Anti-Inflammatory and Active Biological Properties of the Plant-Derived Bioactive Compounds Luteolin and Luteolin 7-Glucoside
Abstract
1. Flavonoids in Nature and Chemical Structure
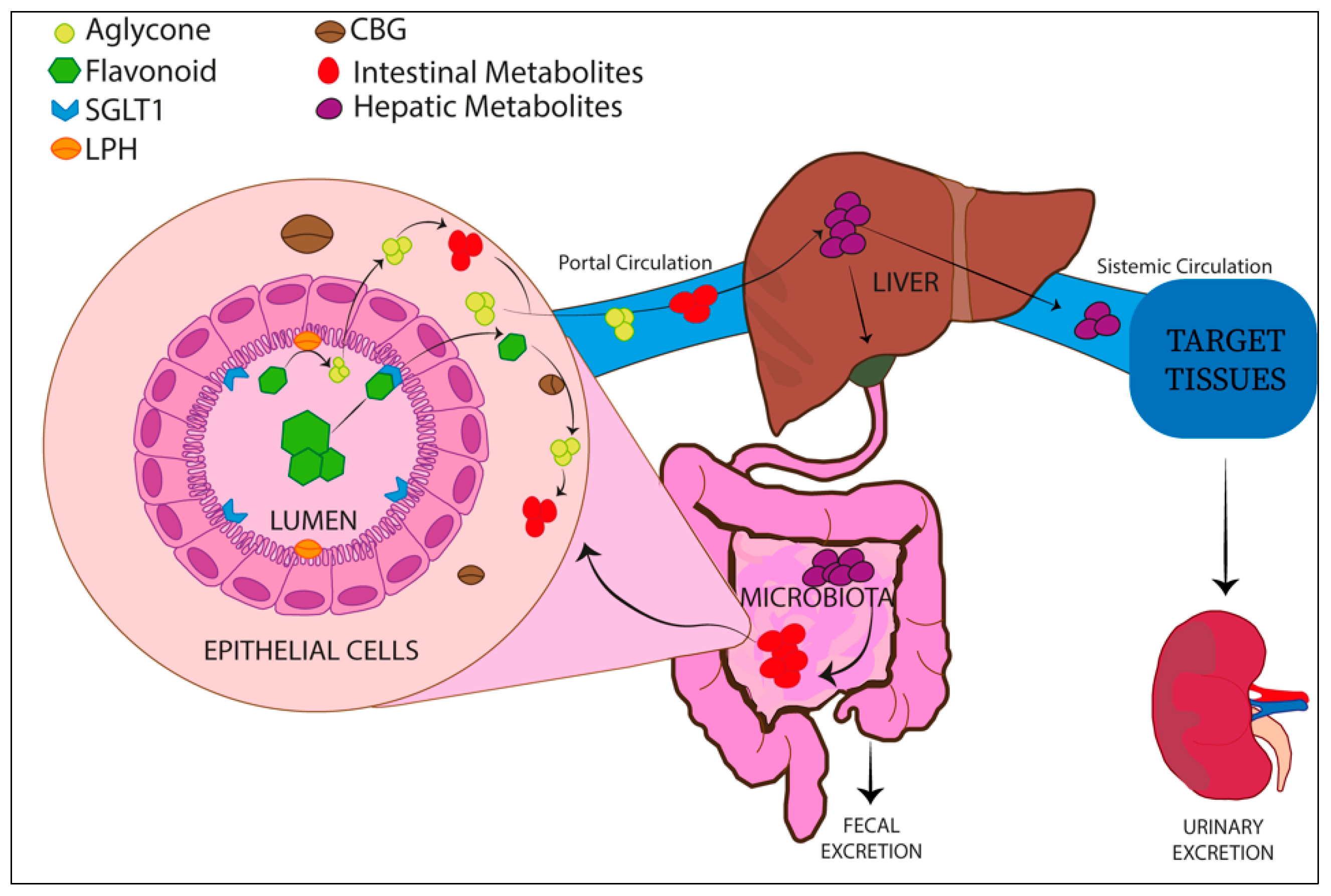
Absorption and Metabolism of Flavonoids
2. Luteolin and Its Glucoside LUT-7G
Luteolin Structure and Natural Plant Sources
3. Luteolin and LUT-7G in Inflammation
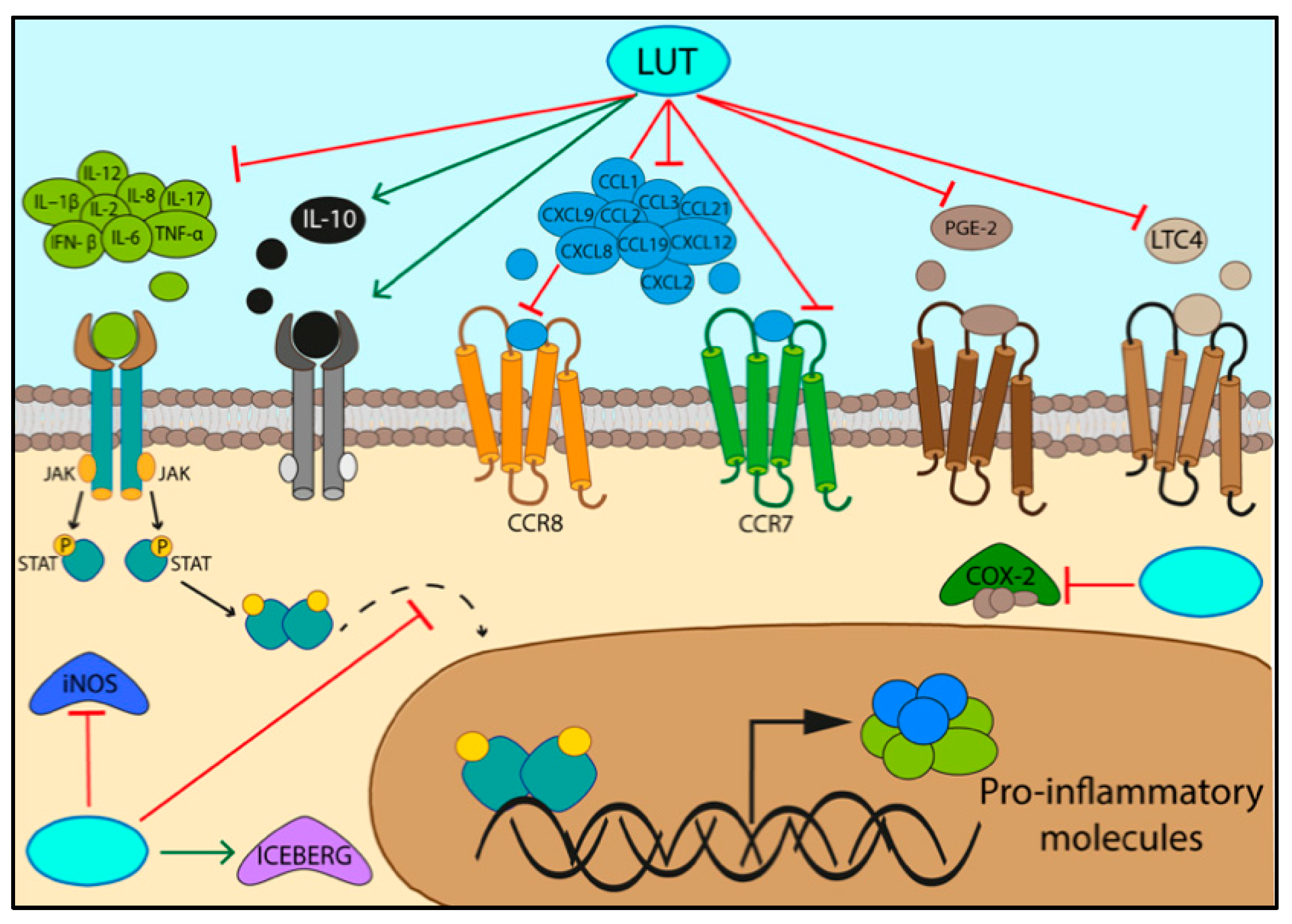
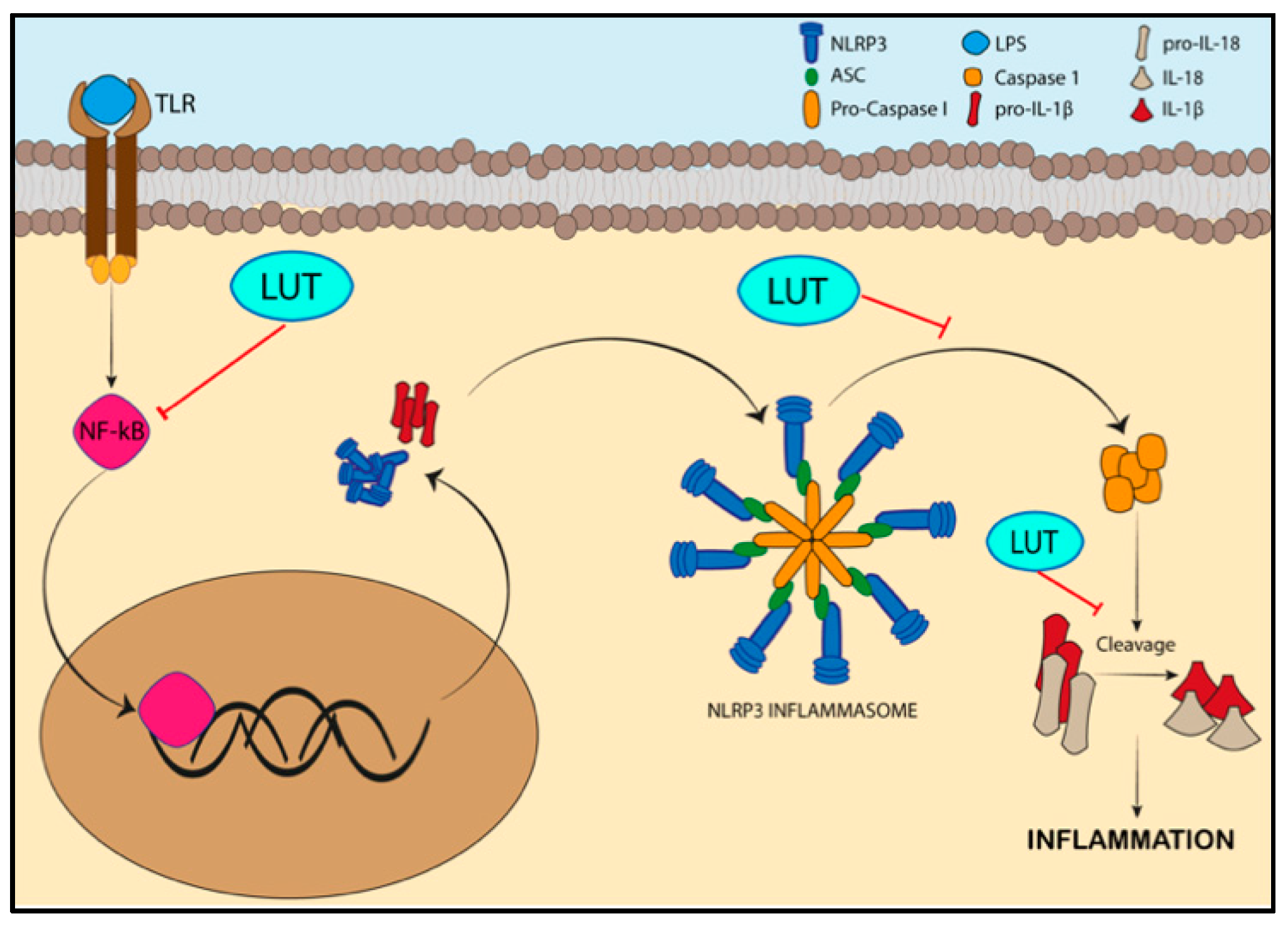
3.1. Specifc Pathways Regulated by the Activity of Luteolin/LUT-7G in Inflammation
| PRR Receptors | PAMP Ligands | DAMPs Ligands | Refs. | |
|---|---|---|---|---|
| TLRs (TLR 1-9) | Transmembrane protein in plasma membrane or in endosome | LPS (lipopolysaccharide of bacteria), proteins, nucleic acids, glycans | HSPs, S-100 proteins, histones, DNA, RNA, mtDNA, heparan sulfate, fibrinogen, LMW hyaluronan, syndecans, glypicans | [40] |
