GLIM-Defined Malnutrition in Patients with Head and Neck Cancer during the Qualification Visit for Home Enteral Nutrition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Institutional Standard to Qualification for Home Enteral Nutrition
Anthropometric Measurements
2.3. Data Collection
Nutritional Assessment
2.4. Laboratory Data
2.5. Statistical Analysis
3. Results
Group Characteristics
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- de Las Peñas, R.; Majem, M.; Perez-Altozano, J.; Virizuela, J.A.; Cancer, E.; Diz, P.; Donnay, O.; Hurtado, A.; Jimenez-Fonseca, P.; Ocon, M.J. SEOM clinical guidelines on nutrition in cancer patients (2018). Clin. Transl. Oncol. 2019, 21, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.S.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.A.E.; Baldwin, C.; Chasen, M.; et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines☆. ESMO Open 2021, 6, 100092. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Liu, G.; Pan, Y.; Li, Y. Prognostic Value of Clinical Biochemistry-Based Indexes in Nasopharyngeal Carcinoma. Front. Oncol. 2020, 10, 146. [Google Scholar] [CrossRef]
- Rosnes, K.S.; Henriksen, C.; Høidalen, A.; Paur, I. Agreement between the GLIM criteria and PG-SGA in a mixed patient population at a nutrition outpatient clinic. Clin. Nutr. 2021, 40, 5030–5037. [Google Scholar] [CrossRef]
- Zhang, Z.; Wan, Z.; Zhu, Y.; Zhang, L.; Zhang, L.; Wan, H. Prevalence of malnutrition comparing NRS2002, MUST, and PG-SGA with the GLIM criteria in adults with cancer: A multi-center study. Nutrition 2021, 83, 111072. [Google Scholar] [CrossRef]
- de van der Schueren, M.A.E.; Keller, H.; Cederholm, T.; Barazzoni, R.; Compher, C.; Correia, M.I.T.D.; Gonzalez, M.C.; Jager-Wittenaar, H.; Pirlich, M.; Steiber, A.; et al. Global Leadership Initiative on Malnutrition (GLIM): Guidance on validation of the operational criteria for the diagnosis of protein-energy malnutrition in adults. Clin. Nutr. 2020, 39, 2872–2880. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Einarsson, S.; Laurell, G.; Tiblom Ehrsson, Y. Mapping the frequency of malnutrition in patients with head and neck cancer using the GLIM Criteria for the Diagnosis of Malnutrition. Clin. Nutr. ESPEN 2020, 37, 100–106. [Google Scholar] [CrossRef]
- Ackerman, D.; Laszlo, M.; Provisor, A.; Yu, A. Nutrition Management for the Head and Neck Cancer Patient. Cancer Treat. Res. 2018, 174, 187–208. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Austin, P.; Boeykens, K.; Chourdakis, M.; Cuerda, C.; Jonkers-Schuitema, C.; Lichota, M.; Nyulasi, I.; Schneider, S.M.; Stanga, Z.; et al. ESPEN guideline on home enteral nutrition. Clin. Nutr. 2020, 39, 5–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bressan, V.; Stevanin, S.; Bianchi, M.; Aleo, G.; Bagnasco, A.; Sasso, L. The effects of swallowing disorders, dysgeusia, oral mucositis and xerostomia on nutritional status, oral intake and weight loss in head and neck cancer patients: A systematic review. Cancer Treat. Rev. 2016, 45, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Alshadwi, A.; Nadershah, M.; Carlson, E.R.; Young, L.S.; Burke, P.A.; Daley, B.J. Nutritional Considerations for Head and Neck Cancer Patients: A Review of the Literature. J. Oral Maxillofac. Surg. 2013, 71, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- National Health and Nutrition Examination Survey (NHANES). Anthropometry Procedures Manual. 2007. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf (accessed on 6 January 2022).
