Reversal of Doxorubicin-Induced Bone Loss and Mineralization by Supplementation of Resveratrol and MitoTEMPO in the Early Development of Sparus aurata
Abstract
1. Introduction
2. Materials and Methods
2.1. Micro Diet Preparation
2.2. Feeding Trial
2.3. Whole-Mount Staining of the Skeleton
2.4. Skeletal Anomalies
- I.
- Incidence of skeletal anomalies;
- II.
- Deformities charge;
- III.
- The average number of affected areas;
- IV.
- Incidence (%) of skeletal anomalies according to the number of areas affected (severity);
- V.
- Incidence (%) of each skeletal anomaly typology according to the region affected.
2.5. Meristic Characters
- I.
- Supernumerary bones were included in the meristic count;
- II.
- Non-completely fused bone elements were counted as distinct elements.
2.6. Developmental Stage of the Skeleton
2.7. Mineral Contents
2.8. Cell Culture and ECM Mineralization Assay
2.9. RNA Extraction and qPCR
2.10. Lipid Peroxidation (MDA) Analysis
2.11. Histology
2.12. Statistical Analysis
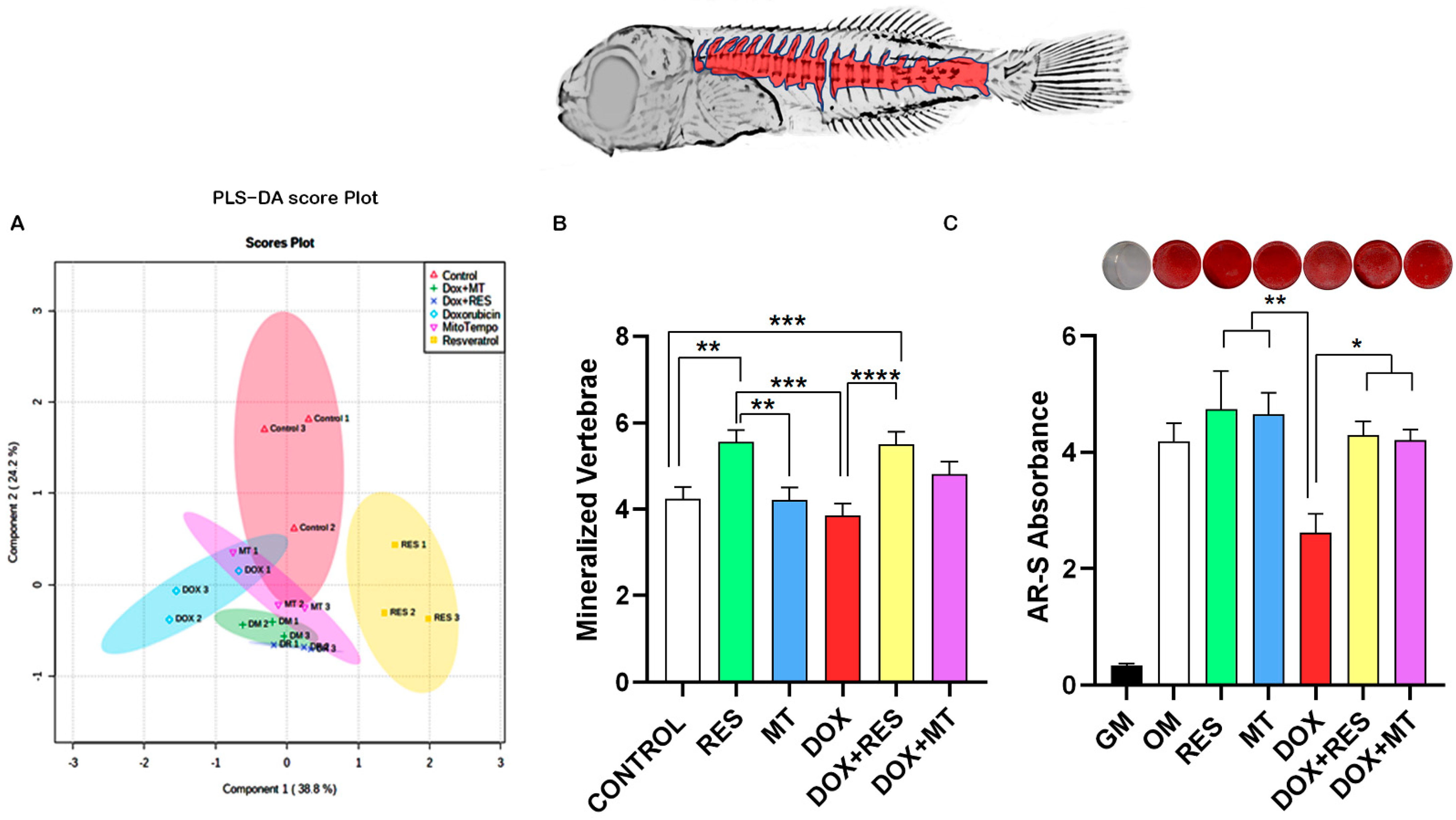
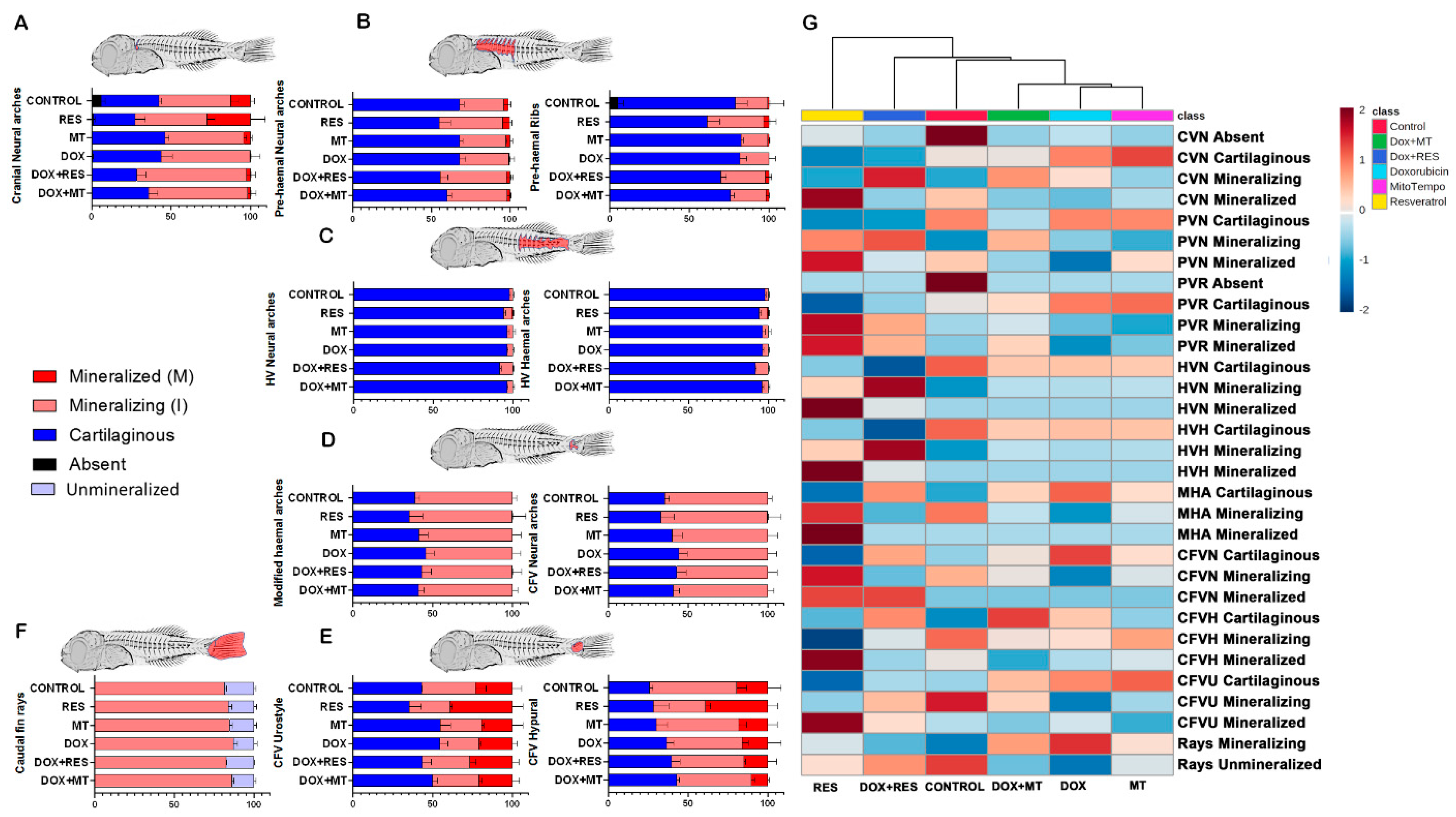
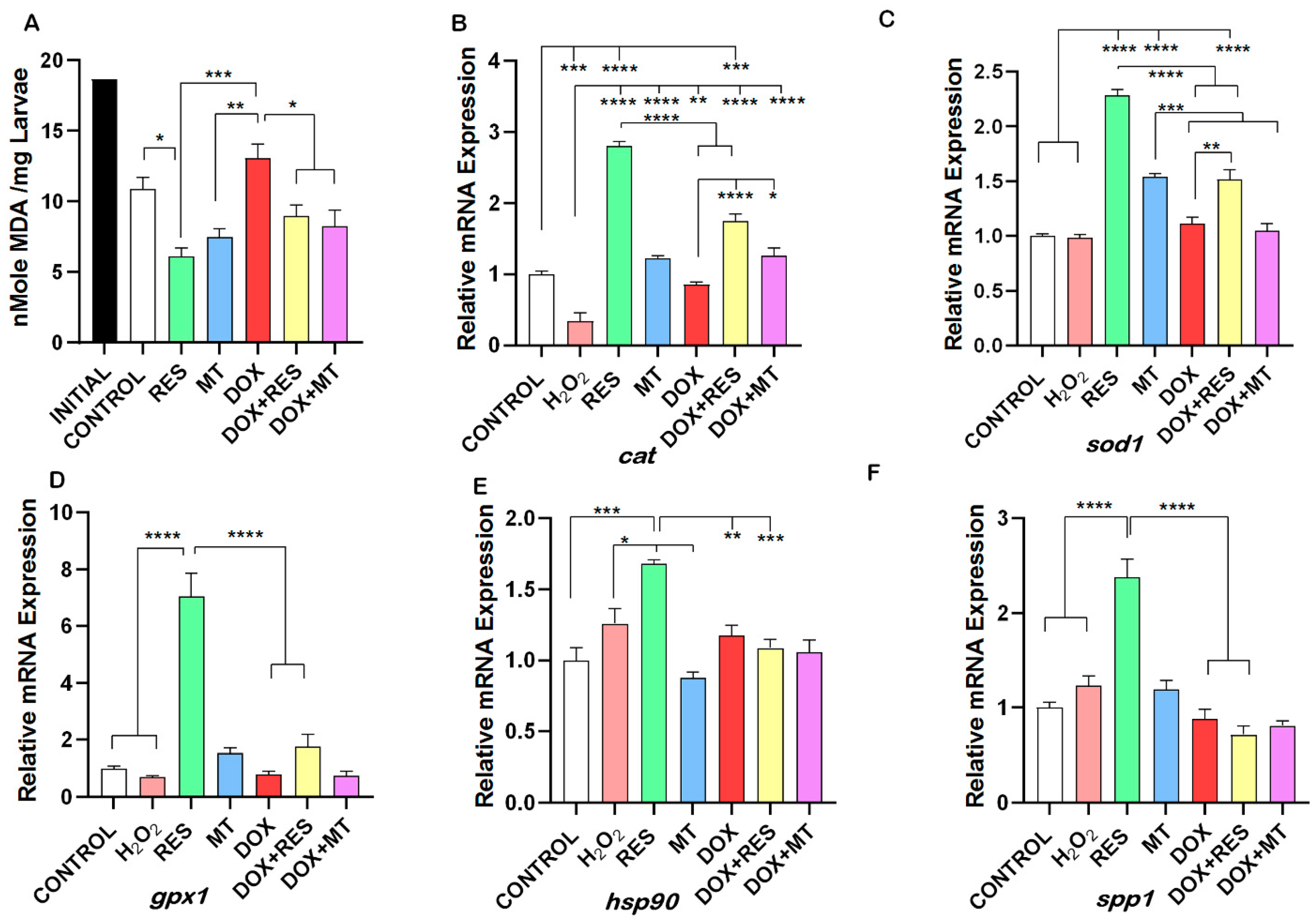
3. Results
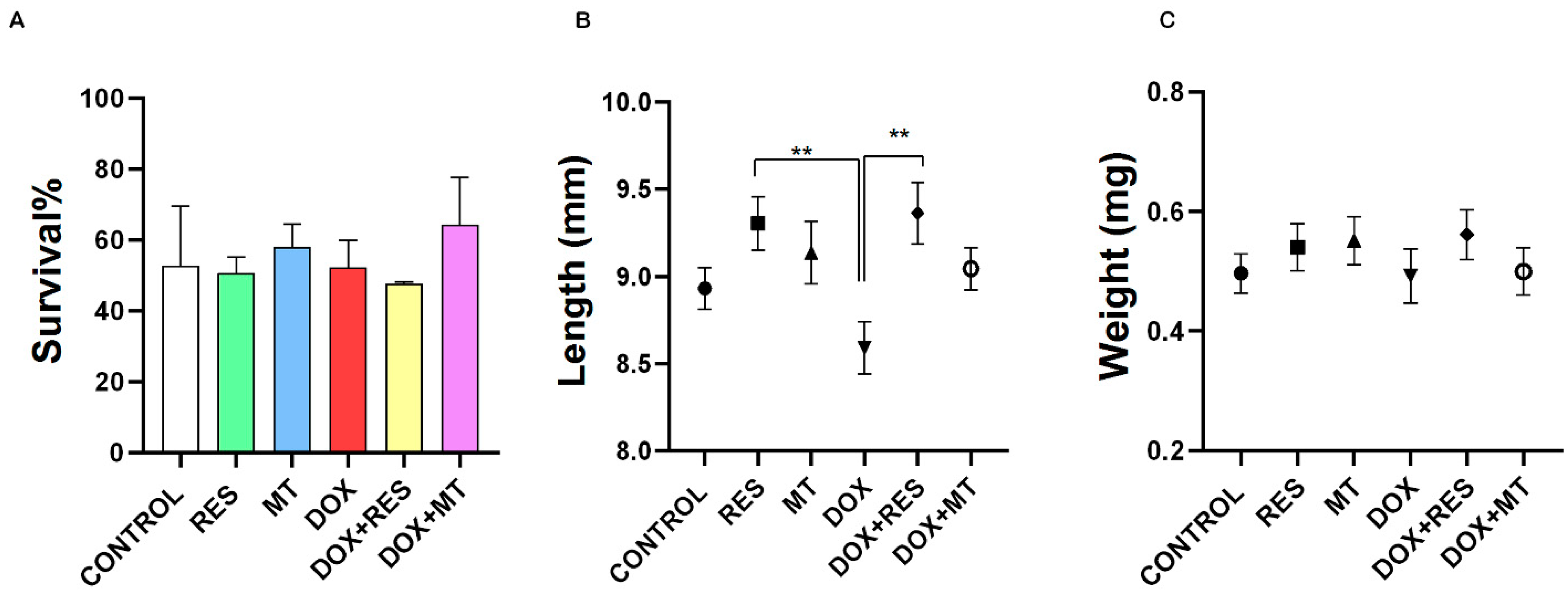
3.1. Doxorubicin Affects Growth and Survival
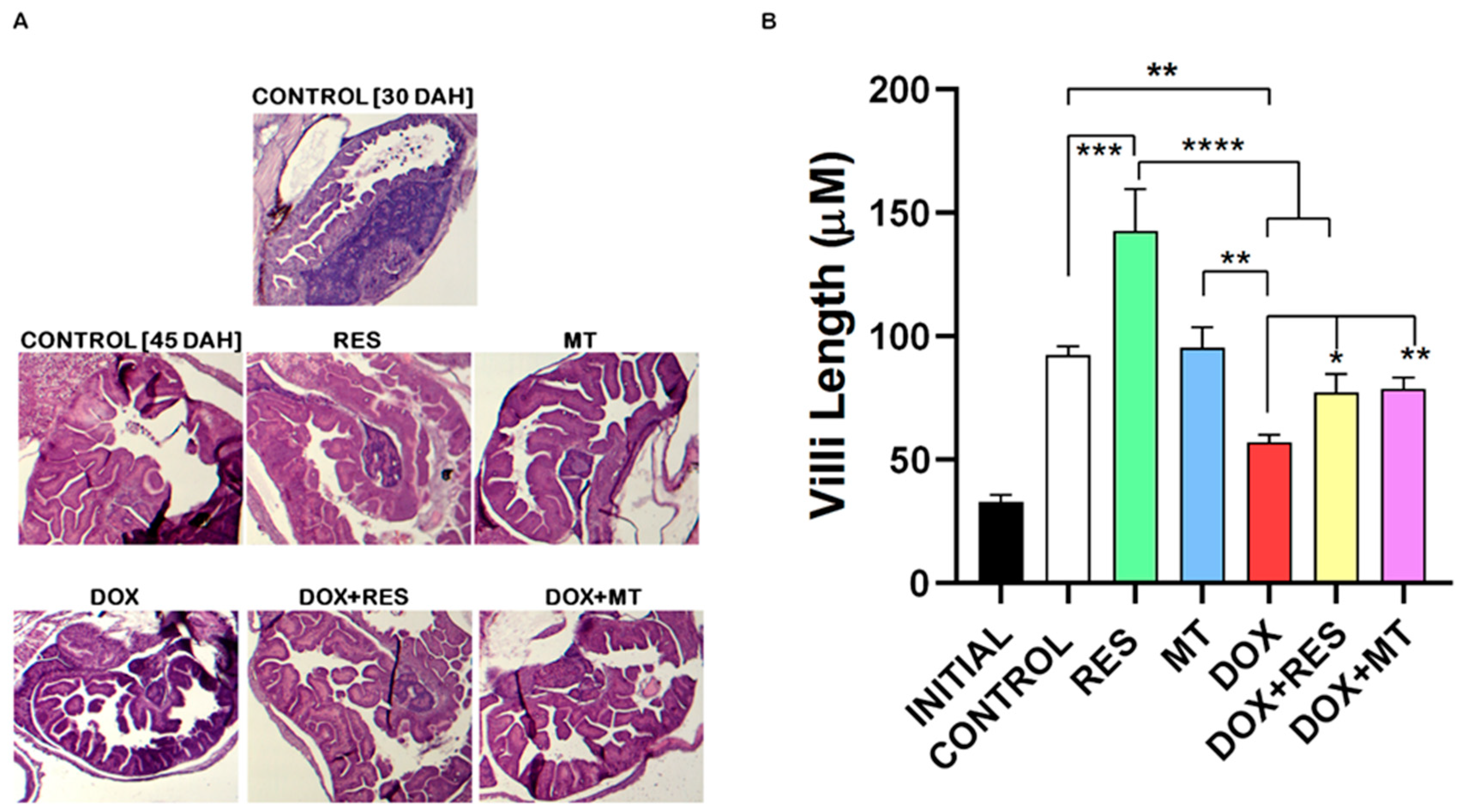
3.2. Histological Changes on Antioxidant and Pro-Oxidants Supplemented Groups
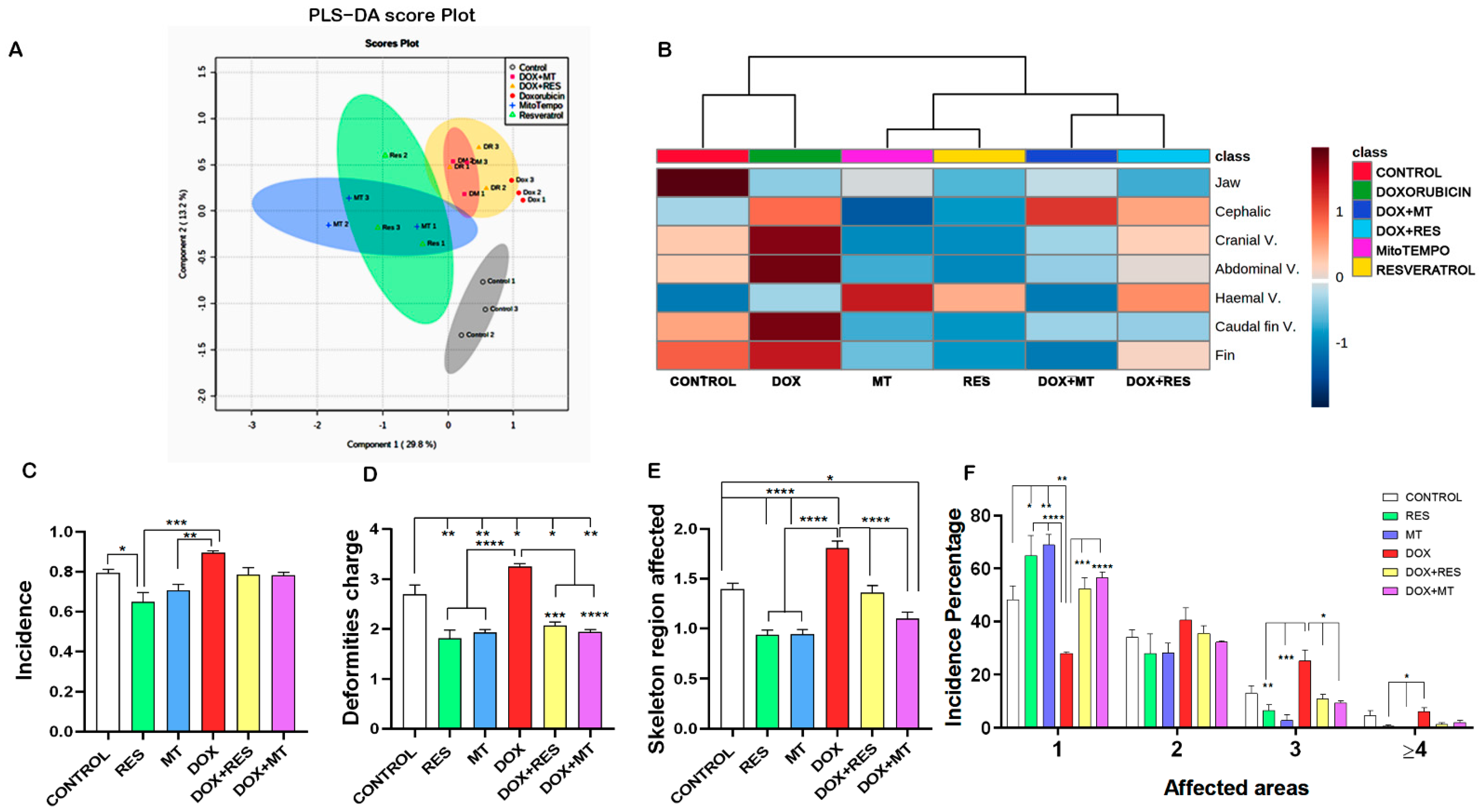
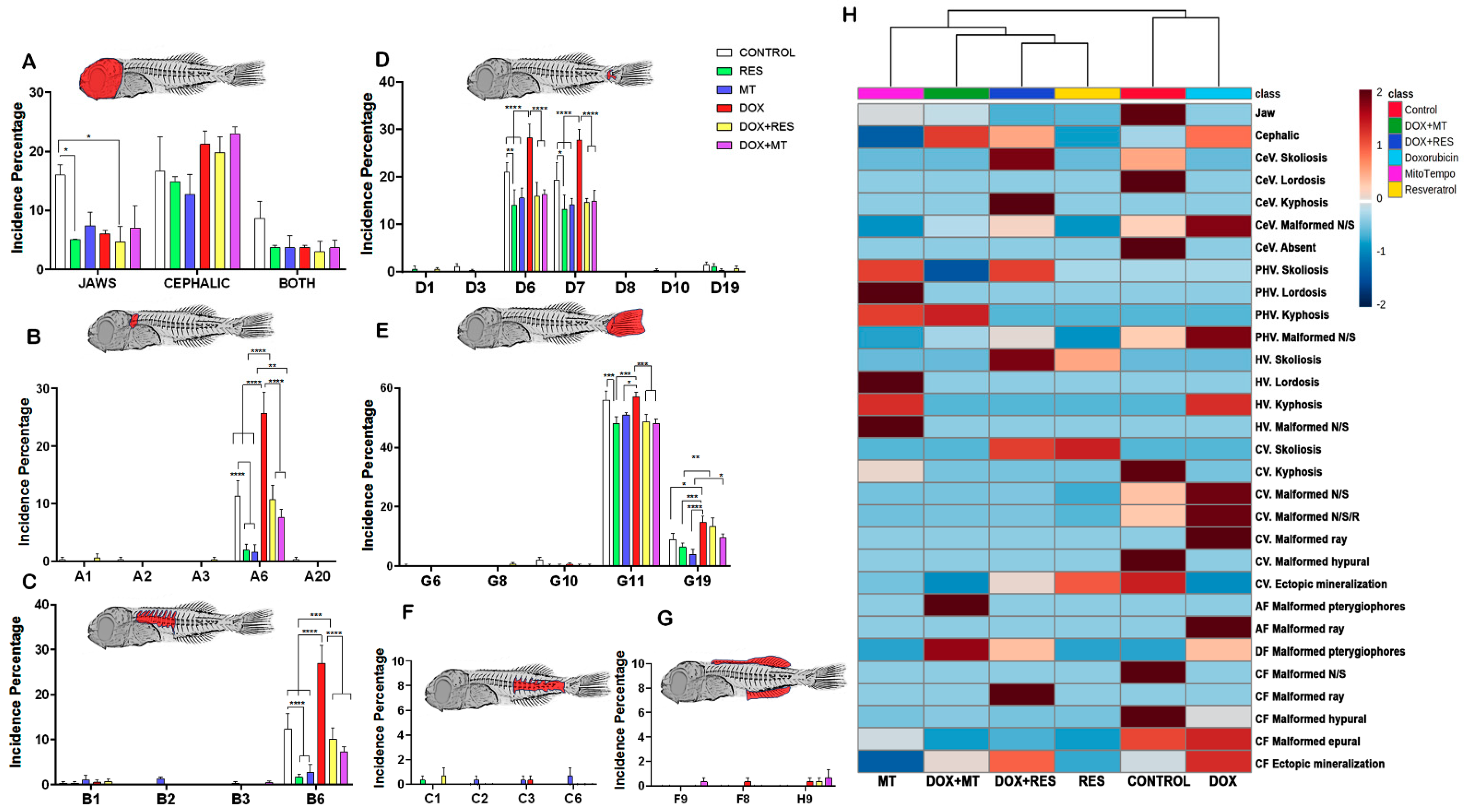
3.3. Antioxidants Prevented the Development of Skeletal Deformities
3.4. Antioxidants Prevent Dox-Induced Delays in Mineralization and Development
3.5. Antioxidant and Pro-Oxidants Alter the Meristic Characters
3.6. Doxorubicin Affects Minerals Content
3.7. Doxorubicin-Induced Oxidative Stress Was Reversed by Antioxidants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ma, X.; Yang, C.; Su, P.; Yin, C.; Qian, A.R. The impact of oxidative stress on the bone system in response to the space special environment. Int. J. Mol. Sci. 2017, 18, 2132. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services. Bone health and osteoporosis: A report of the Surgeon General. US Health Hum. Serv. 2004, 437, NBK45513. [Google Scholar] [CrossRef]
- Baek, K.H.; Oh, K.W.; Lee, W.Y.; Lee, S.S.; Kim, M.K.; Kwon, H.S.; Rhee, E.J.; Han, J.H.; Song, K.H.; Cha, B.Y.; et al. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif. Tissue Int. 2010, 87, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Maggio, D.; Barabani, M.; Pierandrei, M.; Polidori, M.C.; Catani, M.; Mecocci, P.; Senin, U.; Pacifici, R.; Cherubini, A. Marked decrease in plasma antioxidants in aged osteoporotic women: Results of a cross-sectional study. J. Clin. Endocrinol. Metab. 2003, 88, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, G.; Larijani, B.; Mohammadirad, A.; Heshmat, R.; Dehghan, G.; Rahimi, R.; Abdollahi, M. Determination of oxidative stress status and concentration of TGF-β1 in the blood and saliva of osteoporotic subjects. Proc. Natl. Acad. Sci. USA 2006, 1091, 142–150. [Google Scholar] [CrossRef]
- Östman, B.; Michaëlsson, K.; Helmersson, J.; Byberg, L.; Gedeborg, R.; Melhus, H.; Basu, S. Oxidative stress and bone mineral density in elderly men: Antioxidant activity of alpha-tocopherol. Free Radic. Biol. Med. 2009, 47, 668–673. [Google Scholar] [CrossRef]
- Prestinicola, L.; Boglione, C.; Makridis, P.; Spanò, A.; Rimatori, V.; Palamara, E.; Scardi, M.; Cataudella, S. Environmental Conditioning of Skeletal Anomalies Typology and Frequency in Gilthead Seabream (Sparus aurata L., 1758) Juveniles. PLoS ONE 2013, 8, e55736. [Google Scholar] [CrossRef]
- Boglione, C.; Gavaia, P.; Koumoundouros, G.; Gisbert, E.; Moren, M.; Fontagné, S.; Witten, P.E. Skeletal anomalies in reared European fish larvae and juveniles. Part 1: Normal and anomalous skeletogenic processes. Rev. Aquac. 2013, 5, S99–S120. [Google Scholar] [CrossRef]
- Boglione, C.; Gisbert, E.; Gavaia, P.; Witten, P.E.; Moren, M.; Fontagné, S.; Koumoundouros, G. Skeletal anomalies in reared European fish larvae and juveniles. Part 2: Main typologies, occurrences and causative factors. Rev. Aquac. 2013, 5, S121–S167. [Google Scholar] [CrossRef]
- Izquierdo, M.S.; Scolamacchia, M.; Betancor, M.; Roo, J.; Caballero, M.J.; Terova, G.; Witten, P.E. Effects of dietary DHA and α-tocopherol on bone development, early mineralisation and oxidative stress in Sparus aurata (Linnaeus, 1758) larvae. Br. J. Nutr. 2013, 109, 1796–1805. [Google Scholar] [CrossRef] [PubMed]
- Hamre, K.; Yúfera, M.; Rønnestad, I.; Boglione, C.; Conceição, L.E.C.; Izquierdo, M. Fish larval nutrition and feed formulation: Knowledge gaps and bottlenecks for advances in larval rearing. Rev. Aquac. 2013, 5, S26–S58. [Google Scholar] [CrossRef]
- Faustino, M.; Power, D.M. Development of the pectoral, pelvic, dorsal and anal fins in cultured sea bream. J. Fish Biol. 1999, 54, 1094–1110. [Google Scholar] [CrossRef]
