Poultry Meat and Eggs as an Alternative Source of n-3 Long-Chain Polyunsaturated Fatty Acids for Human Nutrition
Abstract
1. Introduction
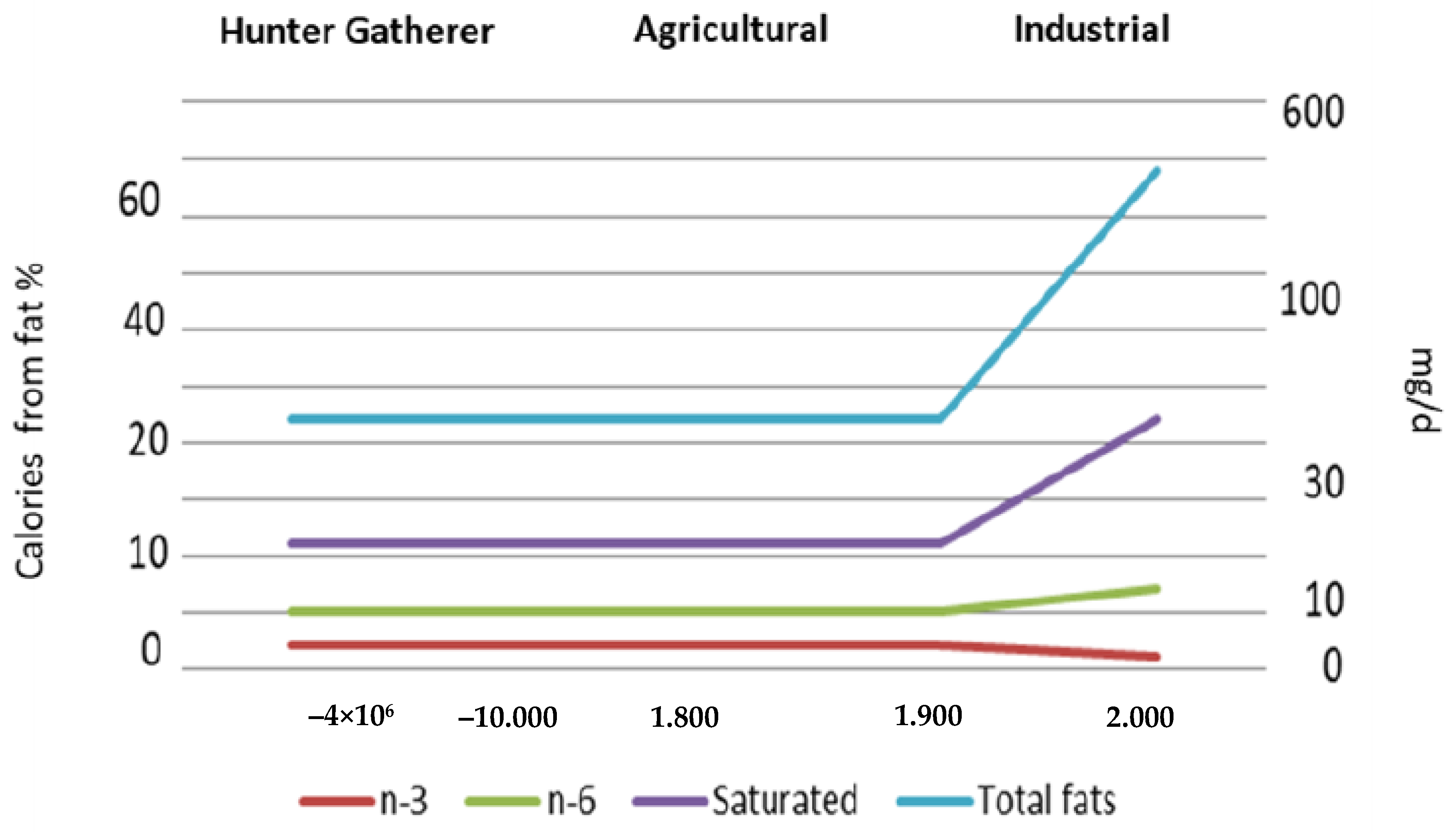
2. Evolution of Human Diet
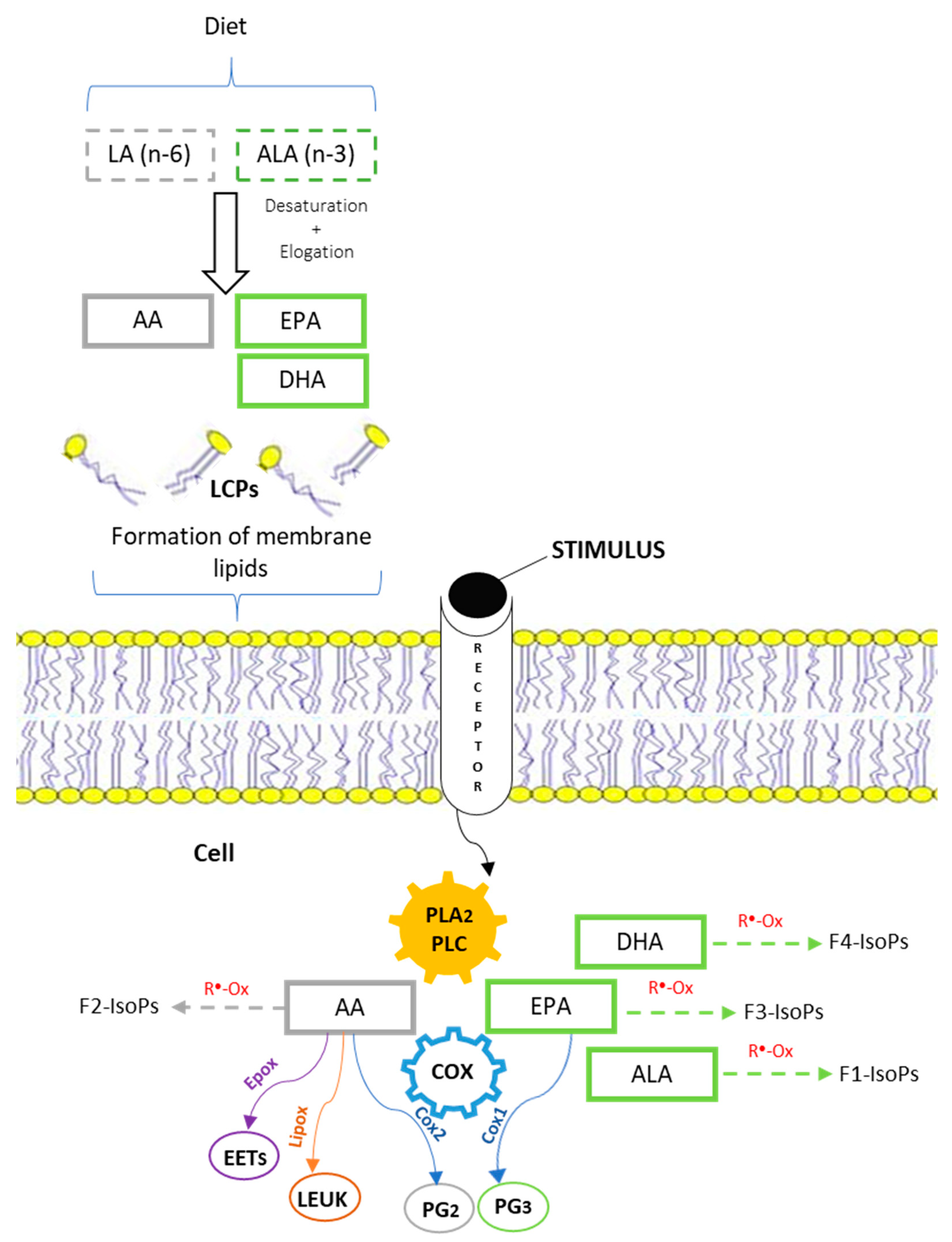
3. Notes on Metabolism of Essential Fatty Acids and Desaturase Activity (Details of Synthesis)
- COX-1, a constitutive enzyme widely expressed in most tissues because it controls the synthesis of PGs involved in the regulation of homeostatic function;
3.1. Relevance of n-3 LC-PUFA in Human Nutrition
3.2. Global Requirements for EPA and DHA
4. Sources of n-3 LC-PUFA and Strategies for Enriching Terrestrial Food
4.1. Vegetable Source
4.2. Fish and Fish Products
4.3. Terrestrial (Farmed) Animals
- Meat and poultry products (sausages, frankfurters, etc.);
- Eggs and egg products (mayonnaise, etc.);
- Milk and milk products (yoghurt, cheese, etc.).
- monogastric animal;
- short breeding cycle; meat-type chickens have an age at slaughtering of about 40 days;
- there are no religious limitations for poultry meat (or not as many as for pork or beef/lamb);
- lower environmental impact than other livestock productive chain [112] due to the high efficiency in converting feed into food;
- it is the most-consumed meat in the world;
- eggs easily meet the EFSA recommendation for n-3-enriched foods.
Poultry Meat and Eggs as Functional Foods
5. n-3 LC-PUFA in Poultry Meat and Eggs, Strategies of Enrichment
5.1. Dietary Strategies for Broilers and Laying Hens
- The n-6/n-3 ratio of the diet. In fact, due the involvement of the same enzymes, the n-3 synthesis is in competition with the synthesis of the n-6 one; thus, the higher LA diet presence could reduce the production of EPA and DHA by favoring the AA synthesis [125];
- The antioxidant supplementation (vitamin E, vitamin C, Selenium, etc.). Due to their double bonds, PUFA are very susceptible to oxidation, resulting in reduced shelf life of feed as well as meat and eggs. This can lead to a poor acceptance of the feed by the animals but also a poor acceptance of n-3 LC-PUFA-enriched products by the final consumers due to the development of unattractive colors or unpleasant tastes and aromas.
5.1.1. Dietary Enrichment for Broilers
5.1.2. Dietary Enrichment for Laying Hens
5.1.3. Use of Insect and Earthworms as a Future Prospective
6. Poultry Genotype
- Fast-growing genotypes (FG) are represented by birds used in intensive rearing systems reaching commercial weight in a very short time and characterized by a high breast yield (> 25% live weight). The most common genotypes are selected for precocity; at about 40 days of age their weight is more than 2.5 kg;
- Medium-growing genotypes (MG), also known as slower-growing genotypes (SrG), are a recently recognized group and comprise some commercial chicken genotypes that are lower-performing then the FG ones, which is why the breed companies define them as SrG. These genotypes are less common in intensive rearing systems, but they are widely used in alternative rearing systems (e.g., free-range and organic);
- Slow-growing genotypes (SG) are represented by breeds that are very important for maintaining biodiversity and genetic variability but, due to their low productive performance (growth rate and breast yield), are not utilized in intensive rearing systems. Thus, they are mainly used for niche-production in small-scale farms [203].