- Lipschitz, D.A. Screening for nutritional status in the elderly. Prim. Care Clin. Off. Pract. 1994, 21, 55–67. [Google Scholar] [CrossRef]
- Calixto-Lima, L.; Reis, N.T. Interpretação de Exames Laboratoriais Aplicados à Nutrição Clínica; Editora Rubio: Rio de Janeiro, Brasil, 2012. [Google Scholar]
- Pourhassan, M.; Sieske, L.; Janssen, G.; Babel, N.; Westhoff, T.H.; Wirth, R. The impact of acute changes of inflammation on appetite and food intake among older hospitalised patients. Br. J. Nutr. 2020, 124, 1069–1075. [Google Scholar] [CrossRef]
- Einarsson, S.; Karlsson, H.-E.; Björ, O.; Haylock, A.-K.; Tiblom Ehrsson, Y. Mapping impact factors leading to the GLIM diagnosis of malnutrition in patients with head and neck cancer. Clin. Nutr. ESPEN 2020, 40, 149–155. [Google Scholar] [CrossRef]
- Citak, E.; Tulek, Z.; Uzel, O. Nutritional status in patients with head and neck cancer undergoing radiotherapy: A longitudinal study. Support. Care Cancer 2019, 27, 239–247. [Google Scholar] [CrossRef]
- Hébuterne, X.; Lemarié, E.; Michallet, M.; de Montreuil, C.B.; Schneider, S.M.; Goldwasser, F. Prevalence of Malnutrition and Current Use of Nutrition Support in Patients With Cancer. J. Parenter. Enter. Nutr. 2014, 38, 196–204. [Google Scholar] [CrossRef]
- Righini, C.A.; Timi, N.; Junet, P.; Bertolo, A.; Reyt, E.; Atallah, I. Assessment of nutritional status at the time of diagnosis in patients treated for head and neck cancer. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2013, 130, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Bak, B.; Skrobala, A.; Adamska, A.; Malicki, J. What information can we gain from performing adaptive radiotherapy of head and neck cancer patients from the past 10 years? Cancer Radiothér. 2021. [Google Scholar] [CrossRef]
- Bento, C.; Neves, P.M.; Ravasco, P. Head-neck cancer under concurrent chemoradiotherapy: Does weight loss affect disease progression and treatment efficacy? Clin. Nutr. ESPEN 2020, 40, 549. [Google Scholar] [CrossRef]
