Effects of Intermittent Fasting on Cardiometabolic Health: An Energy Metabolism Perspective
Abstract
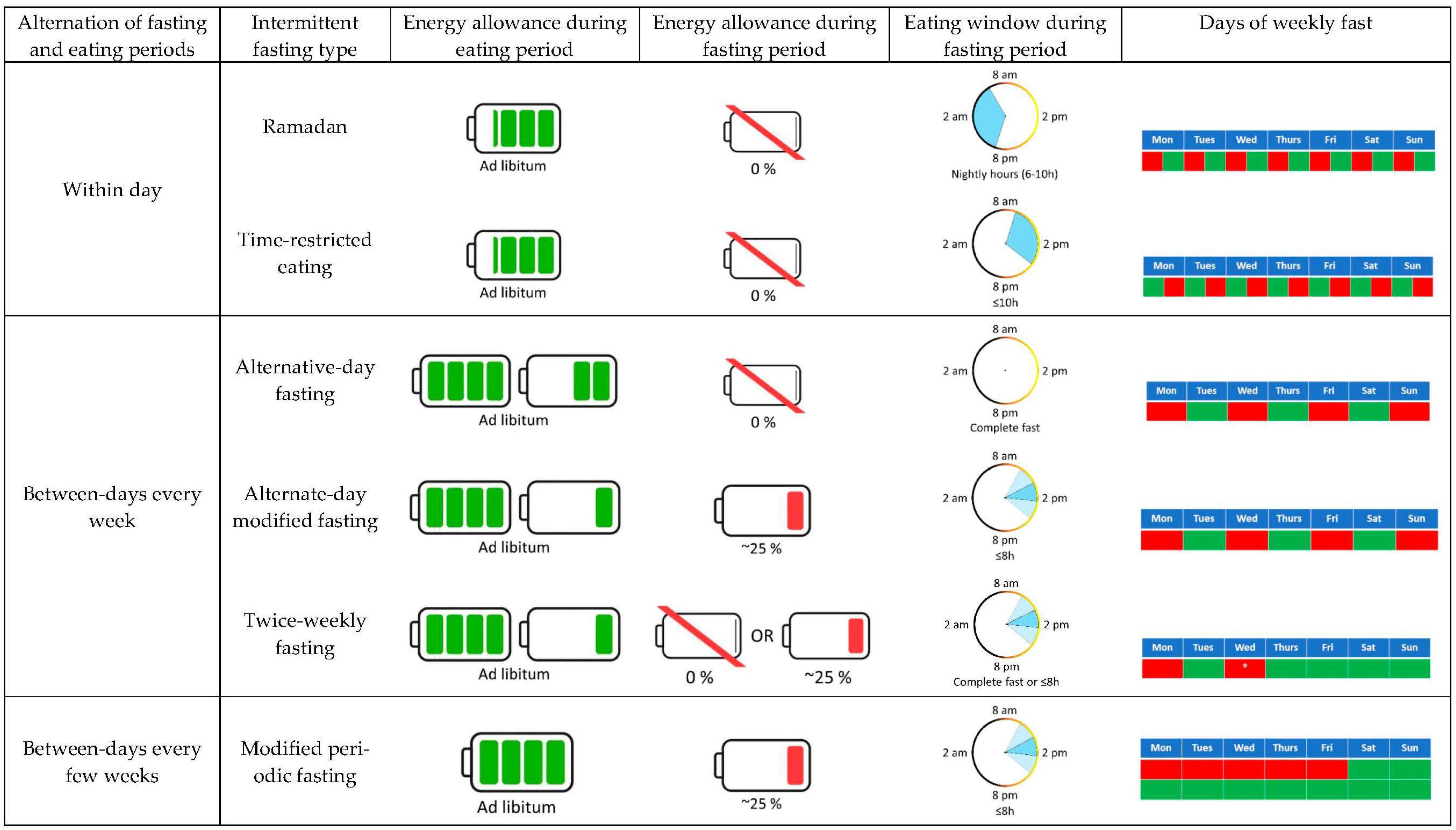
:1. Introduction
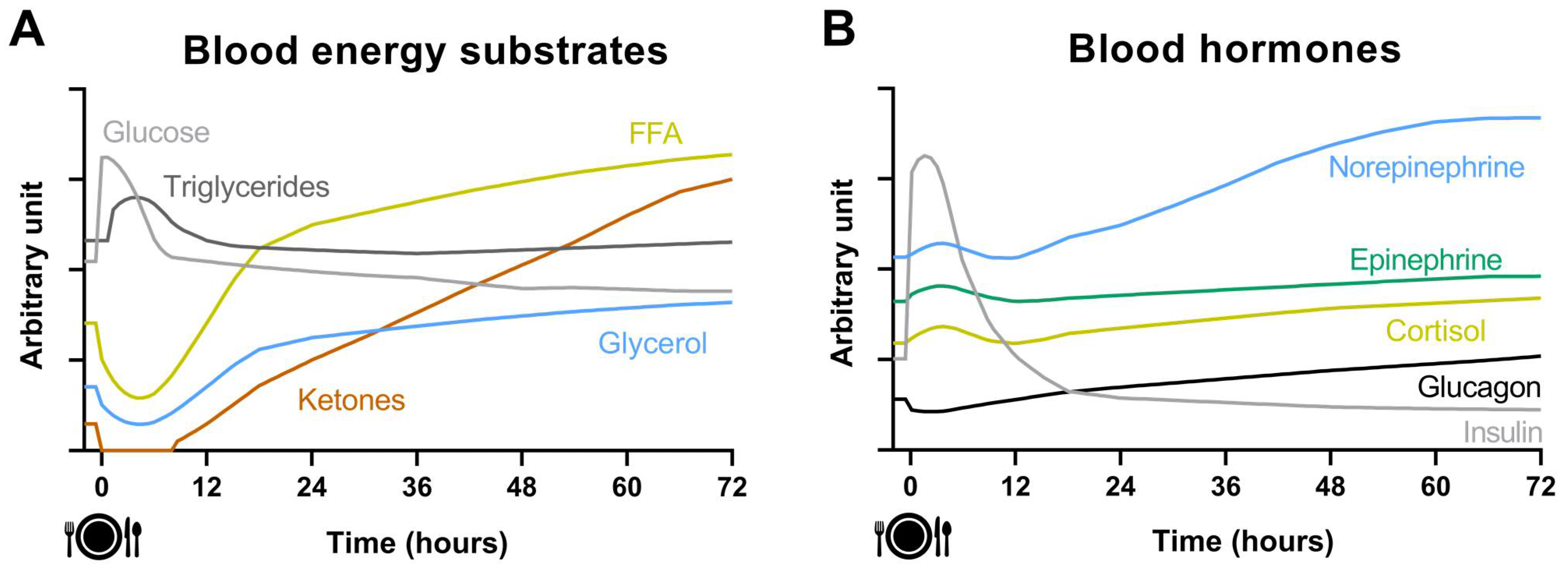
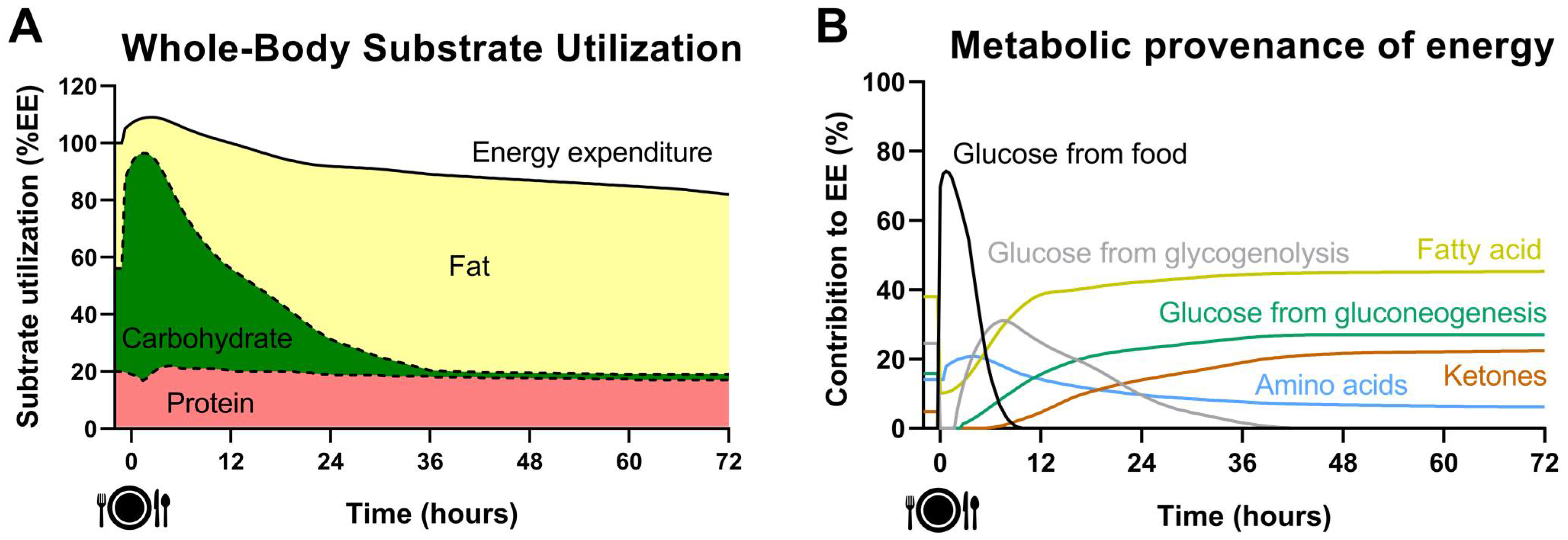
2. Acute Effect of Fasting on Energy Metabolism
3. Impact of IF on Cardiometabolic Health
3.1. Body Weight and Composition
3.2. Ectopic Fat
3.3. Cardiometabolic Risk Factors
3.4. Impact of IF on Cardiometabolic Health: Conclusion and Gaps
4. Effects of IF on Energy Metabolism: Energy Expenditure and Substrate Oxidation
4.1. Ramadan
4.2. Alternate-Day Fasting (ADF)
4.3. Alternate-Day Modified Fasting (ADMF)
4.4. Twice-Weekly Fasting
| Study | Population | Design | Fasting Regimen | Eating Regimen | Assessment | No Change | Increase | Decrease |
|---|---|---|---|---|---|---|---|---|
| Acute alternative-day fasting | ||||||||
| [27] | Healthy normal weight/obesity n = 14 (14 M/0 F) | 48 h Cross-over (a) Control (b) ADF | (2) 48 h fasting | (1) 100% Energy needs | Metabolic chamber | AEE | 24 h EE and SEE 24 h RER | |
| [83] | Healthy normal weight/obesity n = 64 (51 M/13 F) | 36 h Cross-over (a) Control (b) ADF | (2) 36 h fasting | (1) 100% Energy needs | Metabolic chamber | SEE | 24 h Fat ox. | 24 h EE 24 h RER and sleep RER 24 h CHO ox. 24 h Protein ox. |
| Chronic alternative-day fasting | ||||||||
| [75] | Healthy normal/overweight n = 8 (8 M/0 F) | 2-week Single-arm (1) ADF | 20 h fasting every other day (From 10 p.m. to 6 p.m.) | Ad libitum | Overnight fasting Metabolic cart (Oxycon Pro, Jaeger) | RER | ||
| [76] | Healthy normal/overweight n = 16 (8 M/8 F) | 22-day Single-arm (1) ADF | 24 h fasting every other day | Ad libitum | Overnight fasting Metabolic cart (DeltaTrac, SensorMedics) | RMR and adjusted RMR * in fed and fast days RER, CHO and Fat ox. In fed day | Fat ox. In fast day | RER and CHO ox. In fast day |
| [77] | Healthy normal/overweight n = 8 (8 M/0 F) | 2-week Cross-over (a) Control (b) ADF | (2) 20 h fasting every other day (From 10 p.m. to 6 p.m.) | 1 and (2) 100% Energy needs | Overnight fasting Metabolic cart | RER, CHO and Fat ox. | RMR | |
| [74] | Obesity (1) n = 12 (3 M/9 F) (2) n = 13 (3 M/10 F) | 8-week RCT (1) CR (2) ADF | (2) 24 h fasting every other day | (1) Prescribed CR −400 kcal/day. Measure 28% CR (2) 100% Energy needs + ad libitum access to 5–7 snacks (200 kcal/serve) during eating days. Measure 47% CR | Overnight fasting Metabolic cart (TrueOne 2400, Parvo Medics) | RMR and adjusted RMR * | ||
| [78] | Healthy normal/overweight (1) n = 29 (12 M/17 F) (2) n = 28 (11 M/17 F) | 4-week RCT (1) Control (2) ADF | (2) 24 h fasting every other day | (1) Ad libitum. Measured 8% CR (2) Ad libitum. Measured 37% CR | Overnight fasting Metabolic cart (MetaMax 3b, Cortex) | RMR | ||
| [78] | Healthy normal/overweight (1) n = 60 (24 M/36 F) (2) n = 30 (14 M/16 F) | Observational (1) Control (2) ADF performed >24 weeks | (2) 24 h fasting every other day | (1) Ad libitum. Measured 0% CR (2) Ad libitum. Measured 29% CR | Overnight fasting Metabolic cart (MetaMax 3b, Cortex) | RMR | ||
| [42] | Healthy normal/overweight (1) n = 12 (5 M/7 F) (2) n = 12 (3 M/9 F) (3) n = 12 (7 M/5 F) | 3-week RCT (1) CR (2) ADF without CR (3) ADF + CR | 2 and (3) 24 h fasting every other day | (1) Daily 25% CR (2) 200% Energy needs in fed days, 0% net CR (3) 150% Energy needs in fed days, 25% net CR | Overnight fasting and 3-h postprandial period Metabolic cart | RMR and adjusted RMR ¥ Fasting CHO, Fat and Protein ox. Postprandial CHO and Protein ox. | Postprandial Fat ox. in both ADF groups vs. CR | |
| Chronic alternative-day modified fasting | ||||||||
| [81] | Overweight/obesity (1) n = 10 (0 M/10 F) (2) n = 10 (0 M/10 F) (3) n = 10 (0 M/10 F) | 4-week Cross-over (a) CR (b) Bread ADMF (c) ADMF | (2) Ad libitum bread, coffee and tea (3) 50% CR | (1) Daily 50% CR (2) 100% Energy needs (3) 100% Energy needs | Metabolic chamber | Adjusted 24 h EE ¥ and AEE Adjusted SEE ¥ in CR vs. ADMF | Adjusted SEE ¥ in ADMF vs. bread ADMF | |
| [80] | Obesity (1) n = 14 (4 M/10 F) (2) n = 14 (2 M/12 F) | 12-week RCT (1) CR (2) ADMF | (2) EI of 660 and 550 kcal/day for men and women, respectively, on 3 non-consecutive days/week | (1) Daily 25% CR (2) 100% Energy needs | Overnight fasting Metabolic cart (Vmax Encore 29N, Care Fusion) | RMR and adjusted RMR α RER | ||
| [79] | Overweight/obesity (1) n = 18 (0 M/18 F) (2) n = 12 (0 M/12 F) | RCT ≥ 5% WL within 12 weeks (1) CR (2) ADMF | (2) 75% CR every other day | (1) Daily 25% CR (2) Ad libitum | Overnight fasting Metabolic cart (GEMNutrition) | RMR | ||
| Chronic twice-weekly fasting (2-WF) | ||||||||
| [54] | Overweight/obesity (1) n = 12 (6 M/6 F) (2) n = 15 (7 M/8 F) | RCT 5% WL target (1) CR (2) 2-WF | (2) 75% CR on two consecutive days/week | (1) Daily 33% CR (2) Ad libitum | Overnight fasting Metabolic cart Φ (ISGEM319, GEMNutrition) | RMR and adjusted RMR £ RER | ||
