Exosomal FZD-7 Expression Is Modulated by Different Lifestyle Interventions in Patients with NAFLD
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients’ Diagnostics Assessment
2.2. Patient Enrollment
2.3. Interventions
2.4. Exosome Isolation
2.5. Dynamic Light Scattering and ζ-Potential Investigation
2.6. Transmission Electronic Microscopy Investigation
2.7. Protein Extraction and Quantification from Exosomes
2.8. Statistical Analysis
3. Results
3.1. Evaluation of the FZD7 Expression Levels in Plasma-Derived Exosomes
3.2. Characteristics of Participants
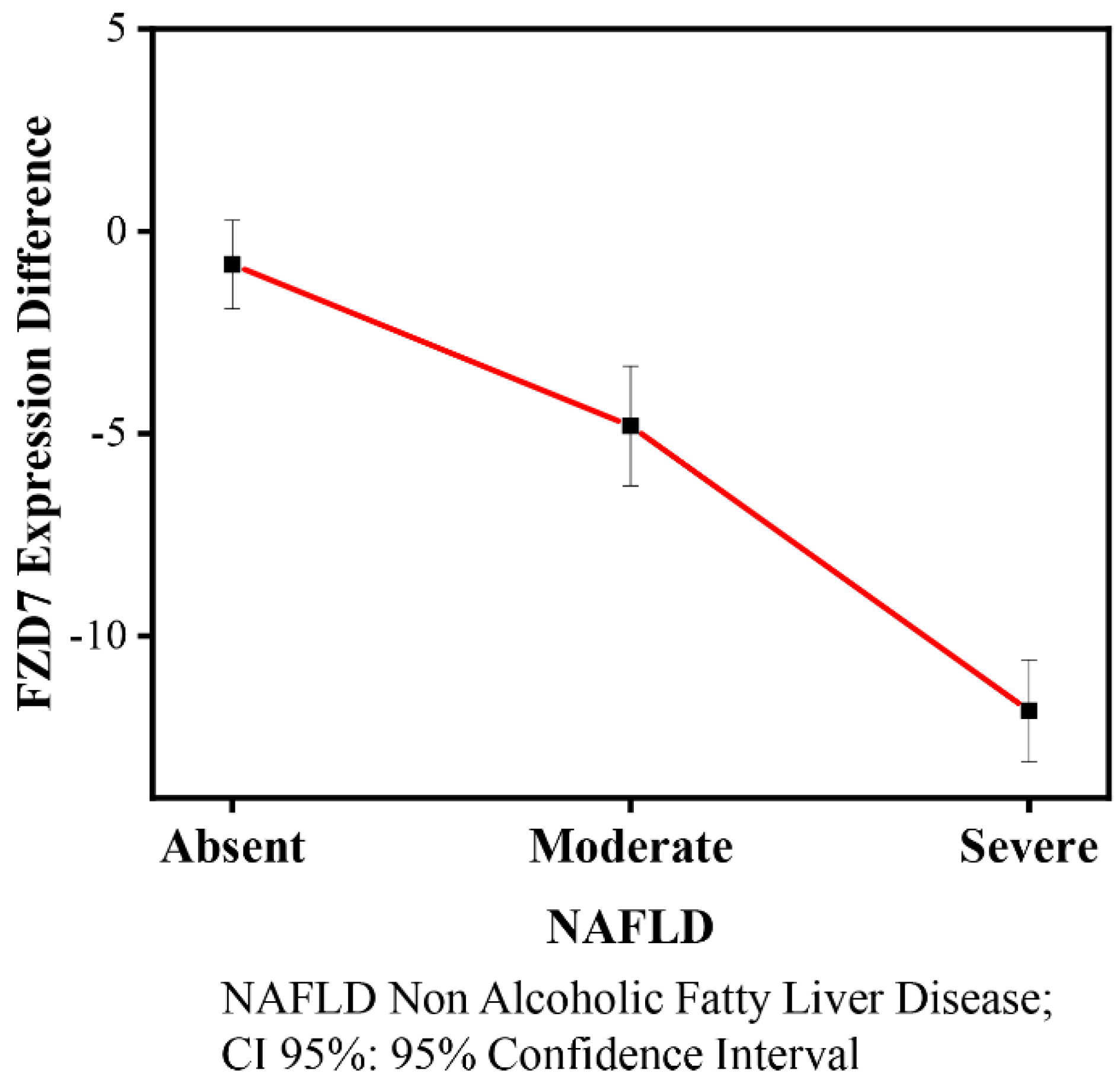
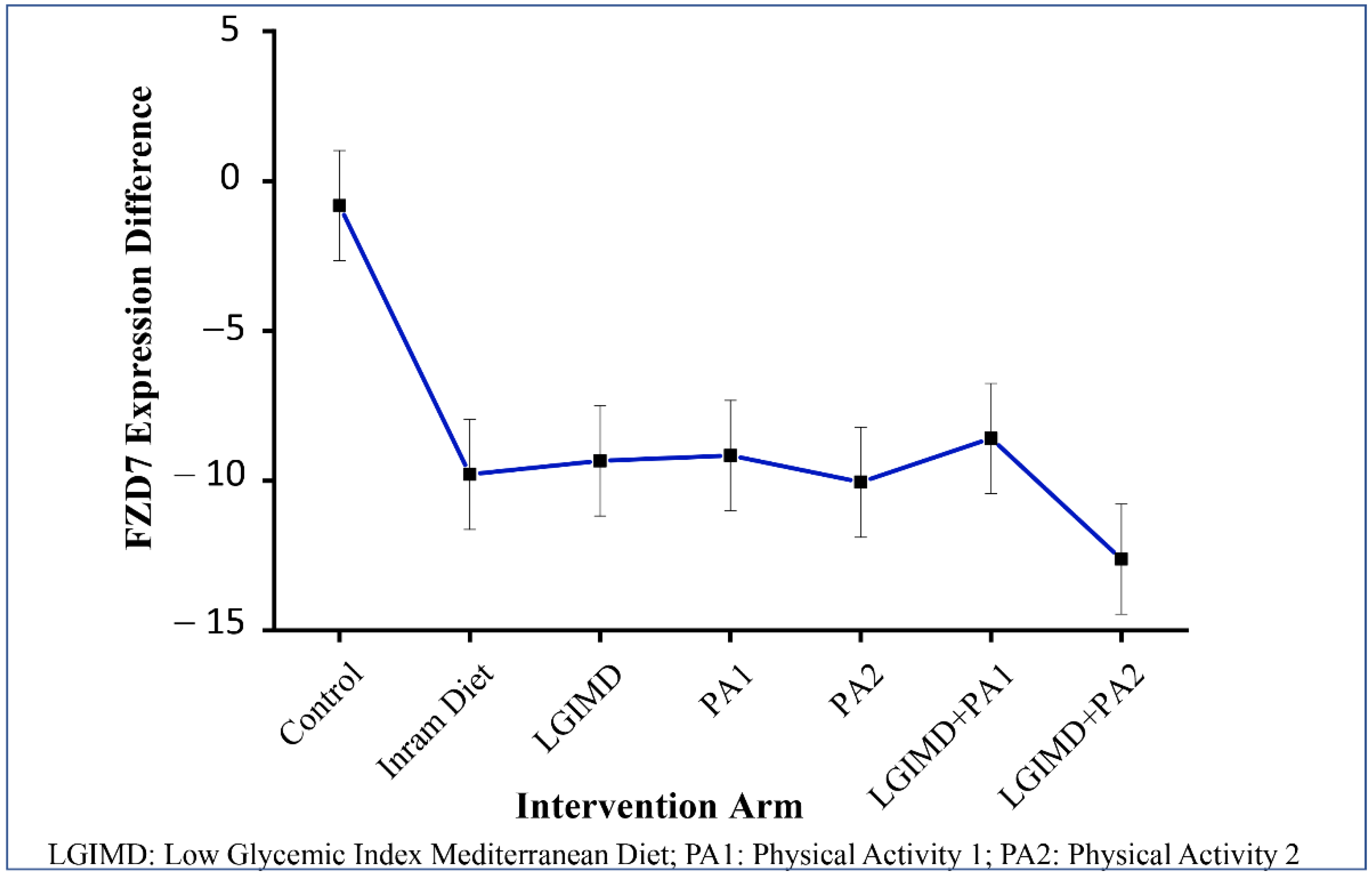
3.3. Effects of Diet, Lifestyle, Sex, and Age on FZD7 Expression
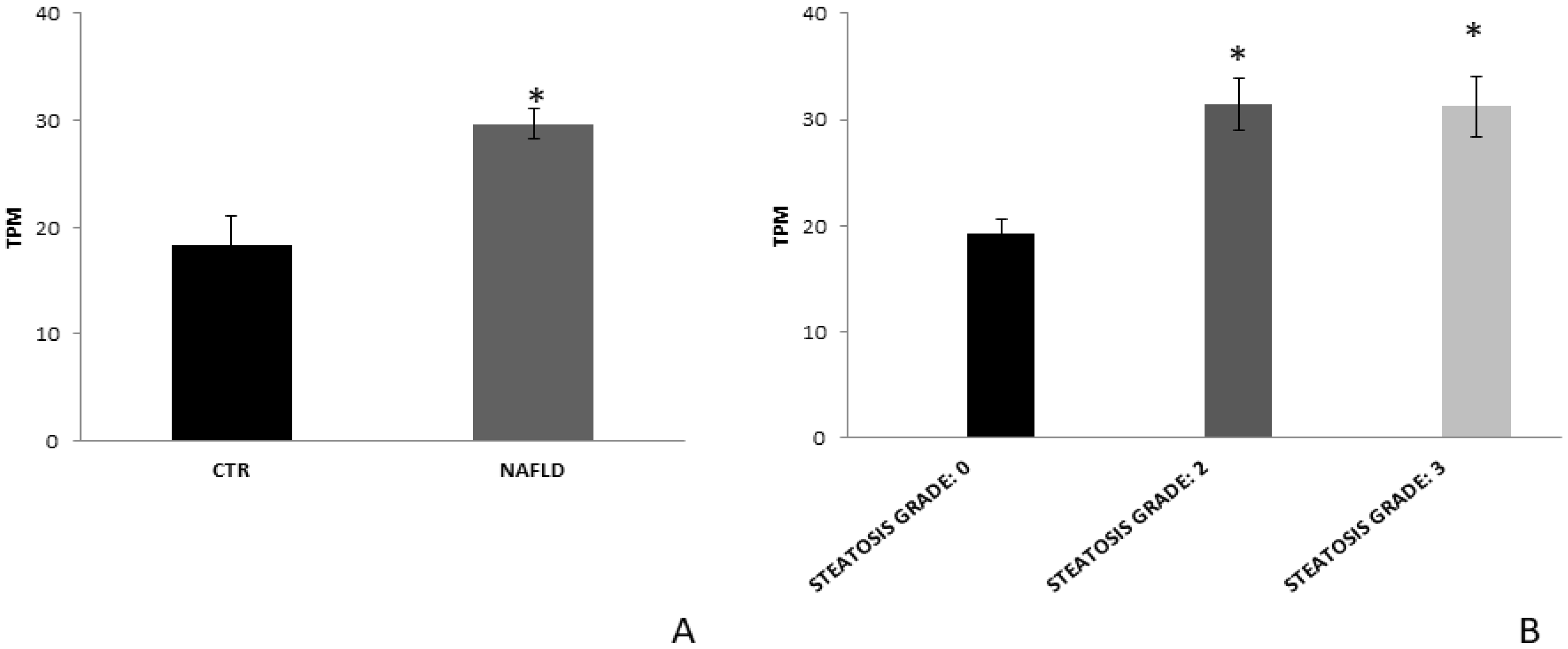
3.4. FZD7 Expression in Liver Tissue Derived from NAFLD Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibrahim, S.H.; Hirsova, P.; Gores, G.J. Non-alcoholic steatohepatitis pathogenesis: Sublethal hepatocyte injury as a driver of liver inflammation. Gut 2018, 67, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Anstee, Q.M.; Tilg, H.; Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017, 66, 1138–1153. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M. Endothelial dysfunction: The first step toward coronary arteriosclerosis. Circ. J. 2009, 73, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; El-Serang, H.B.; Sada, Y.H.; Kanwal, F.; Duan, Z.; Temple, S.; May, S.B.; Kramer, J.R.; Richardson, P.A.; Davila, J.A. Hepatocellular carcinoma in the absence of cirrhosis in United States Veterans is associated with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2016, 14, 124–131.e1. [Google Scholar] [CrossRef] [PubMed]
- Nejak-Bowen, K.; Monga, S.P. Wnt/beta-catenin signaling in hepatic organogenesis. Organogenesis 2008, 4, 92–99. [Google Scholar] [CrossRef]
- Bi, Y.; Huang, J.; He, Y.; Zhu, G.H.; Su, Y.; He, B.C.; Luo, J.; Wang, Y.; Kang, Q.; Luo, Q.; et al. Wnt antagonist SFRP3 inhibits the differentiation of mouse hepatic progenitor cells. J. Cell Biochem. 2009, 108, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Kwan-Shuen Chan, K.; Cheuk-Lam Lo, R. Deregulation of Frizzled Receptors in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2018, 19, 313. [Google Scholar] [CrossRef]
