Human Serum Betaine and Associated Biomarker Concentrations Following a 14 Day Supplemental Betaine Loading Protocol and during a 28 Day Washout Period: A Pilot Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Approach and Betaine Supplementation
2.3. Anthropometrics, Whole-Body Hydration, and Body Composition Analysis
2.4. Dietary Tracking and Records
2.5. Venipuncture
2.6. Serum Betaine Analysis
2.7. Serum Growth Hormone (GH), Insulin-like Growth Factor-1 (IGF-1), and Homocysteine (HCY)
2.8. Statistical Analyses
3. Results
3.1. Participant Descriptives and Body Composition Analyses
3.2. Dietary Assessments
3.3. Hydration and Serum Analyses
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apicella, J.M.; Lee, E.C.; Bailey, B.L.; Saenz, C.; Anderson, J.M.; Craig, S.A.; Kraemer, W.J.; Volek, J.S.; Maresh, C.M. Betaine supplementation enhances anabolic endocrine and Akt signaling in response to acute bouts of exercise. Eur. J. Appl. Physiol. 2013, 113, 793–802. [Google Scholar] [CrossRef] [PubMed]
- McRae, M.P. Betaine supplementation decreases plasma homocysteine in healthy adult participants: A meta-analysis. J. Chiropr. Med. 2013, 12, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, M.D.; Zeisel, S.H. Diet, methyl donors and DNA methylation: Interactions between dietary folate, methionine and choline. J. Nutr. 2002, 132, 2333S–2335S. [Google Scholar] [CrossRef] [PubMed]
- Najib, S.; Sanchez-Margalet, V. Homocysteine thiolactone inhibits insulin-stimulated DNA and protein synthesis: Possible role of mitogen-activated protein kinase (MAPK), glycogen synthase kinase-3 (GSK-3) and p70 S6K phosphorylation. J. Mol. Endocrinol. 2005, 34, 119–126. [Google Scholar] [CrossRef]
- Cholewa, J.M.; Guimaraes-Ferreira, L.; Zanchi, N.E. Effects of betaine on performance and body composition: A review of recent findings and potential mechanisms. Amino Acids 2014, 46, 1785–1793. [Google Scholar] [CrossRef]
- Willingham, B.D.; Ragland, T.J.; Ormsbee, M.J. Betaine supplementation may improve heat tolerance: Potential mechanisms in humans. Nutrients 2020, 12, 2939. [Google Scholar] [CrossRef]
- Gropper, S.; Smith, J. Advanced Nutrition and Human Metabolism, 6th ed.; Cengage Learning: Belmont, CA, USA, 2013. [Google Scholar]
- Ismaeel, A. Effects of betaine supplementation on muscle strength and power: A systematic review. J. Strength Cond. Res. 2017, 31, 2338–2346. [Google Scholar] [CrossRef]
- Machek, S.B.; Cardaci, T.D.; Willoughby, D.S. Blood flow restriction training and betaine supplementation as a novel combined modality to augment skeletal muscle adaptation: A short review. Strength Cond. J. 2020, 43, 50–63. [Google Scholar] [CrossRef]
- Lee, E.C.; Maresh, C.M.; Kraemer, W.J.; Yamamoto, L.M.; Hatfield, D.L.; Bailey, B.L.; Armstrong, L.E.; Volek, J.S.; McDermott, B.P.; Craig, S.A. Ergogenic effects of betaine supplementation on strength and power performance. J. Int. Soc. Sports Nutr. 2010, 7, 27. [Google Scholar] [CrossRef]
- Pryor, J.L.; Craig, S.A.; Swensen, T. Effect of betaine supplementation on cycling sprint performance. J. Int. Soc. Sports Nutr. 2012, 9, 12. [Google Scholar] [CrossRef]
- Cholewa, J.M.; Wyszczelska-Rokiel, M.; Glowacki, R.; Jakubowski, H.; Matthews, T.; Wood, R.; Craig, S.A.; Paolone, V. Effects of betaine on body composition, performance, and homocysteine thiolactone. J. Int. Soc. Sports Nutr. 2013, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Cholewa, J.M.; Hudson, A.; Cicholski, T.; Cervenka, A.; Barreno, K.; Broom, K.; Barch, M.; Craig, S.A.S. The effects of chronic betaine supplementation on body composition and performance in collegiate females: A double-blind, randomized, placebo controlled trial. J. Int. Soc. Sports Nutr. 2018, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Nobari, H.; Cholewa, J.M.; Castillo-Rodríguez, A.; Kargarfard, M.; Pérez-Gómez, J. Effects of chronic betaine supplementation on performance in professional young soccer players during a competitive season: A double blind, randomized, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2021, 18, 67. [Google Scholar] [CrossRef]