- Faustino, M.; Power, D.M. Development of osteological structures in the sea bream: Vertebral column and caudal fin complex. J. Fish Biol. 1998, 52, 11–22. [Google Scholar] [CrossRef]
- Matsuoka, M. Comparison of Meristic Variations and Bone Abnormalities between Wild and Laboratory-Reared Red Sea Bream. Jpn. Agric. Res. Q. JARQ 2003, 37, 21–30. [Google Scholar] [CrossRef][Green Version]
- Vijayakumar, P.; Laizé, V.; Cardeira, J.; Trindade, M.; Cancela, M.L. Development of an in vitro cell system from zebrafish suitable to study bone cell differentiation and extracellular matrix mineralization. Zebrafish 2013, 10, 500–509. [Google Scholar] [CrossRef]
- Marques, C.L.; Rafael, M.S.; Cancela, M.L.; Laizé, V. Establishment of primary cell cultures from fish calcified tissues. Cytotechnology 2007, 55, 9–13. [Google Scholar] [CrossRef][Green Version]
- Tiago, D.M.; Laizé, V.; Cancela, M.L.; Aureliano, M. Impairment of mineralization by metavanadate and decavanadate solutions in a fish bone-derived cell line. Cell Biol. Toxicol. 2008, 24, 253–263. [Google Scholar] [CrossRef][Green Version]
- Saleh, R.; Betancor, M.B.; Roo, J.; Benítez-Santana, T.; Zamorano, M.J.; Izquierdo, M. Biomarkers of bone development and oxidative stress in gilthead sea bream larvae fed microdiets with several levels of polar lipids and α-tocopherol. Aquac. Nutr. 2015, 21, 341–354. [Google Scholar] [CrossRef]
- Filho, D.W.; Giulivi, C.; Boveris, A. Antioxidant defences in marine fish—I. Teleosts. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1993, 106, 409–413. [Google Scholar] [CrossRef]
- Winston, G.W.; Di Giulio, R.T. Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat. Toxicol. 1991, 19, 137–161. [Google Scholar] [CrossRef]
- Tou, J.C. Resveratrol supplementation affects bone acquisition and osteoporosis: Pre-clinical evidence toward translational diet therapy. Biochim. Biophys. Acta-Mol. Basis Dis. 2014, 1852, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Cottart, C.-H.; Nivet-Antoine, V.; Beaudeux, J.-L. Review of recent data on the metabolism, biological effects, and toxicity of resveratrol in humans. Mol. Nutr. Food Res. 2014, 58, 7–21. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, S.; Xie, L.; Yu, Y.; Zhou, L.; Feng, Y.; Cai, D. Resveratrol Ameliorates Glucocorticoid-Induced Bone Damage in a Zebrafish Model. Front. Pharmacol. 2019, 10, 195. [Google Scholar] [CrossRef]
- Giordo, R.; Nasrallah, G.K.; Al-Jamal, O.; Paliogiannis, P.; Pintus, G. Resveratrol Inhibits Oxidative Stress and Prevents Mitochondrial Damage Induced by Zinc Oxide Nanoparticles in Zebrafish (Danio rerio). Int. J. Mol. Sci. 2020, 21, 3838. [Google Scholar] [CrossRef]
- Dai, Z.; Li, Y.; Quarles, L.D.; Song, T.; Pan, W.; Zhou, H.; Xiao, Z. Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine 2007, 14, 806–814. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, Y.S.; Lee, S.Y.; Kim, G.H.; Kim, B.J.; Lee, S.H.; Lee, K.U.; Kim, G.S.; Kim, S.W.; Koh, J.M. AMP kinase acts as a negative regulator of RANKL in the differentiation of osteoclasts. Bone 2010, 47, 926–937. [Google Scholar] [CrossRef]
- Miura, S.; Saitoh, S.I.; Kokubun, T.; Owada, T.; Yamauchi, H.; Machii, H.; Takeishi, Y. Mitochondrial-targeted antioxidant maintains blood flow, mitochondrial function, and redox balance in old mice following prolonged limb ischemia. Int. J. Mol. Sci. 2017, 18, 1897. [Google Scholar] [CrossRef]
- Ni, R.; Cao, T.; Xiong, S.; Ma, J.; Fan, G.C.; Lacefield, J.C.; Lu, Y.; Tissier, S.L.; Peng, T. Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy. Free Radic. Biol. Med. 2016, 90, 12–23. [Google Scholar] [CrossRef]
- Dai, P.; Mao, Y.; Sun, X.; Li, X.; Muhammad, I.; Gu, W.; Zhang, D.; Zhou, Y.; Ma, J.; Ni, Z.; et al. Attenuation of Oxidative Stress-Induced Osteoblast Apoptosis by Curcumin is Associated with Preservation of Mitochondrial Functions and Increased Akt-GSK3β Signaling. Cell. Physiol. Biochem. 2017, 41, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Knowles, H. Hypoxic regulation of osteoclast differentiation and bone resorption activity. Hypoxia 2015, 3, 73–82. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Harris, N.; Ren, J.; Han, X. Mitochondria-targeted antioxidant prevents cardiac dysfunction induced by tafazzin gene knockdown in cardiac myocytes. Oxid. Med. Cell. Longev. 2014, 2014, 654198. [Google Scholar] [CrossRef] [PubMed]
- Cappetta, D.; De Angelis, A.; Sapio, L.; Prezioso, L.; Illiano, M.; Quaini, F.; Rossi, F.; Berrino, L.; Naviglio, S.; Urbanek, K. Oxidative stress and cellular response to doxorubicin: A common factor in the complex milieu of anthracycline cardiotoxicity. Oxid. Med. Cell. Longev. 2017, 2017, 1521020. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C.; Quinlan, G.J. Free radical damage to deoxyribose by anthracycline, aureolic acid and aminoquinone antitumour antibiotics: An essential requirement for iron, semiquinones and hydrogen peroxide. Biochem. Pharmacol. 1985, 34, 4099–4103. [Google Scholar] [CrossRef]