| NLRs | Cytoplasmic sensor | Viral DNA, bacterial DNA, bacterial peptidoglycan | Uric Acid, mROS, Histones, LMW hyaluronan | [40] |
| CLRs | Transmembrane protein in plasma membrane | Glycans of bacteria, glycans of fungi | F-actin, SAP130 | [40] |
| RLRs | Cytoplasmic sensor | Viral RNA | RNA | [40] |
| CDSs | Cytosolic DNA sensor | Bacterial and viral DNA | DNA | [40,46] |
| FPRs | Mitochondrial formyl peptide sensor | Pathogen peptides | Formyl peptide | [40,47] |
3.2. Activation of Inflammatory Pathways
3.3. Metabolism and Energy Production
3.4. Lipid Pathways Involved in Inflammation
3.5. Glucose Homeostasis
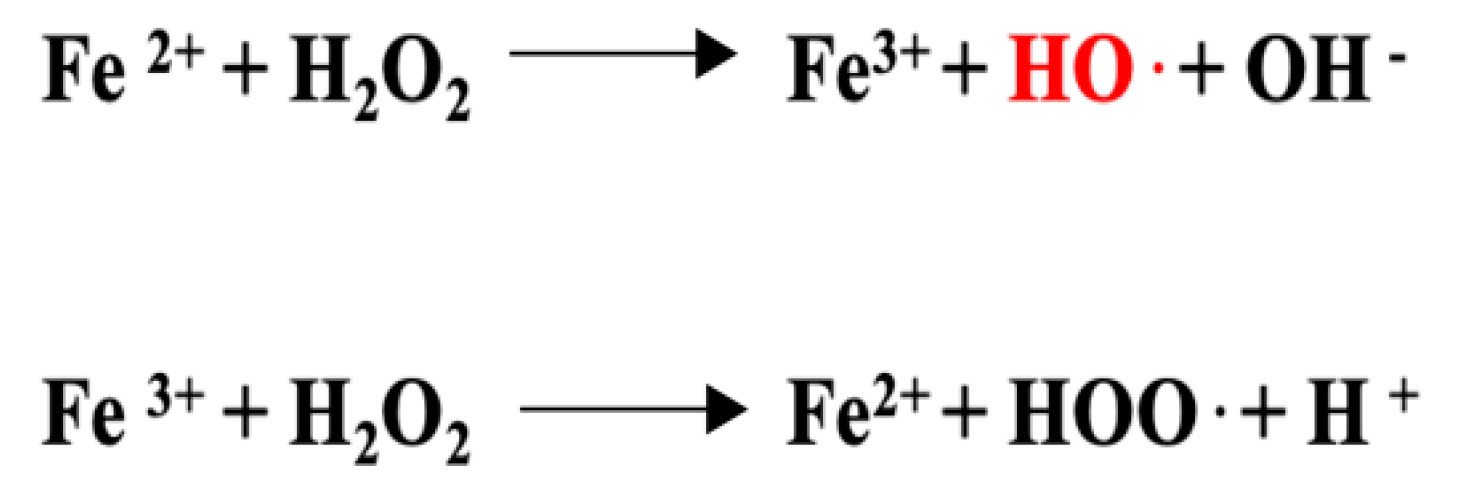
3.6. Anti-Inflammatory Properties Connected to the Anti-Oxidant Activity of Luteolin
4. Anti-Aging Properties of Luteolin
5. Anticancer Activity of Luteolin/LUT-7G
- The JAK/STAT signaling pathway plays opposite roles in the carcinogenesis process depending on the ligand/receptor and member of the STAT (STAT1, STAT2, STAT3, STAT4) family involved. In the IFN alpha/beta (ligand)-IFNRA1-2 (receptor) activation pathway, luteolin is able to maintain the phosphorylated status of STAT1 by inhibiting the SHP-2 dephosphorylase. In this context, activated STAT1 signaling arrests the cancer growth. In contrast, luteolin can reduce the phosphorylated levels of STAT3, leading to tumor suppression in breast cancer since STAT3 is a transcription factor for S100 calcium-binding protein A7 (S100A7) required in the metastasis formation [57,92].
- Tumor cells frequently report a dysregulation of wnt/β-catenin and Notch signaling, leading to EMT transition and metastasis. Luteolin is able to modulate both these pathways by downregulating β-catenin and Notch-1 [92].