- Anas, M.-K.I.; Lee, M.B.; Zhou, C.; Hammer, M.-A.; Slow, S.; Karmouch, J.; Liu, X.J.; Broer, S.; Lever, M.; Baltz, J. SIT1 is a betaine/proline transporter that is activated in mouse eggs after fertilization and functions until the 2-cell stage. Development 2008, 135, 4123–4130. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, A.; Uchida, S.; Kwon, H.M.; Preston, A.S.; Robey, R.B.; Garcia-Perez, A.; Burg, M.B.; Handler, J.S. Cloning of a Na(+)- and Cl(-)-dependent betaine transporter that is regulated by hypertonicity. J. Biol. Chem. 1992, 267, 649–652. [Google Scholar] [CrossRef]
- Kempson, S.A.; Vovor-Dassu, K.; Day, C. Betaine transport in kidney and liver: Use of betaine in liver injury. Cell Physiol. Biochem. 2013, 32, 32–40. [Google Scholar] [CrossRef]
- Kempson, S.A.; Zhou, Y.; Danbolt, N.C. The betaine/GABA transporter and betaine: Roles in brain, kidney, and liver. Front. Physiol. 2014, 5, 159. [Google Scholar] [CrossRef]
- Schwab, U.; Törrönen, A.; Meririnne, E.; Saarinen, M.; Alfthan, G.; Aro, A.; Uusitupa, M. Orally administered betaine has an acute and dose-dependent effect on serum betaine and plasma homocysteine concentrations in healthy humans. J. Nutr. 2006, 136, 34–38. [Google Scholar] [CrossRef][Green Version]
- Schwahn, B.C.; Hafner, D.; Hohlfeld, T.; Balkenhol, N.; Laryea, M.D.; Wendel, U. Pharmacokinetics of oral betaine in healthy subjects and patients with homocystinuria. Br. J. Clin. Pharmacol. 2003, 55, 6–13. [Google Scholar] [CrossRef]
- Clow, K.A.; Treberg, J.R.; Brosnan, M.E.; Brosnan, J.T. Elevated tissue betaine contents in developing rats are due to dietary betaine, not to synthesis. J. Nutr. 2008, 138, 1641–1646. [Google Scholar] [CrossRef]
- Slow, S.; Lever, M.; Chambers, S.T.; George, P.M. Plasma dependent and independent accumulation of betaine in male and female rat tissues. Physiol. Res. 2009, 58, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Schwab, U.; Alfthan, G.; Aro, A.; Uusitupa, M. Long-term effect of betaine on risk factors associated with the metabolic syndrome in healthy subjects. Eur. J. Clin. Nutr. 2011, 65, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Hecksteden, A.; Faude, O.; Meyer, T.; Donath, L. How to construct, conduct and analyze an exercise training study? Front. Physiol. 2018, 9, 1007. [Google Scholar] [CrossRef]
- Betts, J.A.; Gonzalez, J.T. True responders in exercise science: Novel insight from replicated crossover designs. Med. Sci. Sports Exerc. 2018, 50, 769. [Google Scholar] [CrossRef] [PubMed]
- Riebe, D.; Franklin, B.A.; Thompson, P.D.; Garber, C.E.; Whitfield, G.P.; Magal, M.; Pescatello, L.S. Updating ACSM’s recommendations for exercise preparticipation health screening. Med. Sci. Sports Exerc. 2015, 47, 2473–2479. [Google Scholar] [CrossRef]
- Pryor, J.L.; Wolf, S.T.; Sforzo, G.; Swensen, T. The effect of betaine on nitrate and cardiovascular response to exercise. Int. J. Exerc. Sci. 2017, 10, 550–559. [Google Scholar]
- Moon, J.R. Body composition in athletes and sports nutrition: An examination of the bioimpedance analysis technique. Eur. J. Clin. Nutr. 2013, 67, S54–S59. [Google Scholar] [CrossRef]
- Sartorio, A.; Malavolti, M.; Agosti, F.; Marinone, P.G.; Caiti, O.; Battistini, N.; Bedogni, G. Body water distribution in severe obesity and its assessment from eight-polar bioelectrical impedance analysis. Eur. J. Clin. Nutr. 2005, 59, 155–160. [Google Scholar] [CrossRef]
- Miller, R.; Chambers, T.; Burns, S. Validating InBody® 570 multi-frequency bioelectrical impedance analyzer versus DXA for body fat percentage analysis. J. Exerc. Physiol. Online 2016, 19, 71–78. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025; USDA; HHS: Washington, DC, USA, 2020.