- Feinstein, E.; Canaani, E.; Weiner, L.M. Dependence of nucleic acid degradation on in situ free-radical production by Adriamycin. Biochemistry 2002, 32, 13156–13161. [Google Scholar] [CrossRef] [PubMed]
- Huertas, J.R.; Battino, M.; Lenaz, G.; Mataix, F.J. Changes in mitochondrial and microsomal rat liver coenzyme Q9, and Q10 content induced by dietary fat and endogenous lipid peroxidation. FEBS Lett. 1991, 287, 89–92. [Google Scholar] [CrossRef]
- Huertas, J.R.; Battino, M.; Mataix, F.J.; Lenaz, G. Cytochrome oxidase induction after oxidative stress induced by adriamycin in liver of rats fed with dietary olive oil. Biochem. Biophys. Res. Commun. 1991, 181, 375–382. [Google Scholar] [CrossRef]
- Huertas, J.R.; Battino, M.; Barzanti, V.; Maranesi, M.; Parenti-Castelli, G.; Littarru, G.P.; Turchetto, E.; Mataix, F.J.; Lenaz, G. Mitochondrial and microsomal cholesterol mobilization after oxidative stress induced by adriamycin in rats fed with dietary olive and corn oil. Life Sci. 1992, 50, 2111–2118. [Google Scholar] [CrossRef]
- Mataix, J.; Mañas, M.; Quiles, J.; Battino, M.; Cassinello, M.; Lopez-Frias, M.; Huertas, J.R. Coenzyme Q content depends upon oxidative stress and dietary fat unsaturation. Mol. Asp. Med. 1997, 18, 129–135. [Google Scholar] [CrossRef]
- Quiles, J.L.; Ramirez-Tortosa, M.C.; Huertas, J.R.; Ibañez, S.; Gomez, J.A.; Battino, M.; Mataix, J. Olive oil supplemented with vitamin E affects mitochondrial coenzyme Q levels in liver of rats after an oxidative stress induced by adriamycin. BioFactors 1999, 9, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.L.; Ramírez-Tortosa, M.C.; Ibéñez, S.; Gonzélez, J.A.; Duthie, G.G.; Fiuertas, J.R.; Mataix, J. Vitamin E Supplementation Increases the Stability and the In Vivo Antioxidant Capacity of Refined Olive Oil. Free Radic. Res. 1999, 31, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Olson, R.D.; Mushlin, P.S. Doxorubicin cardiotoxicity: Analysis of prevailing hypotheses. FASEB J. 1990, 4, 3076–3086. [Google Scholar] [CrossRef] [PubMed]
- De Beer, E.L.; Bottone, A.E.; Voest, E.E. Doxorubicin and mechanical performance of cardiac trabeculae after acute and chronic treatment: A review. Eur. J. Pharmacol. 2001, 415, 1–11. [Google Scholar] [CrossRef]
- Singal, P.; Li, T.; Kumar, D.; Danelisen, I.; Iliskovic, N. Adriamycin-Induced heart failure: Mechanisms and modulation. Mol. Cell. Biochem. 2000, 207, 77–86. [Google Scholar] [CrossRef]
- Gianni, L.; Zweier, J.L.; Levy, A.; Myers, C.E. Characterization of the cycle of iron-mediated electron transfer from Adriamycin to molecular oxygen. J. Biol. Chem. 1985, 260, 6820–6826. [Google Scholar] [CrossRef]
- Sinha, B.K.; Politi, P.M. Anthracyclines. Cancer Chemother. Biol. Response Modif. 1990, 11, 45–57. [Google Scholar]
- Hadji, P.; Ziller, M.; Maskow, C.; Albert, U.; Kalder, M. The influence of chemotherapy on bone mineral density, quantitative ultrasonometry and bone turnover in pre-menopausal women with breast cancer. Eur. J. Cancer 2009, 45, 3205–3212. [Google Scholar] [CrossRef]
- Van Leeuwen, B.L.; Kamps, W.A.; Hartel, R.M.; Veth, R.P.H.; Sluiter, W.J.; Hoekstra, H.J. Effect of single chemotherapeutic agents on the growing skeleton of the rat. Ann. Oncol. 2000, 11, 1121–1126. [Google Scholar] [CrossRef]
- Friedlaender, G.E.; Tross, R.B.; Doganis, A.C.; Kirkwood, J.M.; Baron, R. Effects of chemotherapeutic agents on bone. I. Short-term methotrexate and doxorubicin (adriamycin) treatment in a rat model. J. Bone Jt. Surg.-Ser. A 1984, 66, 602–607. [Google Scholar] [CrossRef]
- Zakaria, Z.Z.; Benslimane, F.M.; Nasrallah, G.K.; Shurbaji, S.; Younes, N.N.; Mraiche, F.; Da’as, S.I.; Yalcin, H.C. Using Zebrafish for Investigating the Molecular Mechanisms of Drug-Induced Cardiotoxicity. Biomed Res. Int. 2018, 2018, 1642684. [Google Scholar] [CrossRef] [PubMed]
- Calienni, M.N.; Cagel, M.; Montanari, J.; Moretton, M.A.; Prieto, M.J.; Chiappetta, D.A.; del Valle Alonso, S. Zebrafish (Danio rerio) model as an early stage screening tool to study the biodistribution and toxicity profile of doxorubicin-loaded mixed micelles. Toxicol. Appl. Pharmacol. 2018, 357, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Wu, S.L.; Zhao, X.D.; Zhao, C.T.; Li, Y.H. Developmental toxicity of doxorubicin hydrochloride in embryo-larval stages of zebrafish. Bio-Med. Mater. Eng. 2014, 24, 909–916. [Google Scholar] [CrossRef]
- Peterman, E.M.; Sullivan, C.; Goody, M.F.; Rodriguez-Nunez, I.; Yoder, J.A.; Kim, C.H. Neutralization of mitochondrial superoxide by superoxide dismutase 2 promotes bacterial clearance and regulates phagocyte numbers in zebrafish. Infect. Immun. 2015, 83, 430–440. [Google Scholar] [CrossRef]
- Saleh, R.; Betancor, M.B.; Roo, J.; Montero, D.; Zamorano, M.J.; Izquierdo, M. Selenium levels in early weaning diets for gilthead seabream larvae. Aquaculture 2014, 426–427, 256–263. [Google Scholar] [CrossRef]
