Creatine as a Promising Component of Paternal Preconception Diet
Abstract
:1. Background
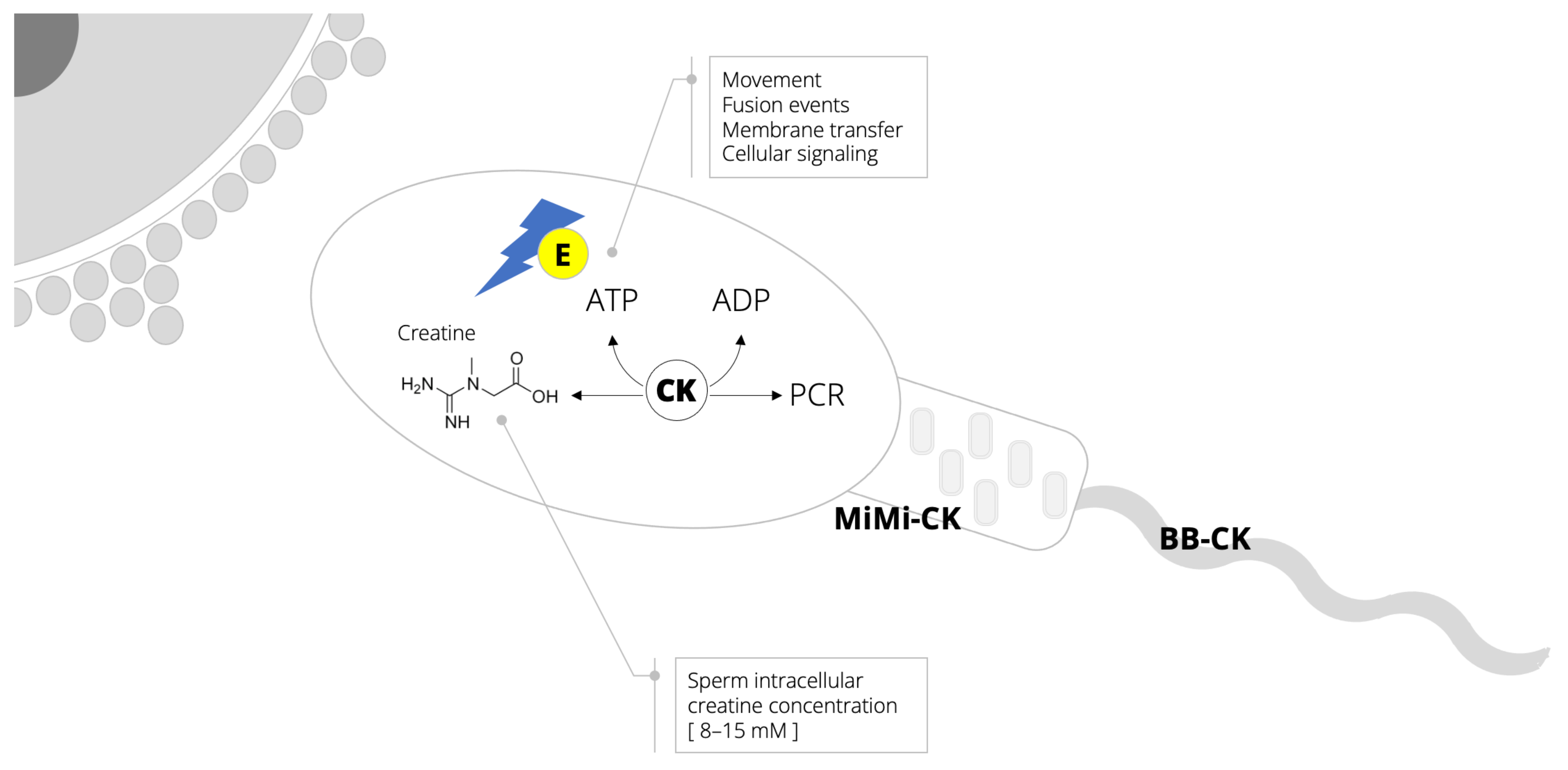
2. Semen: An Energy-Demanding Fluid
3. Biomarkers of Creatine Metabolism and Sperm Quality
4. Exogenous Creatine and Sperm Viability
5. Paternal Preconception Diet with Creatine: The Future Steps
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Demographics Collaborators. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950–2019: A comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1160–1203. [Google Scholar] [CrossRef]
- Mann, U.; Shiff, B.; Patel, P. Reasons for worldwide decline in male fertility. Curr. Opin. Urol. 2020, 30, 296–301. [Google Scholar] [CrossRef]
- Virtanen, H.E.; Jørgensen, N.; Toppari, J. Semen quality in the 21st century. Nat. Rev. Urol. 2017, 14, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Nassan, F.L.; Chavarro, J.E.; Tanrikut, C. Diet and men’s fertility: Does diet affect sperm quality? Fertil. Steril. 2018, 110, 570–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas-Huetos, A.; James, E.R.; Aston, K.I.; Jenkins, T.G.; Carrell, D.T. Diet and sperm quality: Nutrients, foods and dietary patterns. Reprod. Biol. 2019, 19, 219–224. [Google Scholar] [CrossRef]
- Skoracka, K.; Eder, P.; Łykowska-Szuber, L.; Dobrowolska, A.; Krela-Kaźmierczak, I. Diet and nutritional factors in male (in)fertility-underestimated factors. J. Clin. Med. 2020, 9, 1400. [Google Scholar] [CrossRef]
- Karayiannis, D.; Kontogianni, M.D.; Mendorou, C.; Douka, L.; Mastrominas, M.; Yiannakouris, N. Association between adherence to the Mediterranean diet and semen quality parameters in male partners of couples attempting fertility. Hum. Reprod. 2017, 32, 215–222. [Google Scholar] [CrossRef]
- Braga, D.P.; Halpern, G.; Figueira Rde, C.; Setti, A.S.; Iaconelli, A., Jr.; Borges, E., Jr. Food intake and social habits in male patients and its relationship to intracytoplasmic sperm injection outcomes. Fertil. Steril. 2012, 97, 53–59. [Google Scholar] [CrossRef]
- Fallah, A.; Mohammad-Hasani, A.; Colagar, A.H. Zinc is an essential element for male fertility: A review of Zn roles in men’s health, germination, sperm quality, and fertilization. J. Reprod. Infertil. 2018, 19, 69–81. [Google Scholar]
- Scott, R.; MacPherson, A.; Yates, R.W.; Hussain, B.; Dixon, J. The effect of oral selenium supplementation on human sperm motility. Br. J. Urol. 1998, 82, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Falsig, A.L.; Gleerup, C.S.; Knudsen, U.B. The influence of omega-3 fatty acids on semen quality markers: A systematic PRISMA review. Andrology 2019, 7, 