- Crowder, S.L.; Douglas, K.G.; Yanina Pepino, M.; Sarma, K.P.; Arthur, A.E. Nutrition impact symptoms and associated outcomes in post-chemoradiotherapy head and neck cancer survivors: A systematic review. J. Cancer Surviv. 2018, 12, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, J.; Sulz, I.; Tarantino, S.; Hiesmayr, M.; Szostak-Węgierek, D. Hospital Malnutrition, Nutritional Risk Factors, and Elements of Nutritional Care in Europe: Comparison of Polish Results with All European Countries Participating in the nDay Survey. Nutrients 2021, 13, 263. [Google Scholar] [CrossRef] [PubMed]
- Magnano, M.; Mola, P.; Machetta, G.; Maffeis, P.; Forestiero, I.; Cavagna, R.; Artino, E.; Boffano, P. The nutritional assessment of head and neck cancer patients. Eur. Arch. Otorhinolaryngol. 2015, 272, 3793–3799. [Google Scholar] [CrossRef]
- Gascón-Ruiz, M.; Casas-Deza, D.; Torres-Ramón, I.; Zapata-García, M.; Alonso, N.; Sesma, A.; Lambea, J.; Álvarez-Alejandro, M.; Quílez, E.; Isla, D.; et al. GLIM vs. ESPEN criteria for the diagnosis of early malnutrition in oncological outpatients. Clin. Nutr. 2021, 40, 3741–3747. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Zhang, X.; Ruan, G.-T.; Zhang, K.-P.; Tang, M.; Zhang, Q.; Song, M.-M.; Zhang, X.-W.; Ge, Y.-Z.; Yang, M.; et al. One-Year Mortality in Patients with Cancer Cachexia: Association with Albumin and Total Protein. Cancer Manag. Res. 2021, 13, 6775–6783. [Google Scholar] [CrossRef]
- Reis, T.G.; Silva, R.A.W.P.d.; Nascimento, E.d.S.; Bessa, J.d.; Oliveira, M.C.; Fava, A.S.; Lehn, C.N. Early postoperative serum albumin levels as predictors of surgical outcomes in head and neck squamous cell carcinoma. Braz. J. Otorhinolaryngol. 2021. [Google Scholar] [CrossRef]
- Evans, D.C.; Corkins, M.R.; Malone, A.; Miller, S.; Mogensen, K.M.; Guenter, P.; Jensen, G.L. The Use of Visceral Proteins as Nutrition Markers: An ASPEN Position Paper. Nutr. Clin. Pract. 2021, 36, 22–28. [Google Scholar] [CrossRef]