- Eryalçın, K.M.; Domínguez, D.; Roo, J.; Hernandez-Cruz, C.M.; Zamorano, M.J.; Castro, P.; Hamre, K.; Izquierdo, M. Effect of dietary microminerals in early weaning diets on growth, survival, mineral contents and gene expression in gilthead sea bream (Sparus aurata, L) larvae. Aquac. Nutr. 2020, 26, 1760–1770. [Google Scholar] [CrossRef]
- Gavaia, P.J.; Sarasquete, C.; Cancela, M.L. Detection of mineralized structures in early stages of development of marine Teleostei using a modified alcian blue-alizarin red double staining technique for bone and cartilage. Biotech. Histochem. 2000, 75, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.B.; Kimmel, C.B. A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech. Histochem. 2007, 82, 23–28. [Google Scholar] [CrossRef]
- Gavaia, P.J.; Dinis, M.T.; Cancela, M.L. Osteological development and abnormalities of the vertebral column and caudal skeleton in larval and juvenile stages of hatchery-reared Senegal sole (Solea senegalensis). Aquaculture 2002, 211, 305–323. [Google Scholar] [CrossRef]
- Pombinho, A.R.; Laizé, V.; Molha, D.M.; Marques, S.M.P.; Cancela, M.L. Development of two bone-derived cell lines from the marine teleost Sparus aurata; evidence for extracellular matrix mineralization and cell-type-specific expression of matrix Gla protein and osteocalcin. Cell Tissue Res. 2004, 315, 393–406. [Google Scholar] [CrossRef]
- Puchtler, H.; Meloan, S.N.; Terry, M.S. On the history and mechanism of alizarin and alizarin red S stains for calcium. J. Histochem. Cytochem. 1969, 17, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Viegas, M.N.; Dias, J.; Cancela, M.L.; Laizé, V. Polyunsaturated fatty acids regulate cell proliferation, extracellular matrix mineralization and gene expression in a gilthead seabream skeletal cell line. J. Appl. Ichthyol. 2012, 28, 427–432. [Google Scholar] [CrossRef]
- Dominguez, D.; Sehnine, Z.; Castro, P.; Zamorano, M.J.; Robaina, L.; Fontanillas, R.; Antony Jesu Prabhu, P.; Izquierdo, M. Dietary manganese levels for gilthead sea bream (Sparus aurata) fingerlings fed diets high in plant ingredients. Aquaculture 2020, 529, 735614. [Google Scholar] [CrossRef]
- Cardiff, R.D.; Miller, C.H.; Munn, R.J. Manual Hematoxylin and Eosin Staining of Mouse Tissue Sections. Cold Spring Harb. Protoc. 2014, 2014, 655–658. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and Eosin Staining of Tissue and Cell Sections. Cold Spring Harb. Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef] [PubMed]
- Baeverfjord, G.; Krogdahl, A. Development and regression of soybean meal induced enteritis in Atlantic salmon, Salmo salar L., distal intestine: A comparison with the intestines of fasted fish. J. Fish Dis. 1996, 19, 375–387. [Google Scholar] [CrossRef]
- Magalhães, R.; Guerreiro, I.; Santos, R.A.; Coutinho, F.; Couto, A.; Serra, C.R.; Olsen, R.E.; Peres, H.; Oliva-Teles, A. Oxidative status and intestinal health of gilthead sea bream (Sparus aurata) juveniles fed diets with different ARA/EPA/DHA ratios. Sci. Rep. 2020, 10, 13824. [Google Scholar] [CrossRef]
- Bijlsma, S.; Bobeldijk, I.; Verheij, E.R.; Ramaker, R.; Kochhar, S.; Macdonald, I.A.; Van Ommen, B.; Smilde, A.K. Large-scale human metabolomics studies: A strategy for data (pre-) processing and validation. Anal. Chem. 2006, 78, 567–574. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Li, S.; Xia, J. MetaboAnalystR 3.0: Toward an Optimized Workflow for Global Metabolomics. Metabolites 2020, 10, 186. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; De Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Yarmohammadi, F.; Rezaee, R.; Karimi, G. Natural compounds against doxorubicin-induced cardiotoxicity: A review on the involvement of Nrf2/ARE signaling pathway. Phyther. Res. 2021, 35, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Rana, T.; Chakrabarti, A.; Freeman, M.; Biswas, S. Doxorubicin-mediated bone loss in breast cancer bone metastases is driven by an interplay between oxidative stress and induction of TGFβ. PLoS ONE 2013, 8, e78043. [Google Scholar] [CrossRef]
- Carvalho, F.S.; Burgeiro, A.; Garcia, R.; Moreno, A.J.; Carvalho, R.A.; Oliveira, P.J. Doxorubicin-Induced Cardiotoxicity: From Bioenergetic Failure and Cell Death to Cardiomyopathy. Med. Res. Rev. 2014, 34, 106–135. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Ashour, O.M.; Ibrahim, Y.F.; EL-Bitar, H.I.; Gomaa, W.; Abdel-Rahim, S.R. Angiotensin-Converting enzyme inhibition and angiotensin AT1-receptor antagonism equally improve doxorubicin-induced cardiotoxicity and nephrotoxicity. Pharmacol. Res. 2009, 60, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, T.; Krause, U.; Thome, U.; Rajewski, A.; Skorzek, M.; Scheulen, M.E. Tissue toxicity of doxorubicin in first and second hyperthermic isolated limb perfusion—an experimental study in dogs. Eur. J. Surg. Oncol. 1997, 23, 439–444. [Google Scholar] [CrossRef]
