From the Bench to the Bedside: Branched Amino Acid and Micronutrient Strategies to Improve Mitochondrial Dysfunction Leading to Sarcopenia
Abstract
:1. Introduction
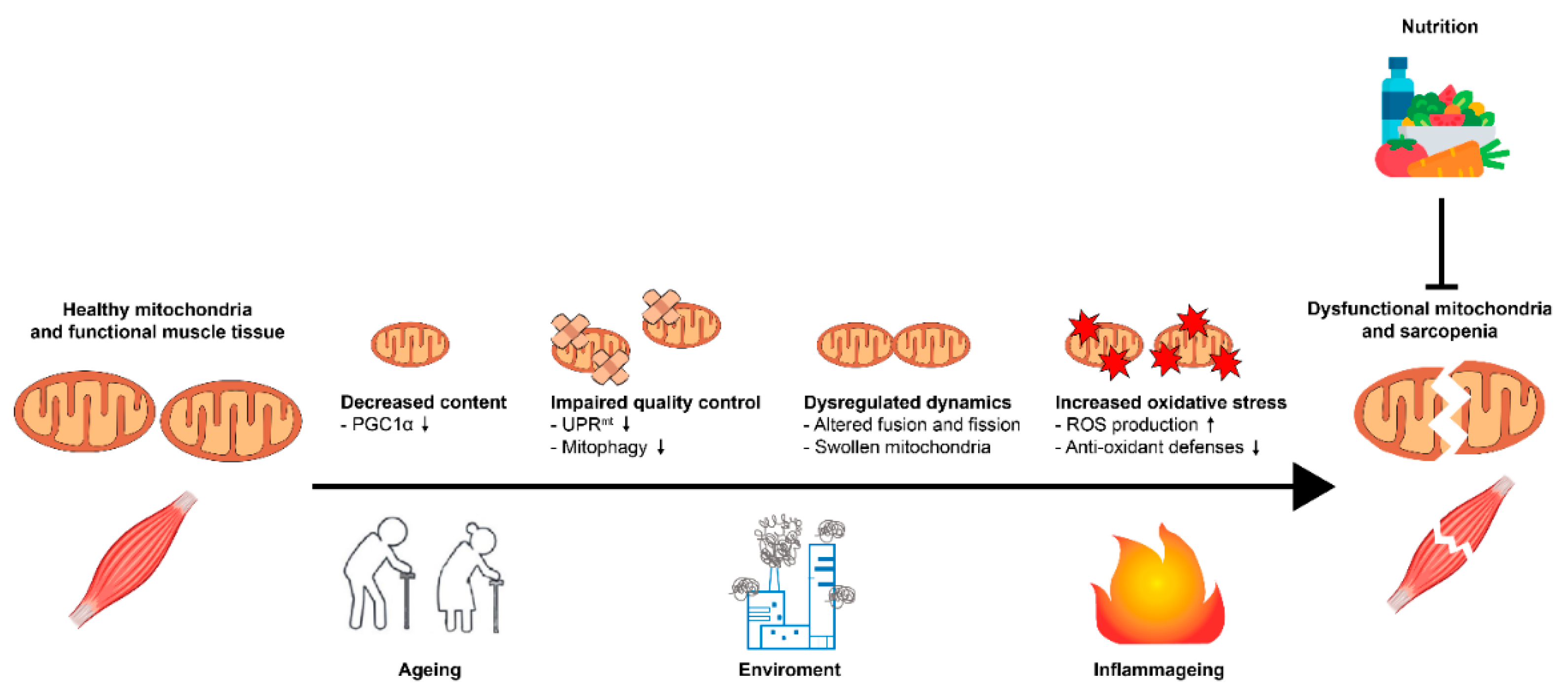
2. Mitochondrial Dysfunction and Oxidative Stress
3. Inflammageing
4. Malnutrition
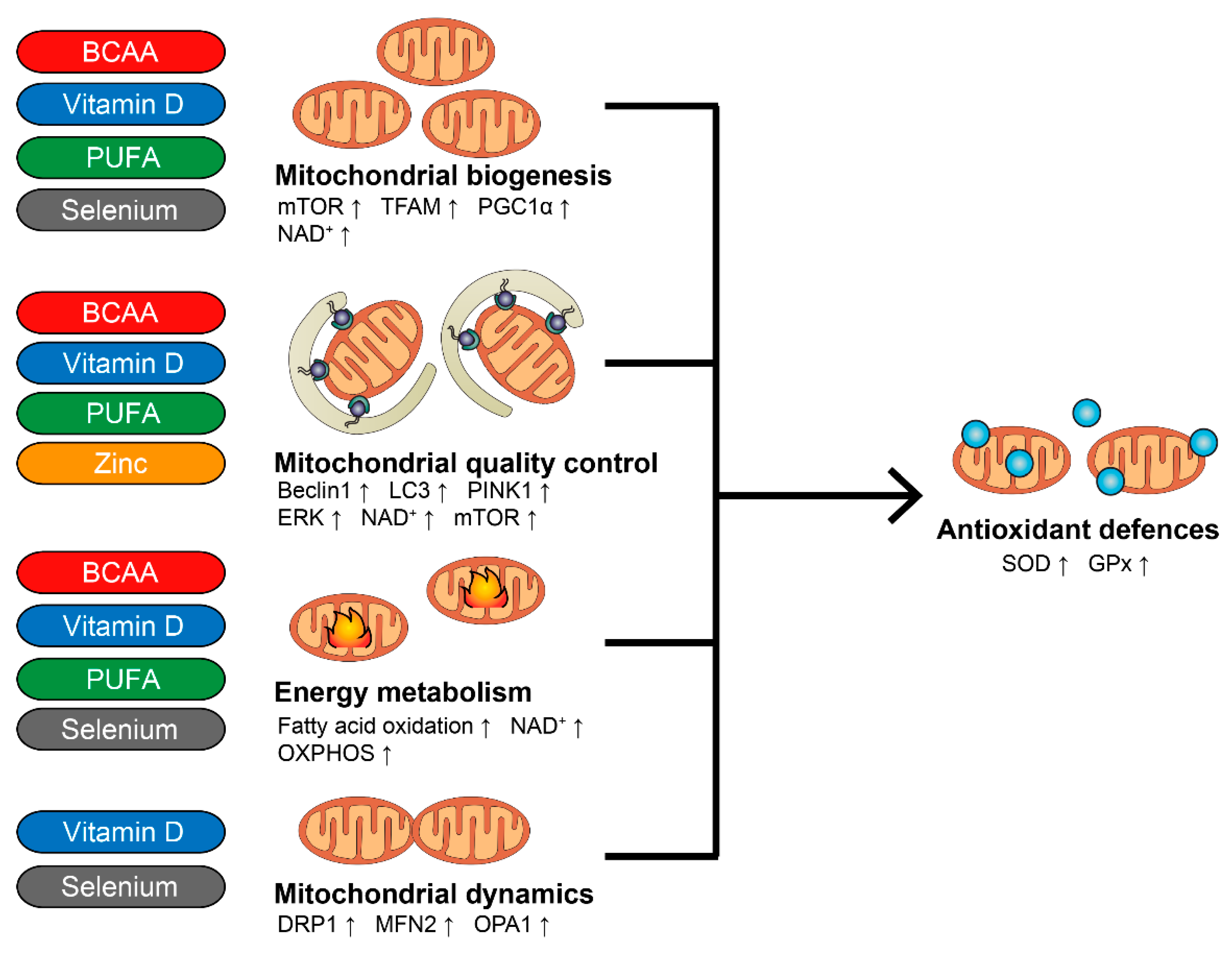
5. BCAAs
6. Omega-3 PUFA
7. Vitamin D
8. Selenium and Zinc
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pacifico, J.; Geerlings, M.A.J.; Reijnierse, E.M.; Phassouliotis, C.; Lim, W.K.; Maier, A.B. Prevalence of sarcopenia as a comorbid disease: A systematic review and meta-analysis. Exp. Gerontol. 2020, 131, 110801. [Google Scholar] [CrossRef] [PubMed]
- Marty, E.; Liu, Y.; Samuel, A.; Or, O.; Lane, J. A review of sarcopenia: Enhancing awareness of an increasingly prevalent disease. Bone 2017, 105, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Antunes, A.C.; Araujo, D.A.; Verissimo, M.T.; Amaral, T.F. Sarcopenia and hospitalisation costs in older adults: A cross-sectional study. Nutr. Diet 2017, 74, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.S.; Guerra, R.S.; Fonseca, I.; Pichel, F.; Ferreira, S.; Amaral, T.F. Financial impact of sarcopenia on hospitalization costs. Eur. J. Clin. Nutr. 2016, 70, 1046–1051. [Google Scholar] [CrossRef]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.; Goodpaster, B.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B.; et al. Sarcopenia: Alternative definitions and associations with lower extremity function. J. Am. Geriatr. Soc. 2003, 51, 1602–1609. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Chumlea, W.C.; Cesari, M.; Evans, W.J.; Ferrucci, L.; Fielding, R.A.; Pahor, M.; Studenski, S.; Vellas, B.; International Working Group on Sarcopenia Task Force Members. Sarcopenia: Designing phase IIB trials. J. Nutr. Health. Aging 2011, 15, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A simple questionnaire to rapidly diagnose sarcopenia. J. Am. Med Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef]
- Dodds, R.M.; Syddall, H.E.; Cooper, R.; Benzeval, M.; Deary, I.J.; Dennison, E.M.; Der, G.; Gale, C.R.; Inskip, H.M.; Jagger, C.; et al. Grip strength across the life course: Normative data from twelve British studies. PLoS ONE 2014, 9, e113637. [Google Scholar] [CrossRef] [Green Version]
- Cesari, M.; Kritchevsky, S.B.; Newman, A.B.; Simonsick, E.M.; Harris, T.B.; Penninx, B.W.; Brach, J.S.; Tylavsky, F.A.; Satterfield, S.; Bauer, D.C.; et al. Added value of physical performance measures in predicting adverse health-related events: Results from the Health, Aging And Body Composition Study. J. Am. Geriatr. Soc. 2009, 57, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Pavasini, R.; Guralnik, J.; Brown, J.C.; di Bari, M.; Cesari, M.; Landi, F.; Vaes, B.; Legrand, D.; Verghese, J.; Wang, C.; et al. Short Physical Performance Battery and all-cause mortality: Systematic review and meta-analysis. BMC Med. 2016, 14, 215. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, H.A.; Stahelin, H.B.; Monsch, A.U.; Iversen, M.D.; Weyh, A.; von Dechend, M.; Akos, R.; Conzelmann, M.; Dick, W.; Theiler, R. Identifying a cut-off point for normal mobility: A comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing 2003, 32, 315–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, A.B.; Simonsick, E.M.; Naydeck, B.L.; Boudreau, R.M.; Kritchevsky, S.B.; Nevitt, M.C.; Pahor, M.; Satterfield, S.; Brach, J.S.; Studenski, S.A.; et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA 2006, 295, 2018–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallengren, O.; Bosaeus, I.; Frandin, K.; Lissner, L.; Falk Erhag, H.; Wetterberg, H.; Rydberg Sterner, T.; Ryden, L.; Rothenberg, E.; Skoog, I. Comparison of the 2010 and 2019 diagnostic criteria for sarcopenia by the European Working Group on Sarcopenia in Older People (EWGSOP) in two cohorts of Swedish older adults. BMC Geriatr. 2021, 21, 600. [Google Scholar] [CrossRef]
- Lu, J.L.; Ding, L.Y.; Xu, Q.; Zhu, S.Q.; Xu, X.Y.; Hua, H.X.; Chen, L.; Xu, H. Screening Accuracy of SARC-F for Sarcopenia in the Elderly: A Diagnostic Meta-Analysis. J. Nutr. Health. Aging 2021, 25, 172–182. [Google Scholar] [CrossRef]
- Dodds, R.M.; Roberts, H.C.; Cooper, C.; Sayer, A.A. The Epidemiology of Sarcopenia. J. Clin. Densitom. 2015, 18, 461–466. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Wan, C.S.; Ktoris, K.; Reijnierse, E.M.; Maier, A.B. Sarcopenia Is Associated with Mortality in Adults: A Systematic Review and Meta-Analysis. Gerontology 2021, 1–16. [Google Scholar] [CrossRef]
- Mitchell, W.K.; Phillips, B.E.; Williams, J.P.; Rankin, D.; Smith, K.; Lund, J.N.; Atherton, P.J. Development of a new Sonovue contrast-enhanced ultrasound approach reveals temporal and age-related features of muscle microvascular responses to feeding. Physiol. Rep. 2013, 1, e00119. [Google Scholar] [CrossRef]
- Kim, Y.; Triolo, M.; Hood, D.A. Impact of Aging and Exercise on Mitochondrial Quality Control in Skeletal Muscle. Oxid. Med. Cell Longev. 2017, 2017, 3165396. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, J.; Chen, X.; Hou, L.; Lin, X.; Yang, M. Prevalence and Associated Factors of Sarcopenia in Nursing Home Residents: A Systematic Review and Meta-analysis. J. Am. Med. Dir. Assoc. 2019, 20, 5–13. [Google Scholar] [CrossRef]