6. The Role of Luteolin on Vascular Function
| Molecular Mechanisms | Treatment | Cell Lines/Animal Model/Humans | Refs. |
|---|---|---|---|
| Antihypertensive effects by inhibition of RAAS | LUT | SHR mice | [95] |
| Anti-inflammatory effects by inhibition of JAK/STAT3 pathway | LUT-7G | Human endothelial cells (HUVEC | [9] |
| Endothelium-independent vasorelaxation | LUT | Pregnant rats (uterine arteries) | [98] |
| Endothelium-dependent vasorelaxation | LUT | Rat aortic rings | [99] |
| Inhibition the ROS and TNF-α action and improvement of vasodilation | LUT | HFD mice | [100] |
| Improvement of flow-mediated dilation of the brachial artery | Altilix | Humans | [101] |

7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wolfle, U. Luteolin as a modulator of skin aging and inflammation. Biofactors 2021, 47, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, F.; Magkos, F.; Fava, F.; Milani, G.P.; Agostoni, C.; Astrup, A.; Saguy, I.S. A Multidisciplinary Perspective of Ultra-Processed Foods and Associated Food Processing Technologies: A View of the Sustainable Road Ahead. Nutrients 2021, 13, 3948. [Google Scholar] [CrossRef] [PubMed]
- Della Guardia, L.; Roggi, C.; Cena, H. Diet-induced acidosis and alkali supplementation. Int. J. Food Sci. Nutr. 2016, 67, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Manuelli, M.; Della Guardia, L.; Cena, H. Enriching Diet with n-3 PUFAs to Help Prevent Cardiovascular Diseases in Healthy Adults: Results from Clinical Trials. Int. J. Mol. Sci. 2017, 18, 1552. [Google Scholar] [CrossRef]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Fruit Polyphenols: A Review of Anti-inflammatory Effects in Humans. Crit. Rev. Food Sci. Nutr. 2016, 56, 419–444. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Kozlowska, A.; Szostak-Wegierek, D. Flavonoids--food sources and health benefits. Rocz. Panstw. Zakl. Hig. 2014, 65, 79–85. [Google Scholar]
- De Stefano, A.; Caporali, S.; Di Daniele, N.; Rovella, V.; Cardillo, C.; Schinzari, F.; Minieri, M.; Pieri, M.; Candi, E.; Bernardini, S.; et al. Anti-Inflammatory and Proliferative Properties of Luteolin-7-O-Glucoside. Int. J. Mol. Sci. 2021, 22, 1321. [Google Scholar] [CrossRef]
- Douglas, C.C.; Johnson, S.A.; Arjmandi, B.H. Soy and its isoflavones: The truth behind the science in breast cancer. Anticancer Agents Med. Chem. 2013, 13, 1178–1187. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Chahar, M.K.; Sharma, N.; Dobhal, M.P.; Joshi, Y.C. Flavonoids: A versatile source of anticancer drugs. Pharmacogn. Rev. 2011, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Leo, C.H.; Woodman, O.L. Flavonols in the Prevention of Diabetes-induced Vascular Dysfunction. J. Cardiovasc. Pharmacol. 2015, 65, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Oyagbemi, A.A.; Omobowale, T.O.; Ola-Davies, O.E.; Asenuga, E.R.; Ajibade, T.O.; Adejumobi, O.A.; Arojojoye, O.A.; Afolabi, J.M.; Ogunpolu, B.S.; Falayi, O.O.; et al. Quercetin attenuates hypertension induced by sodium fluoride via reduction in oxidative stress and modulation of HSP 70/ERK/PPARgamma signaling pathways. Biofactors 2018, 44, 465–479. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hernandez Bautista, R.J.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxid. Med. Cell. Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef]
- Krizova, L.; Dadakova, K.; Kasparovska, J.; Kasparovsky, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Andersen, O.; Markham, R. Flavonoids, Chemistry, Biochemistry and Applications; CRC Press: Boca Raton, FL, USA, 2005; Volume 1, p. 1256. [Google Scholar]
- Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Hyun, Y.J.; Park, J.E.; Shilnikova, K.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Jeong, Y.J.; et al. Luteolin induces apoptotic cell death via antioxidant activity in human colon cancer cells. Int. J. Oncol. 2017, 51, 1169–1178. [Google Scholar] [CrossRef]
- Mu, J.; Ma, H.; Chen, H.; Zhang, X.; Ye, M. Luteolin Prevents UVB-Induced Skin Photoaging Damage by Modulating SIRT3/ROS/MAPK Signaling: An in vitro and in vivo Studies. Front. Pharmacol. 2021, 12, 728261. [Google Scholar] [CrossRef]
- Kou, J.J.; Shi, J.Z.; He, Y.Y.; Hao, J.J.; Zhang, H.Y.; Luo, D.M.; Song, J.K.; Yan, Y.; Xie, X.M.; Du, G.H.; et al. Luteolin alleviates cognitive impairment in Alzheimer’s disease mouse model via inhibiting endoplasmic reticulum stress-dependent neuroinflammation. Acta Pharmacol. Sin. 2021. [Google Scholar] [CrossRef]
- Masraksa, W.; Tanasawet, S.; Hutamekalin, P.; Wongtawatchai, T.; Sukketsiri, W. Luteolin attenuates migration and invasion of lung cancer cells via suppressing focal adhesion kinase and non-receptor tyrosine kinase signaling pathway. Nutr. Res. Pract. 2020, 14, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, Y.; Zhang, Z.; Chen, M.; Zhang, D.; Tian, C.; Liu, M.; Jiang, G. The Antibacterial Activity and Mechanism of Action of Luteolin Against Trueperella pyogenes. Infect. Drug Resist. 2020, 13, 1697–1711. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Liu, M.; Fu, Y.; Zhang, J.; Liu, W.; Li, J.; Li, X.; Li, Y.; Wang, T. Antimicrobial mechanism of luteolin against Staphylococcus aureus and Listeria monocytogenes and its antibiofilm properties. Microb. Pathog. 2020, 142, 104056. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Venegas, G.; Gomez-Mora, J.A.; Meraz-Rodriguez, M.A.; Flores-Sanchez, M.A.; Ortiz-Miranda, L.F. Effect of flavonoids on antimicrobial activity of microorganisms present in dental plaque. Heliyon 2019, 5, e03013. [Google Scholar] [CrossRef]