- Wilburn, D.T.; Machek, S.B.; Cardaci, T.D.; Hwang, P.S.; Willoughby, D.S. Acute maltodextrin supplementation during resistance exercise. J. Sports Sci. Med. 2020, 19, 282–288. [Google Scholar] [PubMed]
- Mlodzik-Czyzewska, M.A.; Szwengiel, A.; Malinowska, A.M.; Chmurzynska, A. Comparison of associations between one-carbon metabolism, lipid metabolism, and fatty liver markers in normal-weight and overweight people aged 20–40 years. Ann. Nutr. Metab. 2021, 77, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Koc, H.; Mar, M.H.; Ranasinghe, A.; Swenberg, J.A.; Zeisel, S.H. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal. Chem. 2002, 74, 4734–4740. [Google Scholar] [CrossRef] [PubMed]
- Steuer, C.; Schütz, P.; Bernasconi, L.; Huber, A.R. Simultaneous determination of phosphatidylcholine-derived quaternary ammonium compounds by a LC-MS/MS method in human blood plasma, serum and urine samples. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1008, 206–211. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, K.; Stip, E.; Pélissier, M.C.; Aardema, F.; Guay, S.; Gaudette, G.; Van Haaster, I.; Robillard, S.; Grenier, S.; Careau, Y.; et al. Treating delusional disorder: A comparison of cognitive-behavioural therapy and attention placebo control. Can. J. Psychiatry 2007, 52, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Deminice, R.; Ribeiro, D.F.; Frajacomo, F.T. The effects of acute exercise and exercise training on plasma homocysteine: A meta-analysis. PLoS ONE 2016, 11, e0151653. [Google Scholar] [CrossRef]
- Dankner, R.; Chetrit, A.; Lubin, F.; Sela, B. A Life-style habits and homocysteine levels in an elderly population. Aging Clin. Exp. Res. 2004, 16, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Maroto-Sanchez, B.; Lopez-Torres, O.; Palacios, G.; González-Gross, M. What do we know about homocysteine and exercise? A review from the literature. Clin. Chem. Lab. Med. 2016, 54, 1561–1577. [Google Scholar] [CrossRef]