- Norman, K.; Hass, U.; Pirlich, M. Malnutrition in Older Adults-Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef] [PubMed]
- Rom, O.; Kaisari, S.; Aizenbud, D.; Reznick, A.Z. Lifestyle and sarcopenia-etiology, prevention, and treatment. Rambam Maimonides Med. J. 2012, 3, e0024. [Google Scholar] [CrossRef] [PubMed]
- Hardee, J.P.; Lynch, G.S. Current pharmacotherapies for sarcopenia. Expert Opin. Pharm. 2019, 20, 1645–1657. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Broccatelli, M.; Savera, G.; D’Elia, M.; Pahor, M.; et al. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 35–42. [Google Scholar] [CrossRef]
- Anton, S.D.; Hida, A.; Mankowski, R.; Layne, A.; Solberg, L.M.; Mainous, A.G.; Buford, T. Nutrition and Exercise in Sarcopenia. Curr. Protein Pept. Sci. 2018, 19, 649–667. [Google Scholar] [CrossRef]
- Xia, X.; Chen, W.; McDermott, J.; Han, J.J. Molecular and phenotypic biomarkers of aging. F1000Research 2017, 6, 860. [Google Scholar] [CrossRef] [Green Version]
- Mankhong, S.; Kim, S.; Moon, S.; Kwak, H.B.; Park, D.H.; Kang, J.H. Experimental Models of Sarcopenia: Bridging Molecular Mechanism and Therapeutic Strategy. Cells 2020, 9, 1385. [Google Scholar] [CrossRef]
- van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buondonno, I.; Sassi, F.; Carignano, G.; Dutto, F.; Ferreri, C.; Pili, F.G.; Massaia, M.; Nisoli, E.; Ruocco, C.; Porrino, P.; et al. From mitochondria to healthy aging: The role of branched-chain amino acids treatment: MATeR a randomized study. Clin. Nutr. 2020, 39, 2080–2091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gioscia-Ryan, R.A.; Clayton, Z.S.; Zigler, M.C.; Richey, J.J.; Cuevas, L.M.; Rossman, M.J.; Battson, M.L.; Ziemba, B.P.; Hutton, D.A.; VanDongen, N.S.; et al. Lifelong voluntary aerobic exercise prevents age- and Western diet- induced vascular dysfunction, mitochondrial oxidative stress and inflammation in mice. J. Physiol. 2021, 599, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, C.; Segala, A.; Valerio, A.; Nisoli, E. Essential amino acid formulations to prevent mitochondrial dysfunction and oxidative stress. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 88–95. [Google Scholar] [CrossRef]
- Wesselink, E.; Koekkoek, W.A.C.; Grefte, S.; Witkamp, R.F.; van Zanten, A.R.H. Feeding mitochondria: Potential role of nutritional components to improve critical illness convalescence. Clin. Nutr. 2019, 38, 982–995. [Google Scholar] [CrossRef] [Green Version]
- Bai, G.H.; Tsai, M.C.; Tsai, H.W.; Chang, C.C.; Hou, W.H. Effects of branched-chain amino acid-rich supplementation on EWGSOP2 criteria for sarcopenia in older adults: A systematic review and meta-analysis. Eur. J. Nutr. 2021. [Google Scholar] [CrossRef]
- McGlory, C.; Calder, P.C.; Nunes, E.A. The Influence of Omega-3 Fatty Acids on Skeletal Muscle Protein Turnover in Health, Disuse, and Disease. Front. Nutr. 2019, 6, 144. [Google Scholar] [CrossRef] [Green Version]
- Romani, M.; Sorrentino, V.; Oh, C.M.; Li, H.; de Lima, T.I.; Zhang, H.; Shong, M.; Auwerx, J. NAD(+) boosting reduces age-associated amyloidosis and restores mitochondrial homeostasis in muscle. Cell Rep. 2021, 34, 108660. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nat. 2006, 443, 787–795. [Google Scholar] [CrossRef]
- D’Amico, D.; Mottis, A.; Potenza, F.; Sorrentino, V.; Li, H.; Romani, M.; Lemos, V.; Schoonjans, K.; Zamboni, N.; Knott, G.; et al. The RNA-Binding Protein PUM2 Impairs Mitochondrial Dynamics and Mitophagy During Aging. Mol. Cell 2019, 73, 775–787.e710. [Google Scholar] [CrossRef] [Green Version]
- Short, K.R.; Bigelow, M.L.; Kahl, J.; Singh, R.; Coenen-Schimke, J.; Raghavakaimal, S.; Nair, K.S. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. USA 2005, 102, 5618–5623. [Google Scholar] [CrossRef] [Green Version]
- Chabi, B.; Ljubicic, V.; Menzies, K.J.; Huang, J.H.; Saleem, A.; Hood, D.A. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell 2008, 7, 2–12. [Google Scholar] [CrossRef]
- Ghosh, S.; Lertwattanarak, R.; Lefort, N.; Molina-Carrion, M.; Joya-Galeana, J.; Bowen, B.P.; Garduno-Garcia Jde, J.; Abdul-Ghani, M.; Richardson, A.; DeFronzo, R.A.; et al. Reduction in reactive oxygen species production by mitochondria from elderly subjects with normal and impaired glucose tolerance. Diabetes 2011, 60, 2051–2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, A.M.; Adhihetty, P.J.; Buford, T.W.; Wohlgemuth, S.E.; Lees, H.A.; Nguyen, L.M.; Aranda, J.M.; Sandesara, B.D.; Pahor, M.; Manini, T.M.; et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell 2012, 11, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Ibebunjo, C.; Chick, J.M.; Kendall, T.; Eash, J.K.; Li, C.; Zhang, Y.; Vickers, C.; Wu, Z.; Clarke, B.A.; Shi, J.; et al. Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia. Mol. Cell Biol. 2013, 33, 194–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koltai, E.; Hart, N.; Taylor, A.W.; Goto, S.; Ngo, J.K.; Davies, K.J.; Radak, Z. Age-associated declines in mitochondrial biogenesis and protein quality control factors are minimized by exercise training. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R127–R134. [Google Scholar] [CrossRef] [Green Version]