- Shawan, M.; Halder, S.K.; Hasan, M.A. Luteolin and abyssinone II as potential inhibitors of SARS-CoV-2: An in silico molecular modeling approach in battling the COVID-19 outbreak. Bull. Natl. Res. Cent. 2021, 45, 27. [Google Scholar] [CrossRef]
- Wu, S.; Wang, H.Q.; Guo, T.T.; Li, Y.H. Luteolin inhibits CVB3 replication through inhibiting inflammation. J. Asian Nat. Prod. Res. 2020, 22, 762–773. [Google Scholar] [CrossRef]
- Nainu, F.; Masyita, A.; Bahar, M.A.; Raihan, M.; Prova, S.R.; Mitra, S.; Emran, T.B.; Simal-Gandara, J. Pharmaceutical Prospects of Bee Products: Special Focus on Anticancer, Antibacterial, Antiviral, and Antiparasitic Properties. Antibiotics 2021, 10, 822. [Google Scholar] [CrossRef]
- Mittra, B.; Saha, A.; Roy Chowdhury, A.; Pal, C.; Mandal, S.; Mukhopadhyay, S.; Bandyopadhyay, S.; Majumder, H.K. Luteolin, an Abundant Dietary Component is a Potent Anti-leishmanial Agent that Acts by Inducing Topoisomerase II-mediated Kinetoplast DNA Cleavage Leading to Apoptosis. Mol. Med. 2000, 6, 527–541. [Google Scholar] [CrossRef]
- Luo, Y.; Shang, P.; Li, D. Luteolin: A Flavonoid that Has Multiple Cardio-Protective Effects and Its Molecular Mechanisms. Front. Pharmacol. 2017, 8, 692. [Google Scholar] [CrossRef]
- Sangeetha, S. Luteolin in the Management of Type 2 Diabetes Mellitus. Curr. Res. Nutr. Food Sci. 2019, 7, 393–398. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Al-Ishaq, R.K.; Liskova, A.; Kubatka, P.; Busselberg, D. Enzymatic Metabolism of Flavonoids by Gut Microbiota and Its Impact on Gastrointestinal Cancer. Cancers 2021, 13, 3934. [Google Scholar] [CrossRef] [PubMed]
- Baroni, L.; Sarni, A.R.; Zuliani, C. Plant Foods Rich in Antioxidants and Human Cognition: A Systematic Review. Antioxidants 2021, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Igarashi, K.; Li, Y. Anti-diabetic effects of luteolin and luteolin-7-O-glucoside on KK-A(y) mice. Biosci. Biotechnol. Biochem. 2016, 80, 1580–1586. [Google Scholar] [CrossRef]
- Park, C.M.; Song, Y.S. Luteolin and luteolin-7-O-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of NF-kappaB/AP-1/PI3K-Akt signaling cascades in RAW 264.7 cells. Nutr. Res. Pract. 2013, 7, 423–429. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Wang, S.; Cao, M.; Xu, S.; Shi, J.; Mao, X.; Yao, X.; Liu, C. Luteolin Alters Macrophage Polarization to Inhibit Inflammation. Inflammation 2020, 43, 95–108. [Google Scholar] [CrossRef]
- Xagorari, A.; Roussos, C.; Papapetropoulos, A. Inhibition of LPS-stimulated pathways in macrophages by the flavonoid luteolin. Br. J. Pharmacol. 2002, 136, 1058–1064. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, X.; Sun, Z.; Ma, Y. Astilbin Inhibits High Glucose-Induced Inflammation and Extracellular Matrix Accumulation by Suppressing the TLR4/MyD88/NF-kappaB Pathway in Rat Glomerular Mesangial Cells. Front. Pharmacol. 2018, 9, 1187. [Google Scholar] [CrossRef]
- Dasu, M.R.; Jialal, I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E145–E154. [Google Scholar] [CrossRef] [PubMed]
- Rosa, D.F.; Sarandy, M.M.; Novaes, R.D.; Freitas, M.B.; do Carmo Gouveia Peluzio, M.; Goncalves, R.V. High-Fat Diet and Alcohol Intake Promotes Inflammation and Impairs Skin Wound Healing in Wistar Rats. Mediat. Inflamm. 2018, 2018, 4658583. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Sun, Z.; Lv, X.; Pei, X.; Manthari, R.K.; Wang, J. Fluoride Induces Autoimmune Orchitis Involved with Enhanced IL-17A Secretion in Mice Testis. J. Agric. Food Chem. 2019, 67, 13333–13343. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.C.; Xu, Z.R.; Xu, C.L.; He, Q.K.; Yang, G.M.; Li, Y.P.; Luo, Y.S.; Wang, H.L.; Qi, Z.Q.; Liu, Y. Nickel sulfate exposure induces ovarian inflammation and fibrosis and decreases oocyte quality in mice. Ecotoxicol. Environ. Saf. 2021, 224, 112634. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Morimoto, N.; Kono, T.; Sakai, M.; Hikima, J.I. Molecular characterization and expression of the teleost cytosolic DNA sensor genes cGAS, LSm14A, DHX9, and DHX36 in Japanese medaka, Oryzias latipes. Dev. Comp. Immunol. 2019, 99, 103402. [Google Scholar] [CrossRef]
- Bufe, B.; Schumann, T.; Kappl, R.; Bogeski, I.; Kummerow, C.; Podgorska, M.; Smola, S.; Hoth, M.; Zufall, F. Recognition of bacterial signal peptides by mammalian formyl peptide receptors: A new mechanism for sensing pathogens. J. Biol. Chem. 2015, 290, 7369–7387. [Google Scholar] [CrossRef]
- Shen, R.; Ma, L.; Zheng, Y. Anti-inflammatory effects of luteolin on acute gouty arthritis rats via TLR/MyD88/NF-kappaB pathway. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2020, 45, 115–122. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, S.Y.; Kim, Y.S.; Lee, W.H.; Hwang, D.H.; Lee, J.Y. Suppression of the TRIF-dependent signaling pathway of Toll-like receptors by luteolin. Biochem. Pharmacol. 2009, 77, 1391–1400. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Aziz, N.; Kim, M.Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, Z.; Koh, Y.-S. Mitogen-activated Protein Kinases in Inflammation. J. Bacteriol. Virol. 2012, 42, 189–195. [Google Scholar] [CrossRef]