7. Rearing System
8. Animals Transport
9. Cooking Procedures
10. Conclusions
- avoiding livestock and human competition for n-3 LC-PUFA;
- developing livestock systems with the best conditions for bio-conversion of n-3 precursors into n-3 LC-PUFA.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Dael, P. Role of N-3 Long-Chain Polyunsaturated Fatty Acids in Human Nutrition and Health: Review of Recent Studies and Recommendations. Nutr. Res. Pract. 2021, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Goyens, P.L.; Spilker, M.E.; Zock, P.L.; Katan, M.B.; Mensink, R.P. Conversion of α-linolenic acid in humans is influenced by the absolute amounts of α-linolenic acid and linoleic acid in the diet and not by their ratio. Am. J. Clin. Nutr. 2006, 84, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Mariamenatu, A.H.; Abdu, E.M. Overconsumption of Omega-6 Polyunsaturated Fatty Acids (PUFAs) versus Deficiency of Omega-3 PUFAs in Modern-Day Diets: The Disturbing Factor for Their “Balanced Antagonistic Metabolic Functions” in the Human Body. J. Lipids 2021, 2021, 8848161. [Google Scholar] [CrossRef]
- Mollica, M.; Trinchese, G.; Cimmino, F.; Penna, E.; Cavaliere, G.; Tudisco, R.; Musco, N.; Manca, C.; Catapano, A.; Monda, M.; et al. Milk Fatty Acid Profiles in Different Animal Species: Focus on the Potential Effect of Selected PUFAs on Metabolism and Brain Functions. Nutrients 2021, 13, 1111. [Google Scholar] [CrossRef] [PubMed]
- Dal Bosco, A.; Castellini, C.; Bianchi, L.; Mugnai, C. Effect of Dietary α-Linolenic Acid and Vitamin E on the Fatty Acid Composition, Storage Stability and Sensory Traits of Rabbit Meat. Meat Sci. 2004, 66, 407–413. [Google Scholar] [CrossRef]
- Ferrier, L.K.; Caston, L.J.; Leeson, S.; Squires, J.; Weaver, B.J.; Holub, B.J. Alpha-Linolenic Acid- and Docosahexaenoic Acid-Enriched Eggs from Hens Fed Flaxseed: Influence on Blood Lipids and Platelet Phospholipid Fatty Acids in Humans. Am. J. Clin. Nutr. 1995, 62, 81–86. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary Aspects of Diet: The Omega-6/Omega-3 Ratio and the Brain. Mol. Neurobiol. 2011, 44, 203–215. [Google Scholar] [CrossRef]
- Naughton, J.M.; O’Dea, K.; Sinclair, A.J. Animal Foods in Traditional Australian Aboriginal Diets: Polyunsaturated and Low in Fat. Lipids 1986, 21, 684–690. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef]
- Salter, A.M. Dietary fatty acids and cardiovascular disease. Animal 2013, 7, 163–171. [Google Scholar] [CrossRef]
- Calder, P.C. Marine Omega-3 Fatty Acids and Inflammatory Processes: Effects, Mechanisms and Clinical Relevance. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Lupton, J.R.; Brooks, J.; Butte, N.F.; Caballero, B.; Flatt, J.P.; Fried, S.K. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academy Press: Washington, DC, USA, 2002; Volume 5, pp. 589–768. [Google Scholar]
- Cordain, L.; Miller, J.B.; Eaton, S.B.; Mann, N.; Holt, S.H.A.; Speth, J.D. Plant-Animal Subsistence Ratios and Macronutrient Energy Estimations in Worldwide Hunter-Gatherer Diets. Am. J. Clin. Nutr. 2000, 71, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Eaton, S.B.; Eaton, S.B.; Sinclair, A.J.; Cordain, L.; Mann, N.J. Dietary Intake of Long-Chain Polyunsaturated Fatty Acids during the Paleolithic. World Rev. Nutr. Diet 1998, 83, 12–23. [Google Scholar]
- Sinclair, A.J. Incorporation of radioactive polyunsaturated fatty acids into liver and brain of developing rat. Lipids 1975, 10, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.A. The Role of Dietary Fatty Acids in Biology: Their Place in the Evolution of the Human Brain. Nutr. Rev. 1992, 50, 3–11. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.; Miller, J.B.; Mann, N.; Hill, K. The paradoxical nature of hunter-gatherer diets: Meat-based, yet non-atherogenic. Eur. J. Clin. Nutr. 2002, 56, S42–S52. [Google Scholar] [CrossRef] [PubMed]
- Raatz, S.K.; Conrad, Z.; Jahns, L. Trends in linoleic acid intake in the United States adult population: NHANES 1999–2014. Prostaglandins Leukot. Essent. Fat. Acids 2018, 133, 23–28. [Google Scholar] [CrossRef]
- Harika, R.K.; Eilander, A.; Alssema, M.; Osendarp, S.J.; Zock, P. Intake of Fatty Acids in General Populations Worldwide Does Not Meet Dietary Recommendations to Prevent Coronary Heart Disease: A Systematic Review of Data from 40 Countries. Ann. Nutr. Metab. 2013, 63, 229–238. [Google Scholar] [CrossRef]
- Sioen, I.; Van Lieshout, L.; Eilander, A.; Fleith, M.; Lohner, S.; Szommer, A.; Petisca, C.; Eussen, S.; Forsyth, S.; Calder, P.C.; et al. Systematic Review on N-3 and N-6 Polyunsaturated Fatty Acid Intake in European Countries in Light of the Current Recommendations—Focus on Specific Population Groups. Ann. Nutr. Metab. 2017, 70, 39–50. [Google Scholar] [CrossRef]
- Schmidhuber, J.; Traill, W.B. The changing structure of diets in the European Union in relation to healthy eating guidelines. Public Health Nutr. 2006, 9, 584–595. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Genetic Variation and Evolutionary Aspects of Diet. In Antioxidant Status, Diet, Nutrition, and Health, 1st ed.; Papas, A., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 64–89. [Google Scholar]
- Stark, A.H.; Crawford, M.A.; Reifen, R. Update on alpha-linolenic acid. Nutr. Rev. 2008, 66, 326–332. [Google Scholar] [CrossRef]
- Castellini, C.; Mattioli, S.; Moretti, E.; Cotozzolo, E.; Perini, F.; Dal Bosco, A.; Signorini, C.; Noto, D.; Belmonte, G.; Lasagna, E.; et al. Expression of Genes and Localization of Enzymes Involved in Polyunsaturated Fatty Acid Synthesis in Rabbit Testis and Epididymis. Sci. Rep. 2022, 12, 2637. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, S.; Dal Bosco, A.; Maranesi, M.; Petrucci, L.; Rebollar, P.G.; Castellini, C. Dietary Fish Oil and Flaxseed for Rabbit Does: Fatty Acids Distribution and Δ6-Desaturase Enzyme Expression of Different Tissues. Animal 2019, 13, 1934–1942. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, S.; Collodel, G.; Signorini, C.; Cotozzolo, E.; Noto, D.; Cerretani, D.; Micheli, L.; Fiaschi, A.; Brecchia, G.; Menchetti, L.; et al. Tissue Antioxidant Status and Lipid Peroxidation Are Related to Dietary Intake of n-3 Polyunsaturated Acids: A Rabbit Model. Antioxidants 2021, 10, 681. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Ba, G.N.; Couvreur, P.; Tew, K. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed. Pharmacother. 2002, 56, 215–222. [Google Scholar] [CrossRef]
- Khan, A.A.; Iadarola, M.; Yang, H.-Y.T.; Dionne, R.A. Expression of COX-1 and COX-2 in a Clinical Model of Acute Inflammation. J. Pain 2007, 8, 349–354. [Google Scholar] [CrossRef]
- Mattos, R.; Staples, C.R.; Thatcher, W.W. Effects of dietary fatty acids on reproduction in ruminants. Rev. Reprod. 2000, 5, 38–45. [Google Scholar] [CrossRef]
- Carneiro, L.C.; Williams, E.J.; Saut, J.P.E.; dos Santos, R.M.; Celeghini, E.C.C. The Effect of N-3 Polyunsaturated Fatty Acid Supplementation Oimmune and Reproductive Parameters in Dairy Cows: A Review. Braz. J. Vet. Res. Anim. Sci. 2021, 58, e175224. [Google Scholar] [CrossRef]
- Kolobarić, N.; Drenjančević, I.; Matić, A.; Šušnjara, P.; Mihaljević, Z.; Mihalj, M. Dietary Intake of N-3 Pufa-Enriched Hen Eggs Changes Inflammatory Markers’ Concentration and Treg/Th17 Cells Distribution in Blood of Young Healthy Adults—A Randomised Study. Nutrients 2021, 13, 1851. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes: From Molecules to Man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Morrow, J.D.; Hill, K.E.; Burk, R.F.; Nammour, T.M.; Badr, K.F.; Roberts, L.J. A Series of Prostaglandin F2-like Compounds Are Produced in Vivo in Humans by a Non-Cyclooxygenase, Free Radical-Catalyzed Mechanism. Proc. Natl. Acad. Sci. USA 1990, 87, 9383–9387. [Google Scholar] [CrossRef]
- Signorini, C.; Moretti, E.; Collodel, G. Role of isoprostanes in human male infertility. Syst. Biol. Reprod. Med. 2020, 66, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Milne, G.L.; Yin, H.; Morrow, J.D. Human Biochemistry of the Isoprostane Pathway. J. Biol. Chem. 2008, 283, 15533–15537. [Google Scholar] [CrossRef] [PubMed]
- Galano, J.M.; Lee, Y.Y.; Oger, C.; Vigor, C.; Vercauteren, J.; Durand, T.; Giera, M.; Lee, J.C.Y. Isoprostanes, Neuroprostanes and Phytoprostanes: An Overview of 25 Years of Research in Chemistry and Biology. Prog. Lipid Res. 2017, 68, 83–108. [Google Scholar] [CrossRef] [PubMed]
- Molnár, P.J.; Der, B.; Borsodi, K.; Balla, H.; Borbás, Z.; Molnár, K.; Ruisanchez, É.; Kenessey, I.; Horváth, A.; Keszthelyi, A.; et al. Isoprostanes Evoke Contraction of the Murine and Human Detrusor Muscle via Activation of the Thromboxane Prostanoid TP Receptor and Rho Kinase. Am. J. Physiol. Ren. Physiol. 2021, 320, F537–F547. [Google Scholar] [CrossRef]
- Wu, J.; Fang, S.; Lu, K.T.; Wackman, K.; Schwartzman, M.L.; Dikalov, S.I.; Grobe, J.L.; Sigmund, C.D. EP3 (E-Prostanoid 3) Receptor Mediates Impaired Vasodilation in a Mouse Model of Salt-Sensitive Hypertension. Hypertension 2021, 77, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Very Long-Chain n-3 Fatty Acids and Human Health: Fact, Fiction and the Future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef] [PubMed]
- Yannakopoulos, A.; Tserveni-Gousi, A.; Christaki, E. Enhanced Egg Production in Practice: The Case of Bio-Omega-3 Egg. Int. J. Poult. Sci. 2005, 4, 531–535. [Google Scholar]
- Tang, A.B.; Nishimura, K.Y.; Phinney, S.D. Preferential Reduction in Adipose Tissue a.Linolenic Acid (18:3 3) During Very Low Calorie Dieting Despite Supplementation with 18:3 31. Lipids 1993, 28, 987–993. [Google Scholar] [CrossRef]
- Scientific Opinion on Dietary Reference Values for Fats, Including Saturated Fatty Acids, Polyunsaturated Fatty Acids, Monounsaturated Fatty Acids, Trans Fatty Acids, and Cholesterol. EFSA J. 2010, 8, 1461.