- Dillon, L.M.; Williams, S.L.; Hida, A.; Peacock, J.D.; Prolla, T.A.; Lincoln, J.; Moraes, C.T. Increased mitochondrial biogenesis in muscle improves aging phenotypes in the mtDNA mutator mouse. Hum. Mol. Genet. 2012, 21, 2288–2297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brault, J.J.; Jespersen, J.G.; Goldberg, A.L. Peroxisome proliferator-activated receptor gamma coactivator 1alpha or 1beta overexpression inhibits muscle protein degradation, induction of ubiquitin ligases, and disuse atrophy. J. Biol. Chem. 2010, 285, 19460–19471. [Google Scholar] [CrossRef] [Green Version]
- Yeo, D.; Kang, C.; Gomez-Cabrera, M.C.; Vina, J.; Ji, L.L. Intensified mitophagy in skeletal muscle with aging is downregulated by PGC-1alpha overexpression in vivo. Free Radic Biol. Med. 2019, 130, 361–368. [Google Scholar] [CrossRef]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jager, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef] [Green Version]
- Garcia, S.; Nissanka, N.; Mareco, E.A.; Rossi, S.; Peralta, S.; Diaz, F.; Rotundo, R.L.; Carvalho, R.F.; Moraes, C.T. Overexpression of PGC-1alpha in aging muscle enhances a subset of young-like molecular patterns. Aging Cell 2018, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haynes, C.M.; Fiorese, C.J.; Lin, Y.F. Evaluating and responding to mitochondrial dysfunction: The mitochondrial unfolded-protein response and beyond. Trends Cell Biol. 2013, 23, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Migliavacca, E.; Tay, S.K.H.; Patel, H.P.; Sonntag, T.; Civiletto, G.; McFarlane, C.; Forrester, T.; Barton, S.J.; Leow, M.K.; Antoun, E.; et al. Mitochondrial oxidative capacity and NAD(+) biosynthesis are reduced in human sarcopenia across ethnicities. Nat. Commun. 2019, 10, 5808. [Google Scholar] [CrossRef]
- Drummond, M.J.; Addison, O.; Brunker, L.; Hopkins, P.N.; McClain, D.A.; LaStayo, P.C.; Marcus, R.L. Downregulation of E3 ubiquitin ligases and mitophagy-related genes in skeletal muscle of physically inactive, frail older women: A cross-sectional comparison. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.M.; Adhihetty, P.J.; Wawrzyniak, N.R.; Wohlgemuth, S.E.; Picca, A.; Kujoth, G.C.; Prolla, T.A.; Leeuwenburgh, C. Dysregulation of mitochondrial quality control processes contribute to sarcopenia in a mouse model of premature aging. PLoS ONE 2013, 8, e69327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, Y.; Matsunaga, Y.; Kitaoka, Y.; Hatta, H. Effects of Heat Stress Treatment on Age-dependent Unfolded Protein Response in Different Types of Skeletal Muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 299–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masiero, E.; Agatea, L.; Mammucari, C.; Blaauw, B.; Loro, E.; Komatsu, M.; Metzger, D.; Reggiani, C.; Schiaffino, S.; Sandri, M. Autophagy is required to maintain muscle mass. Cell Metab. 2009, 10, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Carnio, S.; LoVerso, F.; Baraibar, M.A.; Longa, E.; Khan, M.M.; Maffei, M.; Reischl, M.; Canepari, M.; Loefler, S.; Kern, H.; et al. Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging. Cell Rep. 2014, 8, 1509–1521. [Google Scholar] [CrossRef]
- Zhou, H.; Yuan, D.; Gao, W.; Tian, J.; Sun, H.; Yu, S.; Wang, J.; Sun, L. Loss of high-temperature requirement protein A2 protease activity induces mitonuclear imbalance via differential regulation of mitochondrial biogenesis in sarcopenia. IUBMB Life 2020, 72, 1659–1679. [Google Scholar] [CrossRef]
- Ryu, D.; Mouchiroud, L.; Andreux, P.A.; Katsyuba, E.; Moullan, N.; Nicolet-Dit-Felix, A.A.; Williams, E.G.; Jha, P.; Lo Sasso, G.; Huzard, D.; et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 2016, 22, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Arribat, Y.; Broskey, N.T.; Greggio, C.; Boutant, M.; Conde Alonso, S.; Kulkarni, S.S.; Lagarrigue, S.; Carnero, E.A.; Besson, C.; Canto, C.; et al. Distinct patterns of skeletal muscle mitochondria fusion, fission and mitophagy upon duration of exercise training. Acta. Physiol (Oxf) 2019, 225, e13179. [Google Scholar] [CrossRef]
- Rana, A.; Rera, M.; Walker, D.W. Parkin overexpression during aging reduces proteotoxicity, alters mitochondrial dynamics, and extends lifespan. Proc. Natl. Acad. Sci. USA 2013, 110, 8638–8643. [Google Scholar] [CrossRef] [Green Version]
- Cordeiro, A.V.; Bricola, R.S.; Braga, R.R.; Lenhare, L.; Silva, V.R.R.; Anaruma, C.P.; Katashima, C.K.; Crisol, B.M.; Simabuco, F.M.; Silva, A.S.R.; et al. Aerobic Exercise Training Induces the Mitonuclear Imbalance and UPRmt in the Skeletal Muscle of Aged Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 2258–2261. [Google Scholar] [CrossRef] [PubMed]
- Andreux, P.A.; Blanco-Bose, W.; Ryu, D.; Burdet, F.; Ibberson, M.; Aebischer, P.; Auwerx, J.; Singh, A.; Rinsch, C. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat. Metab. 2019, 1, 595–603. [Google Scholar] [CrossRef]
- Impacts of Nicotinamide Riboside on Functional Capacity and Muscle Physiology in Older Veterans. Available online: https://ClinicalTrials.gov/show/NCT04691986 (accessed on 6 December 2021).
- Trial of Nicotinamide Riboside and Co-enzyme Q10 in Chronic Kidney Disease. Available online: https://ClinicalTrials.gov/show/NCT03579693 (accessed on 6 December 2021).
- Resistance Exercise and Low-Intensity Physical Activity Breaks in Sedentary Time to Improve Muscle and Cardiometabolic Health. Available online: https://ClinicalTrials.gov/show/NCT03771417 (accessed on 6 December 2021).
