Manganese Exposure and Metabolic Syndrome: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
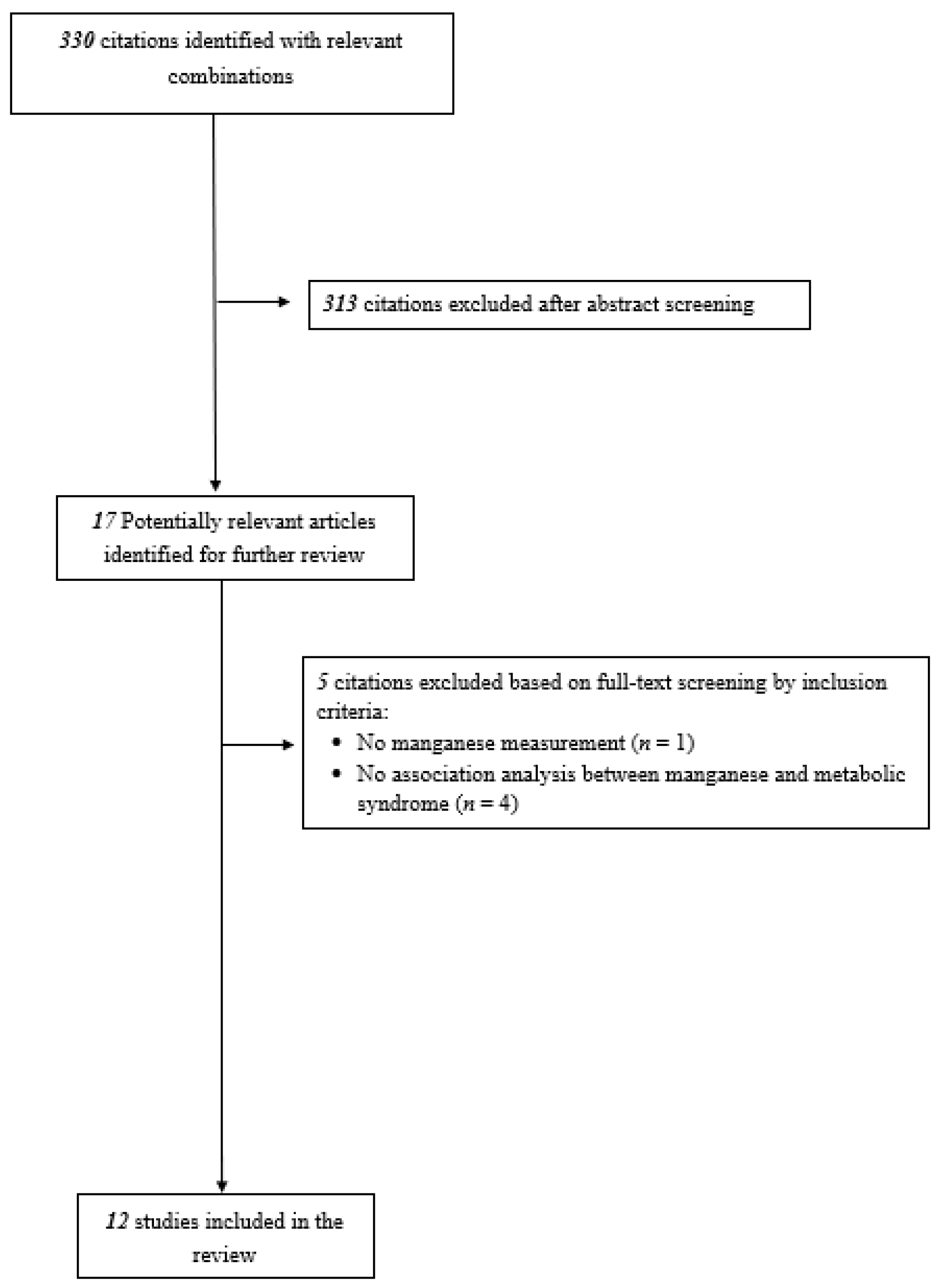
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Analysis
3. Results
3.1. Characteristics of Included Studies
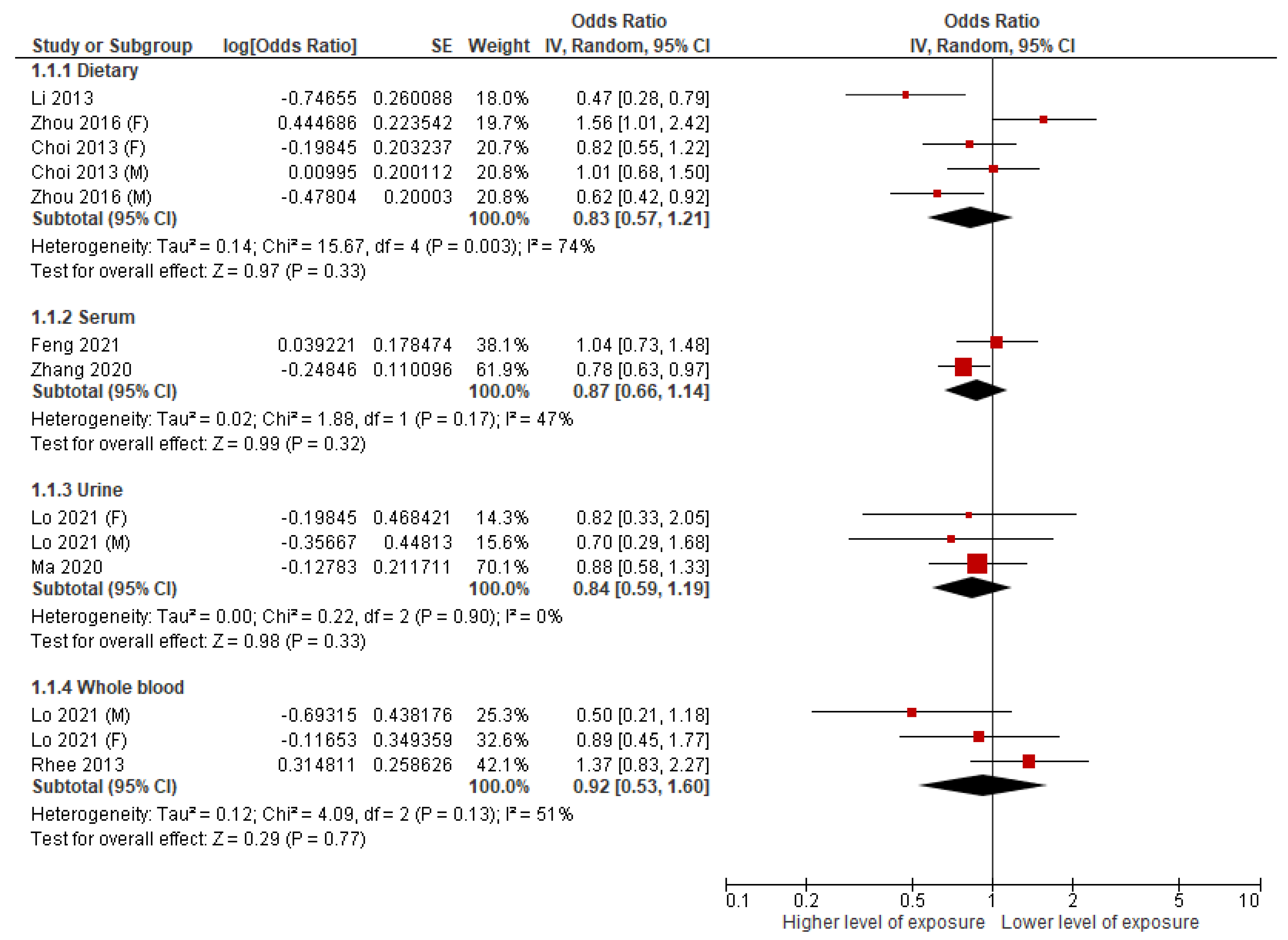
3.2. Dietary Mn and MetS
3.3. Serum Mn and MetS
3.4. Urinary Mn and MetS
3.5. Whole Blood Mn and MetS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malik, S.; Wong, N.D.; Franklin, S.S.; Kamath, T.V.; L’Italien, G.J.; Pio, J.R.; Williams, G.R. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation 2004, 110, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Osborn, M.F.; Miller, C.C.; Badr, A.; Zhang, J. Metabolic syndrome associated with ischemic stroke among the Mexican Hispanic population in the El Paso/US-Mexico border region. J. Stroke Cerebrovasc. Dis. 2014, 23, 1477–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirode, G.; Wong, R.J. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011–2016. JAMA 2020, 323, 2526–2528. [Google Scholar] [CrossRef] [PubMed]
- Pfalzer, A.C.; Bowman, A.B. Relationships Between Essential Manganese Biology and Manganese Toxicity in Neurological Disease. Curr. Environ. Health Rep. 2017, 4, 223–228. [Google Scholar] [CrossRef]
- Juttukonda, L.J.; Berends, E.T.M.; Zackular, J.P.; Moore, J.L.; Stier, M.T.; Zhang, Y.; Schmitz, J.E.; Beavers, W.N.; Wijers, C.D.; Gilston, B.A.; et al. Dietary Manganese Promotes Staphylococcal Infection of the Heart. Cell Host Microbe 2017, 22, 531–542 e538. [Google Scholar] [CrossRef] [Green Version]
- Burlet, E.; Jain, S.K. Manganese supplementation reduces high glucose-induced monocyte adhesion to endothelial cells and endothelial dysfunction in Zucker diabetic fatty rats. J. Biol. Chem. 2013, 288, 6409–6416. [Google Scholar] [CrossRef] [Green Version]
- Owumi, S.E.; Dim, U.J. Manganese suppresses oxidative stress, inflammation and caspase-3 activation in rats exposed to chlorpyrifos. Toxicol. Rep. 2019, 6, 202–209. [Google Scholar] [CrossRef]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid. Med. Cell Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef] [Green Version]