- Jahanshiri, Z.; Manifar, S.; Moosa, H.; Asghari-Paskiabi, F.; Mahmoodzadeh, H.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Oropharyngeal candidiasis in head and neck cancer patients in Iran: Species identification, antifungal susceptibility and pathogenic characterization. J. Mycol. Méd. 2018, 28, 361–366. [Google Scholar] [CrossRef]
- Liu, T.; Li, Q.; Lin, Z.; Wang, P.; Chen, Y.; Fu, Y.; Ding, Z. Viral infections and the efficacy of PD-(L)1 inhibitors in virus-related cancers: Head and neck squamous cell carcinoma and hepatocellular carcinoma. Int. Immunopharmacol. 2021, 100, 108128. [Google Scholar] [CrossRef]
- Keller, H.; de van der Schueren, M.A.E.; Jensen, G.L.; Barazzoni, R.; Compher, C.; Correia, M.; Gonzalez, M.C.; Jager-Wittenaar, H.; Pirlich, M.; Steiber, A.; et al. Global Leadership Initiative on Malnutrition (GLIM): Guidance on Validation of the Operational Criteria for the Diagnosis of Protein-Energy Malnutrition in Adults. JPEN J. Parenter. Enteral Nutr. 2020, 44, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.J.; Sowa, D.; Keim, K.S.; Kinnare, K.; Peterson, S. The use of prealbumin and C-reactive protein for monitoring nutrition support in adult patients receiving enteral nutrition in an urban medical center. JPEN J. Parenter. Enteral Nutr. 2012, 36, 197–204. [Google Scholar] [CrossRef] [PubMed]
| Group of GLIM Criteria | Description of GLIM Criteria | Criteria Used in the Study |
|---|---|---|
| Phenotypic | Weight loss | |
| >5% within past 6 months | Present weight compared to self-estimated weight 6 months earlier | |
| >10% beyond 6 months | Not available | |
| BMI 1 (kg/m2) | ||
| <20 if <70 years | <20 if <70 years | |
| <22 if ≥70 years | <22 if ≥70 years | |
| Reduced Muscle Mass | ||
| FFMI 2 < 17 (males) | Not available | |
| FFMI < 15 (females) | ||
| Etiologic | Reduced food intake | |
| ≤50% for >1 week, or any reduction for >2 weeks | Artificial (enteral) nutrition 3 | |
| Inflammation | ||
| Acute disease/injury or chronic disease-related | Head and neck cancer 2 |