- Banerjee, S.; Biehl, A.; Gadina, M.; Hasni, S.; Schwartz, D.M. JAK-STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects. Drugs 2017, 77, 521–546. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Liou, C.J.; Shen, S.C.; Hu, S.; Hsiao, C.Y.; Wu, S.J. Luteolin Attenuates IL-1beta-Induced THP-1 Adhesion to ARPE-19 Cells via Suppression of NF-kappaB and MAPK Pathways. Mediat. Inflamm. 2020, 2020, 9421340. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Almeida, L.; Barbosa, R.M.; Laranjinha, J. Luteolin suppresses the JAK/STAT pathway in a cellular model of intestinal inflammation. Food Funct. 2017, 8, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Palombo, R.; Savini, I.; Avigliano, L.; Madonna, S.; Cavani, A.; Albanesi, C.; Mauriello, A.; Melino, G.; Terrinoni, A. Luteolin-7-glucoside inhibits IL-22/STAT3 pathway, reducing proliferation, acanthosis, and inflammation in keratinocytes and in mouse psoriatic model. Cell Death Dis. 2016, 7, e2344. [Google Scholar] [CrossRef]
- Fei, J.; Liang, B.; Jiang, C.; Ni, H.; Wang, L. Luteolin inhibits IL-1beta-induced in fl ammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed. Pharmacother. 2019, 109, 1586–1592. [Google Scholar] [CrossRef]
- Xia, N.; Chen, G.; Liu, M.; Ye, X.; Pan, Y.; Ge, J.; Mao, Y.; Wang, H.; Wang, J.; Xie, S. Anti-inflammatory effects of luteolin on experimental autoimmune thyroiditis in mice. Exp. Ther. Med. 2016, 12, 4049–4054. [Google Scholar] [CrossRef]
- Jang, S.; Kelley, K.W.; Johnson, R.W. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc. Natl. Acad. Sci. USA 2008, 105, 7534–7539. [Google Scholar] [CrossRef]
- Hytti, M.; Szabo, D.; Piippo, N.; Korhonen, E.; Honkakoski, P.; Kaarniranta, K.; Petrovski, G.; Kauppinen, A. Two dietary polyphenols, fisetin and luteolin, reduce inflammation but augment DNA damage-induced toxicity in human RPE cells. J. Nutr. Biochem. 2017, 42, 37–42. [Google Scholar] [CrossRef]
- Jin, M.; Son, K.H.; Chang, H.W. Luteolin-7-O-glucoside suppresses leukotriene C(4) production and degranulation by inhibiting the phosphorylation of mitogen activated protein kinases and phospholipase Cgamma1 in activated mouse bone marrow-derived mast cells. Biol. Pharm. Bull. 2011, 34, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Chai, Y.S.; Lin, S.H.; Xu, F.; Wang, C.J. Luteolin Regulates the Differentiation of Regulatory T Cells and Activates IL-10-Dependent Macrophage Polarization against Acute Lung Injury. J. Immunol. Res. 2021, 2021, 8883962. [Google Scholar] [CrossRef] [PubMed]
- Druilhe, A.; Srinivasula, S.M.; Razmara, M.; Ahmad, M.; Alnemri, E.S. Regulation of IL-1beta generation by Pseudo-ICE and ICEBERG, two dominant negative caspase recruitment domain proteins. Cell Death Differ. 2001, 8, 649–657. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, M.N.; Lee, Y.; Wu, D.; Pae, M. Luteolin inhibits NLRP3 inflammasome activation via blocking ASC oligomerization. J. Nutr. Biochem. 2021, 92, 108614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.C.; Li, Z.; Xu, W.; Xiang, C.H.; Ma, Y.F. Luteolin alleviates NLRP3 inflammasome activation and directs macrophage polarization in lipopolysaccharide-stimulated RAW264.7 cells. Am. J. Transl. Res. 2018, 10, 265–273. [Google Scholar]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Kwon, Y. Luteolin as a potential preventive and therapeutic candidate for Alzheimer’s disease. Exp. Gerontol. 2017, 95, 39–43. [Google Scholar] [CrossRef]
- Yan, Y.; Jun, C.; Lu, Y.; Jiangmei, S. Combination of metformin and luteolin synergistically protects carbon tetrachloride-induced hepatotoxicity: Mechanism involves antioxidant, anti-inflammatory, antiapoptotic, and Nrf2/HO-1 signaling pathway. Biofactors 2019, 45, 598–606. [Google Scholar] [CrossRef]
- Al-Megrin, W.A.; Alkhuriji, A.F.; Yousef, A.O.S.; Metwally, D.M.; Habotta, O.A.; Kassab, R.B.; Abdel Moneim, A.E.; El-Khadragy, M.F. Antagonistic Efficacy of Luteolin against Lead Acetate Exposure-Associated with Hepatotoxicity is Mediated via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Activities. Antioxidants 2019, 9, 10. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, X.; Yang, D.; Lu, J.; Liu, B.; Baiyun, R.; Zhang, Z. Dietary luteolin attenuates chronic liver injury induced by mercuric chloride via the Nrf2/NF-kappaB/P53 signaling pathway in rats. Oncotarget 2017, 8, 40982–40993. [Google Scholar] [CrossRef]
- Li, B.; Du, P.; Du, Y.; Zhao, D.; Cai, Y.; Yang, Q.; Guo, Z. Luteolin alleviates inflammation and modulates gut microbiota in ulcerative colitis rats. Life Sci. 2021, 269, 119008. [Google Scholar] [CrossRef] [PubMed]
- Palombo, R.; Caporali, S.; Falconi, M.; Iacovelli, F.; Morozzo Della Rocca, B.; Lo Surdo, A.; Campione, E.; Candi, E.; Melino, G.; Bernardini, S.; et al. Luteolin-7-O-beta-d-Glucoside Inhibits Cellular Energy Production Interacting with HEK2 in Keratinocytes. Int. J. Mol. Sci. 2019, 20, 2689. [Google Scholar] [CrossRef] [PubMed]
- Vejux, A.; Malvitte, L.; Lizard, G. Side effects of oxysterols: Cytotoxicity, oxidation, inflammation, and phospholipidosis. Braz. J. Med. Biol. Res. 2008, 41, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Vejux, A.; Abed-Vieillard, D.; Hajji, K.; Zarrouk, A.; Mackrill, J.J.; Ghosh, S.; Nury, T.; Yammine, A.; Zaibi, M.; Mihoubi, W.; et al. 7-Ketocholesterol and 7beta-hydroxycholesterol: In vitro and animal models used to characterize their activities and to identify molecules preventing their toxicity. Biochem. Pharmacol. 2020, 173, 113648. [Google Scholar] [CrossRef] [PubMed]