- Harischandra, D.S.; Ghaisas, S.; Zenitsky, G.; Jin, H.; Kanthasamy, A.; Anantharam, V.; Kanthasamy, A.G. Manganese-Induced Neurotoxicity: New Insights into the Triad of Protein Misfolding, Mitochondrial Impairment, and Neuroinflammation. Front. Neurosci. 2019, 13, 654. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.H.; Lo, K.; Liu, Q.; Li, J.; Lai, S.; Shadyab, A.H.; Arcan, C.; Snetselaar, L.; Liu, S. Dietary Manganese, Plasma Markers of Inflammation, and the Development of Type 2 Diabetes in Postmenopausal Women: Findings From the Women’s Health Initiative. Diabetes Care 2020, 43, 1344–1351. [Google Scholar] [CrossRef]
- Nelson, K.; Golnick, J.; Korn, T.; Angle, C. Manganese encephalopathy: Utility of early magnetic resonance imaging. Br. J. Ind. Med. 1993, 50, 510–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Sarrafzadegan, N.; Khosravi-Boroujeni, H.; Lotfizadeh, M.; Pourmogaddas, A.; Salehi-Abargouei, A. Magnesium status and the metabolic syndrome: A systematic review and meta-analysis. Nutrition 2016, 32, 409–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, B.; He, D.; Zhang, M.; Xue, J.; Zhou, D. Short sleep duration predicts risk of metabolic syndrome: A systematic review and meta-analysis. Sleep. Med. Rev. 2014, 18, 293–297. [Google Scholar] [CrossRef]
- Gami, A.S.; Witt, B.J.; Howard, D.E.; Erwin, P.J.; Gami, L.A.; Somers, V.K.; Montori, V.M. Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. J. Am. Coll. Cardiol. 2007, 49, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Fatima, Y.; Doi, S.A.; Mamun, A.A. Sleep quality and obesity in young subjects: A meta-analysis. Obes. Rev. 2016, 17, 1154–1166. [Google Scholar] [CrossRef]
- Lo, C.K.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Li, L.; Huang, L.; Zhang, H.; Mo, Z.; Yang, X. Associations Between Serum Multiple Metals Exposures and Metabolic Syndrome: A Longitudinal Cohort Study. Biol. Trace Elem. Res. 2021, 199, 2444–2455. [Google Scholar] [CrossRef]
- Bulka, C.M.; Persky, V.W.; Daviglus, M.L.; Durazo-Arvizu, R.A.; Argos, M. Multiple metal exposures and metabolic syndrome: A cross-sectional analysis of the National Health and Nutrition Examination Survey 2011–2014. Environ. Res. 2019, 168, 397–405. [Google Scholar] [CrossRef]
