Novel Horizons in Postbiotics: Lactobacillaceae Extracellular Vesicles and Their Applications in Health and Disease
Abstract
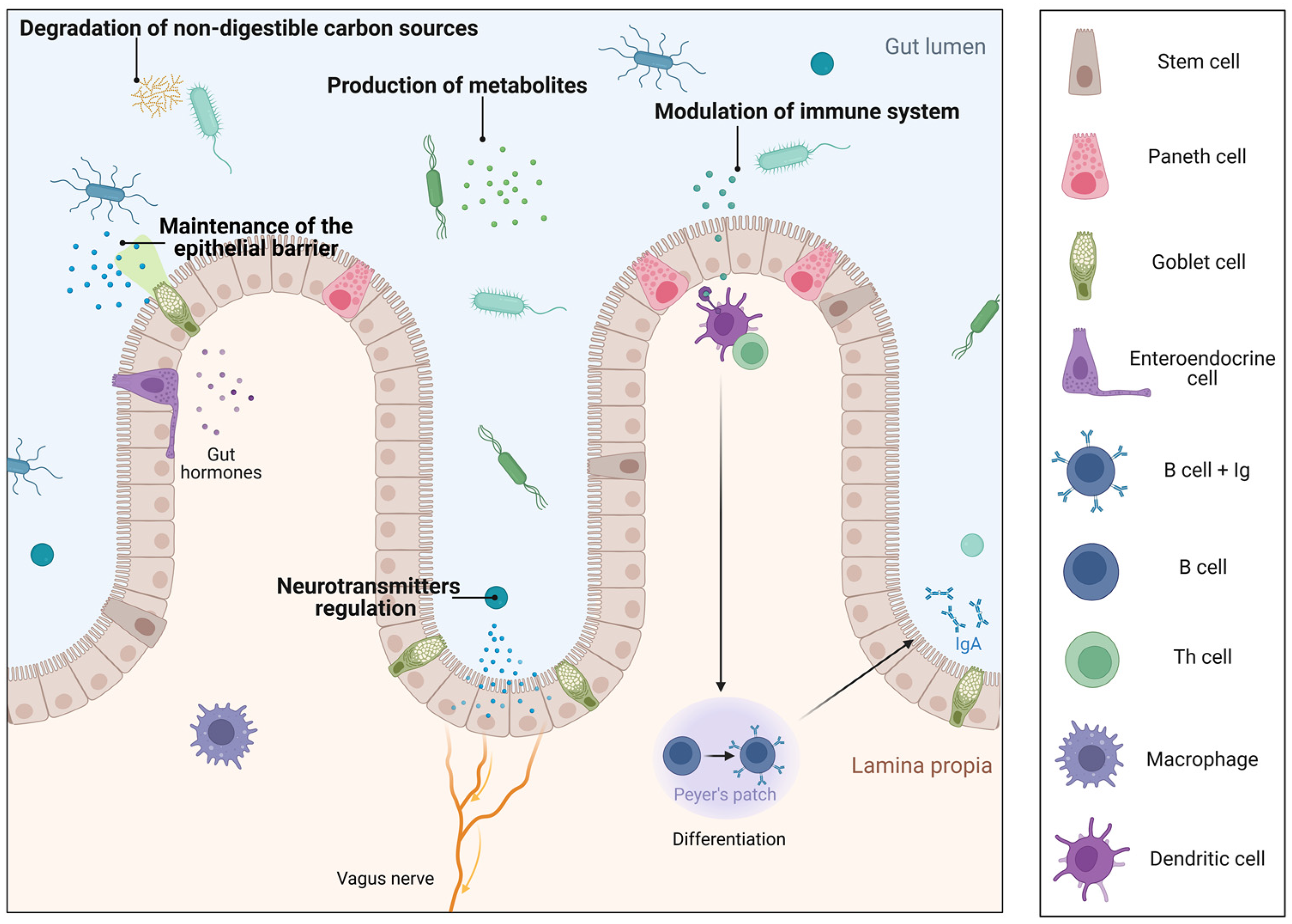
1. Introduction
- Extracellular vesicles
2. Lactobacillus
3. Preventive and/or Therapeutic Application of Lactobacillus MVs
3.1. Inflammatory Bowel Disease (IBD)
3.2. Infectious Diseases
3.3. Neurological Disorders
3.4. Cancer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bäumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.U.; Hayab, H.; Shafique, F.; Mike, P.W.; Almansouri, F.S.; Asim, N.; Shafi, N.; Attacha, S.; Khalid, M.; Ali, N.; et al. Probiotic Properties of Lactobacillus helveticus and Lactobacillus plantarum Isolated from Traditional Pakistani Yoghurt. BioMed Res. Int. 2020, 2020, 8889198. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, B.; Delgaso, S.; Blanco-Míguez, A.; Luorenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Orwen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut microbiota’s effect on mental health: The gut-brain axis. Clin. Pract. 2017, 7, 987. [Google Scholar] [CrossRef]
- WHO. Health and Nutritional Properties and Guidelines for Evaluation; WHO: Geneva, Switzerland; FAO: Rome, Italy, 2006. [Google Scholar]
- Molina-Tijeras, J.A.; Gálvez, J.; Rodríguez-Cabezas, M.E. The Immunomodulatory Properties of Extracellular Vesicles Derived from Probiotics: A Novel Approach for the Management of Gastrointestinal Diseases. Nutrients 2019, 11, 1038. [Google Scholar] [CrossRef]
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Shukla, G. Metabiotics: One Step ahead of Probiotics; an Insight into Mechanisms Involved in Anticancerous Effect in Colorectal Cancer. Front. Microbiol. 2016, 7, 1940. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Park, J.H.; Jung, H.K. Potential Health-Promoting Benefits of Paraprobiotics, Inactivated Probiotic Cells. J. Microbiol. Biotechnol. 2020, 30, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-González, P.F.; Liceaga, A.M.; Aguilar-Toalá, J.E. Postbiotics and paraprobiotics: From concepts to applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef]
- Barros, C.P.; Guimarães, J.T.; Esmerino, E.A.; Duarte, M.C.K.H.; Silva, M.C.; Silva, R.; Ferreira, B.M.; Sant’Ana, A.; Freitas, M.Q.; Cruz, A.G. Paraprobiotics and postbiotics: Concepts and potential applications in dairy products. Curr. Opin. Food Sci. 2020, 32, 1–8. [Google Scholar] [CrossRef]
- Martyniak, A.; Medyńska-Przęczek, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prebiotics, Probiotics, Synbiotics, Paraprobiotics and Postbiotic Compounds in IBD. Biomolecules 2021, 11, 1903. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Konstantakis, C.; Assimakopoulos, S.F.; Triantos, C. The role of the gut microbiota in the treatment of inflammatory bowel diseases. Microb. Pathog. 2019, 137, 103774. [Google Scholar] [CrossRef]
- Gill, S.; Catchpole, R.; Forterre, P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol. Rev. 2019, 43, 273–303. [Google Scholar] [CrossRef]
- Zomer, A.; Vendrig, T.; Hopmans, E.S.; van Eijndhoven, M.; Middeldorp, J.M.; Pegtel, D.M. Exosomes: Fit to deliver small RNA. Commun. Integr. Biol. 2010, 3, 447–450. [Google Scholar] [CrossRef]
- Liu, Y.; Defourny, K.A.Y.; Smid, E.J.; Abee, T. Gram-Positive Bacterial Extracellular Vesicles and Their Impact on Health and Disease. Front. Microbiol. 2018, 9, 1502. [Google Scholar] [CrossRef]
- Gurung, M.; Moon, D.C.; Choi, C.W.; Lee, J.H.; Bae, Y.C.; Kim, J.; Lee, Y.C.; Seol, S.Y.; Cho, D.T.; Kim, S.I.; et al. Staphylococcus aureus produces membrane-derived vesicles that induce host cell death. PLoS ONE 2011, 6, e27958. [Google Scholar] [CrossRef] [PubMed]
- Kesty, N.C.; Kuehn, M.J. Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles. J. Biol. Chem. 2004, 279, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Bomberger, J.M.; Maceachran, D.P.; Coutermarsh, B.A.; Ye, S.; O’Toole, G.A.; Stanton, B.A. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 2009, 5, e1000382. [Google Scholar] [CrossRef]