| [82] | Adults with central obesity (1) n = 22 (6 M/16 F) (2) n = 21 (6 M/15 F) | 4-week RCT (1) CR (2) 2-WF | (2) EI of 600 kcal on two consecutive days/week | (1) Daily 500 kcal CR (2) Energy-controlled diet to target the same weekly energy deficit as the CR | Overnight fasting Metabolic cart (FitMate, Cosmed) | RMR | ||
| [55] | Healthy normal weight (1) n = 8 (4 M/4 F) (2) n = 8 (4 M/4 F) | 2-week RCT (1) CR (2) 2-WF | (2) 70% CR on two non-consecutive days/week | (1) Daily 20% CR (2) 100% Energy needs | Overnight fasting Metabolic cart (Quark CPFT, Cosmed) | RMR RER | ||
4.5. Time-Restricted Eating (TRE)
| Study | Population | Design and Intervention | Assessment | No Change | Increase | Decrease |
|---|---|---|---|---|---|---|
| Acute time-restricted eating | ||||||
| [88] | Healthy normal/overweight n = 13 (2 M/11 F) | 48 h Cross-over (a) 13 h Control (7:30 a.m.–8:30 p.m., 7 meals/day, 100% energy needs) (b) 6 h TRE (12 p.m.–6 p.m., 2 meals/day, 100% energy needs) | Metabolic chamber | 24 h EE 24 h RER Fat ox. from 6 p.m.–9 p.m. | Fat ox. from 9 a.m.–12 p.m. CHO ox. from 6 p.m.–9 p.m. | CHO ox. from 9 a.m.–12 p.m. |
| [91] | Overweight/Obesity n = 10 (0 M/10 F) | 48 h Cross-over (a) 10 h Control (9 a.m.–7 p.m., 6 meals/day,1000 kcal/day) (b) 8 h TRE (11 a.m.–7 p.m., 2 meals/day, 1000 kcal/day) | Metabolic chamber | 24 h EE | Night EE | |
| [87] | Healthy normal weight n = 14 (0 M/14 F) | 36 h Cross-over (a) 8:30 h TRE (8 a.m.–4:30 p.m., 3 meals/day, 100% energy needs) (b) 8:30 h TRE (8 a.m.–4:30 p.m., 2 meals/day, 100% energy needs) | Metabolic chamber | 24 h EE, SMR, TEF and AEE 24 h Fat, 24 h CHO and 24 h protein balance | 24 h Fat ox. in 3 meals/day vs. 2 meals/day | 24 h and night RER in 3 meals/day vs. 2 meals/day 24 h CHO ox. in 3 meals/day vs. 2 meals/day |
| [85] | Healthy normal weight n = 12 (12 M/0 F) | 36 h Cross-over (a) 13 h Control (8 a.m.–9 p.m., 14 meals/day, 100% energy needs) (b) 9 h TRE (8 a.m.–5 p.m., 3 meals/day, 100% energy needs) | Metabolic chamber | 24 h EE, SMR, TEF and AEE 24 h RER, 24 h CHO and 24 h Fat ox. | RMR 24 h Protein ox. | |
| [84] | Healthy normal/overweight n = 8 (8 M/0 F) | 24 h Cross-over (a) 11 h Control (8 a.m.–7 p.m., 3 meals/day, 100% energy needs) (b) 7 h TRE (12 p.m.–7 p.m., 2 meals/day, 100% energy needs) | Metabolic chamber | 24 h EE, RMR and TEF 24 h RER, 24 h CHO, 24 h Fat and 24 h Protein ox. | SMR Morning Fat ox. Sleeping CHO ox. | Morning CHO ox. Sleeping Fat ox. |
| [86] | Healthy normal/overweight n = 15 (7 M/8 F) | 24 h Cross-over (a) 12:30 h Control (9 a.m.–9:30 p.m., 6 meals/day, 100% energy needs) (b) 10 h TRE (9 a.m.–7 p.m., 3 meals/day, 100% energy needs) | Metabolic chamber | 24 h EE 24 h RER and 24 h Fat ox. | ||
| [90] | Healthy normal/overweight n = 17 (8 M/9 F) | 24 h Cross-over (a) 12 h control (7 a.m.–7 p.m., 3 meals/day, 100% energy needs) (b) 6 h TRE (7 a.m.–1 p.m., 2 meals/day, 100% energy needs) (c) 6 h TRE (1 p.m.–7 p.m., 2 meals/day, 100% energy needs) | Metabolic chamber Φ | 24 h Protein ox. and balance in both 6 h TRE No significant differences between both 6 h TRE | 24 h EE in both 6 h TRE 24 h Fat ox. and 24 h CHO balance in 1 p.m.–7 p.m. TRE | 24 h RER, 24 h CHO ox. and Fat balance in 1 p.m.–7 p.m. TRE |
| [89] | Healthy normal weight n = 12 (2 M/10 F) | 72 h Cross-over (a) 13 h Control (8 a.m.–9 p.m., 4 meals/day, 100% energy needs) (b) 10 h TRE (8 a.m.–6 p.m., 4 meals/day, 100% energy needs) | Overnight fasting and 4-h postprandial period Douglas bags | RMR and RER | RER 30 and 60 min after a meal | |
| Chronic time-restricted eating | ||||||
| [94] | Healthy normal/overweight n = 8 (8 M/0 F) | 2-week Cross-over (a) 10 h Control (9 a.m.–7 p.m., 6 meals/day, 100% energy needs) (b) 8 h TRE (11 a.m.–7 p.m., 2 meals/day, 100% energy needs) | Metabolic chamber | 24 h EE and RMR | Night EE | Day EE |
| [96] | Healthy normal/overweight n = 10 (10 M/0 F) | 7-day Cross-over (a) 13 h Control (7:30 a.m.–8:30 p.m., 7 meals/day, 100% energy needs) (b) 6 h TRE (12 p.m.–6 p.m., 2 meals/day, 100% energy needs) | Metabolic chamber Doubly-labeled water during 7 days | 24 h EE, TDEE, RMR, TEF and AEE | ||
| [95] | Overweight/Obesity n = 14 (0 M/14 F) | 4-week RCT (1) 13:30 h CR (7:30 a.m.–9 p.m., 3–5 meals/day, 1000 kcal/day) (2) 6 h TRE (12 p.m.–6 p.m., 2 meals/day, 1000 kcal/day) | Metabolic chamber | 24 h EE, SEE and TEF | ||
| [97] | Healthy normal/overweight (1) n = 16 (6 M/10 F) (2) n = 17 (6 M/11 F) | 6-week RCT (1) Control (Ad libitum energy intake starting within 2 h of waking) (2) TRE (Ad libitum energy intake starting after 12 p.m.) | Overnight fasting and 2-h postprandial period Metabolic cart | RMR | TEF | |
| [98] | T2DM n = 54 (29 M/25 F) | 12-week Cross-over (a) CR (6 meals/day, measured −380 kcal/day) (b) 10 h TRE + CR (6 a.m.–4 p.m., measured −420 kcal/day) | Overnight fasting Metabolic cart α (VMAX, SensorMedics) | RMR | ||
| [99] | (1) Normal weight n = 9 (4 M/5 F) (2) Overweight/obesity n = 10 (3 M/7 F) (3) Normal weight n = 9 (5 M/4 F) (4) Overweight/obesity n = 9 (4 M/5 F) | 7-day Cross-over (a) Control (Ad libitum energy intake starting within 2 h of waking) (b) TRE (Ad libitum with breakfast skipping) | Overnight fasting and 2-h postprandial period Douglas bag | RMR and 2 h TEF | ||