- Choi, M.K.; Bae, Y.J. Relationship between dietary magnesium, manganese, and copper and metabolic syndrome risk in Korean adults: The Korea National Health and Nutrition Examination Survey (2007–2008). Biol. Trace Elem. Res. 2013, 156, 56–66. [Google Scholar] [CrossRef]
- Ghaedrahmat, Z.; Cheraghian, B.; Jaafarzadeh, N.; Takdastan, A.; Shahbazian, H.B.; Ahmadi, M. Relationship between urinary heavy metals with metabolic syndrome and its components in population from Hoveyzeh cohort study: A case-control study in Iran. J. Trace Elem. Med. Biol. 2021, 66, 126757. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, H.; Wu, M.; Liu, M. Serum and dietary antioxidant status is associated with lower prevalence of the metabolic syndrome in a study in Shanghai, China. Asia Pac. J. Clin. Nutr. 2013, 22, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.; Yang, J.L.; Chen, C.L.; Liu, L.; Huang, Y.Q.; Feng, Y.Q.; Yang, A.M. Associations between blood and urinary manganese with metabolic syndrome and its components: Cross-sectional analysis of National Health and Nutrition Examination Survey 2011–2016. Sci. Total Environ. 2021, 780, 146527. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhou, Y.; Wang, D.; Guo, Y.; Wang, B.; Xu, Y.; Chen, W. Associations between essential metals exposure and metabolic syndrome (MetS): Exploring the mediating role of systemic inflammation in a general Chinese population. Environ. Int. 2020, 140, 105802. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.Y.; Hwang, Y.C.; Woo, J.T.; Sinn, D.H.; Chin, S.O.; Chon, S.; Kim, Y.S. Blood lead is significantly associated with metabolic syndrome in Korean adults: An analysis based on the Korea National Health and Nutrition Examination Survey (KNHANES), 2008. Cardiovasc. Diabetol. 2013, 12, 9. [Google Scholar] [CrossRef] [Green Version]
- Wen, W.L.; Wang, C.W.; Wu, D.W.; Chen, S.C.; Hung, C.H.; Kuo, C.H. Associations of Heavy Metals with Metabolic Syndrome and Anthropometric Indices. Nutrients 2020, 12, 2666. [Google Scholar] [CrossRef]
- Zhang, W.; Du, J.; Li, H.; Yang, Y.; Cai, C.; Gao, Q.; Xing, Y.; Shao, B.; Li, G. Multiple-element exposure and metabolic syndrome in Chinese adults: A case-control study based on the Beijing population health cohort. Environ. Int. 2020, 143, 105959. [Google Scholar] [CrossRef]