| Characteristics | Female (n = 55) | Male (n = 169) | p-Value * | MD (95% CI) | <65 (n = 124) | ≥65 (n = 100) | p-Value * | MD (95% CI) | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | M | SD | |||||
| Age | 62.49 | 11.49 | 62.76 | 10.90 | 0.98 | −0.27 (−3.65; 3.11) | NA | NA | NA | NA | NA | NA | 62.69 | 11.02 |
| Body mass (kg) | 52.73 | 12.58 | 62.64 | 12.77 | <0.001 | −9.91 (−13.80; −6.01) | 59.60 | 12.29 | 60.20 | 13.39 | 0.55 | −1.36 (−4.91; 2.19) | 60.20 | 13.39 |
| Height (m) | 1.59 | 0.06 | 1.73 | 0.07 | <0.001 | −0.134 (−0.16; −0.11) | 1.71 | 0.09 | 1.68 | 0.09 | 0.01 | 0.03 (0.00; 0.06) | 1.70 | 0.09 |
| BMI kg/m2 | 20.58 | 4.62 | 20.83 | 3.96 | 0.77 | −0.25 (−1.52; 1.01) | 20.77 | 3.90 | 20.77 | 4.12 | 0.04 | −1.15 (−2.23; −0.07) | 20.77 | 4.12 |
| Loss of body mass (%) | 16.19 | 10.90 | 17.30 | 9.13 | 0.55 | −1.11 (−4.05; 1.82) | 17.03 | 9.22 | 17.03 | 9.58 | 0.88 | 0.36 (−2.19; 2.90) | 17.03 | 9.58 |
| Characteristic | M | SD | Minimum | Maximum | CV (%) |
|---|---|---|---|---|---|
| Body weight loss (kg) | 12.62 | 7.98 | 0.00 | 40.00 | 63.2 |
| Leukocytes (thousand/mm3) | 10.59 | 7.77 | 2.63 | 64.43 | 73.4 |
| Lymphocytes (%) | 13.64 | 8.67 | 0.30 | 48.00 | 63.6 |
| Total protein (g/L) | 7.43 | 6.06 | 2.70 | 97.00 | 81.6 |
| Albumin (g/L) | 3.18 | 0.58 | 1.40 | 4.50 | 18.2 |
| CRP (mg/L) | 43.45 | 43.99 | 1.34 | 256.98 | 101.2 |
| Total lymphocyte count (cells/mm3) | 1224.99 | 792.85 | 35.37 | 5202.01 | 64.7 |
| No Malnutrition | Moderate Malnutriton | Severe Malnutrition | p-Value * | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Sex | 0.173 | ||||||
| Female | 6 | 42.9 | 10 | 29.4 | 39 | 22.2 | |
| Male | 8 | 57.1 | 24 | 70.6 | 137 | 77.8 | |
| Tumor Site | 0.225 | ||||||
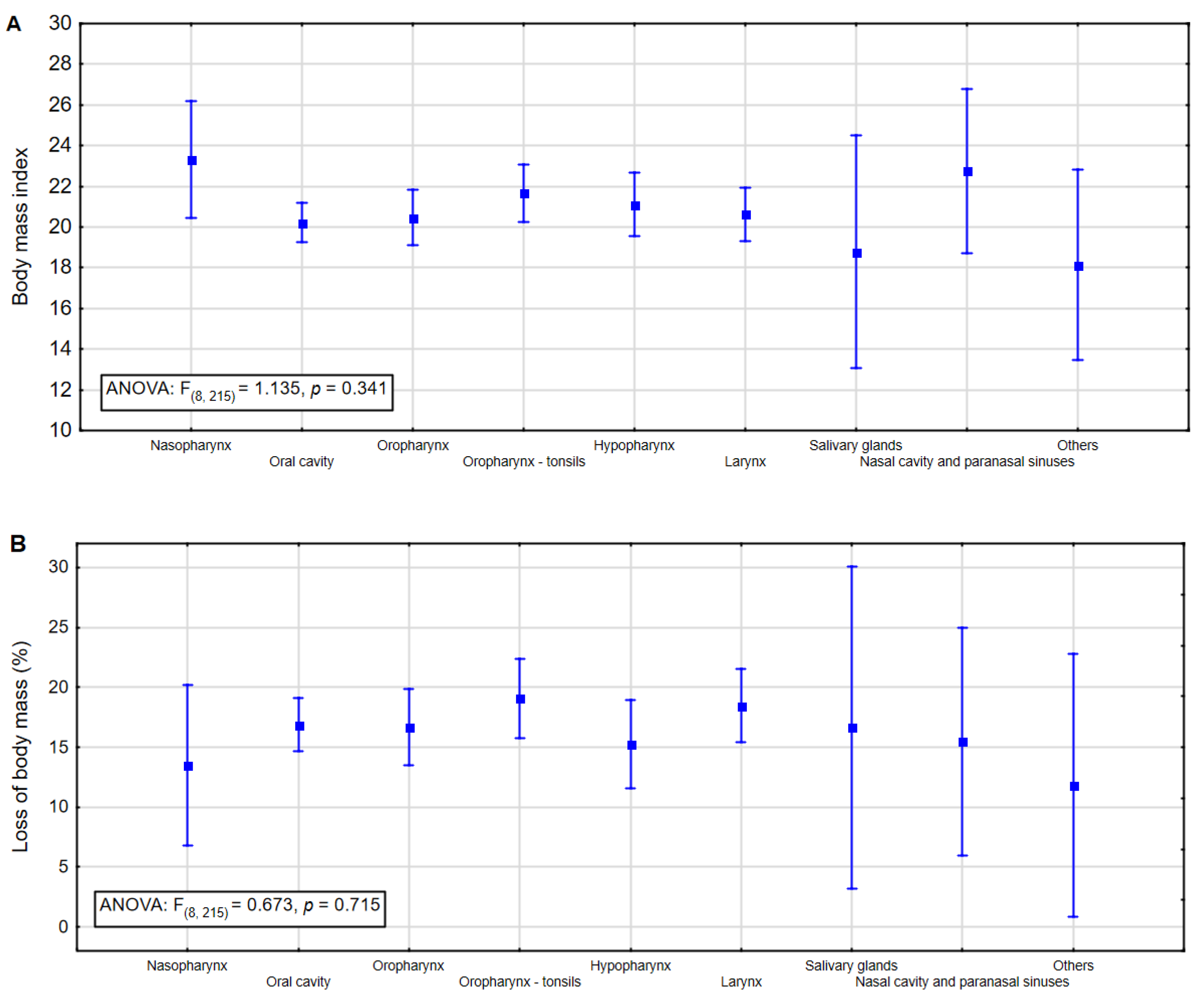
| Nasopharynx | 2 | 14.3 | 0 | 0.0 | 6 | 3.4 | |
| Oral cavity | 1 | 7.1 | 17 | 50.0 | 55 | 31.3 | |
| Oropharynx | 3 | 21.4 | 5 | 14.7 | 28 | 15.9 | |
| Oropharynx-tonsils | 3 | 21.4 | 3 | 8.8 | 27 | 15.3 | |
| Hypopharynx | 3 | 21.4 | 6 | 17.7 | 18 | 10.2 | |