- Helland, I.B.; Smith, L.; Saarem, K.; Saugstad, O.D.; Drevon, C.A. Maternal Supplementation with Very-Long-Chain n-3 Fatty Acids During Pregnancy and Lactation Augments Children’s IQ at 4 Years of Age. Pediatrics 2003, 111, e39–e44. [Google Scholar] [CrossRef] [PubMed]
- Leyrolle, Q.; Decoeur, F.; Briere, G.; Amadieu, C.; Quadros, A.R.A.A.; Voytyuk, I.; Lacabanne, C.; Benmamar-Badel, A.; Bourel, J.; Aubert, A.; et al. Maternal dietary omega-3 deficiency worsens the deleterious effects of prenatal inflammation on the gut-brain axis in the offspring across lifetime. Neuropsychopharmacology 2021, 46, 579–602. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.C.; Freedman, R.; Law, A.J.; Clark, A.M.; Hunter, S.K. Maternal nutrients and effects of gestational COVID-19 infection on fetal brain development. Clin. Nutr. ESPEN 2021, 43, 1–8. [Google Scholar] [CrossRef]
- Castro-Gómez, P.; Garcia-Serrano, A.; Visioli, F.; Fontecha, J. Relevance of dietary glycerophospholipids and sphingolipids to human health. Prostaglandins Leukot. Essent. Fat. Acids 2015, 101, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Hussain, S.; Drevon, C.A.; Nagelhus, E.; Hvalby, D.; Jensen, V.; Walaas, S.I.; Davanger, S. Omega-3 fatty acids regulate plasticity in distinct hippocampal glutamatergic synapses. Eur. J. Neurosci. 2019, 49, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.; Nelson, V.A.; Davila, H.; Ratner, E.; Fink, H.A.; Hemmy, L.S.; McCarten, J.R.; Barclay, T.R.; Brasure, M.; Kane, R.L. Over-the-Counter Supplement Interventions to Prevent Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer-Type Dementia. Ann. Intern. Med. 2018, 168, 52–62. [Google Scholar] [CrossRef]
- Moore, K.; Hughes, C.F.; Ward, M.; Hoey, L.; McNulty, H. Diet, Nutrition and the Ageing Brain: Current Evidence and New Directions. Proc. Nutr. Soc. 2018, 77, 152–163. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; Agrón, E.; Launer, L.J.; Grodstein, F.; Bernstein, P.S. Effect of Omega-3 Fatty Acids, Lutein/Zeaxanthin, or Other Nutrient Supplementation on Cognitive Function: The AREDS2 Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2015, 314, 791–801. [Google Scholar] [CrossRef]
- Wood, A.H.R.; Chappell, H.F.; Zulyniak, M.A. Dietary and Supplemental Long-Chain Omega-3 Fatty Acids as Moderators of Cognitive Impairment and Alzheimer’s Disease. Eur. J. Nutr. 2022, 61, 589–604. [Google Scholar] [CrossRef]
- Bernasconi, A.A.; Wiest, M.M.; Lavie, C.J.; Milani, R.V.; Laukkanen, J.A. Effect of Omega-3 Dosage on Cardiovascular Outcomes: An Updated Meta-Analysis and Meta-Regression of Interventional Trials. Mayo Clin. Proc. 2021, 96, 304–313. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA) Advice about Eating Fish. Available online: https://www.fda.gov/food/consumers/advice-about-eating-fish (accessed on 25 January 2022).
- Meyer, B.J.; de Groot, R.H.M. Effects of Omega-3 Long Chain Polyunsaturated Fatty Acid Supplementation on Cardiovascular Mortality: The Importance of the Dose of DHA. Nutrients 2017, 9, 1305. [Google Scholar] [CrossRef] [PubMed]
- Van der Wurff, I.S.M.; Meyer, B.J.; de Groot, R.H.M. Effect of Omega-3 Long Chain Polyunsaturated Fatty Acids (N-3 LCPUFA) Supplementation on Cognition in Children and Adolescents: A Systematic Literature Review with a Focus on n-3 LCPUFA Blood Values and Dose of DHA and EPA. Nutrients 2020, 12, 3115. [Google Scholar] [CrossRef] [PubMed]
- Scientific Opinion on the Tolerable Upper Intake Level of Eicosapentaenoic Acid (EPA), Docosahexaenoic Acid (DHA) and Docosapentaenoic Acid (DPA). EFSA J. 2012, 10, 2815.
- Poulos, A.; Darin-Bennett, A.; White, I. The phospholipid-bound fatty acids and aldehydes of mammalian spermatozoa. Comp. Biochem. Physiol. Part B Comp. Biochem. 1973, 46, 541–549. [Google Scholar] [CrossRef]
- Rodríguez, M.; Rebollar, P.G.; Mattioli, S.; Castellini, C. N-3 PUFA Sources (Precursor/Products): A Review of Current Knowledge on Rabbit. Animals 2019, 9, 806. [Google Scholar] [CrossRef]
- Signorini, C.; Moretti, E.; Noto, D.; Mattioli, S.; Castellini, C.; Pascarelli, N.A.; Durand, T.; Oger, C.; Galano, J.M.; de Felice, C.; et al. F4-Neuroprostanes: A Role in Sperm Capacitation. Life 2021, 11, 655. [Google Scholar] [CrossRef]
- Yuxin, L.; Chen, L.; Xiaoxia, L.; Yue, L.; Junjie, L.; Youzhu, L.; Huiliang, Z.; Qicai, L. Research Progress on the Relationship between Obesity-Inflammation-Aromatase Axis and Male Infertility. Oxid. Med. Cell. Longev. 2021, 2021, 6612796. [Google Scholar] [CrossRef]
- Agarwal, A.; Parekh, N.; Selvam, M.K.P.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Men’s Health 2019, 37, 296–312. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Chaves, H.; Singh, R.B.; Khan, S.; Wilczynska, A.; Takahashi, T. High Omega-6/Omega-3 Fatty Acid Ratio Diets and Risk of Noncommunicable Diseases: Is the Tissue, the Main Issue? In The Role of Functional Food Security in Global Health, 1st ed.; Watson, R.R., Singh, R.B., Takahashi, T., Eds.; Academic Press: London, UK, 2018; pp. 217–259. [Google Scholar]
- World Population Prospect: The 2019 Revision—United Nations, Department of Economic and Social Affairs, Population Division (June 2019). Available online: https://population.un.org/wpp (accessed on 26 January 2022).
- International Programs Center at the U.S. Census Bureau, Population Division. Available online: https://www.census.gov/programs-surveys/international-programs.html (accessed on 29 January 2022).
- Salem, N.; Eggersdorfer, M. Is the World Supply of Omega-3 Fatty Acids Adequate for Optimal Human Nutrition? Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 147–154. [Google Scholar] [CrossRef]
- Cherif, A.; Dubacq, J.; Mache, R.; Oursel, A.; Tremolieres, A. Biosynthesis of α-Linolenic Acid by Desaturation of Oleic and Linoleic Acids in Several Organs of Higher and Lower Plants and in Algae. Phytochemistry 1975, 14, 703–706. [Google Scholar] [CrossRef]
- Anai, T.; Yamada, T.; Kinoshita, T.; Rahman, S.M.; Takagi, Y. Identification of Corresponding Genes for Three Low-α-Linolenic Acid Mutants and Elucidation of Their Contribution to Fatty Acid Biosynthesis in Soybean Seed. Plant Sci. 2005, 168, 1615–1623. [Google Scholar] [CrossRef]
- Somerville, C.; Browse, J. Plant Lipids: Metabolism, Mutants, and Membranes. Science 1991, 252, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Harwood, J.L. Fatty acid metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 101–138. [Google Scholar] [CrossRef]
- Harwood, J.L. Recent advances in the biosynthesis of plant fatty acids. Biochim. Biophys. Acta (BBA)-Lipids Lipid. Metab. 1996, 1301, 7–56. [Google Scholar] [CrossRef]
- Millar, A.A.; Wrischer, M.; Kunst, L. Accumulation of Very-Long-Chain Fatty Acids in Membrane Glycerolipids Is Associated with Dramatic Alterations in Plant Morphology. Plant Cell 1998, 10, 1889–1902. [Google Scholar] [CrossRef]
- Spychalla, J.P.; Kinney, A.J.; Browse, J. Identification of an Animal-3 Fatty Acid Desaturase by Heterologous Expression in Arabidopsis. Proc. Natl. Acad. Sci. USA 1997, 94, 1142–1147. [Google Scholar] [CrossRef]
- Arao, T.; Yamada, M. Biosynthesis of Polyunsaturated Fatty Acids in the Marine Diatom, Phaeodactylum Tricornutum. Phytochemistry 1994, 35, 1177–1181. [Google Scholar] [CrossRef]
- Arao, T.; Sakaki, T.; Yamada, M. Biosynthesis of Polyunsaturated Lipids in the Diatom, Phaeodactylum Tricornutum. Phytochemistry 1994, 36, 629–635. [Google Scholar] [CrossRef]
- Bajpai, P.; Bajpai, P.K. Eicosapentaenoic Acid (EPA) Production from Microorganisms: A Review. J. Biotechnol. 1993, 30, 161–183. [Google Scholar] [CrossRef]
- Girke, T.; Schmidt, H.; Zä Hringer, U.; Reski, R.; Heinz, E. Identification of a novel D6-acyl-group desaturase by targeted gene disruption in Physcomitrella patens. Plant J. 1998, 15, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Twining, C.W.; Brenna, J.T.; Hairston, N.G.; Flecker, A.S. Highly Unsaturated Fatty Acids in Nature: What We Know and What We Need to Learn. Oikos 2016, 125, 749–760. [Google Scholar] [CrossRef]
- Twining, C.W.; Parmar, T.P.; Mathieu-Resuge, M.; Kainz, M.J.; Shipley, J.R.; Martin-Creuzburg, D. Use of Fatty Acids from Aquatic Prey Varies with Foraging Strategy. Front. Ecol. Evol. 2021, 9, 735350. [Google Scholar] [CrossRef]
- Gill, I.; Valivety, R. Polyunsaturated Fatty Acids, Part 1: Occurrence, Biological Activities and Applications. Trends Biotechnol. 1997, 15, 401–409. [Google Scholar] [CrossRef]
- Gunstone, F.D. Gammar linolenic acid occurrence and physical and chemical properties. Prog. Lipid Res. 1992, 31, 145–161. [Google Scholar] [CrossRef]
- Gunstone, F. Movements towards tailor-made fats. Prog. Lipid Res. 1998, 37, 277–305. [Google Scholar] [CrossRef]
- US Department of Agriculture. National Nutrient Database. Available online: http://www.nal.usda.gov/fnic/foodcomp/search/ (accessed on 25 January 2022).