- Kato, S.; Kowashi, Y.; Demuth, D.R. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb. Pathog. 2002, 32, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.T. Outer Membrane Vesicles (OMVs) of Gram-negative Bacteria: A Perspective Update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the wall: Extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.; Park, J.; Gho, Y.S. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin. Cell Dev. Biol. 2015, 40, 97–104. [Google Scholar] [CrossRef]
- Caruana, J.C.; Dean, S.N.; Walper, S.A. Isolation and Characterization of Membrane Vesicles from Lactobacillus Species. Bio-protocol 2021, 11, e4145. [Google Scholar] [CrossRef]
- Pollack, J.H.; Ntamere, A.S.; Neuhaus, F.C. D-alanyl-lipoteichoic acid in Lactobacillus casei: Secretion of vesicles in response to benzylpenicillin. J. Gen. Microbiol. 1992, 138, 849–859. [Google Scholar] [CrossRef]
- Besselink, M.G.; van Santvoort, H.C.; Buskens, E.; Boermeester, M.A.; van Goor, H.; Timmerman, H.M.; Nieuwenhuijs, V.B.; Bollen, T.L.; van Ramshorst, B.; Witteman, B.J.; et al. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 371, 651–659. [Google Scholar] [CrossRef]
- Salvetti, E.; Torriani, S.; Felis, G.E. The Genus Lactobacillus: A Taxonomic Update. Probiotics Antimicrob Proteins 2012, 4, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Slavetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O‘Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Haakensen, M.; Dobson, C.M.; Hill, J.E.; Ziola, B. Reclassification of Pediococcus dextrinicus (Coster and White 1964) back 1978 (Approved Lists 1980) as Lactobacillus dextrinicus comb. nov., and emended description of the genus Lactobacillus. Int. J. Syst. Evol. Microbiol. 2009, 59 Pt 3, 615–621. [Google Scholar] [CrossRef]
- Kleerebezem, M.; Vaughan, E.E. Probiotic and gut lactobacilli and bifidobacteria: Molecular approaches to study diversity and activity. Annu. Rev. Microbiol. 2009, 63, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef]
- Turroni, F.; Ventura, M.; Buttó, L.F.; Duranti, S.; O’Toole, P.W.; Motherway, M.O.; van Sinderen, D. Molecular dialogue between the human gut microbiota and the host: A Lactobacillus and Bifidobacterium perspective. Cell Mol. Life Sci. 2014, 71, 183–203. [Google Scholar] [CrossRef]
- De Gregorio, P.R.; Tomás, M.S.J.; Terraf, M.C.L.; Nader-Macías, M.E.F. In vitro and in vivo effects of beneficial vaginal lactobacilli on pathogens responsible for urogenital tract infections. J. Med. Microbiol. 2014, 63 Pt 5, 685–696. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, J.; Pan, L.; Zhang, Y. Roles and applications of probiotic Lactobacillus strains. Appl. Microbiol. Biotechnol. 2018, 102, 8135–8143. [Google Scholar] [CrossRef]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef]
- Lebeer, S.; Vanderleyden, J.; de Keersmaecker, S.C. Genes and molecules of lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 2008, 72, 728–764. [Google Scholar] [CrossRef] [PubMed]
- Vicariotto, F.; Mogna, L.; del Piano, M. Effectiveness of the two microorganisms Lactobacillus fermentum LF15 and Lactobacillus plantarum LP01, formulated in slow-release vaginal tablets, in women affected by bacterial vaginosis: A pilot study. J. Clin. Gastroenterol. 2014, 48 (Suppl. S1), S106–S112. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Ning, H.; Shen, M.; Li, J.; Zhang, J.; Chen, X. Probiotics for the Treatment of Atopic Dermatitis in Children: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Cell. Infect. Microbiol. 2017, 7, 392. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Dong, B.R.; Wu, T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2015, 2, Cd006895. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Martinez, E.G.; Gregorio, G.V.; Dans, L.F. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst. Rev. 2010, 2010, Cd003048. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, K.P.; Choi, E.; Yim, J.H.; Choi, C.; Yun, H.S.; Ahn, H.Y.; Oh, J.Y.; Cho, Y. Effects of Lactobacillus plantarum CJLP55 on Clinical Improvement, Skin Condition and Urine Bacterial Extracellular Vesicles in Patients with Acne Vulgaris: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2021, 13, 1368. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeun, E.-J.; Hong, C.-P.; Kim, S.-H.; Jang, M.S.; Lee, E.-J.; Moon, S.J.; Yun, C.H.; Im, S.-H.; Jeong, S.-G.; et al. Extracellular vesicle-derived protein from Bifidobacterium longum alleviates food allergy through mast cell suppression. J. Allergy Clin. Immunol. 2016, 137, 507–516.e8. [Google Scholar] [CrossRef]