- Cadoret, A.; Ovejero, C.; Terris, B.; Souil, E.; Levy, L.; Lamers, W.H.; Kitajewski, J.; Kahn, A.; Perret, C. New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene 2002, 21, 8293–8301. [Google Scholar] [CrossRef]
- Cavard, C.; Terris, B.; Grimber, G.; Christa, L.; Audard, V.; Radenen-Bussiere, B.; Simon, M.T.; Renard, C.A.; Buendia, M.A.; Perret, C. Overexpression of regenerating islet-derived 1 alpha and 3 alpha genes in human primary liver tumors with beta-catenin mutations. Oncogene 2006, 25, 599–608. [Google Scholar] [CrossRef]
- Scavo, M.P.; Depalo, N.; Tutino, V.; De Nunzio, V.; Ingrosso, C.; Rizzi, F.; Notarnicola, M.; Curri, M.L.; Giannelli, G. Exosomes for Diagnosis and Therapy in Gastrointestinal Cancers. Int. J. Mol. Sci. 2020, 21, 367. [Google Scholar] [CrossRef]
- Sasaki, R.; Kanda, T.; Yokosuka, O.; Kato, N.; Matsuoka, S.; Moriyama, M. Exosomes and Hepatocellular Carcinoma: From Bench to Bedside. Int. J. Mol. Sci. 2019, 20, 1406. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Osella, A.R.; Caruso, M.G.; Pesole, P.L.; Lippolis, A.; Tutino, V.; Bonfiglio, C.; De Nunzio, V.; Scavo, M.P.; Mirizzi, A.; et al. Nonalcoholic Fatty Liver Disease: Focus on New Biomarkers and Lifestyle Interventions. Int. J. Mol. Sci. 2021, 22, 3899. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.; Arab, J.P.; Reyes, D.; Lapitz, A.; Moshage, H.; Bañales, J.M.; Arrese, M. Extracellular Vesicles in NAFLD/ALD: From Pathobiology to Therapy. Cells 2020, 9, 817. [Google Scholar] [CrossRef]
- Heyens, L.J.M.; Busschots, D.; Koek, G.H.; Robaeys, G.; Francque, S. Liver Fibrosis in Non-alcoholic Fatty Liver Disease: From Liver Biopsy to Non-invasive Biomarkers in Diagnosis and Treatment. Front. Med. 2021, 8, 615978. [Google Scholar] [CrossRef] [PubMed]
- Sasso, M.; Beaugrand, M.; de Ledinghen, V.; Douvin, C.; Marcellin, P.; Poupon, R.; Sandrin, L.; Miette, V. Controlled Attenuation Parameter (CAP): A Novel VCTE™ Guided Ultrasonic Attenuation Measurement for the Evaluation of Hepatic Steatosis: Preliminary Study and Validation in a Cohort of Patients with Chronic Liver Disease from Various Causes. Ultrasound Med. Biol. 2010, 36, 1825–1835. [Google Scholar] [CrossRef]