- Erridge, C.; Webb, D.J.; Spickett, C.M. 25-Hydroxycholesterol, 7beta-hydroxycholesterol and 7-ketocholesterol upregulate interleukin-8 expression independently of Toll-like receptor 1, 2, 4 or 6 signalling in human macrophages. Free Radic. Res. 2007, 41, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.F.; Cazarolli, L.H.; Lavado, C.; Mengatto, V.; Figueiredo, M.S.; Guedes, A.; Pizzolatti, M.G.; Silva, F.R. Effects of flavonoids on alpha-glucosidase activity: Potential targets for glucose homeostasis. Nutrition 2011, 27, 1161–1167. [Google Scholar] [CrossRef]
- Kim, A.; Lee, W.; Yun, J.M. Luteolin and fisetin suppress oxidative stress by modulating sirtuins and forkhead box O3a expression under in vitro diabetic conditions. Nutr. Res. Pract. 2017, 11, 430–434. [Google Scholar] [CrossRef]
- Cho, H.; Kim, K.; Jang, D.J.; Kim, H.Y. Effect of six Korean plants on glucagon like peptide-1 release. Food Sci. Biotechnol. 2019, 28, 1571–1576. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012, 2012, 137289. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gerardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J. Food Process. Pres. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Özgen, U.; Mavi, A.; Terzi, Z.; Kazaz, C.; Asci, A.; Kaya, Y.; Seçen, H. Relationship Between Chemical Structure and Antioxidant Activity of Luteolin and Its Glycosides Isolated from T. sipyleus subsp. sipyleus var. sipyleus. Rec. Nat. Prod. 2011, 5, 12–21. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, C.; Li, X.; Zhou, S.; Hua, J.; Huang, J.; Li, Y.; Yang, K.; Zhang, P.; Zhang, Y.; et al. Luteolin alleviates ochratoxin A induced oxidative stress by regulating Nrf2 and HIF-1alpha pathways in NRK-52E rat kidney cells. Food Chem. Toxicol. 2020, 141, 111436. [Google Scholar] [CrossRef]
- Kalbolandi, S.M.; Gorji, A.V.; Babaahmadi-Rezaei, H.; Mansouri, E. Luteolin confers renoprotection against ischemia-reperfusion injury via involving Nrf2 pathway and regulating miR320. Mol. Biol. Rep. 2019, 46, 4039–4047. [Google Scholar] [CrossRef]
- Alekhya Sita, G.J.; Gowthami, M.; Srikanth, G.; Krishna, M.M.; Rama Sireesha, K.; Sajjarao, M.; Nagarjuna, K.; Nagarjuna, M.; Chinnaboina, G.K.; Mishra, A.; et al. Protective role of luteolin against bisphenol A-induced renal toxicity through suppressing oxidative stress, inflammation, and upregulating Nrf2/ARE/ HO-1 pathway. IUBMB Life 2019, 71, 1041–1047. [Google Scholar] [CrossRef]
- Samec, M.; Liskova, A.; Koklesova, L.; Samuel, S.M.; Zhai, K.; Buhrmann, C.; Varghese, E.; Abotaleb, M.; Qaradakhi, T.; Zulli, A.; et al. Flavonoids against the Warburg phenotype-concepts of predictive, preventive and personalised medicine to cut the Gordian knot of cancer cell metabolism. EPMA J. 2020, 11, 377–398. [Google Scholar] [CrossRef]
- Bader, A.; Tuccinardi, T.; Granchi, C.; Martinelli, A.; Macchia, M.; Minutolo, F.; De Tommasi, N.; Braca, A. Phenylpropanoids and flavonoids from Phlomis kurdica as inhibitors of human lactate dehydrogenase. Phytochemistry 2015, 116, 262–268. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Butt, G.; El-Zahaby, S.A.; Attar, R.; Sabitaliyevich, U.Y.; Jovic, J.J.; Tang, K.F.; Naureen, H.; Xu, B. Luteolin mediated targeting of protein network and microRNAs in different cancers: Focus on JAK-STAT, NOTCH, mTOR and TRAIL-mediated signaling pathways. Pharmacol. Re.s 2020, 160, 105188. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Chen, L.; Li, H. The dietary compound luteolin inhibits pancreatic cancer growth by targeting BCL-2. Food Funct. 2018, 9, 3018–3027. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Q.; Li, M.H.; Qin, Y.M.; Jiang, H.Y.; Zhang, X.; Wu, M.H. Luteolin Inhibits Tumorigenesis and Induces Apoptosis of Non-Small Cell Lung Cancer Cells via Regulation of MicroRNA-34a-5p. Int. J. Mol. Sci. 2018, 19, 447. [Google Scholar] [CrossRef]
- Su, J.; Xu, H.T.; Yu, J.J.; Gao, J.L.; Lei, J.; Yin, Q.S.; Li, B.; Pang, M.X.; Su, M.X.; Mi, W.J.; et al. Luteolin Ameliorates Hypertensive Vascular Remodeling through Inhibiting the Proliferation and Migration of Vascular Smooth Muscle Cells. Evid. Based Complement. Alternat. Med. 2015, 2015, 364876. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, A.; Mannucci, L.; Massoud, R.; Bernardini, S.; Cortese, C. Performance characteristics of lipoprotein-associated phospholipase A2 activity assay on the Dimension Vista analyser and preliminary study of a healthy Italian population. Biochem. Med. 2017, 27, 030701. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.A.; Viola, F.G.; Minieri, M.; Caporali, S.; Copponi, A.; Sancesario, G.; Angeletti, S.; Massoud, R.; Romeo, F.; Bernardini, S.; et al. The Von Willebrand Factor Antigen Plasma Concentration: A Monitoring Marker in the Treatment of Aortic and Mitral Valve Diseases. Folia Biol. (Praha) 2020, 66, 133–141. [Google Scholar]
- Yang, W.; Li, Q.; Duncan, J.W.; Bakrania, B.A.; Bradshaw, J.L.; Granger, J.P.; Rana, S.; Spradley, F.T. Luteolin-induced vasorelaxation in uterine arteries from normal pregnant rats. Pregnancy Hypertens. 2021, 23, 11–17. [Google Scholar] [CrossRef]
- Si, H.; Wyeth, R.P.; Liu, D. The flavonoid luteolin induces nitric oxide production and arterial relaxation. Eur. J. Nutr. 2014, 53, 269–275. [Google Scholar] [CrossRef]
