Impact on Glucose Homeostasis: Is Food Biofortified with Molybdenum a Workable Solution? A Two-Arm Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Setup, Plant Materials, Nutraceutical Traits and Crop Management
2.2. Study Design
2.3. Molybdenum Determination
2.4. Calibration
2.5. Biochemical Analysis
2.6. Statistical Analyses
3. Results
3.1. Molybdenum Concentration in Leaf Tissues
3.2. Participants, Study Design and Compliance
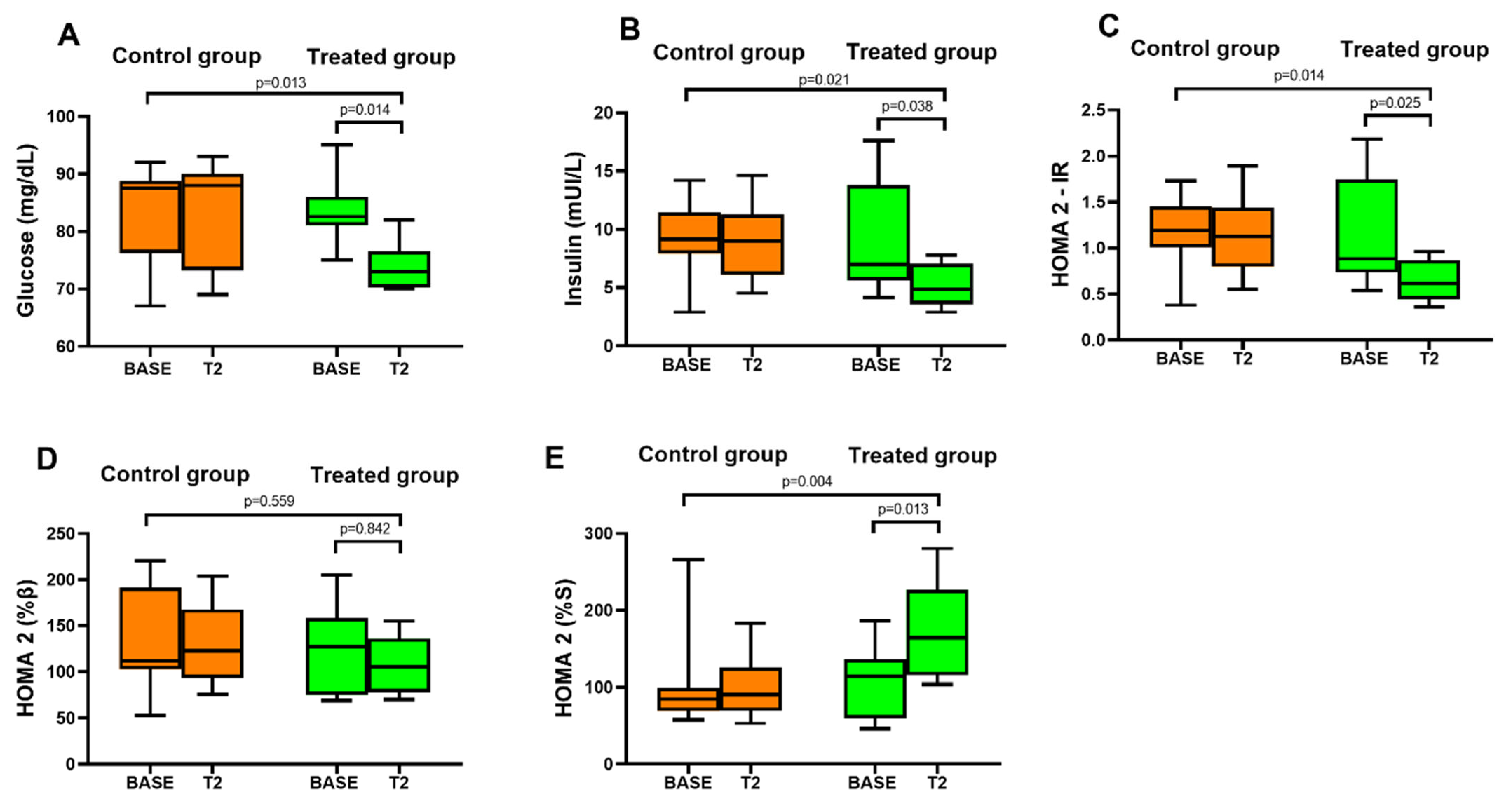
3.3. Lettuce Biofortified with Molybdenum and Glucose Metabolism
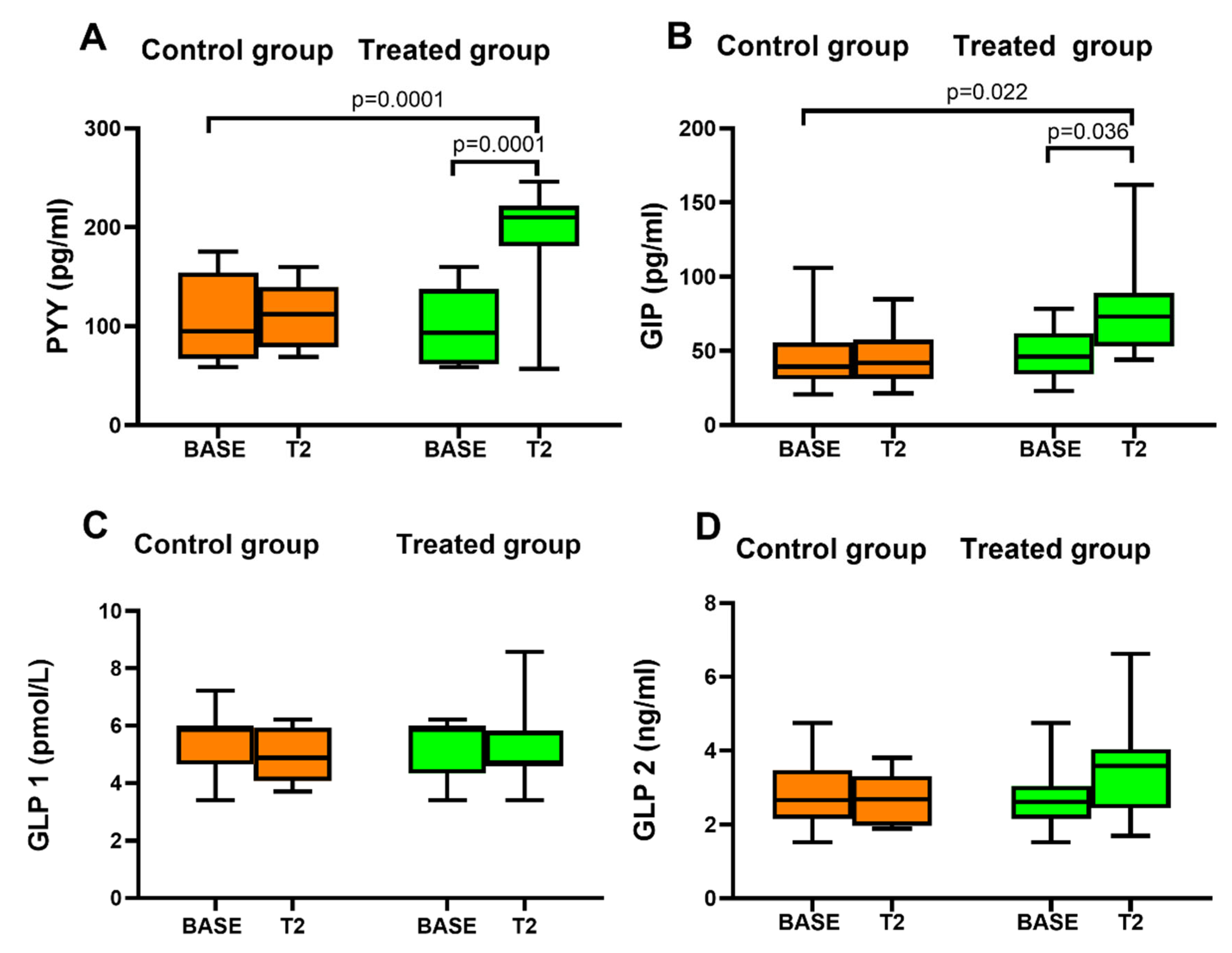
3.4. Gastrointestinal Hormones
3.5. Urinary Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dendup, T.; Feng, X.; Clingan, S.; Astell-Burt, T. Environmental Risk Factors for Developing Type 2 Diabetes Mellitus: A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, A.M.; Considine, R.V.; Mattes, R.D. Acute and second-meal effects of almond form in impaired glucose tolerant adults: A randomized crossover trial. Nutr. Metab. 2011, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Buturi, C.V.; Mauro, R.P.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Mineral Biofortification of Vegetables as a Tool to Improve Human Diet. Foods 2021, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S. Sources and Deficiency Diseases of Mineral Nutrients in Human Health and Nutrition: A Review. Pedosphere 2014, 24, 13–38. [Google Scholar] [CrossRef]
- Mitra, S.; Paul, S.; Roy, S.; Sutradhar, H.; Bin Emran, T.; Nainu, F.; Khandaker, M.U.; Almalki, M.; Wilairatana, P.; Mubarak, M.S. Exploring the Immune-Boosting Functions of Vitamins and Minerals as Nutritional Food Bioactive Compounds: A Comprehensive Review. Molecules 2022, 27, 555. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cai, Z.; Pan, Z.; Yang, Y.; Zhang, J. The effects of vitamin and mineral supplementation on women with gestational diabetes mellitus. BMC Endocr. Disord. 2021, 21, 106. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Hofmeyr, G.J.; Cormick, G.; Garcia-Casal, M.N.; Peña-Rosas, J.P.; Betrán, A.P. Current calcium fortification experiences: A review. Ann. N. Y. Acad. Sci. 2021, 1484, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Samson, K.L.I.; Fischer, J.A.J.; Roche, M.L. Iron Status, Anemia, and Iron Interventions and Their Associations with Cognitive and Academic Performance in Adolescents: A Systematic Review. Nutrients 2022, 14, 224. [Google Scholar] [CrossRef] [PubMed]