- Zhou, B.; Su, X.; Su, D.; Zeng, F.; Wang, M.H.; Huang, L.; Huang, E.; Zhu, Y.; Zhao, D.; He, D.; et al. Dietary intake of manganese and the risk of the metabolic syndrome in a Chinese population. Br. J. Nutr. 2016, 116, 853–863. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.L.; Meng, X.J.; Deng, L.G.; Liu, N. Non-linear associations between metabolic syndrome and four typical heavy metals: Data from NHANES 2011–2018. Chemosphere 2021, 291, 132953. [Google Scholar] [CrossRef]
- Liu, S.; Tinker, L.; Song, Y.; Rifai, N.; Bonds, D.E.; Cook, N.R.; Heiss, G.; Howard, B.V.; Hotamisligil, G.S.; Hu, F.B.; et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch. Intern. Med. 2007, 167, 1676–1685. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Manson, J.E.; Tinker, L.; Rifai, N.; Cook, N.R.; Hu, F.B.; Hotamisligil, G.S.; Ridker, P.M.; Rodriguez, B.L.; Margolis, K.L.; et al. Circulating levels of endothelial adhesion molecules and risk of diabetes in an ethnically diverse cohort of women. Diabetes 2007, 56, 1898–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, N.C.; Croft, K.D. Hypertension and oxidative stress. Clin. Exp. Pharmacol. Physiol. 2006, 33, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Nakamura, H.; Kwon, Y.W.; Teratani, A.; Masutani, H.; Shioji, K.; Kishimoto, C.; Ohira, A.; Horie, R.; Yodoi, J. Enhanced oxidative stress and impaired thioredoxin expression in spontaneously hypertensive rats. Antioxid. Redox Signal 2004, 6, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Greenland, S.; Longnecker, M.P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am. J. Epidemiol. 1992, 135, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Spratlen, M.J.; Grau-Perez, M.; Best, L.G.; Yracheta, J.; Lazo, M.; Vaidya, D.; Balakrishnan, P.; Gamble, M.V.; Francesconi, K.A.; Goessler, W.; et al. The Association of Arsenic Exposure and Arsenic Metabolism with the Metabolic Syndrome and Its Individual Components: Prospective Evidence from the Strong Heart Family Study. Am. J. Epidemiol. 2018, 187, 1598–1612. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.; Zong, G.; Seely, E.W.; Weisskopf, M.; James-Todd, T. Urinary cadmium concentrations and metabolic syndrome in U.S. adults: The National Health and Nutrition Examination Survey 2001–2014. Environ. Int. 2018, 121, 349–356. [Google Scholar] [CrossRef]