- Chon, Y.E.; Jung, K.S.; Kim, S.U.; Park, J.Y.; Park, Y.N.; Kim, D.Y.; Ahn, S.H.; Chon, C.Y.; Lee, H.W.; Park, Y. Controlled attenuation parameter (CAP) for detection of hepatic steatosis in patients with chronic liver diseases: A prospective study of a native Korean population. Liver Int. 2013, 34, 102–109. [Google Scholar] [CrossRef]
- Berzigotti, A. Non-invasive assessment of non-alcoholic fatty liver disease: Ultrasound and transient elastography. Rev. Rec. Clin. Trials. 2014, 9, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A. Getting closer to a point-of-care diagnostic assessment in patients with chronic liver disease: Controlled attenuation parameter for steatosis. J. Hepatol. 2014, 60, 910–912. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Caruso, M.G.; Tutino, V.; Bonfiglio, C.; Cozzolongo, R.; Giannuzzi, V.; De Nunzio, V.; De Leonardis, G.; Abbrescia, D.I.; Franco, I.; et al. Significant decrease of saturation index in erythrocytes membrane from subjects with non-alcoholic fatty liver disease (NAFLD). Lipids Heal. Dis. 2017, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mirizzi, A.; Franco, I.; Leone, C.M.; Bonfiglio, C.; Cozzolongo, R.; Notarnicola, M.; Giannuzzi, V.; Tutino, V.; De Nunzio, V.; Bruno, I.; et al. Effects of Some Food Components on Non-Alcoholic Fatty Liver Disease Severity: Results from a Cross-Sectional Study. Nutrients 2019, 11, 2744. [Google Scholar] [CrossRef]
- Tutino, V.; De Nunzio, V.; Caruso, M.G.; Bonfiglio, C.; Franco, I.; Mirizzi, A.; De Leonardis, G.; Cozzolongo, R.; Giannuzzi, V.; Giannelli, G.; et al. Aerobic Physical Activity and a Low Glycemic Diet Reduce the AA/EPA Ratio in Red Blood Cell Membranes of Patients with NAFLD. Nutrients 2018, 10, 1299. [Google Scholar] [CrossRef] [PubMed]
- Cozzolongo, R.; Osella, A.R.; Elba, S.; Petruzzi, J.; Buongiorno, G.; Giannuzzi, V.; Leone, G.; Bonfiglio, C.; Lanzilotta, E.; Manghisi, O.G.; et al. Epidemiology of HCV infection in the general population: A survey in a southern Italian town. Am. J. Gastroenterol. 2009, 104, 2740–2746. [Google Scholar] [CrossRef] [PubMed]
- Misciagna, G.; Leoci, C.; Guerra, V.; Chiloiro, M.; Elba, S.; Petruzzi, J.; Mossa, A.; Noviello, M.R.; Coviello, A.; Minutolo, M.C.; et al. Epidemiology of cholelithiasis in southern Italy. Part II: Risk factors. Eur. J. Gastroenterol. Hepatol. 1996, 8, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, R.; Oja, P.; Pasanen, M.; Vuori, I. Validity of a two kilometers walking test for estimating maximal aerobic power in overweight adults. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1992, 16, 263–268. [Google Scholar]