794–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzick, D.S.; Overstreet, J.W.; Factor-Litvak, P.; Brazil, C.K.; Nakajima, S.T.; Coutifaris, C.; Carson, S.A.; Cisneros, P.; Steinkampf, M.P.; Hill, J.A.; et al. Sperm morphology, motility, and concentration in fertile and infertile men. N. Engl. J. Med. 2001, 345, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M.; Forbes, S.C. Perspective: Creatine, a conditionally essential nutrient: Building the case. Adv. Nutr. 2021, 18, nmab111. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E. Sperm bioenergetics in a nutshell. Biol. Reprod. 2012, 87, 72. [Google Scholar] [CrossRef] [PubMed]
- Wallimann, T.; Tokarska-Schlattner, M.; Schlattner, U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids 2011, 40, 1271–1296. [Google Scholar] [CrossRef] [Green Version]
- Nasrallah, F.; Hammami, M.B.; Omar, S.; Aribia, H.B.; Sanhaji, H.; Feki, M. Semen creatine and creatine kinase activity as an indicator of sperm quality. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef]
- Wallimann, T.; Moser, H.; Zurbriggen, B.; Wegmann, G.; Eppenberger, H.M. Creatine kinase isoenzymes in spermatozoa. J. Muscle Res. Cell Motil. 1986, 7, 25–34. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef]
- Banihani, S.A.; Abu-Alhayjaa, R.F. The activity of seminal creatine kinase is increased in the presence of pentoxifylline. Andrologia 2016, 48, 603–604. [Google Scholar] [CrossRef]
- Tombes, R.M.; Shapiro, B.M. Metabolite channeling: A phosphorylcreatine shuttle to mediate high energy phosphate transport between sperm mitochondrion and tail. Cell 1985, 41, 325–334. [Google Scholar] [CrossRef]
- Grow, D.; Oehninger, S. Strict criteria for the evaluation of human sperm morphology and its impact on assisted reproduction. Andrologia 1995, 27, 325–333. [Google Scholar] [CrossRef]
- Patel, A.S.; Leong, J.Y.; Ramasamy, R. Prediction of male infertility by the World Health Organization laboratory manual for assessment of semen analysis: A systematic review. Arab. J. Urol. 2017, 16, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, H.; Griffiths, P.D. Creatine-phosphokinase activity in semen. Lancet 1963, 2, 498. [Google Scholar] [CrossRef]
- Gonzalez Buitrago, J.M.; Miralles, J.M.; Muńoz, M.H.; Meza, S.; Alonso, M.T.; Garcia Diez, L.C. Seminal plasma creatine kinase activity in fertility studies. Arch. Androl. 1980, 5, 355–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asseo, P.P.; Panidis, D.K.; Papadimas, J.S.; Ikkos, D.G. Creatine kinase in seminal plasma of infertile men: Activity and isoenzymes. Int. J. Androl. 1981, 4, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Buitrago, J.M.; Garcia Diez, L.C. Enzyme levels in semen of men with different types of azoospermia. Andrologia 1982, 14, 77–80. [Google Scholar] [CrossRef]