- Roy, C.; Tremblay, P.Y.; Ayotte, P. Is mercury exposure causing diabetes, metabolic syndrome and insulin resistance? A systematic review of the literature. Environ. Res. 2017, 156, 747–760. [Google Scholar] [CrossRef]
- Bobb, J.F.; Claus Henn, B.; Valeri, L.; Coull, B.A. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health 2018, 17, 67. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Gao, D.; Zhang, G.; Zhang, X.; Li, Q.; Gao, Q.; Chen, R.; Xu, S.; Huang, L.; Zhang, Y.; et al. Exposure to multiple metals in early pregnancy and gestational diabetes mellitus: A prospective cohort study. Environ. Int. 2020, 135, 105370. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiao, Y.; Yu, Y.; Liu, Y.; Feng, W.; Qiu, G.; Wang, H.; Liu, B.; Wang, J.; Zhou, L.; et al. Associations of multiple plasma metals with incident type 2 diabetes in Chinese adults: The Dongfeng-Tongji Cohort. Environ. Pollut. 2018, 237, 917–925. [Google Scholar] [CrossRef]
- Eshak, E.S.; Muraki, I.; Imano, H.; Yamagishi, K.; Tamakoshi, A.; Iso, H. Manganese intake from foods and beverages is associated with a reduced risk of type 2 diabetes. Maturitas 2021, 143, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Wu, X.; Han, T.; Duan, W.; Liu, L.; Qi, J.; Niu, Y.; Na, L.; Sun, C. Dietary manganese and type 2 diabetes mellitus: Two prospective cohort studies in China. Diabetologia 2018, 61, 1985–1995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Zeng, L.; Zhao, J.; Wu, Q.; Dong, Y.; Zou, F.; Gan, L.; Wei, Y.; Zhang, W. Association of magnesium intake with type 2 diabetes and total stroke: An updated systematic review and meta-analysis. BMJ Open 2020, 10, e032240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Naumova, E.N.; Bobb, J.F.; Claus Henn, B.; Singh, G.M. Joint Associations of Multiple Dietary Components With Cardiovascular Disease Risk: A Machine-Learning Approach. Am. J. Epidemiol. 2021, 190, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