- Moro, T.; Badiali, F.; Fabbri, I.; Paoli, A. Betaine supplementation does not improve muscle hypertrophy or strength following 6 weeks of cross-fit training. Nutrients 2020, 12, 1688. [Google Scholar] [CrossRef]
- Wilburn, D.T.; Machek, S.B.; Zechmann, B.; Willoughby, D.S. Comparison of skeletal muscle ultrastructural changes between normal and blood flow restricted resistance exercise: A case report. Exp. Physiol. 2021, 106, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Nobari, H.; Cholewa, J.M.; Pérez-Gómez, J.; Castillo-Rodríguez, A. Effects of 14-weeks betaine supplementation on pro-inflammatory cytokines and hematology status in professional youth soccer players during a competition season: A double blind, randomized, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2021, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Raina, S.K. Limitations of 24-hour recall method: Micronutrient intake and the presence of the metabolic syndrome. N. Am. J. Med. Sci. 2013, 5, 498. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, R.J.; Whyte, G.P.; Buckley, J.; Quinlivan, R. The role of lactate in the exercise-induced human growth hormone response: Evidence from McArdle disease. Br. J. Sports Med. 2009, 43, 521–525. [Google Scholar] [CrossRef]

| Mean ± SD | PRE | POST | p-Value; η2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 5 | -0 | -4 | -7 | -10 | -13 | -16 | -19 | -22 | -25 | -28 | ||
| Age (years) | 26 ± 6 | n/a | ||||||||||
| Training Age * (years) | 7.8 ± 3.1 | n/a | ||||||||||
| Height (cm) | 171.7 ± 5.0 | n/a | ||||||||||
| Weight (kg) | 93.4 ± 15.7 | 93.2 ± 15.4 | 93.0 ± 15.6 | 93.2 ± 15.8 | 93.4 ± 15.7 | 93.1 ± 15.7 | 92.9 ± 15.8 | 93.2 ± 15.9 | 93.3 ± 15.8 | 93.1 ± 15.9 | 82.9 ± 16.0 | 0.655; 0.101 |
| Body Fat (%) | 18.6 ± 4.8 | 18.0 ± 4.6 | 16.0 ± 4.2 * | 0.003; 0.816 | ||||||||
| Mean ± SD | PRE | POST | p-Value; η2 | ||||
|---|---|---|---|---|---|---|---|
| n = 5 | -0 | -7 | -13 | -22 | -28 | ||
| PRO (g·kg−1) | 1.81 ± 0.40 | 1.74 ± 0.53 | 2.21 ± 0.79 | 1.66 ± 0.63 | 1.86 ± 0.51 | 1.78 ± 0.53 | 0.356; 0.224 |
| CHO (g·kg−1) | 2.68 ± 0.82 | 3.26 ± 0.96 | 3.21 ± 1.00 | 3.08 ± 0.97 | 3.13 ± 1.12 | 3.46 ± 1.07 | 0.330; 0.243 |