- Valenzuela, C.A.; Escobar-Aguirre, S.; Zuloaga, R.; Vera-Tobar, T.; Mercado, L.; Björnsson, B.T.; Valdés, J.A.; Molina, A. Stocking density induces differential expression of immune-related genes in skeletal muscle and head kidney of fine flounder (Paralichthys adspersus). Vet. Immunol. Immunopathol. 2019, 210, 23–27. [Google Scholar] [CrossRef]
- Torno, C.; Staats, S.; de Pascual-Teresa, S.; Rimbach, G.; Schulz, C. Effects of resveratrol and genistein on growth, nutrient utilization and fatty acid composition of rainbow trout. Animal 2019, 13, 933–940. [Google Scholar] [CrossRef]
- Wilson, W.N.; Baumgarner, B.L.; Watanabe, W.O.; Alam, M.S.; Kinsey, S.T. Effects of resveratrol on growth and skeletal muscle physiology of juvenile southern flounder. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 183, 27–35. [Google Scholar] [CrossRef]
- Yang, S.-G.; Park, H.-J.; Kim, J.-W.; Jung, J.-M.; Kim, M.-J.; Jegal, H.-G.; Kim, I.-S.; Kang, M.-J.; Wee, G.; Yang, H.-Y.; et al. Mito-TEMPO improves development competence by reducing superoxide in preimplantation porcine embryos. Sci. Rep. 2018, 8, 10130. [Google Scholar] [CrossRef]
- Kiela, P.R.; Ghishan, F.K. Physiology of Intestinal Absorption and Secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef]
- Kiskova, T.; Kubatka, P.; Büsselberg, D.; Kassayova, M. The Plant-Derived Compound Resveratrol in Brain Cancer: A Review. Biomolecules 2020, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Rigby, R.J.; Carr, J.; Orgel, K.; King, S.L.; Lund, P.K.; Dekaney, C.M. Intestinal bacteria are necessary for doxorubicin-induced intestinal damage but not for doxorubicin-induced apoptosis. Gut Microbes 2016, 7, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, A.; Brinkman, B.M.; Heyndrickx, L.; Vandenabeele, P.; Krysko, D.V. Severity of doxorubicin-induced small intestinal mucositis is regulated by the TLR-2 and TLR-9 pathways. J. Pathol. 2012, 226, 598–608. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Alam, M.A.; Ahmad, F.J. Enhancement of oral bioavailability of doxorubicin through surface modified biodegradable polymeric nanoparticles. Chem. Cent. J. 2018, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.H.; Zhao, K.L.; Chen, X.L.; Xu, J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012, 90, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Weisbrode, S.E. Bone and Joints. Tomson’s Special Veterinary Pathology; Mosby: Maryland, MI, USA, 2001. [Google Scholar]
- Moro, L.; Romanello, M.; Favia, A.; Lamanna, M.P.; Lozupone, E. Posttranslational Modifications of Bone Collagen Type I are Related to the Function of Rat Femoral Regions. Calcif. Tissue Int. 2000, 66, 151–156. [Google Scholar] [CrossRef]
- Lim, C.; Lovell, R.T. Pathology of the Vitamin C Deficiency Syndrome in Channel Catfish (Ictalurus punctatus). J. Nutr. 1978, 108, 1137–1146. [Google Scholar] [CrossRef]
- Santamaría, J.A.; Andrades, J.A.; Herráez, P.; Fernández-Llebrez, P.; Becerra, J. Perinotochordal connective sheet of gilthead sea bream larvae (Sparus aurata, L.) affected by axial malformations: An histochemical and immunocytochemical study. Anat. Rec. 1994, 240, 248–254. [Google Scholar] [CrossRef]
- Berillis, P.; Panagiotopoulos, N.; Boursiaki, V.; Karapanagiotidis, I.T.; Mente, E. Vertebrae length and ultra-structure measurements of collagen fibrils and mineral content in the vertebrae of lordotic gilthead seabreams (Sparus aurata). Micron 2015, 75, 27–33. [Google Scholar] [CrossRef]
- Fernández, I.; Hontoria, F.; Ortiz-Delgado, J.B.; Kotzamanis, Y.; Estévez, A.; Zambonino-Infante, J.L.; Gisbert, E. Larval performance and skeletal deformities in farmed gilthead sea bream (Sparus aurata) fed with graded levels of Vitamin A enriched rotifers (Brachionus plicatilis). Aquaculture 2008, 283, 102–115. [Google Scholar] [CrossRef]
- Boglione, C.; Pulcini, D.; Scardi, M.; Palamara, E.; Russo, T.; Cataudella, S. Skeletal Anomaly Monitoring in Rainbow Trout (Oncorhynchus mykiss, Walbaum 1792) Reared under Different Conditions. PLoS ONE 2014, 9, e96983. [Google Scholar] [CrossRef] [PubMed]
- Koumoundouros, G.; Gagliardi, F.; Divanach, P.; Stefanakis, S.; Kentouri, M. Osteological study of the origin and development of the abnormal caudal fin in gilthead sea bream (Sparus aurata) fry. In Proceedings of the Quality in Aquaculture. Aquaculture Europe ’95 International Conference, Trondheim, Norway, 7–10 August 1995; p. 413. [Google Scholar]
- Hall, B.K. Bones and Cartilage: Developmental and Evolutionary Skeletal Biology; Elsevier: Amsterdam, The Netherlands, 2005; ISBN 9780124166851. [Google Scholar]
- Fragkoulis, S.; Printzi, A.; Geladakis, G.; Katribouzas, N.; Koumoundouros, G. Recovery of haemal lordosis in Gilthead seabream (Sparus aurata L.). Sci. Rep. 2019, 9, 9832. [Google Scholar] [CrossRef]