| Authors, Year | Country | Study Name | Study Design | Sample Size | Mean Age | Type of Mn Exposure | Definition of MetS | % of Male | Quality Assessment Scores a |
|---|---|---|---|---|---|---|---|---|---|
| Bulka 2019 [20] | U.S. | U.S. NHANES 2011–2014 | CS | 1088 | ≥20 | Whole blood | 2009 Joint Scientific Statement of IDF, AHA/NHLBI, WFH, IASO | 52.7 | 8 |
| Choi 2013 [21] | Korea | The Korea NHANES 2007–2008 | CS | 5136 | ≥19 | Diet | NCEP ATP III | 40.6 | 8 |
| Feng 2021 [19] | China | FAMHES | CS | 1970 | 37.53 | Serum | AHA/NHLBI | 100 | 8 |
| Ghaedrahmat 2021 [22] | Iran | Hoveyzeh cohort study | Nested CC | 150 | 36–70 | Urine | AHA/NHLBI | 35.0 | 6 |
| Li 2013 [23] | China | Nil | CC | 544 | 53.7 | Diet | NCEP ATP III | 38.4 | 6 |
| Lo 2021 [24] | U.S. | U.S. NHANES 2011–2016 | CS | 3335 | ≥18 | Whole blood, Urine | NCEP ATP III | 48.1 | 8 |
| Ma 2020 [25] | China | Wuhan–Zhuhai cohort | CS | 3272 | 53.2 | Urine | NCEP ATP III | 31.5 | 8 |
| Rhee 2013 [26] | Korea | The Korea NHANES 2008 | CS | 1405 | ≥20 | Whole blood | NCEP ATP III | 49.3 | 8 |
| Wen 2020 [27] | China (Taiwan) | Nil | CS | 2444 | 55.1 | Urine | NCEP ATP III | 39.9 | 7 |
| Zhang 2020 [28] | China | Beijing Population Health Cohort study | Nested CC | 4134 | 60.0 | Serum | IDF | 49.5 | 6 |
| Zhou 2016 [29] | China | CNNHS 2010–2012 | CS | 2111 | 53.1 | Diet | AHA/NHLBI | 47.2 | 8 |
| Zhou 2021 [30] | U.S. | U.S. NHANES 2011–2018 | CS | 23,825 | ≥18 | Whole blood | IDF | 48.4 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, M.M.H.; Chan, K.Y.; Lo, K. Manganese Exposure and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 825. https://doi.org/10.3390/nu14040825
Wong MMH, Chan KY, Lo K. Manganese Exposure and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Nutrients. 2022; 14(4):825. https://doi.org/10.3390/nu14040825
Chicago/Turabian StyleWong, Martin Ming Him, Kwan Yi Chan, and Kenneth Lo. 2022. "Manganese Exposure and Metabolic Syndrome: A Systematic Review and Meta-Analysis" Nutrients 14, no. 4: 825. https://doi.org/10.3390/nu14040825
APA StyleWong, M. M. H., Chan, K. Y., & Lo, K. (2022). Manganese Exposure and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Nutrients, 14(4), 825. https://doi.org/10.3390/nu14040825