- Baldassano, S.; Di Gaudio, F.; Sabatino, L.; Caldarella, R.; De Pasquale, C.; Di Rosa, L.; Nuzzo, D.; Picone, P.; Vasto, S. Biofortification: Effect of Iodine fortified food in the healthy population, double-arm nutritional study. Front. Nutr. 2022, 871638, 1–8. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Otten, J.; Hellwig, J.; Meyers, L. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; The National Academies Press: Washington, DC, USA, 2007; Volume 85, p. 924. [Google Scholar]
- Ott, G.; Havemeyer, A.; Clement, B. The mammalian molybdenum enzymes of mARC. JBIC J. Biol. Inorg. Chem. 2014, 20, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Reiss, J.; Hahnewald, R. Molybdenum cofactor deficiency: Mutations in GPHN, MOCS1, and MOCS2. Hum. Mutat. 2010, 32, 10–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abumrad, N.N.; Schneider, A.J.; Steel, D.; Rogers, L.S. Amino acid intolerance during prolonged total parenteral nutrition reversed by molybdate therapy. Am. J. Clin. Nutr. 1981, 34, 2551–2559. [Google Scholar] [CrossRef] [PubMed]
- Bayram, E.; Topcu, Y.; Karakaya, P.; Yis, U.; Cakmakci, H.; Ichida, K.; Kurul, S.H. Molybdenum cofactor deficiency: Review of 12 cases (MoCD and review). Eur. J. Paediatr. Neurol. 2013, 17, 1–6. [Google Scholar] [CrossRef]
- Hays, S.M.; Macey, K.; Poddalgoda, D.; Lu, M.; Nong, A.; Aylward, L. Biomonitoring Equivalents for molybdenum. Regul. Toxicol. Pharmacol. 2016, 77, 223–229. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, C.; Weisskopf, M.; Williams, P.L.; Parsons, P.J.; Palmer, C.D.; Louis, G.M.B.; James-Todd, T. A Prospective Study of Early Pregnancy Essential Metal(loid)s and Glucose Levels Late in the Second Trimester. J. Clin. Endocrinol. Metab. 2019, 104, 4295–4303. [Google Scholar] [CrossRef]