- Hemarajata, P.; Gao, S.; Pflughoeft, K.J.; Thomas, C.M.; Saulnier, D.M.; Spinler, J.K.; Versalovic, J. Lactobacillus reuteri-specific immunoregulatory gene rsiR modulates histamine production and immunomodulation by Lactobacillus reuteri. J. Bacteriol. 2013, 195, 5567–5576. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.Y.; Li, Q.H.; Su, H.; Sun, X. Lactobacillus rhamnosus GG induced protective effect on allergic airway inflammation is associated with gut microbiota. Cell. Immunol. 2018, 332, 77–84. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 2005, 17, 1–14. [Google Scholar] [CrossRef]
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 2015, 15, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Rossi, O.; Necchi, F.; Micoli, F. OMV Vaccines and the Role of TLR Agonists in Immune Response. Int. J. Mol. Sci. 2020, 21, 4416. [Google Scholar] [CrossRef]
- Bhar, S.; Edelmann, M.J.; Jones, M.K. Characterization and proteomic analysis of outer membrane vesicles from a commensal microbe, Enterobacter cloacae. J. Proteom. 2021, 231, 103994. [Google Scholar] [CrossRef] [PubMed]
- Al-Nedawi, K.; Mian, M.F.; Hossain, N.; Karimi, K.; Mao, Y.-K.; Forsythe, P.; Min, K.K.; Stanisz, A.M.; Kunze, W.A.; Bienenstock, J. Gut commensal microvesicles reproduce parent bacterial signals to host immune and enteric nervous systems. FASEB J. 2015, 29, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Mata Forsberg, M.; Björkander, S.; Pang, Y.; Lundqvist, L.; Ndi, M.; Ott, M.; Buesa, I.; Jaeger, M.-C.; Roos, S.; Sverremark-Ekström, E. Extracellular Membrane Vesicles from Lactobacilli Dampen IFN-γ Responses in a Monocyte-Dependent Manner. Sci. Rep. 2019, 9, 17109. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Lee, E.J.; Bae, I.-H.; Myoung, K.; Kim, S.T.; Park, P.J.; Lee, H.-H.; Ko, J.; Oh, S.H.; Cho, E.G.; et al. Lactobacillus plantarum-derived extracellular vesicles induce anti-inflammatory M2 macrophage polarization in vitro. J. Extracell. Vesicles 2020, 9, 1793514. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Rao, S.-S.; Yue, T.; Tan, Y.-J.; Yin, H.; Chen, L.-J.; Luo, M.-J.; Wang, Z.; Wang, Y.-Y.; Hong, C.-G.; et al. Glucocorticoid-induced loss of beneficial gut bacterial extracellular vesicles is associated with the pathogenesis of osteonecrosis. Sci. Adv. 2022, 8, eabg8335. [Google Scholar] [CrossRef]
- Nakase, H.; Uchino, M.; Shinzaki, S.; Matsuura, M.; Matsuoka, K.; Kobayashi, T.; Saruta, M.; Hirai, F.; Hata, K.; Hiraoka, S.; et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J. Gastroenterol. 2021, 56, 489–526. [Google Scholar] [CrossRef]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Burisch, J.; Jess, T.; Martinato, M.; Lakatos, P.L.; ECCO-EpiCom. The burden of inflammatory bowel disease in Europe. J. Crohns Colitis 2013, 7, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Faye, A.S.; Holmer, A.K.; Axelrad, J.E. Cancer in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2022, 51, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend Your Fences: The Epithelial Barrier and its Relationship with Mucosal Immunity in Inflammatory Bowel Disease. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 33–46. [Google Scholar] [CrossRef]
- Miner-Williams, W.M.; Moughan, P.J. Intestinal barrier dysfunction: Implications for chronic inflammatory conditions of the bowel. Nutr. Res. Rev. 2016, 29, 40–59. [Google Scholar] [CrossRef]
- Schmitz, H.; Barmeyer, C.; Fromm, M.; Runkel, N.; Foss, H.D.; Bentzel, C.J.; Riecken, E.O.; Schulzke, J.D. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 1999, 116, 301–309. [Google Scholar] [CrossRef]
- Merga, Y.; Campbell, B.J.; Rhodes, J.M. Mucosal barrier, bacteria and inflammatory bowel disease: Possibilities for therapy. Dig. Dis. 2014, 32, 475–483. [Google Scholar] [CrossRef]
- Hazel, K.; O’Connor, A. Emerging treatments for inflammatory bowel disease. Ther. Adv. Chronic Dis. 2020, 11, 2040622319899297. [Google Scholar] [CrossRef]