- Hoeger, W.W.; Hopkins, D.R. A comparison of the sit and reach and the modified sit and reach in the measurement of flexibility in women. Res. Q. Exerc. Sport 1992, 63, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Cialfa, E.; D’amicis, A.; Leclercq, C.; Quaglia, G.; Sette, S.; Ticca, M.; Tomassi, G. Linee guida per una sana alimentazione italiana. Rome Ist. Naz. Ric. Gli. Aliment. Nutr. 2003, 86, 7–94. [Google Scholar]
- Misciagna, G.; Del Pilar Diaz, M.; Caramia, D.V.; Bonfiglio, C.; Franco, I.; Noviello, M.R.; Chiloiro, M.; Abbrescia, D.I.; Mirizzi, A.; Tanzi, M.; et al. Effect of a Low Glycemic Index Mediterranean Diet on Non-Alcoholic Fatty Liver Disease. A Randomized Controlled Clinici Trial. J. Nutr. Health Aging 2017, 21, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Scavo, M.P.; Cigliano, A.; Depalo, N.; Fanizza, E.; Bianco, M.G.; Denora, N.; Laquintana, V.; Curri, M.L.; Lorusso, D.; Lotesoriere, C.; et al. Frizzled-10 Extracellular Vesicles Plasma Concentration Is Associated with Tumoral Progression in Patients with Colorectal and Gastric Cancer. J. Oncol. 2019, 2019, 2715968. [Google Scholar] [CrossRef] [PubMed]
- Whitham, B.L.; Parker, I.; Friedrichsen, M. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. 2018, 27, 237–251. [Google Scholar] [CrossRef]
- Scavo, M.P.; Rizzi, F.; Depalo, N.; Armentano, R.; Coletta, S.; Serino, G.; Fanizza, E.; Pesole, P.L.; Cervellera, A.; Carella, N.; et al. Exosome Released FZD10 Increases Ki-67 Expression via Phospho-ERK1/2 in Colorectal and Gastric Cancer. Front. Oncol. 2021, 11, 3797. [Google Scholar] [CrossRef] [PubMed]
- Latronico, T.; Rizzi, F.; Panniello, A.; Laquintana, V.; Arduino, I.; Denora, N.; Fanizza, E.; Milella, S.; Mastroianni, C.M.; Striccoli, M.; et al. Luminescent PLGA Nanoparticles for Delivery of Darunavir to the Brain and Inhibition of Matrix Metalloproteinase-9, a Relevant Therapeutic Target of HIV-Associated Neurological Disorders. ACS Chem. Neurosci. 2021, 12, 4286–4301. [Google Scholar] [CrossRef] [PubMed]