- Li, B.; Huang, Y.; Luo, C.; Peng, X.; Jiao, Y.; Zhou, L.; Yin, J.; Liu, L. Inverse Association of Plasma Molybdenum with Metabolic Syndrome in a Chinese Adult Population: A Case-Control Study. Nutrients 2021, 13, 4544. [Google Scholar] [CrossRef]
- Sabatino, L.; Di Gaudio, F.; Consentino, B.; Rouphael, Y.; El-Nakhel, C.; La Bella, S.; Vasto, S.; Mauro, R.; D’Anna, F.; Iapichino, G.; et al. Iodine Biofortification Counters Micronutrient Deficiency and Improve Functional Quality of Open Field Grown Curly Endive. Horticulturae 2021, 7, 58. [Google Scholar] [CrossRef]
- Sabatino, L.; Ntatsi, G.; Iapichino, G.; D’Anna, F.; De Pasquale, C. Effect of Selenium Enrichment and Type of Application on Yield, Functional Quality and Mineral Composition of Curly Endive Grown in a Hydroponic System. Agronomy 2019, 9, 207. [Google Scholar] [CrossRef] [Green Version]
- Consentino, B.B.; Rouphael, Y.; Ntatsi, G.; De Pasquale, C.; Iapichino, G.; D’Anna, F.; La Bella, S.; Sabatino, L. Agronomic performance and fruit quality in greenhouse grown eggplant are interactively modulated by iodine dosage and grafting. Sci. Hortic. 2022, 295, 110891. [Google Scholar] [CrossRef]
- Sabatino, L.; La Bella, S.; Ntatsi, G.; Iapichino, G.; D’Anna, F.; De Pasquale, C.; Consentino, B.B.; Rouphael, Y. Selenium biofortification and grafting modulate plant performance and functional features of cherry tomato grown in a soilless system. Sci. Hortic. 2021, 285, 110095. [Google Scholar] [CrossRef]
- Sabatino, L.; Consentino, B.; Rouphael, Y.; De Pasquale, C.; Iapichino, G.; D’Anna, F.; La Bella, S. Protein Hydrolysates and Mo-Biofortification Interactively Modulate Plant Performance and Quality of ‘Canasta’ Lettuce Grown in a Protected Environment. Agronomy 2021, 11, 1023. [Google Scholar] [CrossRef]
- La Bella, S.; Consentino, B.; Rouphael, Y.; Ntatsi, G.; De Pasquale, C.; Iapichino, G.; Sabatino, L. Impact of Ecklonia maxima Seaweed Extract and Mo Foliar Treatments on Biofortification, Spinach Yield, Quality and NUE. Plants 2021, 10, 1139. [Google Scholar] [CrossRef]