- Kranenbarg, S.; Waarsing, J.H.; Muller, M.; Weinans, H.; van Leeuwen, J.L. Lordotic vertebrae in sea bass (Dicentrarchus labrax L.) are adapted to increased loads. J. Biomech. 2005, 38, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Koumoundouros, G.; Gagliardi, F.; Divanach, P.; Boglione, C.; Cataudella, S.; Kentouri, M. Normal and abnormal osteological development of caudal fin in Sparus aurata L. fry. Aquaculture 1997, 149, 215–226. [Google Scholar] [CrossRef]
- Divanach, P.; Boglione, C.; Menu, B.; Koumoundouros, G.; Kentouri, M.; Cataudella, S. Abnormalities in Finfish Mariculture: An Overview of the Problem, Causes and Solutions; CINECA IRIS: Trento, Italy, 1996. [Google Scholar]
- Gavaia, P.J.; Simes, D.C.; Ortiz-Delgado, J.B.; Viegas, C.S.B.; Pinto, J.P.; Kelsh, R.N.; Sarasquete, M.C.; Cancela, M.L. Osteocalcin and matrix Gla protein in zebrafish (Danio rerio) and Senegal sole (Solea senegalensis): Comparative gene and protein expression during larval development through adulthood. Gene Expr. Patterns 2006, 6, 637–652. [Google Scholar] [CrossRef]
- Witten, P.E.; Gil-Martens, L.; Hall, B.K.; Huysseune, A.; Obach, A. Compressed vertebrae in Atlantic salmon Salmo salar: Evidence for metaplastic chondrogenesis as a skeletogenic response late in ontogeny. Dis. Aquat. Organ. 2005, 64, 237–246. [Google Scholar] [CrossRef]
- Holm, E.; Gleberzon, J.S.; Liao, Y.; Sørensen, E.S.; Beier, F.; Hunter, G.K.; Goldberg, H.A. Osteopontin mediates mineralization and not osteogenic cell development in vitro. Biochem. J. 2014, 464, 355–364. [Google Scholar] [CrossRef]
- Chen, J.; McKee, M.D.; Nanci, A.; Sodek, J. Bone sialoprotein mRNA expression and ultrastructural localization in fetal porcine calvarial bone: Comparisons with osteopontin. Histochem. J. 1994, 26, 67–78. [Google Scholar] [CrossRef]
- Sodek, J.; Chen, J.; Nagata, T.; Kasugai, S.; Todescan, R.J.; Li, I.W.; Kim, R.H. Regulation of osteopontin expression in osteoblasts. Ann. N. Y. Acad. Sci. 1995, 760, 223–241. [Google Scholar] [CrossRef]
- Shen, C.; Yang, C.; Xu, S.; Zhao, H. Comparison of osteogenic differentiation capacity in mesenchymal stem cells derived from human amniotic membrane (AM), umbilical cord (UC), chorionic membrane (CM), and decidua (DC). Cell Biosci. 2019, 9, 17. [Google Scholar] [CrossRef]
- Morinobu, M.; Ishijima, M.; Rittling, S.R.; Tsuji, K.; Yamamoto, H.; Nifuji, A.; Denhardt, D.T.; Noda, M. Osteopontin Expression in Osteoblasts and Osteocytes During Bone Formation Under Mechanical Stress in the Calvarial Suture In Vivo. J. Bone Miner. Res. 2003, 18, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.; Prestinicola, L.; Scardi, M.; Palamara, E.; Cataudella, S.; Boglione, C. Progress in modeling quality in aquaculture: An application of the Self-Organizing Map to the study of skeletal anomalies and meristic counts in gilthead seabream (Sparus aurata, L. 1758). J. Appl. Ichthyol. 2010, 26, 360–365. [Google Scholar] [CrossRef]
- Kong, D.; Yan, Y.; He, X.-Y.; Yang, H.; Liang, B.; Wang, J.; He, Y.; Ding, Y.; Yu, H. Effects of Resveratrol on the Mechanisms of Antioxidants and Estrogen in Alzheimer’s Disease. Biomed Res. Int. 2019, 2019, 8983752. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, J.; Lin, T.; Huang, S.; Ma, J.; Xu, X. Excessive production of mitochondrion-derived reactive oxygen species induced by titanium ions leads to autophagic cell death of osteoblasts via the SIRT3/SOD2 pathway. Mol. Med. Rep. 2020, 22, 257–264. [Google Scholar] [CrossRef]
- Lall, S.P. The Minerals. Fish Nutr. 2003, 259–308. [Google Scholar] [CrossRef]
- Lall, S.P.; Lewis-McCrea, L.M. Role of nutrients in skeletal metabolism and pathology in fish—An overview. Aquaculture 2007, 267, 3–19. [Google Scholar] [CrossRef]
- Oliveira, P.J.; Bjork, J.A.; Santos, M.S.; Leino, R.L.; Froberg, M.K.; Moreno, A.J.; Wallace, K.B. Carvedilol-Mediated antioxidant protection against doxorubicin-induced cardiac mitochondrial toxicity. Toxicol. Appl. Pharmacol. 2004, 200, 159–168. [Google Scholar] [CrossRef]
- Ascensão, A.; Lumini-Oliveira, J.; Machado, N.G.; Ferreira, R.M.; Gonçalves, I.O.; Moreira, A.C.; Marques, F.; Sardão, V.A.; Oliveira, P.J.; Magalhães, J. Acute exercise protects against calcium-induced cardiac mitochondrial permeability transition pore opening in doxorubicin-treated rats. Clin. Sci. 2010, 120, 37–49. [Google Scholar] [CrossRef]
- Santos, D.; Moreno, A.; Leino, R.; Froberg, M.; Wallace, K. Carvedilol protects against doxorubicin-induced mitochondrial cardiomyopathy. Toxicol. Appl. Pharmacol. 2002, 185, 218–227. [Google Scholar] [CrossRef]
- Clementi, M.E.; Giardina, B.; Di Stasio, E.; Mordente, A.; Misiti, F. Doxorubicin-Derived metabolites induce release of cytochrome C and inhibition of respiration on cardiac isolated mitochondria. Anticancer Res. 2003, 23, 2445–2450. [Google Scholar]
- Zhou, S.; Starkov, A.; Kent, F.M.; And, L.L.R.; Kendall, W.B. Cumulative and Irreversible Cardiac Mitochondrial Dysfunction Induced by Doxorubicin|Cancer Research. Cancer Res. 2001, 61, 771–777. [Google Scholar] [PubMed]