- Sebastian, D.; Sorianello, E.; Segales, J.; Irazoki, A.; Ruiz-Bonilla, V.; Sala, D.; Planet, E.; Berenguer-Llergo, A.; Munoz, J.P.; Sanchez-Feutrie, M.; et al. Mfn2 deficiency links age-related sarcopenia and impaired autophagy to activation of an adaptive mitophagy pathway. EMBO J. 2016, 35, 1677–1693. [Google Scholar] [CrossRef] [PubMed]
- Tezze, C.; Romanello, V.; Desbats, M.A.; Fadini, G.P.; Albiero, M.; Favaro, G.; Ciciliot, S.; Soriano, M.E.; Morbidoni, V.; Cerqua, C.; et al. Age-Associated Loss of OPA1 in Muscle Impacts Muscle Mass, Metabolic Homeostasis, Systemic Inflammation, and Epithelial Senescence. Cell Metab. 2017, 25, 1374–1389.e1376. [Google Scholar] [CrossRef] [PubMed]
- Dulac, M.; Leduc-Gaudet, J.P.; Reynaud, O.; Ayoub, M.B.; Guerin, A.; Finkelchtein, M.; Hussain, S.N.; Gouspillou, G. Drp1 knockdown induces severe muscle atrophy and remodelling, mitochondrial dysfunction, autophagy impairment and denervation. J. Physiol. 2020, 598, 3691–3710. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Lorenzi, M.; Menghi, A.; Galli, M.; Vitiello, R.; Randisi, F.; Bernabei, R.; Landi, F.; Marzetti, E. Mitochondrial dynamics signaling is shifted toward fusion in muscles of very old hip-fractured patients: Results from the Sarcopenia in HIp FracTure (SHIFT) exploratory study. Exp. Gerontol. 2017, 96, 63–67. [Google Scholar] [CrossRef]
- Genova, M.L.; Lenaz, G. Functional role of mitochondrial respiratory supercomplexes. Biochim. Biophys. Acta. 2014, 1837, 427–443. [Google Scholar] [CrossRef] [Green Version]
- Sakellariou, G.K.; Pearson, T.; Lightfoot, A.P.; Nye, G.A.; Wells, N.; Giakoumaki, I.I.; Vasilaki, A.; Griffiths, R.D.; Jackson, M.J.; McArdle, A. Mitochondrial ROS regulate oxidative damage and mitophagy but not age-related muscle fiber atrophy. Sci. Rep. 2016, 6, 33944. [Google Scholar] [CrossRef] [Green Version]
- Sullivan-Gunn, M.J.; Lewandowski, P.A. Elevated hydrogen peroxide and decreased catalase and glutathione peroxidase protection are associated with aging sarcopenia. BMC Geriatr. 2013, 13, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deepa, S.S.; Van Remmen, H.; Brooks, S.V.; Faulkner, J.A.; Larkin, L.; McArdle, A.; Jackson, M.J.; Vasilaki, A.; Richardson, A. Accelerated sarcopenia in Cu/Zn superoxide dismutase knockout mice. Free Radic. Biol. Med. 2019, 132, 19–23. [Google Scholar] [CrossRef]
- Jang, Y.C.; Lustgarten, M.S.; Liu, Y.; Muller, F.L.; Bhattacharya, A.; Liang, H.; Salmon, A.B.; Brooks, S.V.; Larkin, L.; Hayworth, C.R.; et al. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010, 24, 1376–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakellariou, G.K.; McDonagh, B.; Porter, H.; Giakoumaki, I.I.; Earl, K.E.; Nye, G.A.; Vasilaki, A.; Brooks, S.V.; Richardson, A.; Van Remmen, H.; et al. Comparison of Whole Body SOD1 Knockout with Muscle-Specific SOD1 Knockout Mice Reveals a Role for Nerve Redox Signaling in Regulation of Degenerative Pathways in Skeletal Muscle. Antioxid. Redox. Signal 2018, 28, 275–295. [Google Scholar] [CrossRef] [Green Version]
- Dobrowolny, G.; Martini, M.; Scicchitano, B.M.; Romanello, V.; Boncompagni, S.; Nicoletti, C.; Pietrangelo, L.; De Panfilis, S.; Catizone, A.; Bouche, M.; et al. Muscle Expression of SOD1(G93A) Triggers the Dismantlement of Neuromuscular Junction via PKC-Theta. Antioxid. Redox. Signal 2018, 28, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Gurney, M.E.; Pu, H.; Chiu, A.Y.; Dal Canto, M.C.; Polchow, C.Y.; Alexander, D.D.; Caliendo, J.; Hentati, A.; Kwon, Y.W.; Deng, H.X.; et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Sci. 1994, 264, 1772–1775. [Google Scholar] [CrossRef] [PubMed]
- Vays, V.B.; Eldarov, C.M.; Vangely, I.M.; Kolosova, N.G.; Bakeeva, L.E.; Skulachev, V.P. Antioxidant SkQ1 delays sarcopenia-associated damage of mitochondrial ultrastructure. Aging (Albany NY) 2014, 6, 140–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, J.R.; Ryan, M.J.; Hao, Y.; Alway, S.E. Mediation of endogenous antioxidant enzymes and apoptotic signaling by resveratrol following muscle disuse in the gastrocnemius muscles of young and old rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1572–R1581. [Google Scholar] [CrossRef]
- Jackson, J.R.; Ryan, M.J.; Alway, S.E. Long-term supplementation with resveratrol alleviates oxidative stress but does not attenuate sarcopenia in aged mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 751–764. [Google Scholar] [CrossRef] [Green Version]
- Sakellariou, G.K.; Pearson, T.; Lightfoot, A.P.; Nye, G.A.; Wells, N.; Giakoumaki, I.I.; Griffiths, R.D.; McArdle, A.; Jackson, M.J. Long-term administration of the mitochondria-targeted antioxidant mitoquinone mesylate fails to attenuate age-related oxidative damage or rescue the loss of muscle mass and function associated with aging of skeletal muscle. FASEB J. 2016, 30, 3771–3785. [Google Scholar] [CrossRef] [Green Version]
- Spate, U.; Schulze, P.C. Proinflammatory cytokines and skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.; Leeuwenburgh, C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005, 19, 668–670. [Google Scholar] [CrossRef]
- Bian, A.L.; Hu, H.Y.; Rong, Y.D.; Wang, J.; Wang, J.X.; Zhou, X.Z. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-alpha. Eur. J. Med. Res. 2017, 22, 25. [Google Scholar] [CrossRef] [Green Version]
- Schaap, L.A.; Pluijm, S.M.; Deeg, D.J.; Visser, M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 2006, 119, 526.e9–526.e17. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Guardia, D.; Palomer, X.; Coll, T.; Davidson, M.M.; Chan, T.O.; Feldman, A.M.; Laguna, J.C.; Vazquez-Carrera, M. The p65 subunit of NF-kappaB binds to PGC-1alpha, linking inflammation and metabolic disturbances in cardiac cells. Cardiovasc. Res. 2010, 87, 449–458. [Google Scholar] [CrossRef] [Green Version]
- Handschin, C.; Choi, C.S.; Chin, S.; Kim, S.; Kawamori, D.; Kurpad, A.J.; Neubauer, N.; Hu, J.; Mootha, V.K.; Kim, Y.B.; et al. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J. Clin. Invest. 2007, 117, 3463–3474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handschin, C.; Chin, S.; Li, P.; Liu, F.; Maratos-Flier, E.; Lebrasseur, N.K.; Yan, Z.; Spiegelman, B.M. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J. Biol. Chem. 2007, 282, 30014–30021. [Google Scholar] [CrossRef] [Green Version]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Soenen, S.; Chapman, I.M. Body weight, anorexia, and undernutrition in older people. J. Am. Med. Dir. Assoc. 2013, 14, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, W.F.; Weenen, H.; Rigby, P.; Hetherington, M.M. Older adults and patients in need of nutritional support: Review of current treatment options and factors influencing nutritional intake. Clin. Nutr. 2010, 29, 160–169. [Google Scholar] [CrossRef]