| Larynx | 2 | 14.3 | 3 | 8.8 | 33 | 18.8 | |
| Salivary glands | 0 | 0.00 | 0 | 0.00 | 2 | 1.1 | |
| Nasal cavity and paranasal sinuses | 0 | 0.00 | 0 | 0.00 | 4 | 2.3 | |
| Others | 0 | 0.00 | 0 | 0.00 | 3 | 1.7 | |
| Age | 0.948 | ||||||
| <65 | 8 | 57.1 | 18 | 52.9 | 98 | 55.7 | |
| ≥65 | 6 | 42.9 | 16 | 47.1 | 78 | 44.3 | |
| GLIM Category | M | Mdn | Min | Max | SD | |
|---|---|---|---|---|---|---|
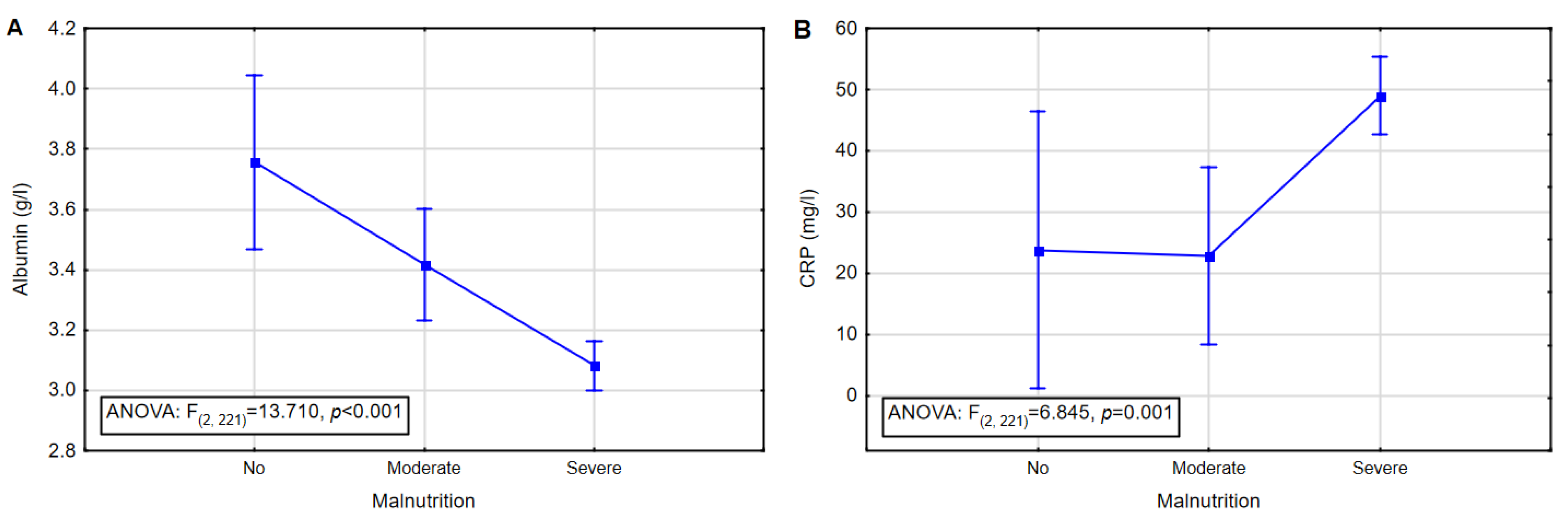
| No malnutrition (n = 14) | Albumin (g/L) | 3.8 | 3.9 | 3.1 | 4.5 | 0.43 |
| Total lymphocyte count (cells/mm3) | 1472 | 1220 | 395 | 3760 | 1034 | |
| CRP (mg/L) | 23.8 | 9.9 | 1.85 | 94.72 | 31.03 | |
| Total protein (g/L) | 7.4 | 7.3 | 5.9 | 9.0 | 0.75 | |
| Moderate malnutrition (n = 34) | Albumin (g/L) | 3.4 | 3.5 | 2.5 | 4.2 | 0.42 |
| Total lymphocyte count (cells/mm3) | 1012 | 964 | 146 | 2042 | 547 | |
| CRP (mg/L) | 22.9 | 13.5 | 2.02 | 94.82 | 23.21 | |
| Total protein (g/L) | 7.20 | 7.2 | 5.6 | 8.4 | 0.67 | |
| Severe malnutrition (n = 176) | Albumin (g/L) | 3.1 | 3.2 | 1.4 | 4.4 | 0.58 |
| Total lymphocyte count (cells/mm3) | 1247 | 998 | 35 | 5202 | 807 | |
| CRP (mg/L) | 49.0 | 34.0 | 1.34 | 256.98 | 46.35 | |
| Total protein (g/L) | 7.5 | 7.0 | 2.7 | 97.0 | 6.83 |
| ROC Results | Albumin | TLC | CRP | Total Protein |
|---|---|---|---|---|
| AUC (CI 95%) | 0.672 (0.58–0.77) | 0.431 (0.32–0.54) | 0.701 (0.61–0.79) | 0.582 (0.48–0.69) |
| SE | 0.049 | 0.056 | 0.047 | 0.054 |
| z | 3.472 | −1.233 | 4.292 | 1.507 |
| p | 0.0005 | 0.218 | 0.0000 | 0.132 |
| Sensitivity (95% CI) | 67% (59% to 73%) | NA | 52% (45% to 60%) | NA |
| Specificity (95% CI) | 62% (44% to 78%) | NA | 82% (65% to 93%) | NA |
| Positive Predictive Value (95% CI) | 90% (85% to 93%) | NA | 94% (88% to 97%) | NA |
| Negative Predictive Value (95% CI) | 26% (20% to 33%) | NA | 25% (21% to 29%) | NA |
| Accuracy (95% CI) | 66% (59% to 72%) | NA | 57% (50% to 64%) | NA |
| GLIM Category |
GLIM Moderate Malnutrition |