| [100] | Obesity (1) n = 16 (6 M/10 F) (2) n = 17 (6 M/11 F) | 6-week RCT (1) Control (Ad libitum energy intake starting within 2 h of waking) (2) TRE (Ad libitum energy intake starting after 12 p.m.) | Overnight fasting and 2-h postprandial period Metabolic cart | RMR and TEF | ||
| [101] | Healthy normal/overweight n = 9 (9 M/0 F) | 6-day Cross-over (a) 11 h Control (7 a.m.–6 p.m., 3 meals/day, 100% energy needs) (b) 5:30 h TRE (12:30 p.m.–6 p.m., 2 meals/day, 100% energy needs) | Metabolic chamber | 24 h EE and 7 h SEE 24 h RER, 24 h CHO, 24 h Fat and 24 h Protein ox. | TEF | |
| [102] | Overweight/Obesity n = 10 (6 M/4 F) | 4-day Cross-over (a) 12 h control (8 a.m.–8 p.m., 3 meals/day, 100% energy needs) (b) 6 h TRE (8 a.m.–2 p.m., 3 meals/day, 100% energy needs) | Metabolic chamber | 24 h EE and RMR Day RER | Day EE and TEF 24 h Protein ox. | Nigh EE and sleep EE 24 h RER, night, rest and sleep RER |
| [48] | Overweight/Obesity (1) n = 25 (15 M/10 F) (2) n = 25 (13 M/12 F) | 13-week RCT (1) ~16 h Control (6–10 a.m. to 5–10 p.m., 3 meals/day, ad libitum) (2) 8 h TRE (12 p.m.–8 p.m., ad libitum) | Overnight fasting Metabolic cart (TrueOne 2400, Parvo Medics) Doubly-labeled water during 7 days | RMR, RER and TDEE | ||
5. Optimizing IF by Combining It with Exercise
5.1. Metabolic Switching: The Role of Endurance Exercise
5.2. Optimizing IF by Combining It with Resistance Exercise
6. Perspectives of the Usefulness of IF on Cardiometabolic Health: Gaps and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Flanagan, E.W.; Most, J.; Mey, J.T.; Redman, L.M. Calorie Restriction and Aging in Humans. Annu. Rev. Nutr. 2020, 40, 105–133. [Google Scholar] [CrossRef] [PubMed]
- Redman, L.M.; Ravussin, E. Caloric restriction in humans: Impact on physiological, psychological, and behavioral outcomes. Antioxid. Redox Signal. 2011, 14, 275–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacLean, P.S.; Wing, R.R.; Davidson, T.; Epstein, L.; Goodpaster, B.; Hall, K.D.; Levin, B.E.; Perri, M.G.; Rolls, B.J.; Rosenbaum, M.; et al. NIH working group report: Innovative research to improve maintenance of weight loss. Obesity 2015, 23, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Hoddy, K.K.; Marlatt, K.L.; Çetinkaya, H.; Ravussin, E. Intermittent Fasting and Metabolic Health: From Religious Fast to Time-Restricted Feeding. Obesity 2020, 28 (Suppl. S1), S29–S37. [Google Scholar] [CrossRef]
- O’Connor, S.G.; Boyd, P.; Bailey, C.P.; Shams-White, M.M.; Agurs-Collins, T.; Hall, K.; Reedy, J.; Sauter, E.R.; Czajkowski, S.M. Perspective: Time-Restricted Eating Compared with Caloric Restriction: Potential Facilitators and Barriers of Long-Term Weight Loss Maintenance. Adv. Nutr. 2021, 12, 325–333. [Google Scholar] [CrossRef]
- Alghafli, Z.; Hatch, T.G.; Rose, A.H.; Abo-Zena, M.M.; Marks, L.D.; Dollahite, D.C. A Qualitative Study of Ramadan: A Month of Fasting, Family, and Faith. Religions 2019, 10, 123. [Google Scholar] [CrossRef] [Green Version]
- Enríquez Guerrero, A.; San Mauro Martín, I.; Garicano Vilar, E.; Camina Martín, M.A. Effectiveness of an intermittent fasting diet versus continuous energy restriction on anthropometric measurements, body composition and lipid profile in overweight and obese adults: A meta-analysis. Eur. J. Clin. Nutr. 2021, 75, 1024–1039. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef] [Green Version]
- Prentice, A.M. Starvation in humans: Evolutionary background and contemporary implications. Mech. Ageing Dev. 2005, 126, 976–981. [Google Scholar] [CrossRef]
- Mattson, M.P. An Evolutionary Perspective on Why Food Overconsumption Impairs Cognition. Trends Cogn. Sci. 2019, 23, 200–212. [Google Scholar] [CrossRef]
- McCue, M.D. Comparative Physiology of Fasting, Starvation, and Food Limitation; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Keys, A.; Brožek, J.; Henschel, A.; Mickelsen, O.; Taylor, H.L. The Biology of Human Starvation; University of Minnesota Press: Minneapolis, MN, USA, 1950. [Google Scholar]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahill, G.F., Jr. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frayn, K.N. Metabolic Regulation: A Human Perspective, 3rd ed.; Wiley-Blackwell Pub.: Chichester, UK, 2010. [Google Scholar]
- Stimson, R.H.; Mohd-Shukri, N.A.; Bolton, J.L.; Andrew, R.; Reynolds, R.M.; Walker, B.R. The postprandial rise in plasma cortisol in men is mediated by macronutrient-specific stimulation of adrenal and extra-adrenal cortisol production. J. Clin. Endocrinol. Metab. 2014, 99, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Knoll, E.; Müller, F.W.; Ratge, D.; Bauersfeld, W.; Wisser, H. Influence of food intake on concentrations of plasma catecholamines and cortisol. J. Clin. Chem. Clin. Biochem. Z. Klin. Chem. Klin. Biochem. 1984, 22, 597–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schutz, Y.; Flatt, J.P.; Jéquier, E. Failure of dietary fat intake to promote fat oxidation: A factor favoring the development of obesity. Am. J. Clin. Nutr. 1989, 50, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Flatt, J.P.; Ravussin, E.; Acheson, K.J.; Jéquier, E. Effects of dietary fat on postprandial substrate oxidation and on carbohydrate and fat balances. J. Clin. Investig. 1985, 76, 1019–1024. [Google Scholar] [CrossRef]