- Rémond, D.; Shahar, D.R.; Gille, D.; Pinto, P.; Kachal, J.; Peyron, M.A.; Dos Santos, C.N.; Walther, B.; Bordoni, A.; Dupont, D.; et al. Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition. Oncotarget 2015, 6, 13858–13898. [Google Scholar] [CrossRef] [Green Version]
- Appleton, K.M. Increases in energy, protein and fat intake following the addition of sauce to an older person’s meal. Appet. 2009, 52, 161–165. [Google Scholar] [CrossRef]
- Denis, S.; Sayd, T.; Georges, A.; Chambon, C.; Chalancon, S.; Sante-Lhoutellier, V.; Blanquet-Diot, S. Digestion of cooked meat proteins is slightly affected by age as assessed using the dynamic gastrointestinal TIM model and mass spectrometry. Food Funct. 2016, 7, 2682–2691. [Google Scholar] [CrossRef]
- Short, K.R.; Nair, K.S. The effect of age on protein metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Davies, K.; Jagger, C.; R, M.D.; Kirkwood, T.B.L.; Sayer, A.A. Initial level and rate of change in grip strength predict all-cause mortality in very old adults. Age Ageing 2017, 46, 970–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulos, C.; Salameh, P.; Barberger-Gateau, P. Malnutrition and frailty in community dwelling older adults living in a rural setting. Clin. Nutr. 2016, 35, 138–143. [Google Scholar] [CrossRef]
- Wilson, D.; Jackson, T.; Sapey, E.; Lord, J.M. Frailty and sarcopenia: The potential role of an aged immune system. Ageing Res. Rev. 2017, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Corish, C.A.; Bardon, L.A. Malnutrition in older adults: Screening and determinants. Proc. Nutr. Soc. 2019, 78, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.R.; Tan, Z.J.; Zhang, Q.; Gui, Q.F.; Yang, Y.M. Clinical effectiveness of protein and amino acid supplementation on building muscle mass in elderly people: A meta-analysis. PLoS ONE 2014, 9, e109141. [Google Scholar] [CrossRef] [Green Version]
- Yaegashi, A.; Kimura, T.; Hirata, T.; Tamakoshi, A. Association of dietary protein intake with skeletal muscle mass in older adults: A systematic review. Geriatr. Gerontol. Int. 2021, 21, 1077–1083. [Google Scholar] [CrossRef]
- Tieland, M.; Franssen, R.; Dullemeijer, C.; van Dronkelaar, C.; Kyung Kim, H.; Ispoglou, T.; Zhu, K.; Prince, R.L.; van Loon, L.J.C.; de Groot, L. The Impact of Dietary Protein or Amino Acid Supplementation on Muscle Mass and Strength in Elderly People: Individual Participant Data and Meta-Analysis of RCT’s. J. Nutr. Health Aging 2017, 21, 994–1001. [Google Scholar] [CrossRef]
- Jang, E.H.; Han, Y.J.; Jang, S.E.; Lee, S. Association between Diet Quality and Sarcopenia in Older Adults: Systematic Review of Prospective Cohort Studies. Life (Basel) 2021, 11, 811. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am. J. Clin. Nutr. 2005, 82, 1065–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Eijk, H.M.; Dejong, C.H.; Deutz, N.E.; Soeters, P.B. Influence of storage conditions on normal plasma amino-acid concentrations. Clin. Nutr. 1994, 13, 374–380. [Google Scholar] [CrossRef]
- Moreau, K.; Walrand, S.; Boirie, Y. Protein redistribution from skeletal muscle to splanchnic tissue on fasting and refeeding in young and older healthy individuals. J. Am. Med. Dir. Assoc. 2013, 14, 696–704. [Google Scholar] [CrossRef]
- Deutz, N.E.P.; Thaden, J.J.; Ten Have, G.A.M.; Walker, D.K.; Engelen, M. Metabolic phenotyping using kinetic measurements in young and older healthy adults. Metab. 2018, 78, 167–178. [Google Scholar] [CrossRef]
- Cheng, H.; Kong, J.; Underwood, C.; Petocz, P.; Hirani, V.; Dawson, B.; O’Leary, F. Systematic review and meta-analysis of the effect of protein and amino acid supplements in older adults with acute or chronic conditions. Br. J. Nutr. 2018, 119, 527–542. [Google Scholar] [CrossRef]
- D’Antona, G.; Ragni, M.; Cardile, A.; Tedesco, L.; Dossena, M.; Bruttini, F.; Caliaro, F.; Corsetti, G.; Bottinelli, R.; Carruba, M.O.; et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010, 12, 362–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morio, A.; Tsutsumi, R.; Satomi, S.; Kondo, T.; Miyoshi, H.; Kato, T.; Kuroda, M.; Kitamura, T.; Hara, K.; Saeki, N.; et al. Leucine imparts cardioprotective effects by enhancing mTOR activity and mitochondrial fusion in a myocardial ischemia/reperfusion injury murine model. Diabetol. Metab. Syndr. 2021, 13, 139. [Google Scholar] [CrossRef]
- Sanchez Canedo, C.; Demeulder, B.; Ginion, A.; Bayascas, J.R.; Balligand, J.L.; Alessi, D.R.; Vanoverschelde, J.L.; Beauloye, C.; Hue, L.; Bertrand, L. Activation of the cardiac mTOR/p70(S6K) pathway by leucine requires PDK1 and correlates with PRAS40 phosphorylation. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E761–E769. [Google Scholar] [CrossRef]
- Zhang, L.; Li, F.; Guo, Q.; Duan, Y.; Wang, W.; Zhong, Y.; Yang, Y.; Yin, Y. Leucine Supplementation: A Novel Strategy for Modulating Lipid Metabolism and Energy Homeostasis. Nutrients 2020, 12, 1299. [Google Scholar] [CrossRef]
- Bifari, F.; Dolci, S.; Bottani, E.; Pino, A.; Di Chio, M.; Zorzin, S.; Ragni, M.; Zamfir, R.G.; Brunetti, D.; Bardelli, D.; et al. Complete neural stem cell (NSC) neuronal differentiation requires a branched chain amino acids-induced persistent metabolic shift towards energy metabolism. Pharmacol. Res. 2020, 158, 104863. [Google Scholar] [CrossRef]
- Morio, A.; Tsutsumi, R.; Kondo, T.; Miyoshi, H.; Kato, T.; Narasaki, S.; Satomi, S.; Nakaya, E.; Kuroda, M.; Sakaue, H.; et al. Leucine induces cardioprotection in vitro by promoting mitochondrial function via mTOR and Opa-1 signaling. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2979–2986. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Rom, O.; Hamoud, S.; Volkova, N.; Hayek, T.; Abu-Saleh, N.; Aviram, M. Leucine supplementation attenuates macrophage foam-cell formation: Studies in humans, mice, and cultured macrophages. Biofactors. 2018, 44, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Solerte, S.B.; Gazzaruso, C.; Bonacasa, R.; Rondanelli, M.; Zamboni, M.; Basso, C.; Locatelli, E.; Schifino, N.; Giustina, A.; Fioravanti, M. Nutritional supplements with oral amino acid mixtures increases whole-body lean mass and insulin sensitivity in elderly subjects with sarcopenia. Am. J. Cardiol. 2008, 101, 69E–77E. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.; Pereira, S.; Hays, N.; Oliver, J.; Edens, N.; Evans, C.; Wolfe, R. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin. Nutr. 2013, 32, 704–712. [Google Scholar] [CrossRef]