- Sabatino, L.; D’Anna, F.; Iapichino, G.; Moncada, A.; D’Anna, E.; De Pasquale, C. Interactive Effects of Genotype and Molybdenum Supply on Yield and Overall Fruit Quality of Tomato. Front. Plant Sci. 2019, 9, 1922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amato, A.; Proia, P.; Caldara, G.; Alongi, A.; Ferrantelli, V.; Baldassano, S. Analysis of Body Perception, Preworkout Meal Habits and Bone Resorption in Child Gymnasts. Int. J. Environ. Res. Public Health 2021, 18, 2184. [Google Scholar] [CrossRef] [PubMed]
- Baldassano, S.; Gasbjerg, L.S.; Kizilkaya, H.S.; Rosenkilde, M.M.; Holst, J.J.; Hartmann, B. Increased Body Weight and Fat Mass After Subchronic GIP Receptor Antagonist, but Not GLP-2 Receptor Antagonist, Administration in Rats. Front. Endocrinol. 2019, 10, 492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasto, S.; Amato, A.; Proia, P.; Caldarella, R.; Cortis, C.; Baldassano, S. Dare to jump: The effect of the new high impact activity SuperJump on bone remodeling. A new tool to maintain fitness during COVID-19 home confinement. Biol. Sport 2022, 39, 1011–1019. [Google Scholar] [CrossRef]
- Baldasano, S.; Accardi, G.; Aiello, A.; Buscemi, S.; Di Miceli, G.; Galimberti, D.; Candore, G.; Ruisi, P.; Caruso, C.; Vasto, S. Fibres as functional foods and the effects on gut hormones: The example of β-glucans in a single arm pilot study. J. Funct. Foods 2018, 47, 264–269. [Google Scholar] [CrossRef]
- Vasto, S.; Amato, A.; Proia, P.; Baldassano, S. Is the Secret in the Gut? SuperJump Activity Improves Bone Remodeling and Glucose Homeostasis by GLP-1 and GIP Peptides in Eumenorrheic Women. Biology 2022, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-K.; Green, B.D.; McClenaghan, N.H.; McCluskey, J.T.; Flatt, P. Long-Term Beneficial Effects of Vanadate, Tungstate, and Molybdate on Insulin Secretion and Function of Cultured Beta Cells. Pancreas 2004, 28, 364–368. [Google Scholar] [CrossRef]
- Reimann, F.; Gribble, F. Mechanisms underlying glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 secretion. J. Diabetes Investig. 2016, 7, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Baldasano, S.; Rappa, F.; Amato, A.; Cappello, F.; Mulè, F. GLP-2 as Beneficial Factor in the Glucose Homeostasis in Mice Fed a High Fat Diet. J. Cell. Physiol. 