| FAT (g·kg−1) | 0.92 ± 0.36 | 1.09 ± 0.29 | 1.19 ± 0.49 | 0.98 ± 0.29 | 0.93 ± 0.27 | 1.12 ± 0.28 | 0.498; 0.160 |
| Fiber (g·kg−1) | 0.28 ± 0.14 | 0.26 ± 0.11 | 0.34 ± 0.14 | 0.26 ± 0.12 | 0.33 ± 0.24 | 0.27 ± 0.10 | 0.624; 0.106 |
| Micronutrient Intakes | RDA/AI | ||||||
| B2 (mg) | 3.38 ± 3.18 | 3.72 ± 2.31 | 4.90 ± 2.46 | 4.51 ± 2.93 | 4.80 ± 3.73 | 2.68 ± 1.15 | 1.30 |
| B6 (mg) | 3.86 ± 2.55 | 5.16 ± 2.44 | 7.10 ± 3.49 | 4.67 ± 4.67 | 7.89 ± 5.78 | 4.15 ± 3.30 | 1.30 |
| B12 (mcg) | 12.34 ± 5.49 | 10.89 ± 5.49 | 12.68 ± 5.17 | 12.89 ± 4.15 | 14.06 ± 5.29 | 10.47 ± 4.08 | 2.40 |
| Folate (mcg) * | 435.31 ± 215.12 | 509.70 ± 279.47 | 509.93 ± 443.12 | 524.15 ± 146.79 | 501.93 ± 250.95 | 445.43 ± 205.02 | 400.00 |
| Choline (mg) | 435.31 ± 152.68 | 509.70 ± 444.30 | 509.93 ± 333.08 | 524.15 ± 303.70 | 501.93 ± 179.46 | 445.43 ± 239.74 | 550.00 |
| Mean ± SD | PRE | POST | p-Value; | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 5 | -0 | -4 | -7 | -10 | -13 | -16 | -19 * | -22 | -25 * | -28 | η2 | |
| PCV (%) | 47.2 ± 1.8 | 46.8 ± 2.4 | 47.2 ± 1.8 | 47.8 ± 2.0 | 46.4 ± 2.3 | 46.6 ± 2.4 | 46.2 ± 2.5 | 47.0 ± 3.0 | 46.8 ± 2.3 | 47.4 ± 1.7 | 46.8 ± 2.3 | 0.540; 0.152 |
| ICW (kg) | 32.3 ± 2.7 | 32.8 ± 2.7 | 32.1 ± 3.2 | 32.8 ± 2.8 | 32.8 ± 2.9 | 32.7 ± 2.6 | 32.8 ± 2.9 | 32.7 ± 3.0 | 32.7 ± 3.1 | 33.0 ± 3.0 | 32.8 ± 2.9 | 0.521; 0.192 |
| ECW (kg) | 18.4 ± 1.5 | 18.7 ± 1.3 | 18.5 ± 1.5 | 18.8 ± 1.6 | 18.8 ± 1.7 | 18.7 ± 1.5 | 18.9 ± 1.7 | 18.8 ± 1.7 | 18.9 ± 1.7 | 18.8 ± 1.7 | 18.8 ± 1.5 | 0.328; 0.241 |
| Serum Markers | ||||||||||||
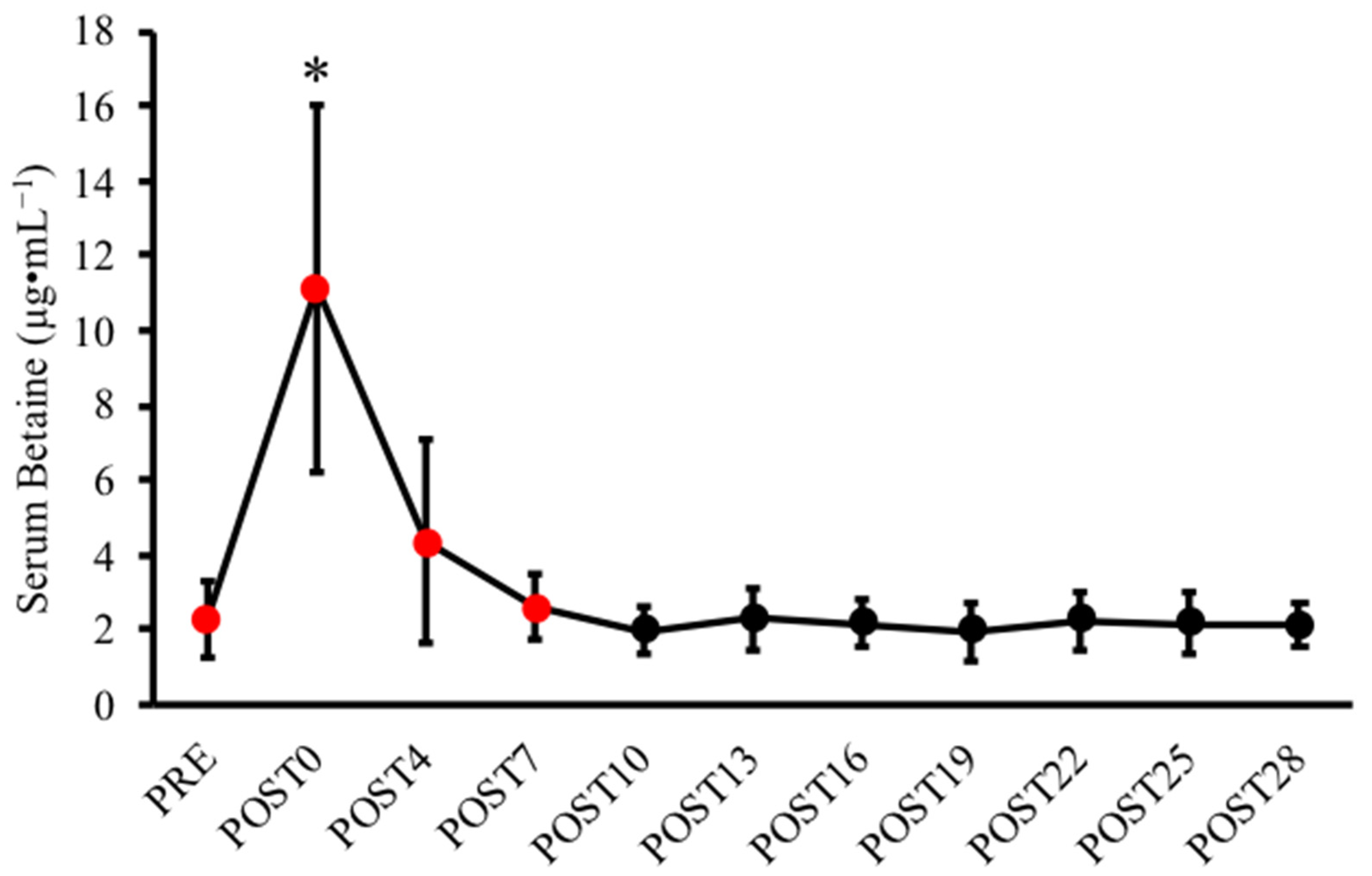
| Betaine (µg·mL−1) | 2.31 ± 1.05 | 11.1 ± 4.91 | 4.38 ± 2.71 | 2.61 ± 0.88 | 1.99 ± 0.66 | 2.29 ± 0.84 | 2.17 ± 0.60 | 1.98 ± 0.77 | 2.25 ± 0.80 | 2.17 ± 0.83 | 2.11 ± 0.60 | 0.010; 0.820 |
| GH (ng·mL−1) | 0.50 ± 0.77 | 0.09 ± 0.10 | 1.26 ± 1.81 | 1.51 ± 2.24 | 0.46 ± 0.76 | 0.26 ± 0.32 | 0.50 ± 0.61 | 0.27 ± 0.22 | 0.16 ± 0.28 | 0.15 ± 0.23 | 0.19 ± 0.29 | 0.279; 0.276 |