- Standley, R.A.; Distefano, G.; Pereira, S.L.; Tian, M.; Kelly, O.J.; Coen, P.M.; Deutz, N.E.P.; Wolfe, R.R.; Goodpaster, B.H. Effects of beta-hydroxy-beta-methylbutyrate on skeletal muscle mitochondrial content and dynamics, and lipids after 10 days of bed rest in older adults. J. Appl. Physiol. (1985) 2017, 123, 1092–1100. [Google Scholar] [CrossRef] [Green Version]
- Kaczka, P.; Michalczyk, M.M.; Jastrzab, R.; Gawelczyk, M.; Kubicka, K. Mechanism of Action and the Effect of Beta-Hydroxy-Beta-Methylbutyrate (HMB) Supplementation on Different Types of Physical Performance—A Systematic Review. J. Hum. Kinet. 2019, 68, 211–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin. Sci. (Lond) 2011, 121, 267–278. [Google Scholar] [CrossRef] [Green Version]
- Lalia, A.Z.; Dasari, S.; Robinson, M.M.; Abid, H.; Morse, D.M.; Klaus, K.A.; Lanza, I.R. Influence of omega-3 fatty acids on skeletal muscle protein metabolism and mitochondrial bioenergetics in older adults. Aging (Albany NY) 2017, 9, 1096–1129. [Google Scholar] [CrossRef] [Green Version]
- Custodero, C.; Mankowski, R.T.; Lee, S.A.; Chen, Z.; Wu, S.; Manini, T.M.; Hincapie Echeverri, J.; Sabba, C.; Beavers, D.P.; Cauley, J.A.; et al. Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflammation in middle-age and older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2018, 46, 42–59. [Google Scholar] [CrossRef]
- Dupont, J.; Dedeyne, L.; Dalle, S.; Koppo, K.; Gielen, E. The role of omega-3 in the prevention and treatment of sarcopenia. Aging Clin. Exp. Res. 2019, 31, 825–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshino, J.; Smith, G.I.; Kelly, S.C.; Julliand, S.; Reeds, D.N.; Mittendorfer, B. Effect of dietary n-3 PUFA supplementation on the muscle transcriptome in older adults. Physiol. Rep. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; El-Hajj Fuleihan, G.; Bouillon, R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, P23–P54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Ashcroft, S.P.; Fletcher, G.; Philp, A.M.; Jenkinson, C.; Das, S.; Hansbro, P.M.; Atherton, P.J.; Philp, A. Diet-induced vitamin D deficiency reduces skeletal muscle mitochondrial respiration. J. Endocrinol. 2021, 249, 113–124. [Google Scholar] [CrossRef]
- Girgis, C.M.; Baldock, P.A.; Downes, M. Vitamin D, muscle and bone: Integrating effects in development, aging and injury. Mol. Cell. Endocrinol. 2015, 410, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Shinchuk, L.M.; Holick, M.F. Vitamin d and rehabilitation: Improving functional outcomes. Nutr. Clin. Pract. 2007, 22, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Moreira-Pfrimer, L.D.; Pedrosa, M.A.; Teixeira, L.; Lazaretti-Castro, M. Treatment of vitamin D deficiency increases lower limb muscle strength in institutionalized older people independently of regular physical activity: A randomized double-blind controlled trial. Ann. Nutr. Metab. 2009, 54, 291–300. [Google Scholar] [CrossRef]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amelio, P.; Quacquarelli, L. Hypovitaminosis D and Aging: Is There a Role in Muscle and Brain Health? Nutrients 2020, 12, 628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodman, J.S.; Baker, T. Changes in the kinetics of muscle contraction in vitamin D-depleted rats. Kidney Int. 1978, 13, 189–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pleasure, D.; Wyszynski, B.; Sumner, A.; Schotland, D.; Feldman, B.; Nugent, N.; Hitz, K.; Goodman, D.B. Skeletal muscle calcium metabolism and contractile force in vitamin D-deficient chicks. J. Clin. Investig. 1979, 64, 1157–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubert, L.; DeLuca, H.F. Hypophosphatemia is responsible for skeletal muscle weakness of vitamin D deficiency. Arch. Biochem. Biophys. 2010, 500, 157–161. [Google Scholar] [CrossRef]
- Girgis, C.M.; Cha, K.M.; Houweling, P.J.; Rao, R.; Mokbel, N.; Lin, M.; Clifton-Bligh, R.J.; Gunton, J.E. Vitamin D Receptor Ablation and Vitamin D Deficiency Result in Reduced Grip Strength, Altered Muscle Fibers, and Increased Myostatin in Mice. Calcif. Tissue Int. 2015, 97, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Aspell, N.; Laird, E.; Healy, M.; Lawlor, B.; O’Sullivan, M. Vitamin D Deficiency Is Associated With Impaired Muscle Strength And Physical Performance In Community-Dwelling Older Adults: Findings From The English Longitudinal Study Of Ageing. Clin. Interv. Aging 2019, 14, 1751–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, H.J.; Seo, Y.G. Differences in Fat-Free Mass According to Serum Vitamin D Level and Calcium Intake: Korea National Health and Nutrition Examination Survey 2008-2011. J. Clin. Med. 2021, 10, 5428. [Google Scholar] [CrossRef]
- Minamino, H.; Katsushima, M.; Torii, M.; Yamamoto, W.; Fujita, Y.; Ikeda, K.; Okamura, E.; Murakami, K.; Watanabe, R.; Murata, K.; et al. Serum vitamin D status inversely associates with a prevalence of severe sarcopenia among female patients with rheumatoid arthritis. Sci. Rep. 2021, 11, 20485. [Google Scholar] [CrossRef]
- Luo, S.; Chen, X.; Hou, L.; Yue, J.; Liu, X.; Wang, Y.; Xia, X.; Dong, B. The Relationship between Sarcopenia and Vitamin D Levels in Adults of Different Ethnicities: Findings from the West China Health and Aging Trend Study. J. Nutr. Health Aging 2021, 25, 909–913. [Google Scholar] [CrossRef]
- Rosendahl-Riise, H.; Spielau, U.; Ranhoff, A.H.; Gudbrandsen, O.A.; Dierkes, J. Vitamin D supplementation and its influence on muscle strength and mobility in community-dwelling older persons: A systematic review and meta-analysis. J. Hum. Nutr. Diet 2017, 30, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.Y.; Bruyere, O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef] [Green Version]
- Tabrizi, R.; Hallajzadeh, J.; Mirhosseini, N.; Lankarani, K.B.; Maharlouei, N.; Akbari, M.; Asemi, Z. The effects of vitamin D supplementation on muscle function among postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. EXCLI J. 2019, 18, 591–603. [Google Scholar] [CrossRef]
- Ryan, Z.C.; Craig, T.A.; Folmes, C.D.; Wang, X.; Lanza, I.R.; Schaible, N.S.; Salisbury, J.L.; Nair, K.S.; Terzic, A.; Sieck, G.C.; et al. 1alpha,25-Dihydroxyvitamin D3 Regulates Mitochondrial Oxygen Consumption and Dynamics in Human Skeletal Muscle Cells. J. Biol. Chem. 2016, 291, 1514–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashcroft, S.P.; Bass, J.J.; Kazi, A.A.; Atherton, P.J.; Philp, A. The vitamin D receptor regulates mitochondrial function in C2C12 myoblasts. Am. J. Physiol. Cell Physiol. 2020, 318, C536–C541. [Google Scholar] [CrossRef] [PubMed]