- Gebauer, S.K.; Psota, T.L.; Harris, W.S.; Kris-Etherton, P.M. N-3 Fatty Acid Dietary Recommendations and Food Sources to Achieve Essentiality and Cardiovascular Benefits. Am. J. Clin. Nutr. 2006, 83, 1526S–1535S. [Google Scholar] [CrossRef]
- Hardy, R.W. Utilization of Plant Proteins in Fish Diets: Effects of Global Demand and Supplies of Fishmeal. Aquac. Res. 2010, 41, 770–776. [Google Scholar] [CrossRef]
- Tocher, D.R. Metabolism and Functions of Lipids and Fatty Acids in Teleost Fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Bell, M.V.; Tocher, D.R. Biosynthesis of Polyunsaturated Fatty Acids in Aquatic Ecosystems: General Pathways and New Directions. In Lipids in Aquatic Ecosystems, 1st ed.; Kainz, M., Brett, M., Arts, M., Eds.; Springer: New York, NY, USA, 2009; pp. 211–236. [Google Scholar]
- Castro, L.F.C.; Tocher, D.R.; Monroig, O. Long-chain polyunsaturated fatty acid biosynthesis in chordates: Insights into the evolution of Fads and Elovl gene repertoire. Prog. Lipid Res. 2016, 62, 25–40. [Google Scholar] [CrossRef]
- Lopes-Marques, M.; Kabeya, N.; Qian, Y.; Ruivo, R.; Santos, M.M.; Venkatesh, B.; Tocher, D.R.; Castro, L.F.C.; Monroig, Ó. Retention of Fatty Acyl Desaturase 1 (Fads1) in Elopomorpha and Cyclostomata Provides Novel Insights into the Evolution of Long-Chain Polyunsaturated Fatty Acid Biosynthesis in Vertebrates. BMC Evol. Biol. 2018, 18, 157. [Google Scholar] [CrossRef]
- Betancor, M.B.; Oboh, A.; Ortega, A.; Mourente, G.; Navarro, J.C.; de la Gándara, F.; Tocher, D.R.; Monroig, Ó. Molecular and Functional Characterisation of a Putative Elovl4 Gene and Its Expression in Response to Dietary Fatty Acid Profile in Atlantic Bluefin Tuna (Thunnus thynnus). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 240, 110372. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal Triacylglycerols as Feedstocks for Biofuel Production: Perspectives and Advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.; Hodac, L.; Friedl, T.; Feussner, I. Fatty Acid Profiles and Their Distribution Patterns in Microalgae: A Comprehensive Analysis of More than 2000 Strains from the SAG Culture Collection. BMC Plant Biol. 2011, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Kabeya, N.; Ikeya, K.; Kakioka, R.; Cech, J.N.; Osada, N.; Leal, M.C.; Inoue, J.; Kume, M.; Toyoda, A.; et al. A Key Metabolic Gene for Recurrent Freshwater Colonization and Radiation in Fishes. Science 2019, 364, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Kriton, G.; Dimitra, K.; Corraze, G.; Jaume, P.-S.; Adorjan, A.; Zsuzsanna, J.S. Impact of Diets Containing Plant Raw Materials as Fish Meal and Fish Oil Replacement on Rainbow Trout (Oncorhynchus mykiss), Gilthead Sea Bream (Sparus aurata), and Common Carp (Cyprinus carpio) Freshness. J. Food Qual. 2018, 2018, 1717465. [Google Scholar] [CrossRef]
- Faudzi, N.M.; Yong, A.S.K.; Shapawi, R.; Senoo, S.; Biswas, A.; Takii, K. Soy protein concentrate as an alternative in replacement of fish meal in the feeds of hybrid grouper, brown-marbled grouper (Epinephelus fuscoguttatus) × giant grouper (E. lanceolatus) juvenile. Aquac. Res. 2017, 49, 431–441. [Google Scholar] [CrossRef]
- Braña, C.B.C.; Cerbule, K.; Senff, P.; Stolz, I.K. Towards Environmental Sustainability in Marine Finfish Aquaculture. Front. Mar. Sci. 2021, 8. [Google Scholar] [CrossRef]
- Hites, R.A.; Foran, J.A.; Carpenter, D.O.; Hamilton, M.C.; Knuth, B.A.; Schwager, S.J. Global Assessment of Organic Contaminants in Farmed Salmon. Science 2004, 303, 226–229. [Google Scholar] [CrossRef]
- Thomsen, S.T.; Assunção, R.; Afonso, C.; Boué, G.; Cardoso, C.; Cubadda, F.; Garre, A.; Kruisselbrink, J.W.; Mantovani, A.; Pitter, J.G.; et al. Human Health Risk–Benefit Assessment of Fish and Other Seafood: A Scoping Review. Crit. Rev. Food Sci. Nutr. 2021, 1–22. [Google Scholar] [CrossRef]
- Adams, K.J.; Drenner, R.W.; Chumchal, M.M.; Donato, D.I. Disparity between State Fish Consumption Advisory Systems for Methylmercury and US Environmental Protection Agency Recommendations: A Case Study of the South Central United States. Environ. Toxicol. Chem. 2016, 35, 247–251. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Guidance for assessing chemical contaminant data for use in fish advisories. Risk Assess. Fish Consum. Limits 2000, 2. [Google Scholar]
- Kuipers, R.S.; Luxwolda, M.F.; Offringa, P.J.; Boersma, E.R.; Dijck-Brouwer, D.J.; Muskiet, F.A. Gestational age dependent changes of the fetal brain, liver and adipose tissue fatty acid compositions in a population with high fish intakes. Prostaglandins Leukot. Essent. Fat. Acids 2012, 86, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Extier, A.; Langelier, B.; Perruchot, M.-H.; Guesnet, P.; Van Veldhoven, P.P.; Lavialle, M.; Alessandri, J.-M. Gender affects liver desaturase expression in a rat model of n−3 fatty acid repletion. J. Nutr. Biochem. 2010, 21, 180–187. [Google Scholar] [CrossRef]
- Burdge, G.; Slater-Jefferies, J.; Grant, R.; Chung, W.-S.; West, A.; Lillycrop, K.; Hanson, M.; Calder, P. Sex, but not maternal protein or folic acid intake, determines the fatty acid composition of hepatic phospholipids, but not of triacylglycerol, in adult rats. Prostaglandins Leukot. Essent. Fat. Acids 2008, 78, 73–79. [Google Scholar] [CrossRef]
- Toral, P.G.; Monahan, F.J.; Hervás, G.; Frutos, P.; Moloney, A.P. Review: Modulating ruminal lipid metabolism to improve the fatty acid composition of meat and milk. Challenges and opportunities. Animal 2018, 12, s272–s281. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Alimon, A.R.; Yaakub, H.; Samsudin, A.A.; Candyrine, S.C.L.; Wan Mohamed, W.N.; Md Noh, A.; Fuat, M.A.; Mookiah, S. Effects of Vegetable Oil Supplementation on Rumen Fermentation and Microbial Population in Ruminant: A Review. Trop. Anim. Health Prod. 2021, 53, 422. [Google Scholar] [CrossRef]
- Scislowski, V.; Bauchart, D.; Gruffat, D.; Laplaud, P.M.; Durand, D. Effects of Dietary N-6 or n-3 Polyunsaturated Fatty Acids Protected or Not against Ruminal Hydrogenation on Plasma Lipids and Their Susceptibility to Peroxidation in Fattening Steers. J. Anim. Sci. 2005, 83, 2162–2174. [Google Scholar] [CrossRef]
- Pereira, G.; Simões, P.; Bexiga, R.; Silva, E.; Mateus, L.; Fernandes, T.; Alves, S.P.; Bessa, R.J.B.; Lopes-da-Costa, L. Effects of Feeding Rumen-Protected Linseed Fat to Postpartum Dairy Cows on Plasma n-3 Polyunsaturated Fatty Acid Concentrations and Metabolic and Reproductive Parameters. J. Dairy Sci. 2022, 105, 361–374. [Google Scholar] [CrossRef]
- Kitessa, S.M.; Gulati, S.K.; Simos, G.C.; Ashes, J.R.; Scott, T.W.; Fleck, E.; Wynn, P.C. Supplementation of Grazing Dairy Cows with Rumen-Protected Tuna Oil Enriches Milk Fat with n -3 Fatty Acids without Affecting Milk Production or Sensory Characteristics. Br. J. Nutr. 2004, 91, 271–277. [Google Scholar] [CrossRef]
- Simopoulos, A.P. New products from the agri-food industry: The return of n-3 fatty acids into the food supply. Lipids 1999, 34, S297–S301. [Google Scholar] [CrossRef] [PubMed]
- FAO (Food and Agriculture Organization of the United Nations). Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation; FAO Food and Nutrition: Geneva, Switzerland, 2010. [Google Scholar]
- Givens, D.; Kliem, K.E.; Gibbs, R.A. The role of meat as a source of n−3 polyunsaturated fatty acids in the human diet. Meat Sci. 2006, 74, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.G.; Audsley, E.; Sandars, D.L. Determining the Environmental Burdens and Resource Use in the Production of Agricultural and Horticultural Commoditites; Defra Project Report IS0205. Available online: http://randd.defra.gov.uk/Default.aspx (accessed on 3 May 2022).
