Living with Overweight, Rather than a History of Gestational Diabetes, Influences Dietary Quality and Physical Activity during Pregnancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Measures
2.3. Data Analysis
3. Results
3.1. Clinical Characteristics
3.2. Dietary Quality and Physical Activity According to the History of GDM Status
3.3. Dietary Quality and Physical Activity According to the Overweight Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shin, D.; Song, W.O. Prepregnancy body mass index is an independent risk factor for gestational hypertension, gestational diabetes, preterm labor, and small- and large-for-gestational-age infants. J. Matern. Fetal Neonatal Med. 2015, 28, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41 (Suppl. S1), S13–S27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffith, R.J.; Alsweiler, J.; Moore, A.E.; Brown, S.; Middleton, P.; Shepherd, E.; Crowther, C.A. Interventions to prevent women from developing gestational diabetes mellitus: An overview of Cochrane reviews. Cochrane Database Syst. Rev. 2020, 6, CD012394. [Google Scholar] [PubMed]
- National Institute for Health and Welfare. Perinatal Statistics—Parturients, Deliveries and Newborns 2017; Report no. 38/2018; National Institute for Health and Welfare: Helsinki, Finland, 2017. [Google Scholar]
- Hilden, K.; Hanson, U.; Persson, M.; Magnuson, A.; Simmons, D.; Fadl, H. Gestational diabetes and adiposity are independent risk factors for perinatal outcomes: A population based cohort study in Sweden. Diabet. Med. 2019, 36, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Tobias, D.K.; Stuart, J.J.; Li, S.; Chavarro, J.; Rimm, E.B.; Rich-Edwards, J.; Hu, F.B.; Manson, J.E.; Zhang, C. Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern. Med. 2017, 177, 1735–1742. [Google Scholar] [CrossRef]
- Lamminpää, R.; Vehviläinen-Julkunen, K.; Schwab, U. A systematic review of dietary interventions for gestational weight gain and gestational diabetes in overweight and obese pregnant women. Eur. J. Nutr. 2018, 57, 1721–1736. [Google Scholar] [CrossRef] [Green Version]
- Muktabhant, B.; Lawrie, T.A.; Lumbiganon, P.; Laopaiboon, M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst. Rev. 2015, 6, CD007145. [Google Scholar] [CrossRef]
- Working Group Set Up by the Finnish Medical Society Duodecim, the Medical Advisory Board of the Finnish Diabetes Association and the Finnish Gynecological Association. Gestational Diabetes. Current Care Guideline. The Finnish Medical Society Duodecim: Helsinki, Finland, 2013. Available online: www.kaypahoito.fi (accessed on 17 January 2022).
- American Diabetes Association. 13. Management of Diabetes in Pregnancy. Diabetes Care 2017, 40 (Suppl. S1), S114–S119. [Google Scholar] [CrossRef] [Green Version]
- Livingstone, K.M.; McNaughton, S.A. Diet quality is associated with obesity and hypertension in Australian adults: A cross sectional study. BMC Public Health 2016, 16, 1037. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.; Lee, K.W.; Song, W.O. Pre-pregnancy weight status is associated with diet quality and nutritional biomarkers during pregnancy. Nutrients 2016, 8, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leppälä, J.; Lagström, H.; Kaljonen, A.; Laitinen, K. Construction and evaluation of a self-contained index for assessment of diet quality. Scand. J. Public Health 2010, 38, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Pahkala, K.; Heinonen, O.J.; Simell, O.; Viikari, J.S.; Rönnemaa, T.; Niinikoski, H.; Raitakari, O.T. Association of physical activity with vascular endothelial function and intima-media thickness. Circulation 2011, 124, 1956–1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Official Statistics of Finland (OSF). Income Distribution Statistics. 2017. Available online: https://www.stat.fi/til/tjt/2017/index_en.html (accessed on 13 November 2019).