- Girgis, C.M.; Cha, K.M.; So, B.; Tsang, M.; Chen, J.; Houweling, P.J.; Schindeler, A.; Stokes, R.; Swarbrick, M.M.; Evesson, F.J.; et al. Mice with myocyte deletion of vitamin D receptor have sarcopenia and impaired muscle function. J. Cachexia Sarcopenia Muscle 2019, 10, 1228–1240. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.J.; Kazi, A.A.; Deane, C.S.; Nakhuda, A.; Ashcroft, S.P.; Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Philp, A.; Tarum, J.; et al. The mechanisms of skeletal muscle atrophy in response to transient knockdown of the vitamin D receptor in vivo. J. Physiol. 2021, 599, 963–979. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Wang, X.; Yang, Y.; Cheng, S. Cardioprotective effect of calcitriol on myocardial injury induced by isoproterenol in rats. J. Cardiovasc. Pharmacol. Ther. 2013, 18, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Longoni, A.; Kolling, J.; dos Santos, T.M.; dos Santos, J.P.; da Silva, J.S.; Pettenuzzo, L.; Goncalves, C.A.; de Assis, A.M.; Quincozes-Santos, A.; Wyse, A.T. 1,25-Dihydroxyvitamin D3 exerts neuroprotective effects in an ex vivo model of mild hyperhomocysteinemia. Int. J. Dev. Neurosci. 2016, 48, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Romeu Montenegro, K.; Carlessi, R.; Cruzat, V.; Newsholme, P. Effects of vitamin D on primary human skeletal muscle cell proliferation, differentiation, protein synthesis and bioenergetics. J. Steroid. Biochem. Mol. Biol. 2019, 193, 105423. [Google Scholar] [CrossRef]
- Gong, Q.; Li, X.; Sun, J.; Ding, G.; Zhou, M.; Zhao, W.; Lu, Y. The effects of calcipotriol on the dendritic morphology of human melanocytes under oxidative stress and a possible mechanism: Is it a mitochondrial protector? J. Dermatol. Sci. 2015, 77, 117–124. [Google Scholar] [CrossRef]
- Lauretani, F.; Semba, R.D.; Bandinelli, S.; Ray, A.L.; Guralnik, J.M.; Ferrucci, L. Association of low plasma selenium concentrations with poor muscle strength in older community-dwelling adults: The InCHIANTI Study. Am. J. Clin. Nutr. 2007, 86, 347–352. [Google Scholar] [CrossRef] [Green Version]
- Chariot, P.; Bignani, O. Skeletal muscle disorders associated with selenium deficiency in humans. Muscle Nerve 2003, 27, 662–668. [Google Scholar] [CrossRef]
- Verlaan, S.; Aspray, T.J.; Bauer, J.M.; Cederholm, T.; Hemsworth, J.; Hill, T.R.; McPhee, J.S.; Piasecki, M.; Seal, C.; Sieber, C.C.; et al. Nutritional status, body composition, and quality of life in community-dwelling sarcopenic and non-sarcopenic older adults: A case-control study. Clin. Nutr. 2017, 36, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ter Borg, S.; de Groot, L.C.; Mijnarends, D.M.; de Vries, J.H.; Verlaan, S.; Meijboom, S.; Luiking, Y.C.; Schols, J.M. Differences in Nutrient Intake and Biochemical Nutrient Status Between Sarcopenic and Nonsarcopenic Older Adults-Results From the Maastricht Sarcopenia Study. J. Am. Med. Dir. Assoc. 2016, 17, 393–401. [Google Scholar] [CrossRef] [Green Version]
- Bodnar, D.; Ruzsnavszky, O.; Olah, T.; Dienes, B.; Balatoni, I.; Ungvari, E.; Benko, I.; Babka, B.; Prokisch, J.; Csernoch, L.; et al. Dietary selenium augments sarcoplasmic calcium release and mechanical performance in mice. Nutr. Metab. (Lond) 2016, 13, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osaki, Y.; Nishino, I.; Murakami, N.; Matsubayashi, K.; Tsuda, K.; Yokoyama, Y.I.; Morita, M.; Onishi, S.; Goto, Y.I.; Nonaka, I. Mitochondrial abnormalities in selenium-deficient myopathy. Muscle Nerve 1998, 21, 637–639. [Google Scholar] [CrossRef]
- Rani, P.; Lalitha, K. Evidence for altered structure and impaired mitochondrial electron transport function in selenium deficiency. Biol. Trace. Elem. Res. 1996, 51, 225–234. [Google Scholar] [CrossRef]
- White, S.H.; Wohlgemuth, S.; Li, C.; Warren, L.K. Rapid Communication: Dietary selenium improves skeletal muscle mitochondrial biogenesis in young equine athletes. J. Anim. Sci. 2017, 95, 4078–4084. [Google Scholar] [CrossRef]
- Hernandez-Camacho, J.D.; Vicente-Garcia, C.; Parsons, D.S.; Navas-Enamorado, I. Zinc at the crossroads of exercise and proteostasis. Redox. Biol. 2020, 35, 101529. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Blizzard, L.; Fell, J.; Giles, G.; Jones, G. Associations between dietary nutrient intake and muscle mass and strength in community-dwelling older adults: The Tasmanian Older Adult Cohort Study. J. Am. Geriatr. Soc. 2010, 58, 2129–2134. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc: An antioxidant and anti-inflammatory agent: Role of zinc in degenerative disorders of aging. J. Trace Elem. Med. Biol. 2014, 28, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc is an Antioxidant and Anti-Inflammatory Agent: Its Role in Human Health. Front. Nutr. 2014, 1, 14. [Google Scholar] [CrossRef] [Green Version]
- Bian, X.; Teng, T.; Zhao, H.; Qin, J.; Qiao, Z.; Sun, Y.; Liun, Z.; Xu, Z. Zinc prevents mitochondrial superoxide generation by inducing mitophagy in the setting of hypoxia/reoxygenation in cardiac cells. Free Radic. Res. 2018, 52, 80–91. [Google Scholar] [CrossRef]
- Yasuda, H.; Tsutsui, T. Infants and elderlies are susceptible to zinc deficiency. Sci Rep 2016, 6, 21850. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, H.; Enomoto, H.; Yoh, K.; Iwata, Y.; Sakai, Y.; Kishino, K.; Ikeda, N.; Takashima, T.; Aizawa, N.; Takata, R.; et al. Serum Zinc Concentration and Sarcopenia: A Close Linkage in Chronic Liver Diseases. J. Clin. Med. 2019, 8, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dillon, E.L.; Sheffield-Moore, M.; Paddon-Jones, D.; Gilkison, C.; Sanford, A.P.; Casperson, S.L.; Jiang, J.; Chinkes, D.L.; Urban, R.J. Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J. Clin. Endocrinol. Metab. 2009, 94, 1630–1637. [Google Scholar] [CrossRef] [Green Version]
- Volpi, E.; Ferrando, A.A.; Yeckel, C.W.; Tipton, K.D.; Wolfe, R.R. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J. Clin. Investig. 1998, 101, 2000–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scognamiglio, R.; Testa, A.; Aquilani, R.; Dioguardi, F.S.; Pasini, E. Impairment in walking capacity and myocardial function in the elderly: Is there a role for nonpharmacologic therapy with nutritional amino acid supplements? Am. J. Cardiol. 2008, 101, 78E–81E. [Google Scholar] [CrossRef] [PubMed]