- Damião, A.; de Azevedo, M.F.C.; Carlos, A.S.; Wada, M.Y.; Silva, T.V.M.; Feitosa, F.C. Conventional therapy for moderate to severe inflammatory bowel disease: A systematic literature review. World J. Gastroenterol. 2019, 25, 1142–1157. [Google Scholar] [CrossRef]
- Choi, J.H.; Moon, C.M.; Shin, T.-S.; Kim, E.K.; McDowell, A.; Jo, M.-K.; Joo, Y.H.; Kim, S.-E.; Jung, H.-K.; Shim, K.-N.; et al. Lactobacillus paracasei-derived extracellular vesicles attenuate the intestinal inflammatory response by augmenting the endoplasmic reticulum stress pathway. Exp. Mol. Med. 2020, 52, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhang, X.; Hao, H.; Liu, Q.; Zhou, Z.; Liang, X.; Liu, T.; Gong, P.; Zhang, L.; Zhai, Z.; et al. Lactobacillus rhamnosus GG Derived Extracellular Vesicles Modulate Gut Microbiota and Attenuate Inflammatory in DSS-Induced Colitis Mice. Nutrients 2021, 13, 3319. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Zhang, X.; Tong, L.; Liu, Q.; Liang, X.; Bu, Y.; Gong, P.; Liu, T.; Zhang, L.; Xia, Y.; et al. Effect of Extracellular Vesicles Derived From Lactobacillus plantarum Q7 on Gut Microbiota and Ulcerative Colitis in Mice. Front. Immunol. 2021, 12, 777147. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, İ.; Dolar, M.E.; Özpınar, H. Effect of administering kefir on the changes in fecal microbiota and symptoms of inflammatory bowel disease: A randomized controlled trial. Turk. J. Gastroenterol. 2019, 30, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Sevencan, N.O.; Isler, M.; Kapucuoglu, F.N.; Senol, A.; Kayhan, B.; Kiztanir, S.; Kockar, M.C. Dose-dependent effects of kefir on colitis induced by trinitrobenzene sulfonic acid in rats. Food Sci. Nutr. 2019, 7, 3110–3118. [Google Scholar] [CrossRef]
- Seo, M.K.; Park, E.J.; Ko, S.Y.; Choi, E.W.; Kim, S. Therapeutic effects of kefir grain Lactobacillus-derived extracellular vesicles in mice with 2,4,6-trinitrobenzene sulfonic acid-induced inflammatory bowel disease. J. Dairy Sci. 2018, 101, 8662–8671. [Google Scholar] [CrossRef]
- Kang, E.A.; Choi, H.I.; Hong, S.W.; Kang, S.; Jegal, H.-Y.; Choi, E.W.; Park, B.-S.; Kim, J.S. Extracellular Vesicles Derived from Kefir Grain Lactobacillus Ameliorate Intestinal Inflammation via Regulation of Proinflammatory Pathway and Tight Junction Integrity. Biomedicines 2020, 8, 522. [Google Scholar] [CrossRef]
- Domínguez Rubio, A.P.; Martínez, J.H.; Martínez-Casillas, D.C.; Coluccio Leskow, F.; Piuri, M.; Pérez, O.E. Lactobacillus casei BL23 Produces Microvesicles Carrying Proteins That Have Been Associated with Its Probiotic Effect. Front. Microbiol. 2017, 8, 1783. [Google Scholar] [CrossRef]
- Habil, N.; Abate, W.; Beal, J.; Foey, A.D. Heat-killed probiotic bacteria differentially regulate colonic epithelial cell production of human β-defensin-2: Dependence on inflammatory cytokines. Benef. Microbes 2014, 5, 483–495. [Google Scholar] [CrossRef]
- Bäuerl, C.; Coll-Marqués, J.M.; Tarazona-González, C.; Pérez-Martínez, G. Lactobacillus casei extracellular vesicles stimulate EGFR pathway likely due to the presence of proteins P40 and P75 bound to their surface. Sci. Rep. 2020, 10, 19237. [Google Scholar] [CrossRef]
- Yan, F.; Cao, H.; Cover, T.L.; Whitehead, R.; Washington, M.K.; Polk, D.B. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 2007, 132, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Bäuerl, C.; Pérez-Martínez, G.; Yan, F.; Polk, D.B.; Monedero, V. Functional Analysis of the p40 and p75 Proteins from Lactobacillus casei BL23. Microb. Physiol. 2010, 19, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Cao, H.; Cover, T.L.; Washington, M.K.; Shi, Y.; Liu, L.; Chaturvedri, R.; Peek, R.M., Jr.; Wilson, K.T.; Polk, D.B. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J. Clin. Investig. 2011, 121, 2242–2253. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Moore, D.J.; Shen, X.; Peek, R.M.; Acra, S.A.; Li, H.; Ren, X.; Polk, D.B.; Yan, F. An LGG-derived protein promotes IgA production through upregulation of APRIL expression in intestinal epithelial cells. Mucosal Immunol. 2017, 10, 373–384. [Google Scholar] [CrossRef]