- Cavani, C.; Petracci, M.; Trocino, A.; Xiccato, G. Advances in Research on Poultry and Rabbit Meat Quality. Ital. J. Anim. Sci. 2009, 8 (Suppl. 2), 741–750. [Google Scholar] [CrossRef]
- Barroeta, A. Nutritive value of poultry meat: Relationship between vitamin E and PUFA. World’s Poult. Sci. J. 2007, 63, 277–284. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ahn, D.U. Egg White Proteins and Their Potential Use in Food Processing or as Nutraceutical and Pharmaceutical Agents—A Review. Poult. Sci. 2013, 92, 3292–3299. [Google Scholar] [CrossRef] [PubMed]
- Marcet, I.; Sáez-Orviz, S.; Rendueles, M.; Díaz, M. Egg Yolk Granules and Phosvitin. Recent Advances in Food Technology and Applications. LWT 2022, 153, 112442. [Google Scholar] [CrossRef]
- Mattioli, S.; Dal Bosco, A.; Martino, M.; Ruggeri, S.; Marconi, O.; Sileoni, V.; Falcinelli, B.; Castellini, C.; Benincasa, P. Alfalfa and Flax Sprouts Supplementation Enriches the Content of Bioactive Compounds and Lowers the Cholesterol in Hen Egg. J. Funct. Foods 2016, 22, 454–462. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, Y.B.; Kim, D.H.; Lee, D.W.; Lee, H.G.; Jha, R.; Lee, K.W. Dietary Soluble Flaxseed Oils as a Source of Omega-3 Polyunsaturated Fatty Acids for Laying Hens. Poult. Sci. 2021, 100, 101276. [Google Scholar] [CrossRef]
- Kwiecień, M.; Winiarska-Mieczan, A.; Danek-Majewska, A.; Kwiatkowska, K.; Krusiński, R. Effects of Dietary Alfalfa Protein Concentrate on Lipid Metabolism and Antioxidative Status of Serum and Composition and Fatty Acid Profile and Antioxidative Status and Dietetic Value of Muscles in Broilers. Poult. Sci. 2021, 100, 100974. [Google Scholar] [CrossRef]
- Wilkinson, S.J. Big data for poultry—What is possible. In Proceedings of the 29th Annual Australian Poultry Science Symposium, Sydney, Australia, 4–7 February 2018. [Google Scholar]
- Schreiner, M.; Hulan, H.W.; Razzazi-Fazeli, E.; Böhm, J.; Moreira, R.G. Effect of Different Sources of Dietary Omega-3 Fatty Acids on General Performance and Fatty Acid Profiles of Thigh, Breast, Liver and Portal Blood of Broilers. J. Sci. Food Agric. 2005, 85, 219–226. [Google Scholar] [CrossRef]
- Chekani-Azar, S.; Shahriar, H.A.; Maheri-Sis, N.; Ahmadzadeh, A.R.; Vahdatpoor, T. Omega-3 Fatty Acids Enrichment and Organoleptic Characteristics of Broiler Meat. Asian J. Anim. Vet. Adv. 2008, 3, 62–69. [Google Scholar] [CrossRef]
- Rizliya, V.; Mendis, E. Biological, Physical, and Chemical Properties of Fish Oil and Industrial Applications. In Seafood Processing By-Products: Trends and Applications, 1st ed.; Kim, S.K., Ed.; Springer: New York, NY, USA, 2013; pp. 285–313. [Google Scholar]
- Harris, W.S. Fish Oils and Plasma Lipid and Lipoprotein Metabolism in Humans: A Critical Review. J. Lipid Res. 1989, 30, 785–807. [Google Scholar] [CrossRef]
- Sprecher, H.W.; Baykousheva, S.P.; Luthria, D.L.; Mohammed, B.S. Differences in the Regulation of Biosynthesis of 20- versus 22-Carbon Polyunsaturated Fatty Acids. Prostaglandins Leukot. Essent. Fat. Acids 1995, 52, 99–101. [Google Scholar] [CrossRef]
- Bonos, E.; Kasapidou, E.; Kargopoulos, A.; Karampampas, A.; Christaki, E.; Florou-Paneri, P.; Nikolakakis, I. Spirulina as a Functional Ingredient in Broiler Chicken Diets. S. Afr. J. Anim. Sci. 2016, 46, 94–102. [Google Scholar] [CrossRef]
- Alfaia, C.M.; Pestana, J.M.; Rodrigues, M.; Coelho, D.; Aires, M.J.; Ribeiro, D.M.; Major, V.T.; Martins, C.F.; Santos, H.; Lopes, P.A.; et al. Influence of Dietary Chlorella Vulgaris and Carbohydrate-Active Enzymes on Growth Performance, Meat Quality and Lipid Composition of Broiler Chickens. Poult. Sci. 2021, 100, 926–937. [Google Scholar] [CrossRef]
- El-Bahr, S.; Shousha, S.; Shehab, A.; Khattab, W.; Ahmed-Farid, O.; Sabike, I.; El-Garhy, O.; Albokhadaim, I.; Albosadah, K. Effect of Dietary Microalgae on Growth Performance, Profiles of Amino and Fatty Acids, Antioxidant Status, and Meat Quality of Broiler Chickens. Animals 2020, 10, 761. [Google Scholar] [CrossRef]
- Costa, M.M.; Pestana, J.M.; Osório, D.; Alfaia, C.M.; Martins, C.F.; Mourato, M.; Gueifão, S.; Rego, A.M.; Coelho, I.; Coelho, D.; et al. Effect of Dietary Laminaria Digitata with Carbohydrases on Broiler Production Performance and Meat Quality, Lipid Profile, and Mineral Composition. Animals 2022, 12, 1007. [Google Scholar] [CrossRef]
- Jankowski, J.; Zduńczyk, Z.; Mikulski, D.; Juśkiewicz, J.; Pomianowski, J.F.; Zduńczyk, P. Fatty Acid Profile, Oxidative Stability and Sensory Quality of Breast Meat from Turkeys Fed Diets with Graded Levels of Flaxseed Oil for Different Periods of Time. Anim. Prod. Sci. 2018, 58, 1164–1174. [Google Scholar] [CrossRef]
- López-Ferrer, S.; Baucells, M.D.; Barroeta, A.C.; Galobart, J.; Grashorn, M.A. N-3 Enrichment of Chicken Meat. 2. Use of Precursors of Long-Chain Polyunsaturated Fatty Acids: Linseed Oil. Poult. Sci. 2001, 80, 753–761. [Google Scholar] [CrossRef]
- Kartikasari, L.R.; Hughes, R.J.; Geier, M.S.; Makrides, M.; Gibson, R.A. Dietary Alpha-Linolenic Acid Enhances Omega-3 Long Chain Polyunsaturated Fatty Acid Levels in Chicken Tissues. Prostaglandins Leukot. Essent. Fat. Acids 2012, 87, 103–109. [Google Scholar] [CrossRef]
- López-Ferrer, S.; Baucells, M.D.; Barroeta, A.C.; Grashorn, M.A. N-3 Enrichment of Chicken Meat Using Fish Oil: Alternative Substitution with Rapeseed and Linseed Oils. Poult. Sci. 1999, 78, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Esquerra, R.; Leeson, S. Effects of Menhaden Oil and Flaxseed in Broiler Diets on Sensory Quality and Lipid Composition of Poultry Meat. Br. Poult. Sci. 2000, 41, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Zelenka, J.; Jarošová, A.; Schneiderová, D. Influence of N-3 and n-6 Polyunsaturated Fatty Acids on Sensory Characteristics of Chicken Meat. Czech J. Anim. Sci. 2008, 53, 299–305. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Romanov, M.N.; Griffin, D.K. Nutritional Modulation of the Antioxidant Capacities in Poultry: The Case of Vitamin E. Poult. Sci. 2019, 98, 4030–4041. [Google Scholar] [CrossRef]
- González-Ortiz, G.; Sala, R.; Cánovas, E.; Abed, N.; Barroeta, A.C. Consumption of Dietary N-3 Fatty Acids Decreases Fat Deposition and Adipocyte Size, but Increases Oxidative Susceptibility in Broiler Chickens. Lipids 2013, 48, 705–717. [Google Scholar] [CrossRef]
- Shin, D.; Choi, S.; Go, G.; Park, J.; Narciso-Gaytán, C.; Morgan, C.; Smith, S.; Sánchez-Plata, M.; Ruiz-Feria, C. Effects of dietary combination of n-3 and n-9 fatty acids on the deposition of linoleic and arachidonic acid in broiler chicken meats. Poult. Sci. 2012, 91, 1009–1017. [Google Scholar] [CrossRef]
- Cortinas, L.; Villaverde, C.; Galobart, J.; Baucells, M.D.; Codony, R.; Barroeta, A.C. Fatty Acid Content in Chicken Thigh and Breast as Affected by Dietary Polyunsaturation Level. Poult. Sci. 2004, 83, 1155–1164. [Google Scholar] [CrossRef]
- Rymer, C.; Givens, D.I. Effect of Species and Genotype on the Efficiency of Enrichment of Poultry Meat with N-3 Polyunsaturated Fatty Acids. Lipids 2006, 41, 445–451. [Google Scholar] [CrossRef]
- Brooks, G.A.; Mercier, J. Balance of Carbohydrate and Lipid Utilization during Exercise: The “crossover” Concept. J. Appl. Physiol. 1994, 76, 2253–2261. [Google Scholar] [CrossRef]
- Schulz, H. Beta oxidation of fatty acids. Biochim. Et Biophys. Acta (BBA) Lipids Lipid Metab. 1991, 1081, 109–120. [Google Scholar] [CrossRef]
- Cartoni Mancinelli, A.; di Veroli, A.; Mattioli, S.; Cruciani, G.; Dal Bosco, A.; Castellini, C. Lipid Metabolism Analysis in Liver of Different Chicken Genotypes and Impact on Nutritionally Relevant Polyunsaturated Fatty Acids of Meat. Sci. Rep. 2022, 12, 1888. [Google Scholar] [CrossRef] [PubMed]
- Marra, C.A.; de Alaniz, M.J.T. Influence of Testosterone Administration on the Biosynthesis of Unsaturated Fatty Acids in Male and Female Rats. Lipids 1989, 24, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Wootton, S.A. Conversion of α-Linolenic Acid to Eicosapentaenoic, Docosapentaenoic and Docosahexaenoic Acids in Young Women. Br. J. Nutr. 2002, 88, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Poureslami, R.; Raes, K.; Turchini, G.M.; Huyghebaert, G.; de Smet, S. Effect of Diet, Sex and Age on Fatty Acid Metabolism in Broiler Chickens: N-3 and n-6 PUFA. Br. J. Nutr. 2010, 104, 189–197. [Google Scholar] [CrossRef]