- Gentile, D.; Fornai, M.; Pellegrini, C.; Colucci, R.; Benvenuti, L.; Duranti, E.; Masi, S.; Carpi, S.; Nieri, P.; Nericcio, A.; et al. Luteolin Prevents Cardiometabolic Alterations and Vascular Dysfunction in Mice With HFD-Induced Obesity. Front. Pharmacol. 2018, 9, 1094. [Google Scholar] [CrossRef]
- Castellino, G.; Nikolic, D.; Magan-Fernandez, A.; Malfa, G.A.; Chianetta, R.; Patti, A.M.; Amato, A.; Montalto, G.; Toth, P.P.; Banach, M.; et al. Altilix((R)) Supplement Containing Chlorogenic Acid and Luteolin Improved Hepatic and Cardiometabolic Parameters in Subjects with Metabolic Syndrome: A 6 Month Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 2580. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Agathokleous, E.; Kapoor, R.; Dhawan, G.; Calabrese, V. Luteolin and hormesis. Mech. Ageing Dev. 2021, 199, 111559. [Google Scholar] [CrossRef] [PubMed]


| DIETARY SOURCES OF FLAVONOIDS | |
|---|---|
| Vegetables | Broccoli, spinach, red cabbage, onion |
| Fruits | Citrus fruits, blackberries, blueberries, strawberries, raspberries, currants, grapes, plumps, apples, nuts |
| Beverages | Tea, red wine |
| Other foods | Cereals, dark chocolate, spices, soy milk |
| FLAVONOID CLASSIFICATION | |
|---|---|
| Flavones | luteolin, luteolin glucosides, apigenin, chrysin, rutin |
| Flavonols | quercetin, kaempferol, myricetin, tamarixetin |
| Flavan-3-ols | catechin, epicatechin, apigallocathechin gallate |
| Flavanones | naringin, taxifolin, hesperidin, eriodityol, naringenin |
| Isoflavones | genistin, genistein, daidzin, daidzein |
| Anthocyanins | apigenidin, cyanidin |
| Phases of Inflammatory Response |
|---|
| Recognition of microbial and endogenous fragments by cell surface receptors |
| Activation of inflammatory pathways |
| Release of inflammatory mediators |
| Recruitment of immune cells |
| Removal of harmful stimuli |
| Initiation of tissue repair |
| Resolution of inflammation |
| Inflammatory Pathways | Primary Stimuli |
|---|---|
| NF-κB | TLRs, TNF, IL-1 |
| MAPK | TNF, IL-1, IL-6 |
| JAK/STAT | IL-6 |
| Downregulated Target | Treatment | Cell Lines/Animal Model | Refs. |
| IL−1β | LUT | Rat chondrocites | [58] |
| TNF-α, COX-2, iNOS | LUT | Mouse alveolar macrophages (MH-S)Mouse macrophages (RAW 264.7) | [59] |
| IL-6 | LUT | Murine model | [60] |
| IL-8 | LUT | Human retinal pigment epithelial cells (h-RPE) | [61] |
| IL-2, IL-12, CXCL9, IL-17, CXCL2, CXCL8, | LUT | Bone marrow-derived macrophages | [51] |
| PGE2, INF- β | LUT | Mouse macrophages RAW 264.7 | [51] |
| CCL1, CCL2, CCL3, CCR7 CCL19, CCL21, CCR8, CXCL12 | LUT-7G | Human endothelial cells (HUVEC) | [9] |
| Leucotriene C4 | LUT-7G | Bone marrow-derived mast cells | [62] |
| Upregulated Target | Treatment | Cell Lines/Animal Model | Refs. |
| IL-10 | Murine model | [63] | |
| IL10-RB | LUT-7G | Human endothelial cells (HUVEC) | [9] |
| ICEBERG level | LUT-7G | Human endothelial cells (HUVEC) | [9] |
| Metabolic Pathways | Effects of LUT-7G Treatment |
|---|---|
| Glycolysis | G6P (↓), F6P (↓), 3PG (↓), PEP (↓), Riboflavin (↑), Thiamin (↑) |
| Krebs Cycle | Succinate (↓), Fumarate (↓), Riboflavin (↑) |
| Pentose Phosphate | Sedoheptulose-7-P (↓), Xylulose-5P (↓), Riboflavin (↑) |
| Oxidative Phosphorylation | Riboflavin (↑) |
| Glycogenolysis | Pyridoxin (↑) |
| Lipid Metabolism | Cobalamin (↑), Riboflavin (↑) |
| Metabolism of Amino Acid | Cobalamin (↑), Riboflavin (↑) |
| Catabolism of Amino Acid | Thiamin (↑), Riboflavin (↑) |
| LUT-7G EFFECTS ON LIPID PROFILE | ||
|---|---|---|
| Cholesterol hydroxylation pathway | Cholesterol | ↑ |
| 7-Alpha hydroxycholesterol (oxysterol) | ↓ | |
| 7-Beta hydroxycholesterol (oxysterol) | ↓ | |
| 7-Ketocholesterol (oxysterol) | ↓ | |
| Fatty acid hydroxylation pathway | Linoleic acid | ↑ |
| 2-Hydroxypalmitate | ↓ | |
| 2-Hydroxystearate | ↓ | |
| 2-Hydroxydecanoate | ↓ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caporali, S.; De Stefano, A.; Calabrese, C.; Giovannelli, A.; Pieri, M.; Savini, I.; Tesauro, M.; Bernardini, S.; Minieri, M.; Terrinoni, A. Anti-Inflammatory and Active Biological Properties of the Plant-Derived Bioactive Compounds Luteolin and Luteolin 7-Glucoside. Nutrients 2022, 14, 1155. https://doi.org/10.3390/nu14061155
Caporali S, De Stefano A, Calabrese C, Giovannelli A, Pieri M, Savini I, Tesauro M, Bernardini S, Minieri M, Terrinoni A. Anti-Inflammatory and Active Biological Properties of the Plant-Derived Bioactive Compounds Luteolin and Luteolin 7-Glucoside. Nutrients. 2022; 14(6):1155. https://doi.org/10.3390/nu14061155
Chicago/Turabian StyleCaporali, Sabrina, Alessandro De Stefano, Cinzia Calabrese, Alfredo Giovannelli, Massimo Pieri, Isabella Savini, Manfredi Tesauro, Sergio Bernardini, Marilena Minieri, and Alessandro Terrinoni. 2022. "Anti-Inflammatory and Active Biological Properties of the Plant-Derived Bioactive Compounds Luteolin and Luteolin 7-Glucoside" Nutrients 14, no. 6: 1155. https://doi.org/10.3390/nu14061155
APA StyleCaporali, S., De Stefano, A., Calabrese, C., Giovannelli, A., Pieri, M., Savini, I., Tesauro, M., Bernardini, S., Minieri, M., & Terrinoni, A. (2022). Anti-Inflammatory and Active Biological Properties of the Plant-Derived Bioactive Compounds Luteolin and Luteolin 7-Glucoside. Nutrients, 14(6), 1155. https://doi.org/10.3390/nu14061155