- Hutchins-Wiese, H.L.; Kleppinger, A.; Annis, K.; Liva, E.; Lammi-Keefe, C.J.; Durham, H.A.; Kenny, A.M. The impact of supplemental n-3 long chain polyunsaturated fatty acids and dietary antioxidants on physical performance in postmenopausal women. J. Nutr. Health Aging 2013, 17, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Sinha, A.; Hollingsworth, K.G.; Ball, S.; Cheetham, T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J. Clin. Endocrinol. Metab. 2013, 98, E509–E513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murad, M.H.; Elamin, K.B.; Abu Elnour, N.O.; Elamin, M.B.; Alkatib, A.A.; Fatourechi, M.M.; Almandoz, J.P.; Mullan, R.J.; Lane, M.A.; Liu, H.; et al. Clinical review: The effect of vitamin D on falls: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2011, 96, 2997–3006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, P.; Marwaha, R.K.; Kumar, P.; Narang, A.; Devi, M.M.; Tripathi, R.P.; Khushu, S. Effect of vitamin D supplementation on muscle energy phospho-metabolites: A (3)(1)P magnetic resonance spectroscopy-based pilot study. Endocr. Res. 2014, 39, 152–156. [Google Scholar] [CrossRef] [PubMed]
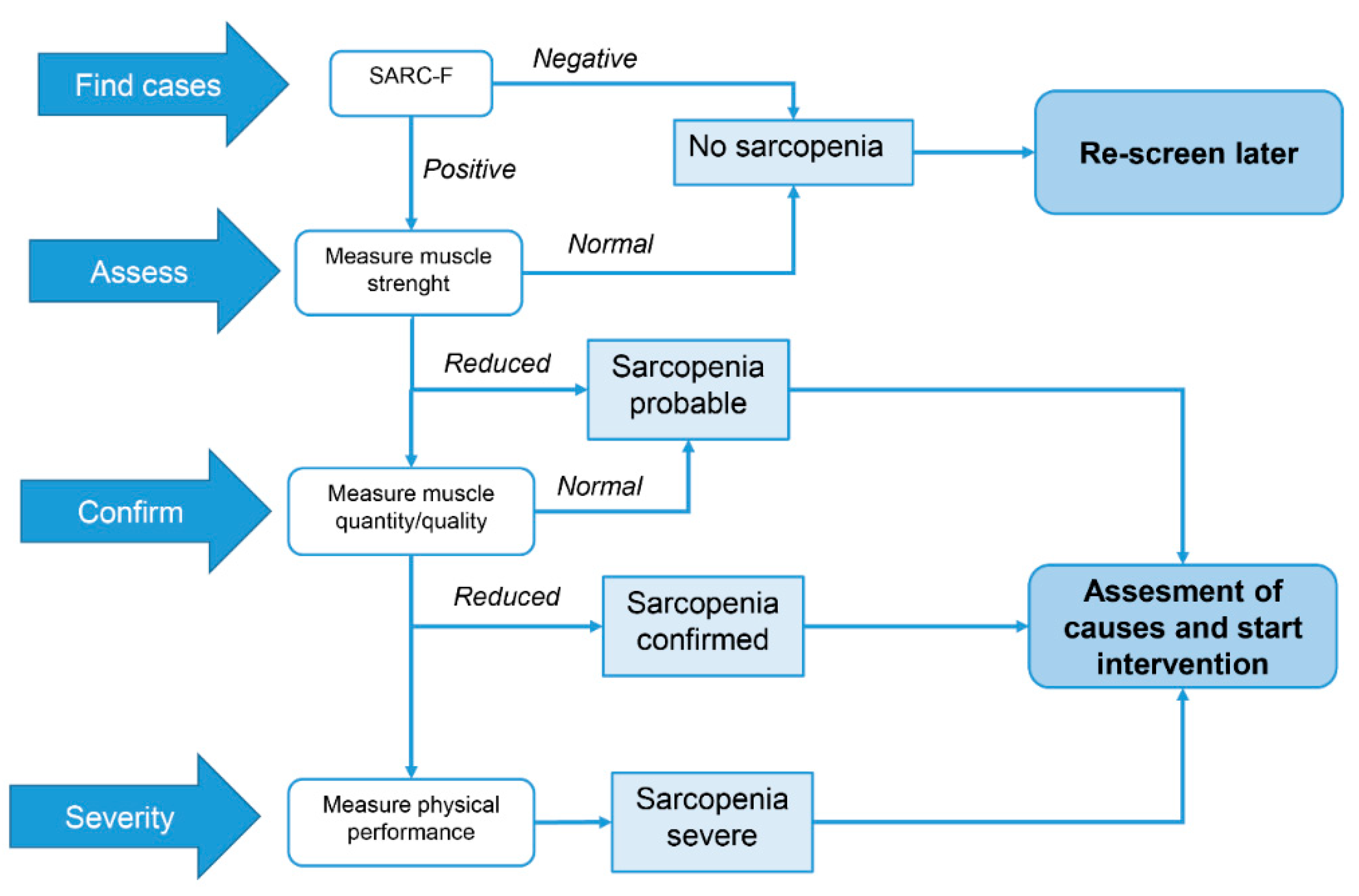
| Criteria | Muscle Performance | Muscle Strenght | Lean Body Mass | Summary Definition |
|---|---|---|---|---|
| International Working Group [7] | Gait speed < 1.0 m/s | Not included | ALM/ht2 ≤ 7.23 kg/m2 | Sarcopenia: slowness and low lean mass |
| EWGSOP-1 [8] | Gait speed ≤ 0.8 m/s | Grip strength < 30 kg | 2 SD < mean reference value | Sarcopenia: low lean mass and slowness or weakness Severe sarcopenia: all three criteria |
| EWSGOP-2 [10] | Gait speed ≤ 0.8 m/s SPPB ≤8 point score TUG ≥ 20 s 400 m walk test ≥6 min | Grip strength <27 kg for men and <16 kg for women Chair stand for five rises > 15 s | ALM/ht2 < 7.0 kg/m2 for men ALM/ht2 < 5.5 kg/m2 for women | Sarcopenia: weakness and low lean mass Severe sarcopenia: all three criteria |
| FNIH [9] | Gait speed ≤ 0.8 m/s | Grip strength < 26 kg | ALM/BMI < 0.789 | Sarcopenia: weakness and low lean mass Severe sarcopenia: all three criteria |
| Baumgartner [6] | Not included | Not included | ALM/ht2 ≤ 7.23 kg/m2 | Low lean mass |
| Newman [5] | Not included | Not included | Residual of actual ALM-predicted ALM from equation | Low lean mass |
| Dose | Subjects | Mitochondria | Muscle | Study Design and References |
|---|---|---|---|---|
| BCAAs | ||||
| Leucine (1250 mg), Lysine (650 mg), Isoleucine (625 mg), Valine (625 mg), Threonine (350 mg), Cystine (150 mg), Histidine (150 mg), Phenylalanine (10 mg), Methionine (50 mg), Tyrosine (30 mg), Tryptophan (20 mg), Vitamin B 6 (0.1 mg), Vitamin B1 (0.15 mg). Twice a day for 2 months. | 116 men and women aged 80 years or older. | ATP ↑ Electron flux ↑ Fusion ↑ Oxidative stress ↓ * | Strength ↑ Walking distance ↑ Balance ↑ Risk of falls ↓ Protein synthesis ↑ Lean mass ↑ Insulin sensitivity ↑ | Randomized Controlled Trial [32] |
| l-leucine (2.5 g), l-lysine (1.3 g), l-isoleucine (1.25 g), l-valine (1.25 g), l-threonine (0.7 g), l-cysteine (0.3 g), l-histidine (0.3 g), l-phenylalanine (0.2 g), l-methionine (0.1 g), l-thyrosine, (0.06 g), l-tryptophan (0.04 g). Twice a day for 8 months | 41 men and women aged 66–84 years with diagnosed sarcopenia. | TNFα ↓ Lean mass ↑ Insulin sensitivity ↑ | Randomized Controlled Trial [118] | |
| Histidine (0.82 g), Isoleucine (0.78 g), Leucine (1.39 g), Lysine (1.17 g), Methionine (0.23 g), Phenylalanine (1.17 g), Threonine (1.10 g), Valine (0.86 g). Twice a day for 3 months. | 14 women aged 68 +/− 2 years. | Fractional synthesis rate ↑ Lean mass ↑ | Randomized, Controlled Trial [169] | |
| (mg · mL−1 and (mmol · l−1), respectively): Alanine 20.7 (232.3), arginine 11.5 (66.0), glycine 10.3 (137.2), histidine 4.8 (30.9), isoleucine 6.0 (45.7), leucine 7.3 (55.6), lysine 5.8 (39.7), methionine 4 (26.8), phenylalanine 5.6 (33.9), proline 6.8 (59.1), serine 5.0 (47.6), threonine 4.2 (35.3), tryptophan 1.8 (8.8), tyrosine 0.4 (2.2), and valine 5.8 (49.5). The total amino acid infusion was 148.5 mg × kg−1 × h−1 for 480 min. | 5 subjects aged 71+/− 2 years. | Fractional synthesis rate ↑ | Longitudinal Clinical Trial [170] | |
| L-leucine (1.3 g), L-lysine (0.66 g), L-isoleucine (0.6 g), L-valine (0.63 g), L-threonine (0.36 g), L-cystine (0.13 g), L-histidine (0.13 g), L-phenylalanine (0.1 g), L-methionine (0.06 g), L-tyrosine (0.03 g), L-triptophane (0.03 g). 3 times a day for 3 months. | One hundred men and women aged >65 years. | Strength ↑ Walking distance ↑ Myocardial performance ↑ | Randomized Controlled Trial [171] | |
| Omega-3 PUFA | ||||
| Ethylesters of eicosapentaenoic acid (1.86 g), docosahexaenoic acid (1.50 g). Once a day for 8 weeks. | 5 men and 4 women aged 25–45 years | Protein concentration ↑ Cell size ↑ | Longitudinal Clinical Trial [122,172] | |
| EPA (1.35 g), DHA (0.6 g). Twice a day for 4 months. | 12 young (18–35 years) and 12 older (65–85 years) men and women. | Biogenesis ↑ Oxidative stress ↓ ** | Fractional synthesis rate↑ | Longitudinal Clinical Trial [123] |
| EPA (0.72 g), DHA (0.24 g). Twice a day for 6 months. | 126 women aged between 64–95 years. | Walking speed ↑ | Randomized Controlled Trial [173] | |
| EPA (0.93 g), DHA (0.75 g). Twice a day for 6 months. | 60 men and women aged 60–85 years. | Thigh muscle volume ↑ Handgrip strength ↑ Upper & lower-body muscle strength ↑ | Randomized Controlled Trial [174] | |
| Vitamin D | ||||
| Vitamin D3 (0.5 mg) on alternate days for 3 months. | 12 individuals with severe vitamin D deficiency aged 18.1–50.4 years and 15 age-matched controls. | OXPHOS ↑ ** | Fatigue ↓ Phosphocreatine recovery half-time ↓ | Longitudinal Clinical trial [175] |
| Vitamin D3 (60,000 IU/week) for 3 months. | 16 females and 3 males, mean age 17–24 years. | ATP ↑ ** | Pi:PCr ↑ | Longitudinal Clinical Trial [176] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romani, M.; Berger, M.M.; D’Amelio, P. From the Bench to the Bedside: Branched Amino Acid and Micronutrient Strategies to Improve Mitochondrial Dysfunction Leading to Sarcopenia. Nutrients 2022, 14, 483. https://doi.org/10.3390/nu14030483
Romani M, Berger MM, D’Amelio P. From the Bench to the Bedside: Branched Amino Acid and Micronutrient Strategies to Improve Mitochondrial Dysfunction Leading to Sarcopenia. Nutrients. 2022; 14(3):483. https://doi.org/10.3390/nu14030483
Chicago/Turabian StyleRomani, Mario, Mette M. Berger, and Patrizia D’Amelio. 2022. "From the Bench to the Bedside: Branched Amino Acid and Micronutrient Strategies to Improve Mitochondrial Dysfunction Leading to Sarcopenia" Nutrients 14, no. 3: 483. https://doi.org/10.3390/nu14030483
APA StyleRomani, M., Berger, M. M., & D’Amelio, P. (2022). From the Bench to the Bedside: Branched Amino Acid and Micronutrient Strategies to Improve Mitochondrial Dysfunction Leading to Sarcopenia. Nutrients, 14(3), 483. https://doi.org/10.3390/nu14030483