- Abedi, E.; Sahari, M.A. Long-Chain Polyunsaturated Fatty Acid Sources and Evaluation of Their Nutritional and Functional Properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef]
- Aymond, W.M.; VAN Elswyk, M.E. Yolk Thiobarbituric Acid Reactive Substances and n-3 Fatty Acids in Response to Whole and Ground Flaxseed. Poult. Sci. 1995, 74, 1388–1394. [Google Scholar] [CrossRef]
- Hayat, Z.; Cherian, G.; Pasha, T.N.; Khattak, F.M.; Jabbar, M.A. Effect of feeding flax and two types of antioxidants on egg production, egg quality, and lipid composition of eggs. J. Appl. Poult. Res. 2009, 18, 541–551. [Google Scholar] [CrossRef]
- Caston, L.J.; Squires, E.J.; Leeson, S. Hen performance, egg quality, and the sensory evaluation of eggs from SCWL hens fed dietary flax. Can. J. Anim. Sci. 1994, 74, 347–353. [Google Scholar] [CrossRef]
- Rizzi, L.; Bochicchio, D.; Bargellini, A.; Parazza, P.; Simioli, M. Effects of dietary microalgae, other lipid sources, inorganic selenium and iodine on yolk n-3 fatty acid composition, selenium content and quality of eggs in laying hens. J. Sci. Food Agric. 2009, 89, 1775–1781. [Google Scholar] [CrossRef]
- Bean, L.; Leeson, S. Long-term effects of feeding flaxseed on performance and egg fatty acid composition of brown and white hens. Poult. Sci. 2003, 82, 388–394. [Google Scholar] [CrossRef]
- Mattioli, S.; Ruggeri, S.; Sebastiani, B.; Brecchia, G.; Dal Bosco, A.; Cartoni Mancinelli, A.; Castellini, C. Performance and Egg Quality of Laying Hens Fed Flaxseed: Highlights on n-3 Fatty Acids, Cholesterol, Lignans and Isoflavones. Animal 2017, 11, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Scheideler, S.E.; Jaroni, D.; Froning, G. Strain and Age Effects on Egg Composition from Hens Fed Diets Rich in N-3 Fatty Acids. Poult. Sci. 1998, 77, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Aguillón-Páez, Y.J.; Romero, L.A.; Diaz, G.J. Effect of Full-Fat Sunflower or Flaxseed Seeds Dietary Inclusion on Performance, Egg Yolk Fatty Acid Profile and Egg Quality in Laying Hens. Anim. Nutr. 2020, 6, 179–184. [Google Scholar] [CrossRef]
- Alzueta, C.; Rodríguez, M.L.; Cutuli, M.T.; Rebolé, A.; Ortiz, L.T.; Centeno, C.; Treviño, J. Effect of Whole and Demucilaged Linseed in Broiler Chicken Diets on Digesta Viscosity, Nutrient Utilisation and Intestinal Microflora. Br. Poult. Sci. 2003, 44, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Anjum, F.M.; Nadeem, M.; Ahmad, N.; Khan, M.K.; Mushtaq, Z.; Hussain, S. Production of Bio-Omega-3 Eggs through the Supplementation of Extruded Flaxseed Meal in Hen Diet. Lipids Health Dis. 2015, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Baurhoo, B.; Mustafa, A. Effects of Feeding Extruded Flaxseed on Layer Performance, Total Tract Nutrient Digestibility, and Fatty Acid Concentrations of Egg Yolk, Plasma and Liver. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1365–1374. [Google Scholar] [CrossRef]
- Westbrook, L.A.; Cherian, G. Egg Quality, Fatty-Acid Composition and Gastrointestinal Morphology of Layer Hens Fed Whole Flaxseed with Enzyme Supplementation. Br. Poult. Sci. 2019, 60, 146–153. [Google Scholar] [CrossRef]
- Kralik, G.; Kralik, Z.; Grčević, M.; Galović, O.; Hanžek, D.; Biazik, E. Fatty Acid Profile of Eggs Produced by Laying Hens Fed Diets Containing Different Shares of Fish Oil. Poult. Sci. 2021, 100, 101379. [Google Scholar] [CrossRef]
- Beynen, A.C. Fatty Acid Composition of Eggs Produced by Hens Fed Diets Containing Groundnut, Soya Bean or Linseed. NJAS Wagening. J. Life Sci. 2004, 52, 3–10. [Google Scholar] [CrossRef]
- Jiang, Z.R.; Ahn, D.U.; Sim, J.S. Effects of Feeding Flax and Two Types of Sunflower Seeds on Fatty Acid Compositions of Yolk Lipid Classes. Poult. Sci. 1991, 70, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Fraeye, I.; Bruneel, C.; Lemahieu, C.; Buyse, J.; Muylaert, K.; Foubert, I. Dietary Enrichment of Eggs with Omega-3 Fatty Acids: A Review. Food Res. Int. 2012, 48, 961–969. [Google Scholar] [CrossRef]
- Irawan, A.; Ningsih, N.; Hafizuddin; Rusli, R.K.; Suprayogi, W.P.S.; Akhirini, N.; Hadi, R.F.; Setyono, W.; Jayanegara, A. Supplementary N-3 Fatty Acids Sources on Performance and Formation of Omega-3 in Egg of Laying Hens: A Meta-Analysis. Poult. Sci. 2022, 101, 101566. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Baurhoo, B.; Mustafa, A. Effects of Extruded Flaxseed on Layer Performance, Nutrient Retention and Yolk Fatty Acid Composition. Br. Poult. Sci. 2018, 59, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Omri, B.; Chalghoumi, R.; Izzo, L.; Ritieni, A.; Lucarini, M.; Durazzo, A.; Abdouli, H.; Santini, A. Effect of Dietary Incorporation of Linseed Alone or Together with Tomato-Red Pepper Mix on Laying Hens’ Egg Yolk Fatty Acids Profile and Health Lipid Indexes. Nutrients 2019, 11, 813. [Google Scholar] [CrossRef] [PubMed]
- Grobas, S.; Mateos, G.G.; Mendez, J. Influence of Dietary Linoleic Acid on Production and Weight of Eggs and Egg Components in Young Brown Hens. J. Appl. Poult. Res. 1999, 8, 177–184. [Google Scholar] [CrossRef]
- Dong, X.; Liu, S.; Tong, J. Comparative Effect of Dietary Soybean Oil, Fish Oil, and Coconut Oil on Performance, Egg Quality and Some Blood Parameters in Laying Hens. Poult. Sci. 2018, 97, 2460–2472. [Google Scholar] [CrossRef]
- Meluzzi, A.; Sirri, F.; Castellini, C.; Roncarati, A.; Melotti, P.; Franchini, A. Influence of genotype and feeding on chemical composition of organic chicken meat. Ital. J. Anim. Sci. 2009, 8, 766–768. [Google Scholar] [CrossRef][Green Version]
- Mugnai, C.; Sossidou, E.N.; Bosco, A.D.; Ruggeri, S.; Mattioli, S.; Castellini, C. The effects of husbandry system on the grass intake and egg nutritive characteristics of laying hens. J. Sci. Food Agric. 2014, 94, 459–467. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Fanatico, A.C.; Arsi, K.; Upadhyaya, I.; Ramos, J.M.; Donoghue, D.; Donoghue, A.M. Sustainable Fish and Invertebrate Meals for Methionine and Protein Feeds in Organic Poultry Production. J. Appl. Poult. Res. 2018, 27, 437–448. [Google Scholar] [CrossRef]
- van der Heide, M.E.; Stødkilde, L.; Nørgaard, J.V.; Studnitz, M. The Potential of Locally-Sourced European Protein Sources for Organic Monogastric Production: A Review of Forage Crop Extracts, Seaweed, Starfish, Mussel, and Insects. Sustainability 2021, 13, 2303. [Google Scholar] [CrossRef]
- Bessa, L.W.; Pieterse, E.; Marais, J.; Hoffman, L.C. Why for feed and not for human consumption? The black soldier fly larvae. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2747–2763. [Google Scholar] [CrossRef] [PubMed]
- Caligiani, A.; Marseglia, A.; Sorci, A.; Bonzanini, F.; Lolli, V.; Maistrello, L.; Sforza, S. Influence of the killing method of the black soldier fly on its lipid composition. Food Res. Int. 2019, 116, 276–282. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Wang, H.; Yang, Q.; Ur Rehman, K.; Li, W.; Cai, M.; Li, Q.; Mazza, L.; Zhang, J.; et al. Dynamic Changes of Nutrient Composition throughout the Entire Life Cycle of Black Soldier Fly. PLoS ONE 2017, 12, e0182601. [Google Scholar] [CrossRef]
- Finke, M.D.; Oonincx, D.G.A.B. Nutrient Content of Insects. In Insects as Food and Feed: From Production to Consumption; van Huis, A., Tomberlin, J.K., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2016; pp. 290–316. [Google Scholar]
- Sánchez-Muros, M.-J.; Barroso, F.; Manzano-Agugliaro, F. Insect meal as renewable source of food for animal feeding: A review. J. Clean. Prod. 2014, 65, 16–27. [Google Scholar] [CrossRef]
- Bbosa, T.; Ndagire, C.T.; Mukisa, I.M.; Fiaboe, K.K.M.; Nakimbugwe, D. Nutritional Characteristics of Selected Insects in Uganda for Use as Alternative Protein Sources in Food and Feed. J. Insect Sci. 2019, 19, 23. [Google Scholar] [CrossRef]
- Schreven, S.; Yener, S.; Van Valenberg, H.; Dicke, M.; Van Loon, J. Life on a piece of cake: Performance and fatty acid profiles of black soldier fly larvae fed oilseed by-products. J. Insects Food Feed 2021, 7, 35–49. [Google Scholar] [CrossRef]