- Bushinsky, D.A.; Riordon, D.R.; Chan, J.S.; Krieger, N.S. Decreased potassium stimulates bone resorption. Am. J. Physiol. 1997, 272, F774–F780. [Google Scholar] [CrossRef] [PubMed]
- Betancor, M.B.; Caballero, M.J.; Terova, G.; Corà, S.; Saleh, R.; Benítez-Santana, T.; Bell, J.G.; Hernández-Cruz, C.M.; Izquierdo, M. Vitamin C Enhances Vitamin E Status and Reduces Oxidative Stress Indicators in Sea Bass Larvae Fed High DHA Microdiets. Lipids 2012, 47, 1193–1207. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Guo, T.; Li, G.; Sun, S.; He, S.; Cheng, B.; Shi, B.; Shan, A. Dietary resveratrol improves antioxidant status of sows and piglets and regulates antioxidant gene expression in placenta by Keap1-Nrf2 pathway and Sirt1. J. Anim. Sci. Biotechnol. 2018, 9, 34. [Google Scholar] [CrossRef]
- Lian, K.; Wang, Q.; Zhao, S.; Yang, M.; Chen, G.; Chen, Y.; Li, C.; Gao, H.; Li, C. Pretreatment of Diabetic Adipose-derived Stem Cells with mitoTEMPO Reverses their Defective Proangiogenic Function in Diabetic Mice with Critical Limb Ischemia. Cell Transplant. 2019, 28, 1652–1663. [Google Scholar] [CrossRef]
- Betancor, M.B.; Nordrum, S.; Atalah, E.; Caballero, M.J.; Benítez-Santana, T.; Roo, J.; Robaina, L.; Izquierdo, M. Potential of three new krill products for seabream larval production. Aquac. Res. 2012, 43, 395–406. [Google Scholar] [CrossRef]
- Zhou, L.; Kuai, F.; Shi, Q.; Yang, H. Doxorubicin restrains osteogenesis and promotes osteoclastogenesis in vitro. Am. J. Transl. Res. 2020, 12, 5640–5654. [Google Scholar]
- McSweeney, K.M.; Bozza, W.P.; Alterovitz, W.-L.; Zhang, B. Transcriptomic profiling reveals p53 as a key regulator of doxorubicin-induced cardiotoxicity. Cell Death Discov. 2019, 5, 102. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, J.; Feng, Z. The regulation of cellular metabolism by tumor suppressor p53. Cell Biosci. 2013, 3, 9. [Google Scholar] [CrossRef]
- Yu, T.; Wang, Z.; You, X.; Zhou, H.; He, W.; Li, B.; Xia, J.; Zhu, H.; Zhao, Y.; Yu, G.; et al. Resveratrol promotes osteogenesis and alleviates osteoporosis by inhibiting p53. Aging 2020, 12, 10359–10369. [Google Scholar] [CrossRef]

| Ingredients (g/kg) | Control | Resveratrol | MitoTEMPO | Doxorubicin |
|---|---|---|---|---|
| Squid powder | 702.00 | 702.00 | 702.00 | 702.00 |
| Gelatin | 30.00 | 30.00 | 30.00 | 30.00 |
| Krill oil | 130 | 130 | 130 | 130 |
| Mineral premix | 45.00 | 45.00 | 45.00 | 45.00 |
| SelPlex | 3.00 | 3.00 | 3.00 | 3.00 |
| Vitamin premix | 60.00 | 60.00 | 60.00 | 60.00 |
| Attractants | 30.00 | 30.00 | 30.00 | 30.00 |
| Resveratrol | --- | 0.034 | --- | --- |
| MitoTEMPO | --- | --- | 0.005 | --- |
| Doxorubicin | --- | --- | --- | 0.030 |
| Vitamin Premix | Mineral Premix | g/kg | |
|---|---|---|---|
| Hydro-soluble Vitamin | g/kg | NaCl | 2.15133 |
| Cyanocobalamin B12 | 0.0003 | MgSO4.7H2O | 6.77545 |
| Astaxanthin | 0.05 | NaH2PO4.H2O | 3.81453 |
| Folate | 0.0544 | K2HPO4 | 7.58949 |
| Pyridoxine B6 | 0.1728 | Ca(H2PO4).2H2O | 6.7161 |
| Thiamine B1 | 0.2177 | FeC6H5O7 | 1.46884 |
| Riboflavin B2 | 0.7253 | C3H5O3.1/2Ca | 16.1721 |
| Pantothenata calcium B5 | 1.0159 | Al2(SO4)3.6H2O | 0.00693 |
| 4-Aminobenzoic acid | 1.45 | ZnSO4.7H2O | 0.14837 |
| Nicotinic acid B3 | 2.9016 | CuSO4.5H2O | 0.01247 |
| Inositol | 14.509 | MnSO4.H2O | 0.02998 |
| Sub Total | 21.097 | KI | 0.00742 |
| Lipo-soluble Vitamin | g/kg | CoSO4.7H2O | 0.10706 |
| Retinoic acid Vit A | 0.0024 | Total | 45.00007 |
| Cholecalciferol Vit D3 | 0.0365 | ||
| Menadione Vit K | 0.1728 | Attractants | g/kg |
| α-Tocopherol acetate (Vit E Acetate) | 1.5 | Inosine-5-monophosphate (Inosinc acid) | 5 |
| Sub total | 1.7117 | Betaine (Trimethylglycine) | 6.6 |
| Ascorbyl polyphosphate (V.C) | 1.8 | L-Serine | 1.7 |
| Choline Chloride | 29.658 | L-Tyrosine | 1.7 |
| L-Phenylalanine | 2.5 | ||
| Vitamin premix Total | 54.2667 | DL-Alanine | 5 |
| L-Aspartic acid | 3.3 | ||
| L-Valine | 2.5 | ||
| Glycine | 1.7 | ||
| Total | 30 |
| Regions affected |
|---|
| A. Cephalic (first–second vertebra; carrying epipleural ribs) |
| B. Pre-haemal or Pleural (With open haemal arches carrying epipleural or pleural ribs, without haemal spines) |
| C. Haemal or pre caudal (With haemal and neural arches closed by spines) |
| D. Caudal (With haemal and neural arches closed by modified spines) |
| E. Pectoral fin |
| F. Anal fin |
| G. Caudal fin |
| H. Dorsal fin |
| Anomalies Considered |
| 1. Skoliosis |
| 2. Lordosis |
| 3. Kyphosis |
| 4. Vertebral fusion |
| 5. Vertebral body malformation |
| 6. Malformed neural arch and/or spine |
| 7. Malformed haemal arch and/or spine and/or rib |
| 8. Malformed ray (deformed, absent, fused, supernumerary) |
| 9. Malformed pterygiophores (deformed, absent, fused, supernumerary) |
| 10. Malformed hypural (deformed, absent, fused, supernumerary) |
| 11. Malformed epural (deformed, absent, fused, supernumerary) |
| 12. Others |