2015, 230, 3029–3036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drucker, D.J. The biology of incretin hormones. Cell Metab. 2006, 3, 153–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koliaki, C.; Liatis, S.; Dalamaga, M.; Kokkinos, A. The Implication of Gut Hormones in the Regulation of Energy Homeostasis and Their Role in the Pathophysiology of Obesity. Curr. Obes. Rep. 2020, 9, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Boey, D.; Sainsbury, A.; Herzog, H. The role of peptide YY in regulating glucose homeostasis. Peptides 2007, 28, 390–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guida, C.; Ramracheya, R. PYY, A Therapeutic Option for Type 2 Diabetes? Clin. Med. Insights Endocrinol. Diabetes 2020, 13, 1179551419892985. [Google Scholar] [CrossRef]
- Nauck, M.A.; Bartels, E.; Orskov, C.; Ebert, R.; Creutzfeldt, W. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J. Clin. Endocrinol. Metab. 1993, 76, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J. The incretin system in healthy humans: The role of GIP and GLP-1. Metabolism 2019, 96, 46–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilsbøll, T.; Krarup, T.; Madsbad, S.; Holst, J.J. Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul. Pept. 2003, 114, 115–121. [Google Scholar] [CrossRef]
- Edwards, C.M.; Todd, J.F.; Mahmoudi, M.; Wang, Z.; Wang, R.M.; Ghatei, M.A.; Bloom, S.R. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: Studies with the antagonist exendin 9-39. Diabetes 1999, 48, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Turnlund, J.R.; Keyes, W.R.; Peiffer, G.L. Molybdenum absorption, excretion, and retention studied with stable isotopes in young men at five intakes of dietary molybdenum. Am. J. Clin. Nutr. 1995, 62, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Giussani, A.; Arogunjo, A.M.; Cantone, M.C.; Tavola, F.; Veronese, I. Rates of intestinal absorption of molybdenum in humans. Appl. Radiat. Isot. 2006, 64, 639–644. [Google Scholar] [CrossRef] [PubMed]
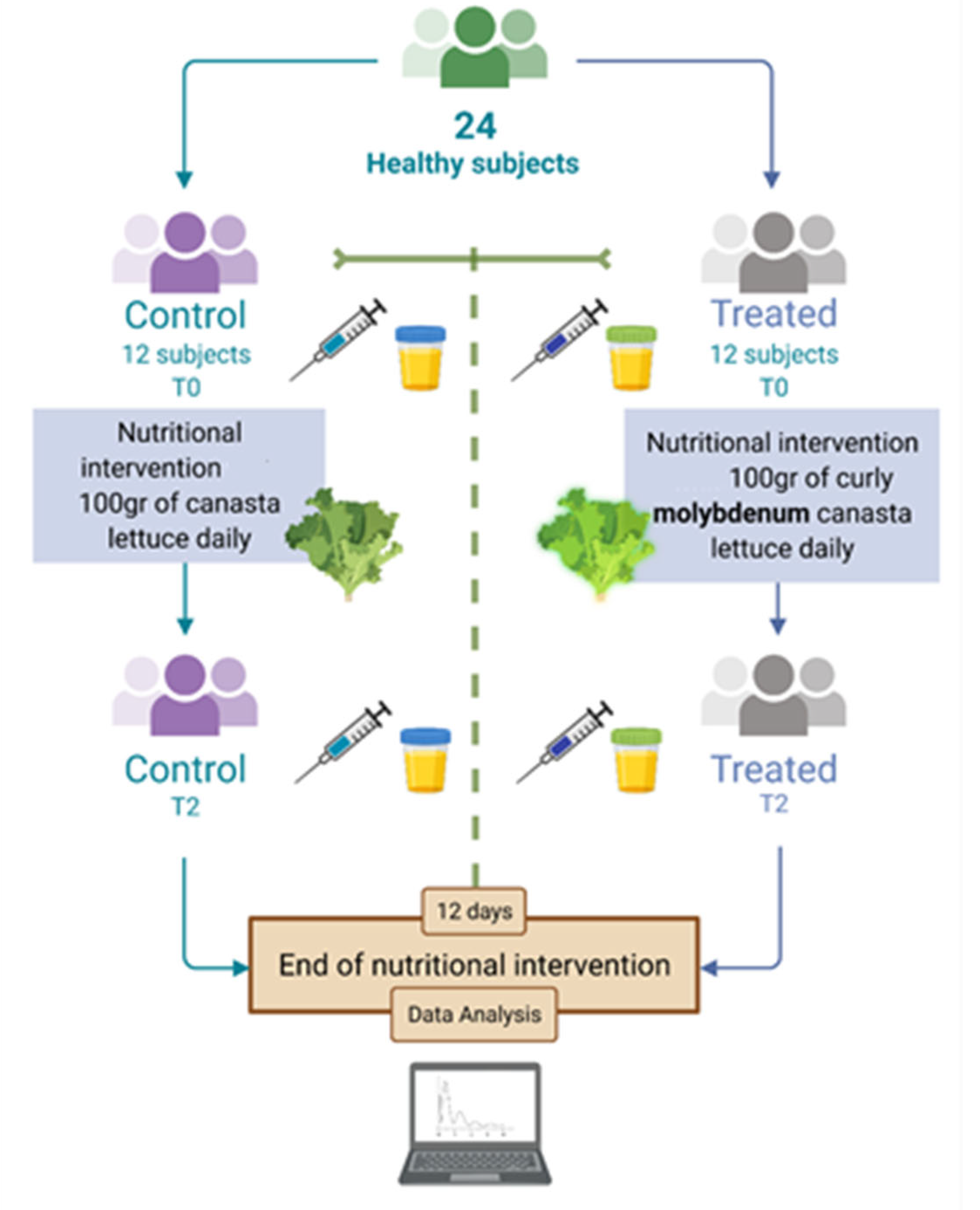
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Italian ethnicity | Chronic disease |