- Hod, M.; Kapur, A.; Sacks, D.A.; Hadar, E.; Agarwal, M.; Di Renzo, G.C.; Roura, L.C.; McIntyre, H.D.; Morris, J.L.; Divakar, H. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int. J. Gynaecol. Obstet. 2015, 131 (Suppl. S3), S173–S211. [Google Scholar] [CrossRef] [Green Version]
- Xiao, R.S.; Simas, T.A.; Person, S.D.; Goldberg, R.J.; Waring, M.E. Diet quality and history of gestational diabetes mellitus among childbearing women, United States, 2007–2010. Prev. Chronic Dis. 2015, 12, E25. [Google Scholar] [CrossRef] [Green Version]
- Persson, M.; Winkvist, A.; Mogren, I. Lifestyle and health status in a sample of Swedish women four years after pregnancy: A comparison of women with a history of normal pregnancy and women with a history of gestational diabetes mellitus. BMC Pregnancy Childbirth 2015, 15, 57. [Google Scholar] [CrossRef] [Green Version]
- Huurre, A.; Laitinen, K.; Hoppu, U.; Isolauri, E. How practice meets guidelines: Evaluation of nutrition counselling in Finnish well-women and well-baby clinics. Acta Paediatr. 2006, 95, 1353–1359. [Google Scholar] [CrossRef]
- Koivusalo, S.B.; Rönö, K.; Klemetti, M.M.; Roine, R.P.; Lindström, J.; Erkkola, M.; Kaaja, R.J.; Pöyhönen-Alho, M.; Tiitinen, A.; Huvinen, E.; et al. Gestational diabetes mellitus can be prevented by lifestyle intervention: The Finnish gestational diabetes prevention study (RADIEL): A randomized controlled trial. Diabetes Care 2016, 39, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Weihua, X.; Pinyuan, D.; Yu, Q.; Ming, W.; Bingquan, Y.; Xiaojin, Y. Effectiveness of telemedicine for pregnant women with gestational diabetes mellitus: An updated meta-analysis of 32 randomized controlled trials with trial sequential analysis. BMC Pregnancy Childbirth 2020, 20, 198. [Google Scholar]
- Laraia, B.A.; Bodnar, L.M.; Siega-Riz, A.M. Pregravid body mass index is negatively associated with diet quality during pregnancy. Public Health Nutr. 2007, 10, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Hillesund, E.R.; Bere, E.; Haugen, M.; Øverby, N.C. Development of a New Nordic Diet score and its association with gestational weight gain and fetal growth—A study performed in the Norwegian Mother and Child Cohort Study (MoBa). Public Health Nutr. 2014, 17, 1909–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charnley, M.; Newson, L.; Weeks, A.; Abayomi, J. Pregnant Women Living with Obesity: A Cross-Sectional Observational Study of Dietary Quality and Pregnancy Outcomes. Nutrients 2021, 13, 1652. [Google Scholar] [CrossRef] [PubMed]
- Skreden, M.; Hillesund, E.R.; Wills, A.K.; Brantsæter, A.L.; Bere, E.; Øverby, N.C. Adherence to the New Nordic Diet during pregnancy and subsequent maternal weight development: A study conducted in the Norwegian Mother and Child Cohort Study (MoBa). Br. J. Nutr. 2018, 119, 1286–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 650: Physical activity and exercise during pregnancy and the postpartum period. Obstet. Gynecol. 2015, 126, e135–e142. [Google Scholar]
- Bacchi, E.; Bonin, C.; Zanolin, M.E.; Zambotti, F.; Livornese, D.; Donà, S.; Tosi, F.; Baldisser, G.; Ihnatava, T.; Di Sarra, D.; et al. Physical activity patterns in normal-weight and overweight/obese pregnant women. PLoS ONE 2016, 11, e0166254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, J.M.; McGowan, C.; Byrne, J.; McAuliffe, F.M. Prevalence of physical activity among healthy pregnant women in Ireland. Int. J. Gynaecol. Obstet. 2011, 114, 154–155. [Google Scholar] [CrossRef]
- Cremona, A.; O’Gorman, C.; Cotter, A.; Saunders, J.; Donnelly, A. Effect of exercise modality on markers of insulin sensitivity and blood glucose control in pregnancies complicated with gestational diabetes mellitus: A systematic review. Obes. Sci. Pract. 2018, 4, 455–467. [Google Scholar] [CrossRef]
- Huvinen, E.; Engberg, E.; Meinilä, J.; Tammelin, T.; Kulmala, J.; Heinonen, K.; Bergman, P.; Stach-Lempinen, B.; Koivusalo, S. Lifestyle and glycemic health 5 years postpartum in obese and non-obese high diabetes risk women. Acta Diabetol. 2020, 57, 1453–1462. [Google Scholar] [CrossRef]
- Official Statistics of Finland (OSF). Educational Structure of Population. 2017. Available online: http://www.stat.fi/til/vkour/2017/vkour_2017_2018-11-02_tie_001_en.html (accessed on 17 January 2022).