| IGF-1 (ng·mL−1) | 144. ± 65.4 | 136.2 ± 54.6 | 155.2 ± 66.5 | 173.8 ± 88.0 | 173.5 ± 81.0 | 179.5 ± 83.4 | 168.4 ± 74.0 | 171.9 ± 98.2 | 154.5 ± 62.2 | 147.0 ± 62.9 | 135.4 ± 53.2 | 0.226; 0.311 |
| HCY (µmol·mL−1) | 26.9 ± 7.4 | 22.9 ± 4.4 | 25.1 ± 5.7 | 26.4 ± 3.3 | 24.9 ± 3.4 | 25.7 ± 7.0 | 25.6 ± 6.3 | 28.2 ± 6.7 | 27.4 ± 12.2 | 28.5 ± 11.7 | 29.1 ± 7.5 | 0.597; 0.173 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machek, S.B.; Zawieja, E.E.; Heileson, J.L.; Harris, D.R.; Wilburn, D.T.; Fletcher, E.A.; Cholewa, J.M.; Szwengiel, A.; Chmurzynska, A.; Willoughby, D.S. Human Serum Betaine and Associated Biomarker Concentrations Following a 14 Day Supplemental Betaine Loading Protocol and during a 28 Day Washout Period: A Pilot Investigation. Nutrients 2022, 14, 498. https://doi.org/10.3390/nu14030498
Machek SB, Zawieja EE, Heileson JL, Harris DR, Wilburn DT, Fletcher EA, Cholewa JM, Szwengiel A, Chmurzynska A, Willoughby DS. Human Serum Betaine and Associated Biomarker Concentrations Following a 14 Day Supplemental Betaine Loading Protocol and during a 28 Day Washout Period: A Pilot Investigation. Nutrients. 2022; 14(3):498. https://doi.org/10.3390/nu14030498
Chicago/Turabian StyleMachek, Steven B., Emilia E. Zawieja, Jeffery L. Heileson, Dillon R. Harris, Dylan T. Wilburn, Emma A. Fletcher, Jason M. Cholewa, Artur Szwengiel, Agata Chmurzynska, and Darryn S. Willoughby. 2022. "Human Serum Betaine and Associated Biomarker Concentrations Following a 14 Day Supplemental Betaine Loading Protocol and during a 28 Day Washout Period: A Pilot Investigation" Nutrients 14, no. 3: 498. https://doi.org/10.3390/nu14030498
APA StyleMachek, S. B., Zawieja, E. E., Heileson, J. L., Harris, D. R., Wilburn, D. T., Fletcher, E. A., Cholewa, J. M., Szwengiel, A., Chmurzynska, A., & Willoughby, D. S. (2022). Human Serum Betaine and Associated Biomarker Concentrations Following a 14 Day Supplemental Betaine Loading Protocol and during a 28 Day Washout Period: A Pilot Investigation. Nutrients, 14(3), 498. https://doi.org/10.3390/nu14030498