- Westerterp, K.R. Diet induced thermogenesis. Nutr. Metab. 2004, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Acheson, K.J.; Ravussin, E.; Wahren, J.; Jéquier, E. Thermic effect of glucose in man. Obligatory and facultative thermogenesis. J. Clin. Investig. 1984, 74, 1572–1580. [Google Scholar] [CrossRef]
- Kratz, A.; Ferraro, M.; Sluss, P.M.; Lewandrowski, K.B. Normal Reference Laboratory Values. N. Engl. J. Med. 2004, 351, 1548–1563. [Google Scholar] [CrossRef]
- Galgani, J.; Ravussin, E. Energy metabolism, fuel selection and body weight regulation. Int. J. Obes. 2008, 32 (Suppl. S7), S109–S119. [Google Scholar] [CrossRef] [Green Version]
- Bobbioni-Harsch, E.; Habicht, F.; Lehmann, T.; James, R.W.; Rohner-Jeanrenaud, F.; Golay, A. Energy expenditure and substrates oxidative patterns, after glucose, fat or mixed load in normal weight subjects. Eur. J. Clin. Nutr. 1997, 51, 370–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurens, C.; Grundler, F.; Damiot, A.; Chery, I.; Le Maho, A.-L.; Zahariev, A.; Le Maho, Y.; Bergouignan, A.; Gauquelin-Koch, G.; Simon, C.; et al. Is muscle and protein loss relevant in long-term fasting in healthy men? A prospective trial on physiological adaptations. J. Cachexia Sarcopenia Muscle 2021, 12, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Rothman, D.L.; Magnusson, I.; Katz, L.D.; Shulman, R.G.; Shulman, G.I. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science 1991, 254, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Vozarova, B.; Ravussin, E.; Tataranni, P.A. Changes in energy metabolism in response to 48 h of overfeeding and fasting in Caucasians and Pima Indians. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2001, 25, 593–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thearle, M.S.; Pannacciulli, N.; Bonfiglio, S.; Pacak, K.; Krakoff, J. Extent and determinants of thermogenic responses to 24 hours of fasting, energy balance, and five different overfeeding diets in humans. J. Clin. Endocrinol. Metab. 2013, 98, 2791–2799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garber, A.J.; Menzel, P.H.; Boden, G.; Owen, O.E. Hepatic ketogenesis and gluconeogenesis in humans. J. Clin. Investig. 1974, 54, 981–989. [Google Scholar] [CrossRef] [Green Version]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G., 3rd; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef]
- Gonzalez, J.T.; Fuchs, C.J.; Betts, J.A.; van Loon, L.J. Liver glycogen metabolism during and after prolonged endurance-type exercise. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E543–E553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinckaers, P.J.; Churchward-Venne, T.A.; Bailey, D.; van Loon, L.J. Ketone Bodies and Exercise Performance: The Next Magic Bullet or Merely Hype? Sports Med. 2017, 47, 383–391. [Google Scholar] [CrossRef] [Green Version]
- Jahrami, H.A.; Alsibai, J.; Clark, C.C.T.; Faris, M.e.A.-I.E. A systematic review, meta-analysis, and meta-regression of the impact of diurnal intermittent fasting during Ramadan on body weight in healthy subjects aged 16 years and above. Eur. J. Nutr. 2020, 59, 2291–2316. [Google Scholar] [CrossRef]
- Allaf, M.; Elghazaly, H.; Mohamed, O.G.; Fareen, M.F.K.; Zaman, S.; Salmasi, A.M.; Tsilidis, K.; Dehghan, A. Intermittent fasting for the prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2021, 1, CD013496. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.; McGarty, A.; Hutchison, L.; Ells, L.; Hankey, C. Short-term intermittent energy restriction interventions for weight management: A systematic review and meta-analysis. Obes. Rev. 2018, 19, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Y.; Cai, T.; Zhou, Z.; Mu, Y.; Lu, Y.; Gao, Z.; Wu, J.; Zhang, Y. Health Effects of Alternate-Day Fasting in Adults: A Systematic Review and Meta-Analysis. Front. Nutr. 2020, 7, 586036. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Kang, J.; Kim, S.H.; Chung, H.S.; Kim, Y.J.; Yu, J.M.; Cho, S.T.; Oh, C.M.; Kim, T. Beneficial Effects of Time-Restricted Eating on Metabolic Diseases: A Systemic Review and Meta-Analysis. Nutrients 2020, 12, 1267. [Google Scholar] [CrossRef]
- Alhamdan, B.A.; Garcia-Alvarez, A.; Alzahrnai, A.H.; Karanxha, J.; Stretchberry, D.R.; Contrera, K.J.; Utria, A.F.; Cheskin, L.J. Alternate-day versus daily energy restriction diets: Which is more effective for weight loss? A systematic review and meta-analysis. Obes. Sci. Pract. 2016, 2, 293–302. [Google Scholar] [CrossRef]
- Cho, Y.; Hong, N.; Kim, K.W.; Cho, S.J.; Lee, M.; Lee, Y.H.; Lee, Y.H.; Kang, E.S.; Cha, B.S.; Lee, B.W. The Effectiveness of Intermittent Fasting to Reduce Body Mass Index and Glucose Metabolism: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1645. [Google Scholar] [CrossRef] [Green Version]