- Yan, F.; Liu, L.; Cao, H.; Moore, D.J.; Washington, M.K.; Wang, B.; Peek, R.M.; Acra, S.A.; Polk, D.B. Neonatal colonization of mice with LGG promotes intestinal development and decreases susceptibility to colitis in adulthood. Mucosal Immunol. 2017, 10, 117–127. [Google Scholar] [CrossRef]
- Yu, S.; Gao, N. Compartmentalizing intestinal epithelial cell toll-like receptors for immune surveillance. Cell. Mol. Life Sci. 2015, 72, 3343–3353. [Google Scholar] [CrossRef]
- Fukata, M.; Arditi, M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. 2013, 6, 451–463. [Google Scholar] [CrossRef]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.-E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef]
- Vargoorani, M.E.; Modarressi, M.H.; Vaziri, F.; Motevaseli, E.; Siadat, S.D. Stimulatory effects of Lactobacillus casei derived extracellular vesicles on toll-like receptor 9 gene expression and cytokine profile in human intestinal epithelial cells. J. Diabetes Metab. Disord. 2020, 19, 223–231. [Google Scholar] [CrossRef]
- Yamasaki-Yashiki, S.; Miyoshi, Y.; Nakayama, T.; Kunisawa, J.; Katakura, Y. IgA-enhancing effects of membrane vesicles derived from Lactobacillus sakei subsp. sakei NBRC15893. Biosci. Microbiota Food Health 2019, 38, 23–29. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Saika, A.; Nagatake, T.; Matsunaga, A.; Kunisawa, J.; Katakura, Y.; Yamasaki-Yashiki, S. Mechanisms underlying enhanced IgA production in Peyer’s patch cells by membrane vesicles derived from Lactobacillus sakei. Biosci. Biotechnol. Biochem. 2021, 85, 1536–1545. [Google Scholar] [CrossRef]
- Hu, R.; Lin, H.; Wang, M.; Zhao, Y.; Liu, H.; Min, Y.; Yang, X.; Gao, Y.; Yang, M. Lactobacillus reuteri-derived extracellular vesicles maintain intestinal immune homeostasis against lipopolysaccharide-induced inflammatory responses in broilers. J. Anim. Sci. Biotechnol. 2021, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Surve, M.V.; Anil, A.; Kamath, K.G.; Buthda, S.; Sthanam, L.K.; Pradhan, A.; Srivastava, R.; Basu, B.; Dutta, S.; Sen, S.; et al. Membrane Vesicles of Group B Streptococcus Disrupt Feto-Maternal Barrier Leading to Preterm Birth. PLoS Pathog. 2016, 12, e1005816. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.; Cordero, R.J.B.; Nakouzi, A.S.; Casadevall, A. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc. Natl. Acad. Sci. USA 2010, 107, 19002–19007. [Google Scholar] [CrossRef] [PubMed]
- Thay, B.; Wai, S.N.; Oscarsson, J. Staphylococcus aureus α-toxin-dependent induction of host cell death by membrane-derived vesicles. PLoS ONE 2013, 8, e54661. [Google Scholar] [CrossRef]
- Olaya-Abril, A.; Prados-Rosales, R.; McConnell, M.J.; Martín-Peña, R.; González-Reyes, J.A.; Jiménez-Munguía, I.; Gómez-Gascón, L.; Fernández, J.; Luque-García, J.L.; García-Lidón, C.; et al. Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae. J. Proteom. 2014, 106, 46–60. [Google Scholar] [CrossRef]
- Jeon, H.; Oh, M.H.; Jun, S.H.; Kim, S.I.; Choi, C.-W.; Kwon, H.I.; Na, S.H.; Kim, Y.J.; Nicholas, A.; Selasi, G.N.; et al. Variation among Staphylococcus aureus membrane vesicle proteomes affects cytotoxicity of host cells. Microb. Pathog. 2016, 93, 185–193. [Google Scholar] [CrossRef]
- Resch, U.; Tsatsaronis, J.A.; Rhun, A.L.; Stübiger, G.; Rohde, M.; Kasvandik, S.; Holzmesiterm, S.; Tinnefeld, P.; Wai, S.N.; Charpentier, E. A Two-Component Regulatory System Impacts Extracellular Membrane-Derived Vesicle Production in Group A Streptococcus. mBio 2016, 7, e00207-16. [Google Scholar] [CrossRef]
- Kim, M.H.; Choi, S.J.; Choi, H.I.; Choi, J.P.; Park, H.K.; Kim, E.K.; Kim, M.J.; Moon, B.S.; Min, T.K.; Rho, M.; et al. Lactobacillus plantarum-derived Extracellular Vesicles Protect Atopic Dermatitis Induced by Staphylococcus aureus-derived Extracellular Vesicles. Allergy Asthma Immunol. Res. 2018, 10, 516–532. [Google Scholar] [CrossRef]
- Li, M.; Lee, K.; Hsu, M.; Nau, G.; Mylonakis, E.; Ramratnam, B. Lactobacillus-derived extracellular vesicles enhance host immune responses against vancomycin-resistant enterococci. BMC Microbiol. 2017, 17, 66. [Google Scholar] [CrossRef]