- Srivastava, A.; Chopra, S.K.; Dasgupta, P.R. Biochemical analysis of human seminal plasma. II. Protein, non-protein nitrogen, urea, uric acid and creatine. Andrologia 1984, 16, 265–268. [Google Scholar] [CrossRef]
- Huszar, G.; Corrales, M.; Vigue, L. Correlation between sperm creatine phosphokinase activity and sperm concentrations in normospermic and oligospermic men. Gamete Res. 1988, 19, 67–75. [Google Scholar] [CrossRef]
- Sidhu, R.S.; Sharma, R.K.; Agarwal, A. Relationship between creatine kinase activity and semen characteristics in subfertile men. Int. J. Fertil. Womens Med. 1998, 43, 192–197. [Google Scholar]
- Hallak, J.; Sharma, R.K.; Pasqualotto, F.F.; Ranganathan, P.; Thomas, A.J., Jr.; Agarwal, A. Creatine kinase as an indicator of sperm quality and maturity in men with oligospermia. Urology 2001, 58, 446–451. [Google Scholar] [CrossRef]
- Durutovic, O.; Lalic, N.; Milenkovic-Petronic, D.; Bojanic, N.; Djordjevic, D.; Milojevic, B.; Ladjevic, N.; Mimic, A.; Tulic, L.; Dzamic, Z.; et al. The correlation of biochemical and morphologic parameters in the assessment of sperm maturity. Urology 2013, 82, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.A.; Rostami, M. The effect of cigarette smoking on human sperm creatine kinase activity: As an ATP buffering system in sperm. Int. J. Fertil. Steril. 2013, 6, 258–265. [Google Scholar] [PubMed]
- Celik-Ozenci, C.; Tasatargil, A.; Tekcan, M.; Sati, L.; Gungor, E.; Isbir, M.; Usta, M.F.; Akar, M.E.; Erler, F. Effect of abamectin exposure on semen parameters indicative of reduced sperm maturity: A study on farmworkers in Antalya (Turkey). Andrologia 2012, 44, 388–395. [Google Scholar] [CrossRef]
- Fakih, H.; MacLusky, N.; DeCherney, A.; Wallimann, T.; Huszar, G. Enhancement of human sperm motility and velocity in vitro: Effects of calcium and creatine phosphate. Fertil. Steril. 1986, 46, 938–944. [Google Scholar] [CrossRef]
- Umehara, T.; Kawai, T.; Goto, M.; Richards, J.S.; Shimada, M. Creatine enhances the duration of sperm capacitation: A novel factor for improving in vitro fertilization with small numbers of sperm. Hum. Reprod. 2018, 33, 1117–1129. [Google Scholar] [CrossRef] [Green Version]
- Umehara, T.; Tsujita, N.; Goto, M.; Tonai, S.; Nakanishi, T.; Yamashita, Y.; Shimada, M. Methyl-beta cyclodextrin and creatine work synergistically under hypoxic conditions to improve the fertilization ability of boar ejaculated sperm. Anim. Sci. J. 2020, 91, e13493. [Google Scholar] [CrossRef] [PubMed]