- Cioffi, I.; Evangelista, A.; Ponzo, V.; Ciccone, G.; Soldati, L.; Santarpia, L.; Contaldo, F.; Pasanisi, F.; Ghigo, E.; Bo, S. Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: A systematic review and meta-analysis of randomized controlled trials. J. Transl. Med. 2018, 16, 371. [Google Scholar] [CrossRef] [Green Version]
- Headland, M.; Clifton, P.M.; Carter, S.; Keogh, J.B. Weight-Loss Outcomes: A Systematic Review and Meta-Analysis of Intermittent Energy Restriction Trials Lasting a Minimum of 6 Months. Nutrients 2016, 8, 354. [Google Scholar] [CrossRef] [Green Version]
- Templeman, I.; Smith, H.A.; Chowdhury, E.; Chen, Y.C.; Carroll, H.; Johnson-Bonson, D.; Hengist, A.; Smith, R.; Creighton, J.; Clayton, D.; et al. A randomized controlled trial to isolate the effects of fasting and energy restriction on weight loss and metabolic health in lean adults. Sci. Transl. Med. 2021, 13, eabd8034. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Holmer, M.; Lindqvist, C.; Petersson, S.; Moshtaghi-Svensson, J.; Tillander, V.; Brismar, T.B.; Hagström, H.; Stål, P. Treatment of NAFLD with intermittent calorie restriction or low-carb high-fat diet—A randomised controlled trial. JHEP Rep. Innov. Hepatol. 2021, 3, 100256. [Google Scholar] [CrossRef] [PubMed]
- Kalam, F.; Gabel, K.; Cienfuegos, S.; Ezpeleta, M.; Wiseman, E.; Varady, K.A. Alternate Day Fasting Combined with a Low Carbohydrate Diet: Effect on Sleep Quality, Duration, Insomnia Severity and Risk of Obstructive Sleep Apnea in Adults with Obesity. Nutrients 2021, 13, 211. [Google Scholar] [CrossRef]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020, 32, 366–378.e3. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men With Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 1491–1499. [Google Scholar] [CrossRef]
- Jahrami, H.A.; Faris, M.E.; Janahi, A.I.; Janahi, M.I.; Abdelrahim, D.N.; Madkour, M.I.; Sater, M.S.; Hassan, A.B.; Bahammam, A.S. Does four-week consecutive, dawn-to-sunset intermittent fasting during Ramadan affect cardiometabolic risk factors in healthy adults? A systematic review, meta-analysis, and meta-regression. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2273–2301. [Google Scholar] [CrossRef]
- Pureza, I.; Macena, M.L.; da Silva Junior, A.E.; Praxedes, D.R.S.; Vasconcelos, L.G.L.; Bueno, N.B. Effect of early time-restricted feeding on the metabolic profile of adults with excess weight: A systematic review with meta-analysis. Clin. Nutr. 2021, 40, 1788–1799. [Google Scholar] [CrossRef]
- Gill, S.; Panda, S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab. 2015, 22, 789–798. [Google Scholar] [CrossRef] [Green Version]
- Blaak, E.E.; Antoine, J.M.; Benton, D.; Björck, I.; Bozzetto, L.; Brouns, F.; Diamant, M.; Dye, L.; Hulshof, T.; Holst, J.J.; et al. Impact of postprandial glycaemia on health and prevention of disease. Obes. Rev. 2012, 13, 923–984. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Benn, M.; Schnohr, P.; Tybjaerg-Hansen, A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007, 298, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Antoni, R.; Johnston, K.L.; Collins, A.L.; Robertson, M.D. Intermittent v. continuous energy restriction: Differential effects on postprandial glucose and lipid metabolism following matched weight loss in overweight/obese participants. Br. J. Nutr. 2018, 119, 507–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Tsintzas, K.; Macdonald, I.A.; Cordon, S.M.; Taylor, M.A. Effects of intermittent (5:2) or continuous energy restriction on basal and postprandial metabolism: A randomised study in normal-weight, young participants. Eur. J. Clin. Nutr. 2022, 76, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Beyl, R.A.; Della Manna, D.L.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parr, E.B.; Devlin, B.L.; Radford, B.E.; Hawley, J.A. A Delayed Morning and Earlier Evening Time-Restricted Feeding Protocol for Improving Glycemic Control and Dietary Adherence in Men with Overweight/Obesity: A Randomized Controlled Trial. Nutrients 2020, 12, 505. [Google Scholar] [CrossRef] [Green Version]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.C.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020, 31, 92–104.e5. [Google Scholar] [CrossRef]
- Chow, L.S.; Manoogian, E.N.C.; Alvear, A.; Fleischer, J.G.; Thor, H.; Dietsche, K.; Wang, Q.; Hodges, J.S.; Esch, N.; Malaeb, S.; et al. Time-Restricted Eating Effects on Body Composition and Metabolic Measures in Humans who are Overweight: A Feasibility Study. Obesity 2020, 28, 860–869. [Google Scholar] [CrossRef]
- Galgani, J.E.; Moro, C.; Ravussin, E. Metabolic flexibility and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1009–E1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Roman, Y.M.; Dominguez, M.C.; Easow, T.M.; Pasupuleti, V.; White, C.M.; Hernandez, A.V. Effects of intermittent versus continuous dieting on weight and body composition in obese and overweight people: A systematic review and meta-analysis of randomized controlled trials. Int. J. Obes. 2019, 43, 2017–2027. [Google Scholar] [CrossRef]