- Ñahui Palomino, R.A.; Vanpouille, C.; Laghi, L.; Parolin, C.; Melikov, K.; Backlund, P.; Vitali, B.; Margolis, L. Extracellular vesicles from symbiotic vaginal lactobacilli inhibit HIV-1 infection of human tissues. Nat. Commun. 2019, 10, 5656. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, P.; Kunze, W.; Bienenstock, J. Moody microbes or fecal phrenology: What do we know about the microbiota-gut-brain axis? BMC Med. 2016, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558 Pt 1, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Salvo-Romero, E.; Stokes, P.; Gareau, M.G. Microbiota-immune interactions: From gut to brain. LymphoSign J. 2020, 7, 1–23. [Google Scholar] [CrossRef]
- Lyte, M.; Li, W.; Opitz, N.; Gaykema, R.P.; Goehler, L.E. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol. Behav. 2006, 89, 350–357. [Google Scholar] [CrossRef]
- Lyte, M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays 2011, 33, 574–581. [Google Scholar] [CrossRef]
- Yong, S.J.; Tong, T.; Chew, J.; Lim, W.L. Antidepressive Mechanisms of Probiotics and Their Therapeutic Potential. Front. Neurosci. 2019, 13, 1361. [Google Scholar] [CrossRef]
- Liang, S.; Wang, T.; Hu, X.; Luo, J.; Li, W.; Duan, X.; Jin, F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 2015, 310, 561–577. [Google Scholar] [CrossRef]
- Maehata, H.; Kobayashi, Y.; Mitsuyama, E.; Kawase, T.; Kuhara, T.; Xiao, J.-Z.; Tsukahara, T.; Toyoda, A. Heat-killed Lactobacillus helveticus strain MCC1848 confers resilience to anxiety or depression-like symptoms caused by subchronic social defeat stress in mice. Biosci. Biotechnol. Biochem. 2019, 83, 1239–1247. [Google Scholar] [CrossRef]
- Dhaliwal, J.; Singh, D.P.; Singh, S.; Pinnaka, A.K.; Boparai, R.K.; Bishnoi, M.; Kondepudi, K.K.; Chopra, K. Lactobacillus plantarum MTCC 9510 supplementation protects from chronic unpredictable and sleep deprivation-induced behaviour, biochemical and selected gut microbial aberrations in mice. J. Appl. Microbiol. 2018, 125, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Chuang, H.-L.; Huang, Y.-T.; Wu, C.-C.; Chou, G.-T.; Wang, S.; Tsai, Y.-C. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav. Brain Res. 2016, 298 Pt B, 202–209. [Google Scholar] [CrossRef]
- Jang, S.C.; Kim, S.R.; Yoon, Y.J.; Park, K.-S.; Kim, J.H.; Lee, J.; Kim, O.Y.; Choi, E.-J.; Kim, D.-K.; Choi, D.-S.; et al. In vivo kinetic biodistribution of nano-sized outer membrane vesicles derived from bacteria. Small 2015, 11, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Stentz, R.; Carvalho, A.L.; Jones, E.J.; Carding, S.R. Fantastic voyage: The journey of intestinal microbiota-derived microvesicles through the body. Biochem. Soc. Trans. 2018, 46, 1021–1027. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, J.; Lee, Y.; Lee, J.-E.; Lee, E.-H.; Kwon, H.-J.; Yang, J.; Jeong, B.-R.; Kim, Y.-K.; Han, P.-L. Metagenome Analysis of Bodily Microbiota in a Mouse Model of Alzheimer Disease Using Bacteria-derived Membrane Vesicles in Blood. Exp. Neurobiol. 2017, 26, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Nikkari, S.; McLaughlin, I.J.; Bi, W.; Dodgem, D.E.; Relman, D.A. Does blood of healthy subjects contain bacterial ribosomal DNA? J. Clin. Microbiol. 2001, 39, 1956–1959. [Google Scholar] [CrossRef]
- Païssé, S.; Valle, C.; Servant, F.; Courtney, M.; Burcelin, R.; Amar, J.; Lelouvier, B. Comprehensive description of blood microbiome from healthy donors assessed by 16S targeted metagenomic sequencing. Transfusion 2016, 56, 1138–1147. [Google Scholar] [CrossRef]
- Zakharzhevskaya, N.B.; Vanyushkina, A.A.; Altukhov, I.A.; Shavarda, A.L.; Butenko, I.O.; Rakitina, D.V.; Nikitina, A.S.; Manolov, A.I.; Egorova, A.N.; Kulikov, E.E.; et al. Outer membrane vesicles secreted by pathogenic and nonpathogenic Bacteroides fragilis represent different metabolic activities. Sci. Rep. 2017, 7, 5008. [Google Scholar] [CrossRef]
- Passani, M.B.; Giannoni, P.; Bucherelli, C.; Baldi, E.; Blandina, P. Histamine in the brain:Beyond sleep and memory. Biochem. Pharmacol. 2007, 73, 1113–1122. [Google Scholar] [CrossRef]
- Choi, J.; Kim, Y.K.; Han, P.L. Extracellular Vesicles Derived from Lactobacillus plantarum Increase BDNF Expression in Cultured Hippocampal Neurons and Produce Antidepressant-like Effects in Mice. Exp. Neurobiol. 2019, 28, 158–171. [Google Scholar] [CrossRef]