- Hoc, B.; Genva, M.; Fauconnier, M.-L.; Lognay, G.; Francis, F.; Megido, R.C. About lipid metabolism in Hermetia illucens (L. 1758): On the origin of fatty acids in prepupae. Sci. Rep. 2020, 10, 11916. [Google Scholar] [CrossRef]
- Rossi, G.; Mattioli, S.; Rondoni Rondoni, G.; Dal Bosco, A.; Servili, M. Characterisation of Fatty Acid Profiles of Tenebrio Molitor Larvae Reared on Diets Enriched with Edible Oils. J. Insects Food Feed. 2022, 1–12. [Google Scholar] [CrossRef]
- Kierończyk, B.; Rawski, M.; Józefiak, A.; Mazurkiewicz, J.; Świątkiewicz, S.; Siwek, M.; Bednarczyk, M.; Szumacher-Strabel, M.; Cieślak, A.; Benzertiha, A.; et al. Effects of Replacing Soybean Oil with Selected Insect Fats on Broilers. Anim. Feed. Sci. Technol. 2018, 240, 170–183. [Google Scholar] [CrossRef]
- Schiavone, A.; Cullere, M.; de Marco, M.; Meneguz, M.; Biasato, I.; Bergagna, S.; Dezzutto, D.; Gai, F.; Dabbou, S.; Gasco, L.; et al. Partial or Total Replacement of Soybean Oil by Black Soldier Fly Larvae (Hermetia Illucens L.) Fat in Broiler Diets: Effect on Growth Performances, Feed-Choice, Blood Traits, Carcass Characteristics and Meat Quality. Ital. J. Anim. Sci. 2017, 16, 93–100. [Google Scholar] [CrossRef]
- Premalatha, M.; Abbasi, T.; Abbasi, T.; Abbasi, S.A. Energy-Efficient Food Production to Reduce Global Warming and Ecodegradation: The Use of Edible Insects. Renew. Sustain. Energy Rev. 2011, 15, 4357–4360. [Google Scholar] [CrossRef]
- Schiavone, A.; Dabbou, S.; de Marco, M.; Cullere, M.; Biasato, I.; Biasibetti, E.; Capucchio, M.T.; Bergagna, S.; Dezzutto, D.; Meneguz, M.; et al. Black Soldier Fly Larva Fat Inclusion in Finisher Broiler Chicken Diet as an Alternative Fat Source. Animal 2018, 12, 2032–2039. [Google Scholar] [CrossRef]
- Barroso, F.G.; Sánchez-Muros, M.J.; Segura, M.; Morote, E.; Torres, A.; Ramos, R.; Guil, J.L. Insects as Food: Enrichment of Larvae of Hermetia Illucens with Omega 3 Fatty Acids by Means of Dietary Modifications. J. Food Compos. Anal. 2017, 62, 8–13. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Laurent, S.; Veenenbos, M.E.; van Loon, J.J.A. Dietary Enrichment of Edible Insects with Omega 3 Fatty Acids. Insect Sci. 2020, 27, 500–509. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Finke, M.D. Nutritional Value of Insects and Ways to Manipulate Their Composition. J. Insects Food Feed. 2021, 7, 639–659. [Google Scholar] [CrossRef]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.J. Modulation of Nutrient Composition of Black Soldier Fly (Hermetia illucens) Larvae by Feeding Seaweed-Enriched Media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef]
- St-Hilaire, S.; Cranfill, K.; McGuire, M.A.; Mosley, E.E.; Tomberlin, J.K.; Newton, L.; Sealey, W.; Sheppard, C.; Irving, S. Fish Offal Recycling by the Black Soldier Fly Produces a Foodstuff High in Omega-3 Fatty Acids. J. World Aquac. Soc. 2007, 38, 309–313. [Google Scholar] [CrossRef]
- Ding, S.; Lin, X.; He, S. Earthworms: A source of protein. J. Food Sci. Eng. 2019, 9, 159–170. [Google Scholar]
- Sampedro, L.; Jeannotte, R.; Whalen, J.K. Trophic Transfer of Fatty Acids from Gut Microbiota to the Earthworm Lumbricus terrestris L. Soil Biol. Biochem. 2006, 38, 2188–2198. [Google Scholar] [CrossRef]
- Khan, S.; Naz, S.; Sultan, A.; Alhidary, I.; Abdelrahman, M.; Khan, R.; Khan, N.; Khan, M.; Ahmad, S. Worm meal: A potential source of alternative protein in poultry feed. World’s Poult. Sci. J. 2016, 72, 93–102. [Google Scholar] [CrossRef]
- Prayogi, H.S. The Effect of Earthworm Meal Supplementation in the Diet on Quail’s Growth Performance in Attempt to Replace the Usage of Fish Meal. Int. J. Poult. Sci. 2011, 10, 804–806. [Google Scholar] [CrossRef]
- Rezaeipour, V.; Nejad, O.A.; Miri, H.Y. Growth Performance, Blood Metabolites and Jejunum Morphology of Broiler Chickens Fed Diets Containing Earthworm (Eisenia foetida) Meal as a Source of Protein. Int. J. Adv. Biol. Biomed. Res. 2014, 2, 2483–2494. [Google Scholar]
- Son, J.H. The study on treatment of poultry waste by earthworms, and the effect of feeding earthworms meal on the performance of broilers and laying hens, and safety of meat and egg. Korean J. Org. Agric. 2009, 17, 17–63. [Google Scholar]
- Son, J.H.; Jo, I.H. Effects of Earthworm Meal Supplementation on the Performance of Broiler Chickens. Korean J. Org. Agric. 2003. [Google Scholar]
- Zang, Y.T.; Bing, S.; Zhang, Y.Z.; Sheng, X.W.; Shu, D.Q. Effects of Dietary Supplementation with Earthworm Powder on Production Performance, Blood Characteristics, and Heavy Metal Residues of Broiler Pullets. J. Appl. Poult. Res. 2018, 27, 609–615. [Google Scholar] [CrossRef]
- Isea-León, F.; Acosta-Balbás, V.; Beatriz Rial-Betancoutd, L.; Luisa Medina-Gallardo, A.; Mélécony Célestin, B. Evaluation of the Fatty Acid Composition of Earthworm Eisenia andrei Meal as an Alternative Lipid Source for Fish Feed. J. Food Nutr. Res. 2019, 7, 696–700. [Google Scholar] [CrossRef]
- EU Commission. Commission Regulation (EU) No. 68/2013 on the Catalogue of Feed Materials. Available online: https://www.ecolex.org/details/legislation/commission-regulation-eu-no-682013-on-the-catalogue-of-feed-materials-lex-faoc119700/ (accessed on 10 February 2022).
- Cartoni Mancinelli, A.; Franzoni, A.; Dal Bosco, A.; Schiavone, A.; Mannelli, F.; Marzoni, M.; Castellini, C. Distribution and Consistency of Ancona and Livorno Poultry Breed in Central Italy. Ital. J. Anim. Sci. 2020, 19, 1297–1303. [Google Scholar] [CrossRef]
- Mancinelli, A.C.; Mattioli, S.; Menchetti, L.; Bosco, A.D.; Ciarelli, C.; Amato, M.G.; Castellini, C. The Assessment of a Multifactorial Score for the Adaptability Evaluation of Six Poultry Genotypes to the Organic System. Animals 2021, 11, 2992. [Google Scholar] [CrossRef]
- Mattioli, S.; Mancinelli, A.C.; Menchetti, L.; Bosco, A.D.; Madeo, L.; Amato, M.G.; Moscati, L.; Cotozzolo, E.; Ciarelli, C.; Angelucci, E.; et al. How the kinetic behavior of organic chickens affects productive performance and blood and meat oxidative status: A study of six poultry genotypes. Poult. Sci. 2021, 100, 101297. [Google Scholar] [CrossRef]
- Van der Waaij, E.H. A Resource Allocation Model Describing Consequences of Artificial Selection under Metabolic Stress. J. Anim. Sci. 2004, 82, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Sirri, F.; Castellini, C.; Bianchi, M.; Petracci, M.; Meluzzi, A.; Franchini, A. Effect of Fast-, Medium- and Slow-Growing Strains on Meat Quality of Chickens Reared under the Organic Farming Method. Animal 2011, 5, 312–319. [Google Scholar] [CrossRef]
- Castellini, C.; Dal Bosco, A.; Mugnai, C.; Bernardini, M. Performance and Behaviour of Chickens with Different Growing Rate Reared According to the Organic System. Ital. J. Anim. Sci. 2003, 6, 561–573. [Google Scholar] [CrossRef]
- Boschetti, E.; Bordoni, A.; Meluzzi, A.; Castellini, C.; Bosco, A.D.; Sirri, F. Fatty acid composition of chicken breast meat is dependent on genotype-related variation of FADS1 and FADS2 gene expression and desaturating activity. Animal 2016, 10, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; Dal Bosco, A.; Mattioli, S.; Davidescu, M.; Corazzi, L.; MacChioni, L.; Rimoldi, S.; Terova, G. Activity, Expression, and Substrate Preference of the Δ6-Desaturase in Slow- or Fast-Growing Rabbit Genotypes. J. Agric. Food Chem. 2016, 64, 792–800. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Mugnai, C.; Ruggeri, S.; Mattioli, S.; Castellini, C. Fatty Acid Composition of Meat and Estimated Indices of Lipid Metabolism in Different Poultry Genotypes Reared under Organic System. Poult. Sci. 2012, 91, 2039–2045. [Google Scholar] [CrossRef]
- Al-Hilal, M.; AlSaleh, A.; Maniou, Z.; Lewis, F.J.; Hall, W.L.; Sanders, T.A.B.; O’Dell, S.D. Genetic Variation at the FADS1-FADS2 Gene Locus Influences Delta-5 Desaturase Activity and LC-PUFA Proportions after Fish Oil Supplement. J. Lipid Res. 2013, 54, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Poultry|European Commission. Available online: https://https://ec.europa.eu/info/food-farming-fisheries/animals-and-animal-products/animal-products_en (accessed on 15 February 2022).