- Dulloo, A.G.; Jacquet, J.; Miles-Chan, J.L.; Schutz, Y. Passive and active roles of fat-free mass in the control of energy intake and body composition regulation. Eur. J. Clin. Nutr. 2017, 71, 353–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravussin, E.; Lillioja, S.; Knowler, W.C.; Christin, L.; Freymond, D.; Abbott, W.G.H.; Boyce, V.; Howard, B.V.; Bogardus, C. Reduced Rate of Energy Expenditure as a Risk Factor for Body-Weight Gain. N. Engl. J. Med. 1988, 318, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Zurlo, F.; Lillioja, S.; Esposito-Del Puente, A.; Nyomba, B.L.; Raz, I.; Saad, M.F.; Swinburn, B.A.; Knowler, W.C.; Bogardus, C.; Ravussin, E. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: Study of 24-h RQ. Am. J. Physiol. 1990, 259, E650–E657. [Google Scholar] [CrossRef] [PubMed]
- Piaggi, P.; Thearle, M.S.; Bogardus, C.; Krakoff, J. Lower energy expenditure predicts long-term increases in weight and fat mass. J. Clin. Endocrinol. Metab. 2013, 98, E703–E707. [Google Scholar] [CrossRef] [Green Version]
- Schlögl, M.; Piaggi, P.; Pannacciuli, N.; Bonfiglio, S.M.; Krakoff, J.; Thearle, M.S. Energy Expenditure Responses to Fasting and Overfeeding Identify Phenotypes Associated with Weight Change. Diabetes 2015, 64, 3680–3689. [Google Scholar] [CrossRef] [Green Version]
- Reinhardt, M.; Thearle, M.S.; Ibrahim, M.; Hohenadel, M.G.; Bogardus, C.; Krakoff, J.; Votruba, S.B. A Human Thrifty Phenotype Associated with Less Weight Loss during Caloric Restriction. Diabetes 2015, 64, 2859–2867. [Google Scholar] [CrossRef] [Green Version]
- Alsubheen, S.A.; Ismail, M.; Baker, A.; Blair, J.; Adebayo, A.; Kelly, L.; Chandurkar, V.; Cheema, S.; Joanisse, D.R.; Basset, F.A. The effects of diurnal Ramadan fasting on energy expenditure and substrate oxidation in healthy men. Br. J. Nutr. 2017, 118, 1023–1030. [Google Scholar] [CrossRef] [Green Version]
- el Ati, J.; Beji, C.; Danguir, J. Increased fat oxidation during Ramadan fasting in healthy women: An adaptative mechanism for body-weight maintenance. Am. J. Clin. Nutr. 1995, 62, 302–307. [Google Scholar] [CrossRef]
- Lessan, N.; Saadane, I.; Alkaf, B.; Hambly, C.; Buckley, A.J.; Finer, N.; Speakman, J.R.; Barakat, M.T. The effects of Ramadan fasting on activity and energy expenditure. Am. J. Clin. Nutr. 2018, 107, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Alharbi, T.J.; Wong, J.; Markovic, T.; Yue, D.; Wu, T.; Brooks, B.; Hetherington, J.; Seimon, R.; Gibson, A.A.; Toth, K.; et al. Brief report: Ramadan as a model of intermittent fasting: Effects on body composition, metabolic parameters, gut hormones and appetite in adults with and without type 2 diabetes mellitus. Obes. Med. 2017, 6, 15–17. [Google Scholar] [CrossRef]
- Catenacci, V.A.; Pan, Z.; Ostendorf, D.; Brannon, S.; Gozansky, W.S.; Mattson, M.P.; Martin, B.; MacLean, P.S.; Melanson, E.L.; Troy Donahoo, W. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity 2016, 24, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Halberg, N.; Henriksen, M.; Söderhamn, N.; Stallknecht, B.; Ploug, T.; Schjerling, P.; Dela, F. Effect of intermittent fasting and refeeding on insulin action in healthy men. J. Appl. Physiol. 2005, 99, 2128–2136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heilbronn, L.K.; Smith, S.R.; Martin, C.K.; Anton, S.D.; Ravussin, E. Alternate-day fasting in nonobese subjects: Effects on body weight, body composition, and energy metabolism. Am. J. Clin. Nutr. 2005, 81, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Soeters, M.R.; Lammers, N.M.; Dubbelhuis, P.F.; Ackermans, M.; Jonkers-Schuitema, C.F.; Fliers, E.; Sauerwein, H.P.; Aerts, J.M.; Serlie, M.J. Intermittent fasting does not affect whole-body glucose, lipid, or protein metabolism. Am. J. Clin. Nutr. 2009, 90, 1244–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stekovic, S.; Hofer, S.J.; Tripolt, N.; Aon, M.A.; Royer, P.; Pein, L.; Stadler, J.T.; Pendl, T.; Prietl, B.; Url, J.; et al. Alternate Day Fasting Improves Physiological and Molecular Markers of Aging in Healthy, Non-obese Humans. Cell Metab. 2020, 31, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, K.; Casanova, N.; Oustric, P.; Turicchi, J.; Gibbons, C.; Hopkins, M.; Varady, K.; Blundell, J.; Finlayson, G. Matched Weight Loss through Intermittent or Continuous Energy Restriction Does Not Lead to Compensatory Increases in Appetite and Eating Behavior in a Randomized Controlled Trial in Women with Overweight and Obesity. J. Nutr. 2020, 150, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, S.R.; Halset, E.H.; Gåsbakk, S.; Rehfeld, J.F.; Kulseng, B.; Truby, H.; Martins, C. Compensatory mechanisms activated with intermittent energy restriction: A randomized control trial. Clin. Nutr. 2018, 37, 815–823. [Google Scholar] [CrossRef]
- De Groot, L.C.; van Es, A.J.; van Raaij, J.M.; Vogt, J.E.; Hautvast, J.G. Adaptation of energy metabolism of overweight women to alternating and continuous low energy intake. Am. J. Clin. Nutr. 1989, 50, 1314–1323. [Google Scholar] [CrossRef] [Green Version]