- Scavo, M.P.; Depalo, N.; Rizzi, F.; Ingrosso, C.; Fanizza, E.; Chieti, A.; Messa, C.; Denora, N.; Laquintana, V.; Striccoli, M. FZD10 Carried by Exosomes Sustains Cancer Cell Proliferation. Cells 2019, 8, 777. [Google Scholar] [CrossRef] [PubMed]
- Hoang, S.A.; Oseini, A.; Feaver, R.E.; Cole, B.K.; Asgharpour, A.; Vincent, R.; Siddiqui, M.; Lawson, M.J.; Day, N.C.; Taylor, J.M.; et al. Gene Expression Predicts Histological Severity and Reveals Distinct Molecular Profiles of Nonalcoholic Fatty Liver Disease. Sci. Rep. 2019, 9, 12541. [Google Scholar] [CrossRef] [PubMed]
- Reccia, I.; Kumar, J.; Akladios, C.; Virdis, F.; Pai, M.; Habib, N.; Spalding, D. Non-alcoholic fatty liver disease: A sign of systemic disease. Metab. Clin. Exp. 2017, 94, 108. [Google Scholar] [CrossRef] [PubMed]
- Trifan, A.; Stanciu, C.; Jurcău, M.; Zenovia, S.; Frunzuc, G.; Timofte, D. Nonalcoholic steatohepatitis. A scientometric analysis of publications during 1980–2018. Medicine 2019, 98, e18221. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Tsukamoto, H. Stearoyl-CoA desaturase and tumorigenesis. Chemico-Biol. Interact. 2020, 316, 108917. [Google Scholar] [CrossRef]
- Pal Monga, S. Beta-catenin signaling and roles in liver homeostasis, injury, and tumorigenesis. Gastroenterology 2015, 148, 1294–1310. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Parsons, C.J.; Stefanovic, B. Gene expression profile of quiescent and activated rat hepatic stellate cells implicates Wnt signaling pathway in activation. J. Hepatol. 2006, 45, 401–409. [Google Scholar] [CrossRef]
- He, S.; Tang, S. WNT/β-catenin signaling in the development of liver cancers. Biomed. Pharmacother. 2020, 132, 110851. [Google Scholar] [CrossRef] [PubMed]
- Merle, P.; de la Monte, S.; Kim, M.; Herrmann, M.; Tanaka, S.; Von Dem Bussche, A.; Kew, M.C.; Trepo, C.; Wands, J.R. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology 2004, 127, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Pode-Shakked, N.; Harari-Steinberg, O.; Haberman-Ziv, Y.; Rom-Gross, E.; Bahar, S.; Omer, D.; Metsuyanim, S.; Buzhor, E.; Jacob-Hirsch, J.; Goldstein, R.S.; et al. Resistance or sensitivity of Wilms’ tumor to anti-FZD7 antibody highlights the Wnt pathway as a possible therapeutic target. Oncogene 2011, 30, 1664–1680. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gong, L.; Xia, Y.; Qu, L.; Li, Q.; Pang, L.; Si, J.; Li, Z. Frizzled-7 promoter is highly active in tumors and promoter-driven Shiga-like toxin I inhibits hepatocellular carcinoma growth. Oncotarget 2015, 6, 39908–39923. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Bai, Y.; Wang, Z.; Sun, Y.; Gao, J.; Na, L.; Zhang, Z.; Wang, W.; Zhao, C. Non-canonical Fzd7 signaling contributes to breast cancer mesenchymal-like stemness involving. Cell Commun. Signal. 2020, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Zhou, J. Frizzled-7 mediates TGF-beta-induced pulmonary fibrosis by transmitting non-canonical Wnt signaling. Exp. Cell Res. 2017, 359, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gómez, M.; Zelber-Sagi, S.; Michael Trenell. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Campanella, A.; Iacovazzi, P.A.; Misciagna, G.; Bonfiglio, C.; Mirizzi, A.; Franco, I.; Bianco, A.; Sorino, P.; Caruso, M.G.; Cisternino, A.M.; et al. The Effect of Three Mediterranean Diets on Remnant Cholesterol and Non-Alcoholic Fatty Liver Disease: A Secondary Analysis. Nutrients 2020, 12, 1674. [Google Scholar] [CrossRef]
- Franco, I.; Bianco, A.; Del Pilar Dìaz, M.; Bonfiglio, C.; Chiloiro, M.; Pou, S.A.; Becaria Coquet, J.; Mirizzi, A.; Nitti, A.; Campanella, A.; et al. Effectiveness of two physical activity programs on non-alcoholic fatty liver disease. a randomized controlled clinical trial. Rev. Fac. Cien. Med. Univ. Nac. Cordoba 2019, 76, 26–36. [Google Scholar] [CrossRef]
- Franco, I.; Bianco, A.; Mirizzi, A.; Campanella, A.; Bonfiglio, C.; Sorino, P.; Notarnicola, M.; Tutino, V.; Cozzolongo, R.; Giannuzzi, V. Physical Activity and Low Glycemic Index Mediterranean Diet: Main and Modification Effects on NAFLD Score. Results from a Randomized Clinical Trial. Nutrients 2021, 13, 66. [Google Scholar] [CrossRef]
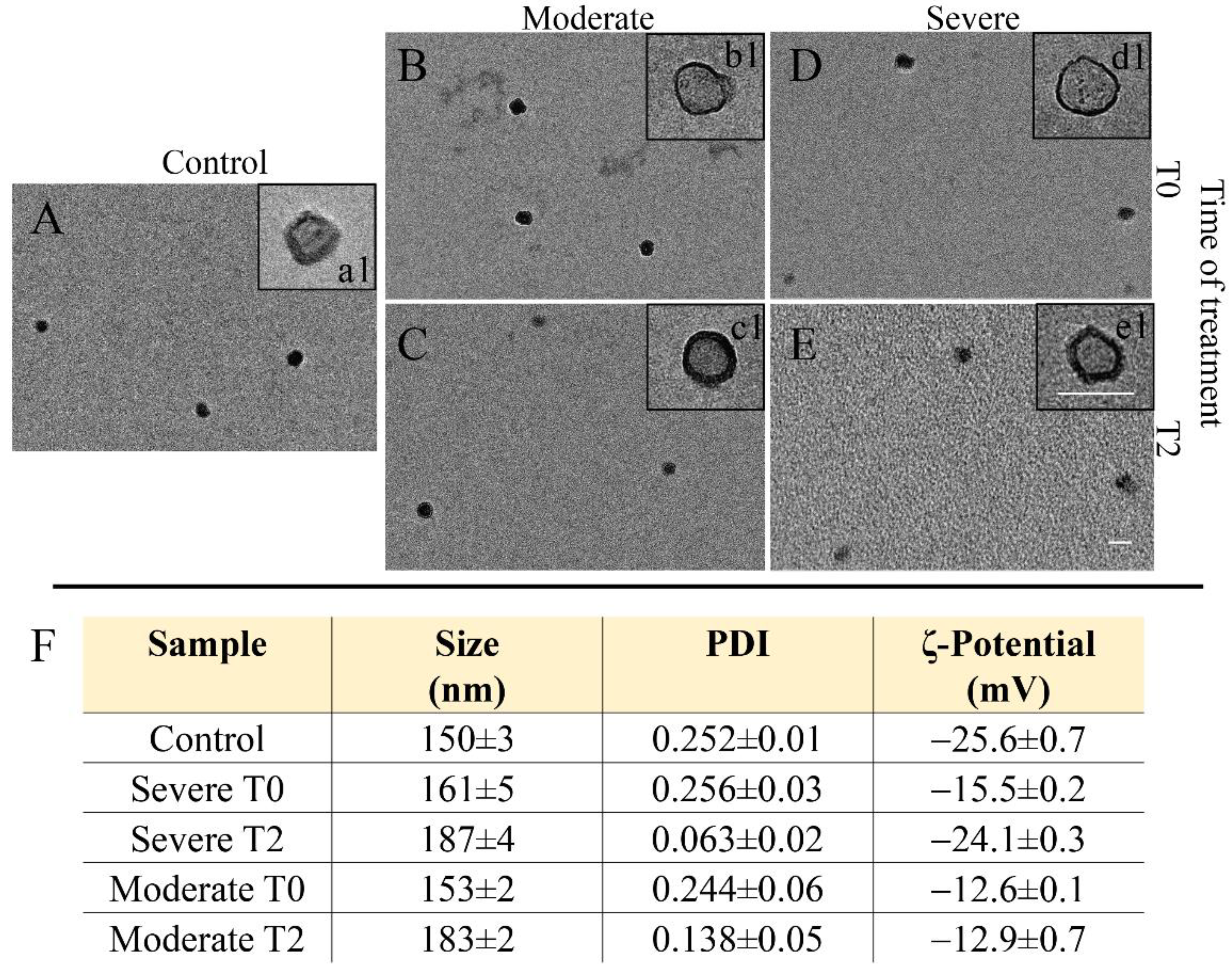
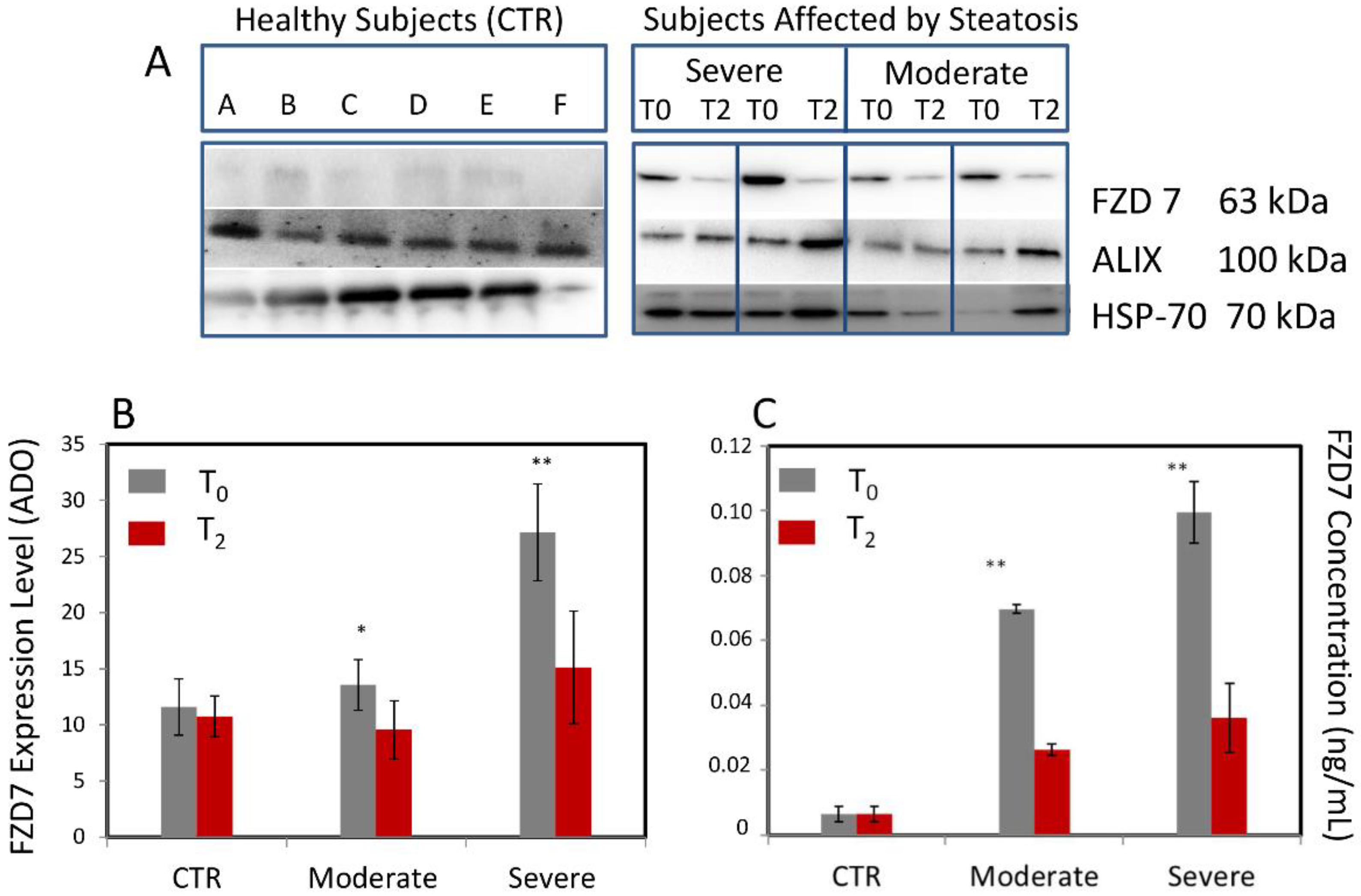
| NAFLD | ||||||||
|---|---|---|---|---|---|---|---|---|
| Absent | Moderate | Severe | Total | |||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Age (categorical, years) | ||||||||
| <40 (n = 19) | 12.30 | (1.05) | 9.85 | (0.97) | 11.56 | (1.18) | 11.71 | (0.73) |
| 40–49 (n = 36) | 10.51 | (0.36) | 9.21 | (0.65) | 16.35 | (1.32) | 13.44 | (0.97) |
| ≥50 (n = 60) | 9.34 | (0.97) | 9.87 | (1.35) | 15.02 | (0.97) | 13.20 | (0.79) |