- Choi, J.; Kwon, H.; Kim, Y.-K.; Han, P.-L. Extracellular Vesicles from Gram-positive and Gram-negative Probiotics Remediate Stress-Induced Depressive Behavior in Mice. Mol. Neurobiol. 2022, 59, 2715–2728. [Google Scholar] [CrossRef] [PubMed]
- West, C.L.; Stanisz, A.M.; Mao, Y.-K.; Champagne-Jorgense, K.; Bienenstock, J.; Kunze, W.A. Microvesicles from Lactobacillus reuteri (DSM-17938) completely reproduce modulation of gut motility by bacteria in mice. PLoS ONE 2020, 15, e0225481. [Google Scholar] [CrossRef]
- Commane, D.; Hughes, R.; Shortt, C.; Rowland, I. The potential mechanisms involved in the anti-carcinogenic action of probiotics. Mutat. Res. 2005, 591, 276–289. [Google Scholar] [CrossRef]
- McIntosh, G.H.; Royle, P.J.; Playne, M.J. A probiotic strain of L. acidophilus reduces DMH-induced large intestinal tumors in male Sprague-Dawley rats. Nutr. Cancer 1999, 35, 153–159. [Google Scholar] [CrossRef]
- Yamazaki, K.; Tsunoda, A.; Sibusawa, M.; Tsunoda, Y.; Kusano, M.; Fukuchi, K.; Yamanaka, M.; Kushima, M.; Nomoto, K.; Morotomi, M. The effect of an oral administration of Lactobacillus casei strain shirota on azoxymethane-induced colonic aberrant crypt foci and colon cancer in the rat. Oncol. Rep. 2000, 7, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Goldin, B.R.; Gualtieri, L.J.; Moore, R.P. The effect of Lactobacillus GG on the initiation and promotion of DMH-induced intestinal tumors in the rat. Nutr. Cancer 1996, 25, 197–204. [Google Scholar] [CrossRef]
- Rafter, J. The effects of probiotics on colon cancer development. Nutr. Res. Rev. 2004, 17, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Keyhani, G.; Hosseini, H.M.; Salimi, A. Effect of extracellular vesicles of Lactobacillus rhamnosus GG on the expression of CEA gene and protein released by colorectal cancer cells. Iran. J. Microbiol. 2022, 14, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, E.; Hosseini, H.M.; Fooladi, A.A.I. The inhibitory impacts of Lactobacillus rhamnosus GG-derived extracellular vesicles on the growth of hepatic cancer cells. Microb. Pathog. 2017, 110, 1–6. [Google Scholar] [CrossRef]

| Specie | Indication | Model | Mechanism | Ref. |
|---|---|---|---|---|
| L. animalis ATCC 35046 | Osteonecrosis | Glucocorticoid- induced osteonecrosis mice | ↑ Angiogenesis and osteogenesis. ↓ Cell apoptosis. | [58] |
| Lc. casei BL23 | IBD | T84 cell line | Anti-apoptotic and anti-inflammatory effects exerted by p40 and p75 proteins. | [81] |
| Lc. casei ATCC 393 | Colitis | Caco-2 cell line | ↑ Anti-inflammatory mediators (TLR9 gene expression and levels of IL-4 and IL-10). ↓ Pro-inflammatory markers (IL-17A and IFN-γ). | [90] |
| L.crispatus BC3 and L. gasseri BC12 | HIV infection | TZM-bl and MT-4 cells | Partial inhibition of viral attachment to target HIV cells and its entry. | [102] |
| Le. kefiri KCT 3611, L. kefiranofaciens KCT 5075, and L. kefirgranum KCT 5086 | Colitis | Caco-2 cell line | ↓ Inflammatory process: ↓ TNF-α pathway. ↓ p65 phosphorylation. | [77] |
| TNBS-IBD-induced mice | ↓ Body weight loss and rectal bleeding. ↓ Infiltration of transmural leukocytes, goblet cells and seric levels of myeloperoxidase. ↑ Stool consistency. | |||
| L. kefirgranum PRCC-1301 | Colitis | DSS-stimulated-Caco-2 cells | ↑ Intestinal cell integrity: recovery of TJs proteins ↓ Pro-inflammatory cytokines (IL-2, IL-8, and TNF-α). | [78] |
| DSS-induced colitis mice | ↓ Body weight loss, colon shortening, and histological damage. ↓ Phosphorylation of NF-κB p65 and IκBα in colon tissue. | |||
| Specie | Indication | Model | Mechanism | Ref. |
| Lc. paracasei | IBD | HT-29 cell line | Anti-inflammatory effect: ↓ Inflammation-associated proteins (COX-2, iNOS, NFκB, and NO). | [72] |
| DSS-induced colitis mice | ↓ Weight loss and DAI. Maintenance of colon length. | |||
| Lp. plantarum APsulloc 331261 | Skin inflammation | THP-1 cells | ↑ Anti-inflammatory M2 phenotype. ↓ M1-associated surface markers and HLA-DRα expression in pro-inflammatory M1 macrophage-favouring conditions. | [57] |
| Human skin organ cultures | ↑ IL-1β, GM-CSF and IL-10. | |||
| Lp. plantarum Q7 | Colitis | DSS-induced colitis mice | ↓ Histological damage. ↓ Pro-inflammatory cytokines. -Modulation of gut microbiota: ↑ Anti-inflammatory bacteria (Muribaculaceae and Bifidobacteria). ↓ Pro-inflammatory bacteria (Proteobacteria). | [74] |