- Pinto, A.M.; Bordoli, C.; Buckner, L.P.; Kim, C.; Kaplan, P.C.; Del Arenal, I.M.; Jeffcock, E.J.; Hall, W.L. Intermittent energy restriction is comparable to continuous energy restriction for cardiometabolic health in adults with central obesity: A randomized controlled trial; the Met-IER study. Clin. Nutr. 2020, 39, 1753–1763. [Google Scholar] [CrossRef]
- Vinales, K.L.; Schlögl, M.; Piaggi, P.; Hohenadel, M.; Graham, A.; Bonfiglio, S.; Krakoff, J.; Thearle, M.S. The Consistency in Macronutrient Oxidation and the Role for Epinephrine in the Response to Fasting and Overfeeding. J. Clin. Endocrinol. Metab. 2017, 102, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, F.; Ogata, H.; Omi, N.; Nagasaka, S.; Yamaguchi, S.; Hibi, M.; Tokuyama, K. Effect of breakfast skipping on diurnal variation of energy metabolism and blood glucose. Obes. Res. Clin. Pract. 2014, 8, e201–e298. [Google Scholar] [CrossRef] [PubMed]
- Munsters, M.J.; Saris, W.H. Effects of meal frequency on metabolic profiles and substrate partitioning in lean healthy males. PLoS ONE 2012, 7, e38632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohkawara, K.; Cornier, M.A.; Kohrt, W.M.; Melanson, E.L. Effects of increased meal frequency on fat oxidation and perceived hunger. Obesity 2013, 21, 336–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeets, A.J.; Westerterp-Plantenga, M.S. Acute effects on metabolism and appetite profile of one meal difference in the lower range of meal frequency. Br. J. Nutr. 2008, 99, 1316–1321. [Google Scholar] [CrossRef]
- Verboeket-van de Venne, W.P.; Westerterp, K.R. Influence of the feeding frequency on nutrient utilization in man: Consequences for energy metabolism. Eur. J. Clin. Nutr. 1991, 45, 161–169. [Google Scholar]
- Nakamura, K.; Tajiri, E.; Hatamoto, Y.; Ando, T.; Shimoda, S.; Yoshimura, E. Eating Dinner Early Improves 24-h Blood Glucose Levels and Boosts Lipid Metabolism after Breakfast the Next Day: A Randomized Cross-Over Trial. Nutrients 2021, 13, 2424. [Google Scholar] [CrossRef]
- Nas, A.; Mirza, N.; Hägele, F.; Kahlhöfer, J.; Keller, J.; Rising, R.; Kufer, T.A.; Bosy-Westphal, A. Impact of breakfast skipping compared with dinner skipping on regulation of energy balance and metabolic risk. Am. J. Clin. Nutr. 2017, 105, 1351–1361. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.A.; Garrow, J.S. Compared with nibbling, neither gorging nor a morning fast affect short-term energy balance in obese patients in a chamber calorimeter. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2001, 25, 519–528. [Google Scholar] [CrossRef] [Green Version]
- Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metab. Clin. Exp. 2018, 84, 11–27. [Google Scholar] [CrossRef] [Green Version]
- Clayton, D.J.; Stensel, D.J.; James, L.J. Effect of breakfast omission on subjective appetite, metabolism, acylated ghrelin and GLP-17-36 during rest and exercise. Nutrition 2016, 32, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Dallosso, H.M.; Murgatroyd, P.R.; James, W.P. Feeding frequency and energy balance in adult males. Hum. Nutr. Clin. Nutr. 1982, 36C, 25–39. [Google Scholar] [PubMed]
- Verboeket-van de Venne, W.P.; Westerterp, K.R. Frequency of feeding, weight reduction and energy metabolism. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1993, 17, 31–36. [Google Scholar]
- Verboeket-van de Venne, W.P.; Westerterp, K.R.; Kester, A.D. Effect of the pattern of food intake on human energy metabolism. Br. J. Nutr. 1993, 70, 103–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betts, J.A.; Richardson, J.D.; Chowdhury, E.A.; Holman, G.D.; Tsintzas, K.; Thompson, D. The causal role of breakfast in energy balance and health: A randomized controlled trial in lean adults. Am. J. Clin. Nutr. 2014, 100, 539–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahleova, H.; Belinova, L.; Malinska, H.; Oliyarnyk, O.; Trnovska, J.; Skop, V.; Kazdova, L.; Dezortova, M.; Hajek, M.; Tura, A.; et al. Eating two larger meals a day (breakfast and lunch) is more effective than six smaller meals in a reduced-energy regimen for patients with type 2 diabetes: A randomised crossover study. Diabetologia 2014, 57, 1552–1560. [Google Scholar] [CrossRef] [Green Version]
- Reeves, S.; Huber, J.W.; Halsey, L.G.; Villegas-Montes, M.; Elgumati, J.; Smith, T. A cross-over experiment to investigate possible mechanisms for lower BMIs in people who habitually eat breakfast. Eur. J. Clin. Nutr. 2015, 69, 632–637. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, E.A.; Richardson, J.D.; Holman, G.D.; Tsintzas, K.; Thompson, D.; Betts, J.A. The causal role of breakfast in energy balance and health: A randomized controlled trial in obese adults. Am. J. Clin. Nutr. 2016, 103, 747–756. [Google Scholar] [CrossRef]
- Ogata, H.; Kayaba, M.; Tanaka, Y.; Yajima, K.; Iwayama, K.; Ando, A.; Park, I.; Kiyono, K.; Omi, N.; Satoh, M.; et al. Effect of skipping breakfast for 6 days on energy metabolism and diurnal rhythm of blood glucose in young healthy Japanese males. Am. J. Clin. Nutr. 2019, 110, 41–52. [Google Scholar] [CrossRef]