| Total (n = 115) | 10.76 | (0.65) | 9.56 | (0.70) | 15.11 | (0.73) | 13.05 | (0.53) |
| Sex | ||||||||
| Male (n = 65) | 10.33 | (0.85) | 10.21 | (1.06) | 14.12 | (1.01) | 12.43 | (0.70) |
| Female (n = 50) | 11.19 | (0.97) | 8.49 | (0.31) | 16.14 | (1.03) | 13.77 | (0.80) |
| Total (n = 115) | 10.76 | (0.65) | 9.56 | (0.70) | 15.11 | (0.73) | 13.05 | (0.53) |
| Intervention | ||||||||
| Control (n = 20) | 10.76 | (0.65) | 10.76 | (0.65) | ||||
| Inram diet (n = 16) | 8.05 | (0.46) | 16.47 | (2.02) | 14.22 | (1.77) | ||
| LGIMD (n = 17) | 10.45 | (0.58) | 15.56 | (1.74) | 14.19 | (1.41) | ||
| PA1 (n = 16) | 12.90 | (4.04) | 14.91 | (1.48) | 14.51 | (1.45) | ||
| PA2 (n = 14) | 9.27 | (1.38) | 15.61 | (2.44) | 14.45 | (2.14) | ||
| LGIMD + PA1 (n = 16) | 8.66 | (1.10) | 14.67 | (1.86) | 12.04 | (1.37) | ||
| LGIMD + PA2 (n = 16) | 9.43 | (0.49) | 13.48 | (1.08) | 12.40 | (0.93) | ||
| Total (n = 115) | 10.76 | (0.65) | 9.56 | (0.70) | 15.11 | (0.73) | 13.05 | (0.53) |
| FZD7 | β | SE | p-Value | (95% CI) |
|---|---|---|---|---|
| NAFLD | ||||
| Absent n = 20 | 0 | |||
| Moderate n = 38 | −9.40 | 9.87 | 0.34 | (−28.98; 10.18) |
| Severe n = 57 | 3.27 | 6.58 | 0.62 | (−9.78; 16.33) |
| Time | ||||
| Baseline | 0 | |||
| 90 days | −0.82 | 1.09 | 0.46 | (−2.99; 1.36) |
| NAFLD × Time | ||||
| Moderate × 90 days | −3.99 | 1.48 | 0.008 | (−6.94; −1.05) |
| Severe × 90 days | −11.03 | 1.26 | 0.000 | (−13.52; −8.53) |
| FZD7 | Contrast (CI 95%) |
|---|---|
| (90daysVs baseline) NAFLD absent | −0.82 (−2.99; 1.35) |
| (90days Vs baseline) NAFLD Moderate | −4.81 (−6.80; −2.83) * |
| (90days Vs baseline) NAFLD Severe | −11.85 (−13.07; −10.62) |
| FDZ7 | β | SE | p-Value | (CI 95%) |
|---|---|---|---|---|
| Working arms | ||||
| Control subjects (n = 20) | 0 | |||
| Inram diet (n = 16) | 12.25 | 12.56 | 0.33 | (−12.67; 37.17) |
| LGIMD a (n = 17) | 19.22 | 11.77 | 0.10 | (−4.14; 42.58) |
| PA1 b (n = 16) | 12.04 | 9.02 | 0.18 | (−5.86; 29.93) |
| PA2 c (n = 14) | 31.53 | 15.60 | 0.04 | (−0.58; 62.48) |
| PA1 + LGIMD d (n = 16) | 12.70 | 16.56 | 0.44 | (−20.16; 45.56) |
| PA2 + LGIMD e (n = 16) | 8.39 | 11.77 | 0.47 | (−14.96; 31.76) |
| Time | ||||
| Baseline | 0 | |||
| 90 days | −0.82 | 1.27 | 0.52 | (−3.34; 1.69) |
| (90 days vs. baseline) Inram diet | −8.97 | 1.93 | 0.000 | (−12.82; −5.13) * |
