmol/L | 139 (136.0–143.0) | - | 140.0 (136.0–143.3) | - |
| K+, mmol/L | 4.9 (4.3–5.2) | - | 4.8 (4.3–5.1) | - |
| Thrombocytes, N × 109/L | 183 (115.5–268.3) | - | 175.0 (109.8–238.5) | - |
| Bilirubine, µmol/L | 0.6 (0.4–0.9) | - | 0.6 (0.4–0.9) | - |
| Procalcitonin, µg/d | 1.3 (0.3–3.8) | - | 1.2 (0.3–3.4) | - |
| C-reaktive protein, mg/L | 98.6 (46.9–205.5) | 1.4 (1.1–2.4) | 102.6 (51.1–182.3) | <0.00001 |
| Leukocytes, N × 109/L | 11.9 (8.9–16.1) | - | 11.5 (8.8–16.4) | - |
| Scores | ||||
| RASS, points | 8 (7–9) | - | 8 (7–9) | - |
| APACHE-II, points | 19 (15–21) | - | 18 (15–21) | - |
| SOFA, points | 7 (4–8) | - | 6 (4–8) | - |
| NUTRIC, points | 5 (4–6) | - | 5 (4–6) | - |
| Mortality, N (%) | 48 (53) | 0 (0) | 31 (53) | <0.00001 |
| Predictive Equation | mREE, kcal/d | cREE, kcal/d | Intraindividual ΔmREE-cREE, kcal | p Value |
|---|---|---|---|---|
| ACCP | 1475 [1251–1892] | 1339 [1158–1574] | 33 [−247–288] | 0.641 |
| IretonJones | 1673 [1443–1773] | 109 [−297–147] | 0.085 | |
| FaisyFagon | 1824 [1653–2080] | −353 [−486–96] | <0.0001 | |
| PennState | 1483 [1244–1759] | 54 [−105–248] | 0.023 | |
| Müller | 1471 [1191–1683] | 105 [−82–334] | <0.0001 | |
| Harris–Benedict | 1339 [1158–1574] | 139 [23–407] | <0.0001 |
| Independent Variables | Dependent Variable: Δ mREE-cREE | ||||
|---|---|---|---|---|---|
| FaisyFagon | PennState | Müller | Harris–Benedict | ||
| Heart rate (min−1) (log) (n = 90) | Change in R2 | 0.106 | 0.093 | - | 0.093 |
| Beta | 0.383 | 0.418 | - | 0.322 | |
| p value * | <0.0001 | <0.0001 | - | 0.001 | |
| Max. body temperature (°C) (log) (n = 90) | Change in R2 | - | - | 0.095 | - |
| Beta | - | - | 0.324 | - | |
| p value * | - | - | 0.001 | - | |
| Days since start of MV (days) (log) (n = 90) | Change in R2 | 0.103 | 0.079 | 0.267 | 0.266 |
| Beta | 0.347 | 0.356 | 0.503 | 0.499 | |
| p value * | 0.001 | 0.001 | <0.0001 | <0.0001 | |
| FIO2 (%) (log) (n = 90) | Change in R2 | 0.119 | 0.119 | 0.107 | 0.09 |
| Beta | 0.356 | 0.355 | 0.306 | 0.27 | |
| p value * | 0.001 | 0.001 | 0.003 | 0.009 | |
| Urea (mmol/L) (log) (n = 88) | Change in R2 | - | - | 0.047 | 0.061 |
| Beta | - | - | 0.226 | 0.258 | |
| p value * | - | - | 0.025 | 0.011 | |
| Hematocrit (%) (log) (n = 88) | Change in R2 | 0.186 | 0.188 | - | - |
| Beta | −0.28 | −0.26 | - | - | |
| p value * | 0.007 | 0.015 | - | - | |
| Na+ (mmol/L) (log) (n = 90) | Change in R2 | 0.217 | 0.083 | 0.169 | 0.175 |
| Beta | 0.458 | 0.415 | 0.399 | 0.414 | |
| p value * | 0.000 | <0.0001 | <0.0001 | <0.0001 | |
| R2 total | 0.732 | 0.704 | 0.685 | 0.685 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindner, M.; Geisler, C.; Rembarz, K.; Hummitzsch, L.; Radke, D.I.; Schulte, D.M.; Müller, M.J.; Bosy-Westphal, A.; Elke, G. Resting Energy Expenditure in the Critically Ill and Healthy Elderly—A Retrospective Matched Cohort Study. Nutrients 2023, 15, 303. https://doi.org/10.3390/nu15020303
Lindner M, Geisler C, Rembarz K, Hummitzsch L, Radke DI, Schulte DM, Müller MJ, Bosy-Westphal A, Elke G. Resting Energy Expenditure in the Critically Ill and Healthy Elderly—A Retrospective Matched Cohort Study. Nutrients. 2023; 15(2):303. https://doi.org/10.3390/nu15020303
Chicago/Turabian StyleLindner, Matthias, Corinna Geisler, Kristina Rembarz, Lars Hummitzsch, David I. Radke, Dominik M. Schulte, Manfred J. Müller, Anja Bosy-Westphal, and Gunnar Elke. 2023. "Resting Energy Expenditure in the Critically Ill and Healthy Elderly—A Retrospective Matched Cohort Study" Nutrients 15, no. 2: 303. https://doi.org/10.3390/nu15020303
APA StyleLindner, M., Geisler, C., Rembarz, K., Hummitzsch, L., Radke, D. I., Schulte, D. M., Müller, M. J., Bosy-Westphal, A., & Elke, G. (2023). Resting Energy Expenditure in the Critically Ill and Healthy Elderly—A Retrospective Matched Cohort Study. Nutrients, 15(2), 303. https://doi.org/10.3390/nu15020303