- Whitaker, C.; Stevelink, S.; Fear, N. The use of Facebook in recruiting participants for health research purposes: A systematic review. J. Med. Internet Res. 2017, 19, e290. [Google Scholar] [CrossRef] [Green Version]
- Topolovec-Vranic, J.; Natarajan, K. The use of social media in recruitment for medical research studies: A scoping review. J. Med. Internet Res. 2016, 18, e286. [Google Scholar] [CrossRef]
| All Women | Women with No History of GDM | Women with a History of GDM | Women with Normal Weight | Women with OverWeight/Obesity | |||
|---|---|---|---|---|---|---|---|
| n = 1034 | n = 383 | n = 86 | n = 656 | n = 378 | p-Value a | p-Value b | |
| Age (years), mean (SD) | 29.4 (4.0) | 30.3 (3.8) | 30.8 (4.3) | 29.2 (3.8) | 29.7 (4.2) | 0.35 c | 0.077 c |
| Pre-pregnancy BMI (kg/m²), mean (SD) | 24.8 (4.9) | 24.5 (4.5) | 28.0 (6.5) | 21.9 (1.8) | 29.9 (4.4) | <0.001 c | <0.001 c |
| Parity, n (%) | 0.97 d | 0.15 d | |||||
| 0 | 560 (54) | 0 (0) | 0 (0) | 371 (57) | 189 (50) | ||
| 1 | 315 (31) | 257 (67) | 58 (67) | 190 (29) | 125 (33) | ||
| 2 | 102 (10) | 84 (22) | 18 (21) | 65 (10) | 37 (10) | ||
| ≥3 | 52 (5) | 42 (11) | 10 (12) | 28 (4) | 24 (6) | ||
| Marital status, n (%) | 0.73 e | 0.92 d | |||||
| Married | 518 (50) | 230 (60) | 52 (61) | 331 (51) | 187 (50) | ||
| Cohabiting | 474 (46) | 143 (37) | 31 (36) | 301 (46) | 173 (46) | ||
| Single | 27 (3) | 4 (1) | 2 (2) | 16 (2) | 11 (3) | ||
| Other | 14 (1) | 6 (12) | 1 (1) | 8 (1) | 6 (2) | ||
| University degree, n (%) | 690 (67) | 229 (60) | 56 (65) | 456 (70) | 234 (62) | 0.39 e | 0.014 e |
| Low income, n (%) | 82 (8) | 28 (7) | 8 (9) | 49 (8) | 33 (9) | 0.50 e | 0.55 e |
| Smoking status, n (%) | |||||||
| Smoked before pregnancy | 169 (16) | 50 (13) | 20 (23) | 84 (13) | 85 (23) | 0.028 e | <0.001 e |
| Smoked during pregnancy | 23 (2) | 9 (2) | 3 (4) | 13 (2) | 10 (3) | 0.46 e | 0.52 e |
| Chronic disease f, n (%) | 120 (12) | 43 (11) | 14 (16) | 70 (11) | 50 (13) | 0.20 e | 0.23 e |
| Special diet g, n (%) | 276 (27) | 97 (25) | 26 (30) | 182 (28) | 94 (25) | 0.35 e | 0.34 e |
| All Women | Women with No History of GDM | Women with a History of GDM | |||
|---|---|---|---|---|---|
| n = 1034 | n = 383 | n = 86 | p-Value a | p-Value b | |
| IDQ score, mean (SD), adjusted mean (95% CI) | 9.3 (2.2) | 9.3 (9.1, 9.5) | 9.5 (9.0, 9.9) | 0.68 c | 0.61 d |
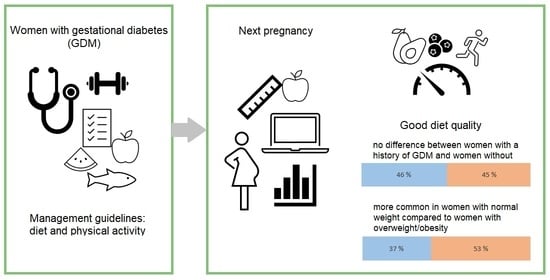
| Good dietary quality, n (%) | 479 (47) | 173 (45) | 38 (46) | 1.0 e | |
| MET-index (MET h/wk), median (IQR) | 7.5 (12.0) | 4.8 (10.1) | 4.8 (10.1) | 0.58 f | |
| Categorized MET-index, n (%) | 0.75 g | ||||
| Light LTPA | 460 (45) | 198 (52) | 44 (52) | ||
| Moderate LTPA | 450 (44) | 146 (38) | 35 (41) | ||