| (90 days vs. baseline) LGIMD a | −8.53 | 1.93 | 0.000 | (−12.37; −4.68) * |
| (90 days vs. baseline) PA1 b | −8.34 | 1.93 | 0.000 | (−12.18; −4.49) * |
| (90 days vs. baseline) PA2 c | −9.23 | 2.13 | 0.000 | (−13.45; −5.01) * |
| (90 days vs. baseline) PA1 + LGIMD d | −7.77 | 1.90 | 0.000 | (−11.54; −3.99) * |
| (90 days vs. baseline) PA2 + LGIMD e | −11.79 | 1.94 | 0.000 | (−15.64; −7.95) * |
| FDZ7 | Contrast | (CI 95%) |
|---|---|---|
| (90 days vs. baseline) control subjects n = 20 | −0.82 | (−3.34; 1.69) |
| (90 days vs. baseline) Inram diet n = 16 | −9.79 | (−12.70; −6.889) * |
| (90 days vs. baseline) LGIMD a n = 17 | −9.34 | (−12.25; −6.44) * |
| (90 days vs. baseline) PA1 b n = 16 | −9.16 | (−12.07; −6.26) * |
| (90 days vs. baseline) PA2 c n = 14 | −10.05 | (−13.44; −6.66) * |
| (90 days vs. baseline) PA1 + LGIMD d n = 16 | −8.59 | (−11.40; −5.78) * |
| (90 days vs. baseline) PA2 + LGIMD e n = 16 | −12.62 | (−15.52; −9.71) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scavo, M.P.; Depalo, N.; Rizzi, F.; Carrieri, L.; Serino, G.; Franco, I.; Bonfiglio, C.; Pesole, P.L.; Cozzolongo, R.; Gianuzzi, V.; et al. Exosomal FZD-7 Expression Is Modulated by Different Lifestyle Interventions in Patients with NAFLD. Nutrients 2022, 14, 1133. https://doi.org/10.3390/nu14061133
Scavo MP, Depalo N, Rizzi F, Carrieri L, Serino G, Franco I, Bonfiglio C, Pesole PL, Cozzolongo R, Gianuzzi V, et al. Exosomal FZD-7 Expression Is Modulated by Different Lifestyle Interventions in Patients with NAFLD. Nutrients. 2022; 14(6):1133. https://doi.org/10.3390/nu14061133
Chicago/Turabian StyleScavo, Maria Principia, Nicoletta Depalo, Federica Rizzi, Livianna Carrieri, Grazia Serino, Isabella Franco, Caterina Bonfiglio, Pasqua Letizia Pesole, Raffaele Cozzolongo, Vito Gianuzzi, and et al. 2022. "Exosomal FZD-7 Expression Is Modulated by Different Lifestyle Interventions in Patients with NAFLD" Nutrients 14, no. 6: 1133. https://doi.org/10.3390/nu14061133
APA StyleScavo, M. P., Depalo, N., Rizzi, F., Carrieri, L., Serino, G., Franco, I., Bonfiglio, C., Pesole, P. L., Cozzolongo, R., Gianuzzi, V., Curri, M. L., Osella, A. R., & Giannelli, G. (2022). Exosomal FZD-7 Expression Is Modulated by Different Lifestyle Interventions in Patients with NAFLD. Nutrients, 14(6), 1133. https://doi.org/10.3390/nu14061133