GLIM Severe Malnutrition | γ * | z | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||
| Albumin | Nourished | 15 | 44.1 | 36 | 20.5 | 0.51 | 4.666 | <0.001 |
| Mild malnutrition | 16 | 47.1 | 90 | 51.1 | ||||
| Moderate malnutrition | 3 | 8.8 | 29 | 16.5 | ||||
| Severe malnutrition | 0 | 0.0 | 21 | 11.9 | ||||
| TLC | No depletion | 1 | 2.9 | 23 | 13.1 | −0.36 | −3.072 | 0.002 |
| Moderate depletion | 17 | 50.0 | 98 | 55.7 | ||||
| Mild depletion | 16 | 47.1 | 55 | 31.3 | ||||
| CRP 1 [17] | Mild inflammation | 2 | 5.9 | 2 | 1.1 | 0.69 | 2.757 | 0.006 |
| Severe inflammation | 32 | 94.1 | 174 | 98.9 | ||||
| CRP 2 [2] | No inflammatory | 12 | 35.3 | 27 | 15.3 | 0.50 | 4.074 | <0.001 |
| Inflammatory | 22 | 64.7 | 149 | 84.7 | ||||
| Total protein | Below norms | 2 | 6.1 | 22 | 12.6 | −0.40 | −2.162 | 0.031 |
| In norm | 30 | 90.9 | 150 | 85.7 | ||||
| Above norms | 1 | 3.0 | 3 | 1.7 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przekop, Z.; Milewska, M.; Szostak-Węgierek, D.; Panczyk, M.; Sobocki, J. GLIM-Defined Malnutrition in Patients with Head and Neck Cancer during the Qualification Visit for Home Enteral Nutrition. Nutrients 2022, 14, 502. https://doi.org/10.3390/nu14030502
Przekop Z, Milewska M, Szostak-Węgierek D, Panczyk M, Sobocki J. GLIM-Defined Malnutrition in Patients with Head and Neck Cancer during the Qualification Visit for Home Enteral Nutrition. Nutrients. 2022; 14(3):502. https://doi.org/10.3390/nu14030502
Chicago/Turabian StylePrzekop, Zuzanna, Magdalena Milewska, Dorota Szostak-Węgierek, Mariusz Panczyk, and Jacek Sobocki. 2022. "GLIM-Defined Malnutrition in Patients with Head and Neck Cancer during the Qualification Visit for Home Enteral Nutrition" Nutrients 14, no. 3: 502. https://doi.org/10.3390/nu14030502
APA StylePrzekop, Z., Milewska, M., Szostak-Węgierek, D., Panczyk, M., & Sobocki, J. (2022). GLIM-Defined Malnutrition in Patients with Head and Neck Cancer during the Qualification Visit for Home Enteral Nutrition. Nutrients, 14(3), 502. https://doi.org/10.3390/nu14030502