- Bosco, A.D.; Mattioli, S.; Mancinelli, A.C.; Cotozzolo, E.; Castellini, C. Extensive Rearing Systems in Poultry Production: The Right Chicken for the Right Farming System. A Review of Twenty Years of Scientific Research in Perugia University, Italy. Animals 2021, 11, 1281. [Google Scholar] [CrossRef]
- Baéza, E.; Guillier, L.; Petracci, M. Review: Production factors affecting poultry carcass and meat quality attributes. Animal 2021, 16, 100331. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Mugnai, C.; Mattioli, S.; Rosati, A.; Ruggeri, S.; Ranucci, D.; Castellini, C. Transfer of bioactive compounds from pasture to meat in organic free-range chickens. Poult. Sci. 2016, 95, 2464–2471. [Google Scholar] [CrossRef]
- Mancinelli, A.C.; Matiolli, S.; Bosco, A.D.; Ciarelli, C.; Castellini, C. Grass intake and meat oxidative status of geese reared in three different agroforestry systems. Acta Fytotech. Zootech. 2020, 23, 308–315. [Google Scholar] [CrossRef]
- Bari, S.; Cohen-Barnhouse, A.M.; Campbell, D.L.M. Early rearing enrichments influenced nest use and egg quality in free-range laying hens. Animal 2020, 14, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, A.C.; Mattioli, S.; Dal Bosco, A.; Piottoli, L.; Ranucci, D.; Branciari, R.; Cotozzolo, E.; Castellini, C. Rearing Romagnola geese in vineyard: Pasture and antioxidant intake, performance, carcass and meat quality. Ital. J. Anim. Sci. 2019, 18, 372–380. [Google Scholar] [CrossRef]
- Popova, T.; Petkov, E.; Ignatova, M. Fatty acid composition of breast meat in two lines of slow-growing chickens reared conventionally or on pasture. Food Sci. Appl. Biotechnol. 2018, 1, 70–76. [Google Scholar] [CrossRef]
- Mugnai, C.; Bosco, A.D.; Castellini, C. Effect of rearing system and season on the performance and egg characteristics of Ancona laying hens. Ital. J. Anim. Sci. 2009, 8, 175–188. [Google Scholar] [CrossRef]
- Gou, Z.; Abouelezz, K.; Fan, Q.; Li, L.; Lin, X.; Wang, Y.; Cui, X.; Ye, J.; Masoud, M.; Jiang, S.; et al. Physiological effects of transport duration on stress biomarkers and meat quality of medium-growing Yellow broiler chickens. Animal 2020, 15, 100079. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, A.C.; Mugnai, C.; Castellini, C.; Mattioli, S.; Moscati, L.; Piottoli, L.; Amato, M.G.; Doretti, M.; Bosco, A.D.; Cordovani, E.; et al. Effect of transport length and genotype on tonic immobility, blood parameters and carcass contamination of free-range reared chickens. Ital. J. Anim. Sci. 2018, 17, 557–564. [Google Scholar] [CrossRef]
- Rimoldi, S.; Lasagna, E.; Sarti, F.M.; Marelli, S.P.; Cozzi, M.C.; Bernardini, G.; Terova, G. Expression profile of six stress-related genes and productive performances of fast and slow growing broiler strains reared under heat stress conditions. Meta Gene 2015, 6, 17–25. [Google Scholar] [CrossRef]
- Berri, C.; le Bihan-Duval, E.; Debut, M.; Santé-Lhoutellier, V.; Baéza, E.; Gigaud, V.; Jégo, Y.; Duclos, M.J. Consequence of Muscle Hypertrophy on Characteristics of Pectoralis Major Muscle and Breast Meat Quality of Broiler Chickens. J. Anim. Sci. 2007, 85, 2005–2011. [Google Scholar] [CrossRef]
- Castellini, C.; Mattioli, S.; Piottoli, L.; Mancinelli, A.C.; Ranucci, D.; Branciari, R.; Amato, M.G.; Dal Bosco, A. Effect of Transport Length on in Vivo Oxidative Status and Breast Meat Characteristics in Outdoor-Reared Chicken Genotypes. Ital. J. Anim. Sci. 2016, 15, 191–199. [Google Scholar] [CrossRef]
- Mancinelli, A.C.; Bosco, A.D.; Mattioli, S.; Ranucci, D.; Castellini, C. Mobile Poultry Processing Unit as a Resource for Small Poultry Farms: Planning and Economic Efficiency, Animal Welfare, Meat Quality and Sanitary Implications. Animals 2018, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Rhee, K.; Anderson, L.; Sams, A. Lipid Oxidation Potential of Beef, Chicken, and Pork. J. Food Sci. 1996, 61, 8–12. [Google Scholar] [CrossRef]
- Amaral, A.B.; da Silva, M.V.; Lannes, S.C.D.S. Lipid oxidation in meat: Mechanisms and protective factors—A review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef]
- Bassam, S.M.; Noleto-Dias, C.; Farag, M.A. Dissecting grilled red and white meat flavor: Its characteristics, production mechanisms, influencing factors and chemical hazards. Food Chem. 2022, 371, 131139. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.M.; Love, J.D.; Shorland, F.B. “Warmed-over” Flavor in Meat, Poultry, and Fish. Adv. Food Res. 1977, 23, 1–74. [Google Scholar]
- Byrne, D.V.; Bredie, W.L.P.; Mottram, D.S.; Martens, M. Sensory and Chemical Investigations on the Effect of Oven Cooking on Warmed-over Flavour Development in Chicken Meat. Meat Sci. 2002, 61, 127–139. [Google Scholar] [CrossRef]
- Mancinelli, A.C.; Silletti, E.; Mattioli, S.; Bosco, A.D.; Sebastiani, B.; Menchetti, L.; Koot, A.; van Ruth, S.; Castellini, C. Fatty acid profile, oxidative status, and content of volatile organic compounds in raw and cooked meat of different chicken strains. Poult. Sci. 2020, 100, 1273–1282. [Google Scholar] [CrossRef]
- Mattioli, S.; Dal Bosco, A.; Ruggeri, S.; Martino, M.; Moscati, L.; Pesca, C.; Castellini, C. Adaptive Response to Exercise of Fast-Growing and Slow-Growing Chicken Strains: Blood Oxidative Status and Non-Enzymatic Antioxidant Defense. Poult. Sci. 2017, 96, 4096–4102. [Google Scholar] [CrossRef]



| n-3 LC-PUFA REQUIREMENT | |||
| Population classes | n | EPA and DHA (mg/d) | EPA and DHA (t/y) |
| Total world population | 7,922,857,397 | ||
| Adult individuals | 7,903,361,753 | 250 | 356 |
| Women in pregnancy and lactation | 9747,822 | 400 | 1423 |
| Young children (<24 months) | 9,747,822 | 100 | 721,182 |
| TOTAL | ~722,960 | ||
| AVAILABILITY | |||
| Wild and farm-raised fish | 100,000,000 | ||
| 50% of fish is suitable for human consumption | 50,000,000 | ||
| 15% of the n-3 represent EPA and DHA | 375,000 | ||
| DEFICIT | ~347,956 | ||
| t/y | mg LC-PUFA/d | t LCP/y | |
|---|---|---|---|
| Poultry | 1.3 × 108 | 0.62 | 40,300 |
| Eggs | 7.7 × 107 | 0.35 | 26,845 |
| Pork | 9.4 × 107 | 0.18 | 8487 |
| Total | 75,632 |
| Tissues | ALA+EPA g/kg of Diet | Time Feeding (d) | Genotype | ALA (C18:3) | EPA (C20:5) | DHA (C22:6) | TOTAL | References |
|---|---|---|---|---|---|---|---|---|
| Breast | 15 | 44 | Ross 308 | 18.0 | 3 | 10 | 31 | Cortinas, 2004 [139] |
| Drumstick | 197 | 7 | 17 | 221 | ||||
| Breast | 25 | 21 | Ross 308 | 147 | 13.5 | 31.5 | 192.0 | Rymer, 2006 [140] |
| Drumstick | 258 | 10.8 | 17.5 | 286.3 | ||||
| Liver | 6.1 | 6 | Cobb × Ross 308 | 67.1 | 280.0 | 120.1 | 467.2 | Shin, 2012 [138] |
| Breast | 52.3 | 36.1 | 20.4 | 108.8 | ||||
| Drumstick | 104.7 | 38.8 | 15.6 | 159.1 | ||||
| Breast | 10 | 30 | Ross 308 | 185 | 56 | 86 | 327 | González-Ortiz, 2013 [137] |
| Liver | 407 | 275 | 335 | 1017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cartoni Mancinelli, A.; Mattioli, S.; Twining, C.; Dal Bosco, A.; Donoghue, A.M.; Arsi, K.; Angelucci, E.; Chiattelli, D.; Castellini, C. Poultry Meat and Eggs as an Alternative Source of n-3 Long-Chain Polyunsaturated Fatty Acids for Human Nutrition. Nutrients 2022, 14, 1969. https://doi.org/10.3390/nu14091969
Cartoni Mancinelli A, Mattioli S, Twining C, Dal Bosco A, Donoghue AM, Arsi K, Angelucci E, Chiattelli D, Castellini C. Poultry Meat and Eggs as an Alternative Source of n-3 Long-Chain Polyunsaturated Fatty Acids for Human Nutrition. Nutrients. 2022; 14(9):1969. https://doi.org/10.3390/nu14091969
Chicago/Turabian StyleCartoni Mancinelli, Alice, Simona Mattioli, Cornelia Twining, Alessandro Dal Bosco, Ann M. Donoghue, Komala Arsi, Elisa Angelucci, Diletta Chiattelli, and Cesare Castellini. 2022. "Poultry Meat and Eggs as an Alternative Source of n-3 Long-Chain Polyunsaturated Fatty Acids for Human Nutrition" Nutrients 14, no. 9: 1969. https://doi.org/10.3390/nu14091969
APA StyleCartoni Mancinelli, A., Mattioli, S., Twining, C., Dal Bosco, A., Donoghue, A. M., Arsi, K., Angelucci, E., Chiattelli, D., & Castellini, C. (2022). Poultry Meat and Eggs as an Alternative Source of n-3 Long-Chain Polyunsaturated Fatty Acids for Human Nutrition. Nutrients, 14(9), 1969. https://doi.org/10.3390/nu14091969