| Age: 18–60 years | Intake of drugs, vitamins or dietary supplements |
| Clinically healthy | Pregnancy, breastfeeding, exogenous hormones |
| Body mass index between 18.5 and 28 kg/m2 |
| Characteristics of the Subjects | Control Group | Treated Group | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | p-Value | |
| W (Kg) | 69 ± 9.8 | 72 ± 13 | ND |
| H (cm) | 168 ± 10 | 172 ± 9 | ND |
| BMI | 24.3 ± 2.5 | 24.2 ± 2.8 | ND |
| FM (%) | 28.6 ± 7.3 | 26.5 ± 7.1 | ND |
| MM (%) | 32.8 ± 5.8 | 33.2 ± 5.4 | ND |
| VF (%) | 6.5 ± 3.3 | 7.2 ± 3.2 | ND |
| Urinary Molybdenum Concentration (μg/L) | ||||||
|---|---|---|---|---|---|---|
| Control Group | ||||||
| BASE | T2 | |||||
| Mean | SD | n | Mean | SD | n | p Value |
| 37.02 | 25.62 | 12 | 45.946 | 29.443 | 12 | ND |
| Treated Group | ||||||
| BASE | T2 | |||||
| Mean | SD | n | Mean | SD | n | p Value |
| 42.253 | 28.035 | 12 | 31.831 | 21.789 | 12 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasto, S.; Di Gaudio, F.; Raso, M.; Sabatino, L.; Caldarella, R.; De Pasquale, C.; Di Rosa, L.; Baldassano, S. Impact on Glucose Homeostasis: Is Food Biofortified with Molybdenum a Workable Solution? A Two-Arm Study. Nutrients 2022, 14, 1351. https://doi.org/10.3390/nu14071351
Vasto S, Di Gaudio F, Raso M, Sabatino L, Caldarella R, De Pasquale C, Di Rosa L, Baldassano S. Impact on Glucose Homeostasis: Is Food Biofortified with Molybdenum a Workable Solution? A Two-Arm Study. Nutrients. 2022; 14(7):1351. https://doi.org/10.3390/nu14071351
Chicago/Turabian StyleVasto, Sonya, Francesca Di Gaudio, Maria Raso, Leo Sabatino, Rosalia Caldarella, Claudio De Pasquale, Luigi Di Rosa, and Sara Baldassano. 2022. "Impact on Glucose Homeostasis: Is Food Biofortified with Molybdenum a Workable Solution? A Two-Arm Study" Nutrients 14, no. 7: 1351. https://doi.org/10.3390/nu14071351
APA StyleVasto, S., Di Gaudio, F., Raso, M., Sabatino, L., Caldarella, R., De Pasquale, C., Di Rosa, L., & Baldassano, S. (2022). Impact on Glucose Homeostasis: Is Food Biofortified with Molybdenum a Workable Solution? A Two-Arm Study. Nutrients, 14(7), 1351. https://doi.org/10.3390/nu14071351