| 13. Jaw deformities |
| 14. Reduced dental/malformed Pre-maxillary and/or maxillary |
| 15. Cephalic deformities (glossohyal, neurocranium, etc.) |
| 16. Vertebral slipping |
| 17. Deformed or reduced opercle |
| 18. Supernumerary vertebra |
| 19. Ectopic mineralization |
| 20. Absent |
| Gene | GenBank (Accession No.) | 5′–3′ Primer Sequence |
|---|---|---|
| catalase (cat) | Q308823.1 | Fw: TGGTCGAGAACTTGAAGGCTGTC |
| Rev: AGGACGCAGAAATGGCAGAGG | ||
| glutathione peroxidase 1 (gpx1) | KC201352.1 | Fw: GAAGGTGGATGTGAATGGAAAAGATG |
| Rev: CTGACGGGACTCCAAATGATGG | ||
| superoxide dismutase 1 (sod1) | XM_030439011.1 | Fw: TGACGCTCACAGGAGAAATCAAAGGG |
| Rev: CAGTAGGACCGCCATGATTCTTACCA | ||
| heat shock protein 90 kDA alpha 1 (hsp90) | KM522802.1 | Fw: TGCCTGGAACTCTTCACCGAACTG |
| Rev: CGCAGCAGATCAGACAACTTCTTCCT | ||
| osteopontin (spp1) | AY651247.1 | Fw: TACCATCGTCACGGACACAGAGACAG |
| Rev: GCTCGTAGGACTTGTAGGGAACAGG | ||
| ß-actin (actb) | AF384096.1 | Fw: CTTCCTCGGTATGGAGTCCTGCGG |
| Rev: TCCTGCTTGCTGATCCACATCTGCT |
| Cranial Vertebrae | Pre-Haemal Vertebrae | Haemal Vertebrae | Caudal Fin | DORSAL FIN | Anal Fin | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mineralized | Mineralizing | Mineralized | Mineralizing | Mineralized | Mineralizing | Epural | Ectopic C. in hypuralia | Rays (Mineralizing) | Rays (Unmineralized) | Pterygiophores (Cartilaginous) | Lepidotrichia (Mineralizing) | Lepidotrichia (Unmineralized) | Pterygiophores (Cartilaginous) | Lepidotrichia (Mineralizing) | Lepidotrichia (Unmineralized) | ||
| CONTROL | Mean | 0.3667 * | 0.2367 * | 2.0633 * | 1.28 * | 0.0533 *δ | 0.15 * | 3.53 * | 0.7567 * | 13.78 * | 2.58 * | 11.92 * | 0.87 * | 6.4533 * | 7.6967 * | 0.8133 *δ | 5.1933 *δ |
| Std. | 0.4827 | 0.42575 | 3.08074 | 1.55014 | 0.37987 | 0.56737 | 0.67623 | 0.81 | 3.24552 | 2.18774 | 9.08085 | 2.87064 | 6.88422 | 5.24624 | 2.72947 | 5.36955 | |
| RES | Mean | 0.5304 * | 0.1791 * | 3.1993 * | 1.3311 * | 0.1824 * | 0.1723 * | 3.4561 * | 0.6723 * | 14.6723 * | 2.0676 * | 12.0946 * | 1.4899 * | 5.8277 * | 7.9122 * | 1.375 * | 4.5946 * |
| Std. | 0.49992 | 0.38405 | 3.42238 | 1.37724 | 0.88327 | 0.62222 | 0.58056 | 0.78 | 2.98085 | 1.96981 | 8.98272 | 3.50785 | 5.91413 | 5.01784 | 3.08379 | 4.88975 | |
| MT | Mean | 0.4047 * | 0.1271 *δ | 2.4314 * | 0.9699 *δ | 0.1271 * | 0.1271 * | 3.5017 * | 0.5585 * | 12.903 * | 2.9431 * | 10.5786 * | 0.9264 *δ | 5.9331 * | 6.6656 * | 0.8361 *δ | 4.5786 * |
| Std. | 0.49165 | 0.33363 | 3.27762 | 1.38148 | 0.75345 | 0.53461 | 0.51406 | 0.708826785 | 3.11318 | 2.1253 | 9.51913 | 2.79524 | 6.15479 | 5.50246 | 2.52967 | 4.88076 | |
| DOX | Mean | 0.4392 * | 0.125 *δ | 2.3547 * | 0.8851 * | 0.0541 *δ | 0.0507 * | 3.4662 *δ | 0.5946 *δ | 13.9189 * | 2.2399 * | 13.5878 * | 1.2568 * | 8.2061 * | 8.3649 *δ | 1.0878 * | 6.1723 * |
| Std. | 0.49713 | 0.33128 | 3.14279 | 1.3405 | 0.46989 | 0.27457 | 0.63689 | 0.677361159 | 2.97564 | 2.04688 | 9.15251 | 3.15717 | 7.18577 | 5.07055 | 2.84737 | 7 | |
| DOX+RES | Mean | 0.5973 * | 0.1107 * | 3.4497 | 0.9564 *δ | 0.1242 * | 0.2483 * | 3.4732 *δ | 0.5906 *δ | 14.4832 * | 2.1745 * | 13.1745 * | 2.2483 * | 6.6946 * | 8.3289 *δ | 1.8658 * | 5.151 * |
| Std. | 0.49126 | 0.31434 | 3.49205 | 1.16115 | 0.6777 | 0.68565 | 0.55135 | 0.756684446 | 2.78187 | 1.59009 | 8.81324 | 4.49452 | 6.41946 | 4.96173 | 3.73654 | 4 | |
| DOX+MT | Mean | 0.5498 * | 0.0859 * | 2.9416 * | 1.055 * | 0.0893 * | 0.1649 * | 3.4777 * | 0.7595 * | 14.0344 * | 2.5636 * | 12.2337 * | 1.488 * | 7.0172 * | 7.7629 * | 1.2749 * | 5.5326 * |
| Std. | 0.49837 | 0.28071 | 3.24622 | 1.22773 | 0.62056 | 0.5933 | 0.52063 | 0.781602051 | 3.05109 | 1.68929 | 9.20871 | 3.71702 | 6.71358 | 5.29822 | 3.03429 | 5.36285 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poudel, S.; Izquierdo, M.; Cancela, M.L.; Gavaia, P.J. Reversal of Doxorubicin-Induced Bone Loss and Mineralization by Supplementation of Resveratrol and MitoTEMPO in the Early Development of Sparus aurata. Nutrients 2022, 14, 1154. https://doi.org/10.3390/nu14061154
Poudel S, Izquierdo M, Cancela ML, Gavaia PJ. Reversal of Doxorubicin-Induced Bone Loss and Mineralization by Supplementation of Resveratrol and MitoTEMPO in the Early Development of Sparus aurata. Nutrients. 2022; 14(6):1154. https://doi.org/10.3390/nu14061154
Chicago/Turabian StylePoudel, Sunil, Marisol Izquierdo, Maria Leonor Cancela, and Paulo J. Gavaia. 2022. "Reversal of Doxorubicin-Induced Bone Loss and Mineralization by Supplementation of Resveratrol and MitoTEMPO in the Early Development of Sparus aurata" Nutrients 14, no. 6: 1154. https://doi.org/10.3390/nu14061154
APA StylePoudel, S., Izquierdo, M., Cancela, M. L., & Gavaia, P. J. (2022). Reversal of Doxorubicin-Induced Bone Loss and Mineralization by Supplementation of Resveratrol and MitoTEMPO in the Early Development of Sparus aurata. Nutrients, 14(6), 1154. https://doi.org/10.3390/nu14061154