- Ravussin, E.; Beyl, R.A.; Poggiogalle, E.; Hsia, D.S.; Peterson, C.M. Early Time-Restricted Feeding Reduces Appetite and Increases Fat Oxidation But Does Not Affect Energy Expenditure in Humans. Obesity 2019, 27, 1244–1254. [Google Scholar] [CrossRef]
- Melanson, E.L.; MacLean, P.S.; Hill, J.O. Exercise improves fat metabolism in muscle but does not increase 24-h fat oxidation. Exerc. Sport Sci. Rev. 2009, 37, 93–101. [Google Scholar] [CrossRef]
- Schrauwen, P.; van Marken Lichtenbelt, W.D.; Saris, W.H.; Westerterp, K.R. Role of glycogen-lowering exercise in the change of fat oxidation in response to a high-fat diet. Am. J. Physiol. 1997, 273, E623–E629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwayam, K.; Ogawa, A.; Tanaka, Y.; Yajima, K.; Park, I.; Ando, A.; Ogata, H.; Kayaba, M.; Zhang, S.; Tanji, F.; et al. Effects of exercise before breakfast on plasma free fatty acid profile and 24-h fat oxidation. Metab. Open 2020, 8, 100067. [Google Scholar] [CrossRef] [PubMed]
- Iwayama, K.; Kawabuchi, R.; Park, I.; Kurihara, R.; Kobayashi, M.; Hibi, M.; Oishi, S.; Yasunaga, K.; Ogata, H.; Nabekura, Y.; et al. Transient energy deficit induced by exercise increases 24-h fat oxidation in young trained men. J. Appl. Physiol. 2015, 118, 80–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwayama, K.; Kurihara, R.; Nabekura, Y.; Kawabuchi, R.; Park, I.; Kobayashi, M.; Ogata, H.; Kayaba, M.; Satoh, M.; Tokuyama, K. Exercise Increases 24-h Fat Oxidation Only When It Is Performed Before Breakfast. EBioMedicine 2015, 2, 2003–2009. [Google Scholar] [CrossRef] [Green Version]
- Lees, M.J.; Hodson, N.; Moore, D.R. A muscle-centric view of time-restricted feeding for older adults. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 521–527. [Google Scholar] [CrossRef]
- Damas, F.; Phillips, S.; Vechin, F.C.; Ugrinowitsch, C. A review of resistance training-induced changes in skeletal muscle protein synthesis and their contribution to hypertrophy. Sports Med. 2015, 45, 801–807. [Google Scholar] [CrossRef]
- Mesinovic, J.; Jansons, P.; Zengin, A.; de Courten, B.; Rodriguez, A.J.; Daly, R.M.; Ebeling, P.R.; Scott, D. Exercise attenuates bone mineral density loss during diet-induced weight loss in adults with overweight and obesity: A systematic review and meta-analysis. J. Sport Health Sci. 2021, 10, 550–559. [Google Scholar] [CrossRef]
- Ashtary-Larky, D.; Bagheri, R.; Tinsley, G.M.; Asbaghi, O.; Paoli, A.; Moro, T. Effects of intermittent fasting combined with resistance training on body composition: A systematic review and meta-analysis. Physiol. Behav. 2021, 237, 113453. [Google Scholar] [CrossRef]
- Ravussin, E.; Smith, S.R.; Ferrante, A.W., Jr. Physiology of Energy Expenditure in the Weight-Reduced State. Obesity 2021, 29 (Suppl. S1), S31–S38. [Google Scholar] [CrossRef]
- Bellisle, F.; McDevitt, R.; Prentice, A.M. Meal frequency and energy balance. Br. J. Nutr. 1997, 77 (Suppl. S1), S57–S70. [Google Scholar] [CrossRef] [Green Version]
- Flatt, J.P. Integration of the overall response to exercise. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1995, 19 (Suppl. S4), S31–S40. [Google Scholar]
- Abbott, W.G.; Howard, B.V.; Christin, L.; Freymond, D.; Lillioja, S.; Boyce, V.L.; Anderson, T.E.; Bogardus, C.; Ravussin, E. Short-term energy balance: Relationship with protein, carbohydrate, and fat balances. Am. J. Physiol. 1988, 255, E332–E337. [Google Scholar] [CrossRef] [PubMed]
| Study | Population | Design and Intervention | Assessment | No Change | Increase | Decrease |
|---|---|---|---|---|---|---|
| [71] | Normal weight n = 16 (0 M/16 F) | 4-week Observational (1) Ramadan | 15 h period Metabolic chamber | RMR and nigh EE Day and evening protein ox. | Fat ox. from 2 p.m.–11 p.m. | RER and CHO ox. from 11 a.m.–11 p.m. EE from 11 a.m.–5 p.m. |
| [73] | (1) Healthy n = 7 (7 M/0 F) (2) TD2M n = 5 (3 M/2 F) | 3-week Observational (1) Ramadan (2) Ramadan | Overnight fasting Metabolic cart | RMR and RER | ||
| [72] | Healthy normal/overweight n = 29 (13 M/16 F) for RMR n = 10 (5 M/5 F) for TDEE | 4-week Observational (1) Ramadan | Overnight fasting Metabolic cart (Quark, Cosmed) Doubly-labeled water during 14 days | RMR and adjusted RMR * TDEE | RER |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dote-Montero, M.; Sanchez-Delgado, G.; Ravussin, E. Effects of Intermittent Fasting on Cardiometabolic Health: An Energy Metabolism Perspective. Nutrients 2022, 14, 489. https://doi.org/10.3390/nu14030489
Dote-Montero M, Sanchez-Delgado G, Ravussin E. Effects of Intermittent Fasting on Cardiometabolic Health: An Energy Metabolism Perspective. Nutrients. 2022; 14(3):489. https://doi.org/10.3390/nu14030489
Chicago/Turabian StyleDote-Montero, Manuel, Guillermo Sanchez-Delgado, and Eric Ravussin. 2022. "Effects of Intermittent Fasting on Cardiometabolic Health: An Energy Metabolism Perspective" Nutrients 14, no. 3: 489. https://doi.org/10.3390/nu14030489
APA StyleDote-Montero, M., Sanchez-Delgado, G., & Ravussin, E. (2022). Effects of Intermittent Fasting on Cardiometabolic Health: An Energy Metabolism Perspective. Nutrients, 14(3), 489. https://doi.org/10.3390/nu14030489