| Vigorous LTPA | 117 (11) | 36 (10) | 6 (7) |
| All Women | Women with No History of GDM | Women with a History of GDM | Women with Normal Weight | Women with OverWeight/Obesity | |||
|---|---|---|---|---|---|---|---|
| n = 1034 | n = 383 | n = 86 | n = 656 | n = 378 | |||
| n (%) | n (%) | n (%) | n (%) | n (%) | p-Value a | p-Value b | |
| Vegetables daily | 709 (69) | 262 (69) | 63 (76) | 466 (72) | 243 (65) | 0.23 c | 0.017 c |
| Fruit or berries daily | 554 (54) | 197 (52) | 46 (55) | 383 (59) | 171 (46) | 0.55 c | <0.001 c |
| Vegetables, fruit or berries ≥5 portions daily | 436 (43) | 157 (41) | 37 (45) | 292 (45) | 144 (38) | 0.62 c | 0.042 c |
| Whole-grain products daily | 610 (60) | 243 (64) | 44 (53) | 425 (66) | 185 (49) | 0.062 c | <0.001 c |
| Vegetable oil -based spread on bread | 331 (32) | 129 (34) | 27 (33) | 212 (33) | 119 (32) | 0.90 c | 0.78 c |
| Fish ≥2 portions weekly | 291 (28) | 107 (28) | 18 (22) | 196 (30) | 95 (25) | 0.28 c | 0.098 c |
| Regular meal pattern | 918 (90) | 335 (88) | 77 (93) | 602 (93) | 316 (84) | 0.25 c | <0.001 c |
| Women with Normal Weight | Women with OverWeight/Obesity | |||
|---|---|---|---|---|
| n = 656 | n = 378 | p-Value a | p-Value b | |
| IDQ score, adjusted mean (95% CI) | 9.6 (9.5, 9.8) | 8.8 (8.6, 9.1) | <0.001 c | <0.001d |
| Good dietary quality, n (%) | 341 (53) | 138 (37) | <0.001 e | |
| MET-index (MET h/wk), median (IQR) | 7.5 (15.8) | 4.8 (10.8) | <0.001 f | |
| Categorized MET-index, n (%) | <0.001 g | |||
| Light LTPA | 255 (39) | 205 (54) | ||
| Moderate LTPA | 309 (48) | 141 (37) | ||
| Vigorous LTPA | 86 (13) | 31 (8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhli, E.; Koivuniemi, E.; Laitinen, K. Living with Overweight, Rather than a History of Gestational Diabetes, Influences Dietary Quality and Physical Activity during Pregnancy. Nutrients 2022, 14, 651. https://doi.org/10.3390/nu14030651
Muhli E, Koivuniemi E, Laitinen K. Living with Overweight, Rather than a History of Gestational Diabetes, Influences Dietary Quality and Physical Activity during Pregnancy. Nutrients. 2022; 14(3):651. https://doi.org/10.3390/nu14030651
Chicago/Turabian StyleMuhli, Ella, Ella Koivuniemi, and Kirsi Laitinen. 2022. "Living with Overweight, Rather than a History of Gestational Diabetes, Influences Dietary Quality and Physical Activity during Pregnancy" Nutrients 14, no. 3: 651. https://doi.org/10.3390/nu14030651
APA StyleMuhli, E., Koivuniemi, E., & Laitinen, K. (2022). Living with Overweight, Rather than a History of Gestational Diabetes, Influences Dietary Quality and Physical Activity during Pregnancy. Nutrients, 14(3), 651. https://doi.org/10.3390/nu14030651