| Lp. plantarum CJLP55 | Atopic dermatitis | HaCaT cells and macrophages treated with S. aureus MVs | Restoration of cell viability. | [100] |
| Human clinical trial | ↑ Proportion of Lactobacillus MVs in the control group. ↓ Epidermal thickening and cytokine IL-4 levels in AD patients. | |||
| Depression | HT22 hippocampal cells | ↑ Expression of BDNF and proBDNF protein. ↑ BDNF regulating factors (Nt4/5 and Mecp2). | [121] | |
| Stress-induced depression mice | Normalisation of BDNF expression. | [122] | ||
| Specie | Indication | Model | Mechanism | Ref. |
| Lp. plantarum WCFS1 | VRE infection | Caco-2 cell line | Modulation of host response. ↑ Expression of host defence genes (CTSB and REG3G). | [101] |
| C. elegans | ↑ Expression of host defence genes (Cpr-1 and clec-60). | |||
| Lc. rhamnosus JB-1 | Immune system | PP-derived DCs | Activation of tolerogenic dendritic cells. ↑ Treg cells. | [55] |
| Brain function | Ex vivo mice model of peak pressure-induced MMC in segments of colon | ↓ Excitability of afferent neurons in the myenteric plexus. | [55] | |
| Enteric nervous system | Ex vivo mice model of peristalsis | ↓ Amplitude of neuronally dependent MMCs. | [55] | |
| Lc. rhamnosus GG | Immune system | PBMCs-derived T and NK cells. | ↓ Pro-inflammatory cytokines (IFN-γ and IL-17A). | [56] |
| Colitis | DSS-induced colitis mice model. | ↓ Colonic tissue damage and colon shortening. Reshape of the gut altered microbiota. ↓ Pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-2). | [73] | |
| Colorectal cancer | SW480 and HT-29 cell lines | Anti-proliferative effect: ↑ Gene expression and protein synthesis of CEA. | [129] | |
| Hepatic cancer | HepG2 cell line | Antiproliferative effect: ↑ Bax/Bcl-2 ratio. | [130] | |
| Li. reuteri BBC3 | IBD | LPS-activated chicken macrophages | ↓ Pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) via the suppression of NF-κB activity. | [93] |
| LPS-induced intestinal inflammation in broilers | ↑ Growth performance. ↓ Intestinal injury and mortality. Anti-inflammatory function: ↓ Pro-inflammatory genes (TNF-α, IL-1β, IL-6, IL-17 and IL-8). ↑ Anti-inflammatory genes (IL-10 and TGF-β). | |||
| Specie | Indication | Model | Mechanism | Ref. |
| Li. reuteri DSM 17938 | Enteric nervous system | Ex vivo model: mouse jejunal and colonic segments | Modulation of velocity and frequency of propagating contractile cluster contractions: ↑ Colon ↓ Jejunum | [123] |
| Immune system | T and NK cells from PBMCs | ↓ Pro-inflammatory cytokines (IFN-γ and IL-17A). | [56] | |
| L. sakei NBRC 15893 | Immune system | Murine bone marrow-derived DCs and murine PP cells | ↑ IgA production in PP cells. ↑ Gene expression of iNOs, RA, and pro-inflammatory cytokines 8IL-6, IL-10, IL-12 and TNF-α). | [91,92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Lozano, E.; García-García, J.; Gálvez, J.; Hidalgo-García, L.; Rodríguez-Nogales, A.; Rodríguez-Cabezas, M.E.; Sánchez, M. Novel Horizons in Postbiotics: Lactobacillaceae Extracellular Vesicles and Their Applications in Health and Disease. Nutrients 2022, 14, 5296. https://doi.org/10.3390/nu14245296
González-Lozano E, García-García J, Gálvez J, Hidalgo-García L, Rodríguez-Nogales A, Rodríguez-Cabezas ME, Sánchez M. Novel Horizons in Postbiotics: Lactobacillaceae Extracellular Vesicles and Their Applications in Health and Disease. Nutrients. 2022; 14(24):5296. https://doi.org/10.3390/nu14245296
Chicago/Turabian StyleGonzález-Lozano, Elena, Jorge García-García, Julio Gálvez, Laura Hidalgo-García, Alba Rodríguez-Nogales, María Elena Rodríguez-Cabezas, and Manuel Sánchez. 2022. "Novel Horizons in Postbiotics: Lactobacillaceae Extracellular Vesicles and Their Applications in Health and Disease" Nutrients 14, no. 24: 5296. https://doi.org/10.3390/nu14245296
APA StyleGonzález-Lozano, E., García-García, J., Gálvez, J., Hidalgo-García, L., Rodríguez-Nogales, A., Rodríguez-Cabezas, M. E., & Sánchez, M. (2022). Novel Horizons in Postbiotics: Lactobacillaceae Extracellular Vesicles and Their Applications in Health and Disease. Nutrients, 14(24), 5296. https://doi.org/10.3390/nu14245296