- Tapeh, R.S.; Zhandi, M.; Zaghari, M.; Akhlaghi, A. Effects of guanidinoacetic acid diet supplementation on semen quality and fertility of broiler breeder roosters. Theriogenology 2017, 89, 178–182. [Google Scholar] [CrossRef]
- Tøttenborg, S.S.; Glazer, C.H.; Hærvig, K.K.; Høyer, B.B.; Toft, G.; Hougaard, K.S.; Flachs, E.M.; Deen, L.; Bonde, J.P.E.; Ramlau-Hansen, C.H. Semen quality among young healthy men taking protein supplements. Fertil. Steril. 2020, 114, 89–96. [Google Scholar] [CrossRef]
- Moore, N.P. The distribution, metabolism and function of creatine in the male mammalian reproductive tract: A review. Int. J. Androl. 2000, 23, 4–12. [Google Scholar] [CrossRef]
- Braissant, O.; Henry, H.; Villard, A.M.; Speer, O.; Wallimann, T.; Bachmann, C. Creatine synthesis and transport during rat embryogenesis: Spatiotemporal expression of AGAT, GAMT and CT1. BMC Dev. Biol. 2005, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Muccini, A.M.; Tran, N.T.; de Guingand, D.L.; Philip, M.; Della Gatta, P.A.; Galinsky, R.; Sherman, L.S.; Kelleher, M.A.; Palmer, K.R.; Berry, M.J.; et al. Creatine metabolism in female reproduction, pregnancy and newborn health. Nutrients 2021, 13, 490. [Google Scholar] [CrossRef] [PubMed]
- Korovljev, D.; Stajer, V.; Ostojic, S.M. Relationship between dietary creatine and growth indicators in children and adolescents aged 2–19 years: A cross-sectional study. Nutrients 2021, 13, 1027. [Google Scholar] [CrossRef] [PubMed]
- Korovljev, D.; Todorovic, N.; Stajer, V.; Ostojic, S.M. Dietary intake of creatine in children aged 0–24 months. Ann. Nutr. Metab. 2021, 77, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, H.; Ellery, S.; Ireland, Z.; LaRosa, D.; Snow, R.; Walker, D.W. Creatine supplementation during pregnancy: Summary of experimental studies suggesting a treatment to improve fetal and neonatal morbidity and reduce mortality in high-risk human pregnancy. BMC Pregnancy Childbirth 2014, 14, 150. [Google Scholar] [CrossRef] [Green Version]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostojic, S.M.; Stea, T.H.; Engeset, D. Creatine as a Promising Component of Paternal Preconception Diet. Nutrients 2022, 14, 586. https://doi.org/10.3390/nu14030586
Ostojic SM, Stea TH, Engeset D. Creatine as a Promising Component of Paternal Preconception Diet. Nutrients. 2022; 14(3):586. https://doi.org/10.3390/nu14030586
Chicago/Turabian StyleOstojic, Sergej M., Tonje Holte Stea, and Dagrun Engeset. 2022. "Creatine as a Promising Component of Paternal Preconception Diet" Nutrients 14, no. 3: 586. https://doi.org/10.3390/nu14030586
APA StyleOstojic, S. M., Stea, T. H., & Engeset, D. (2022). Creatine as a Promising Component of Paternal Preconception Diet. Nutrients, 14(3), 586. https://doi.org/10.3390/nu14030586







