Fatty Acids and Eicosanoids Change during High-Fiber Diet in NAFLD Patients—Randomized Control Trials (RCT)
Abstract
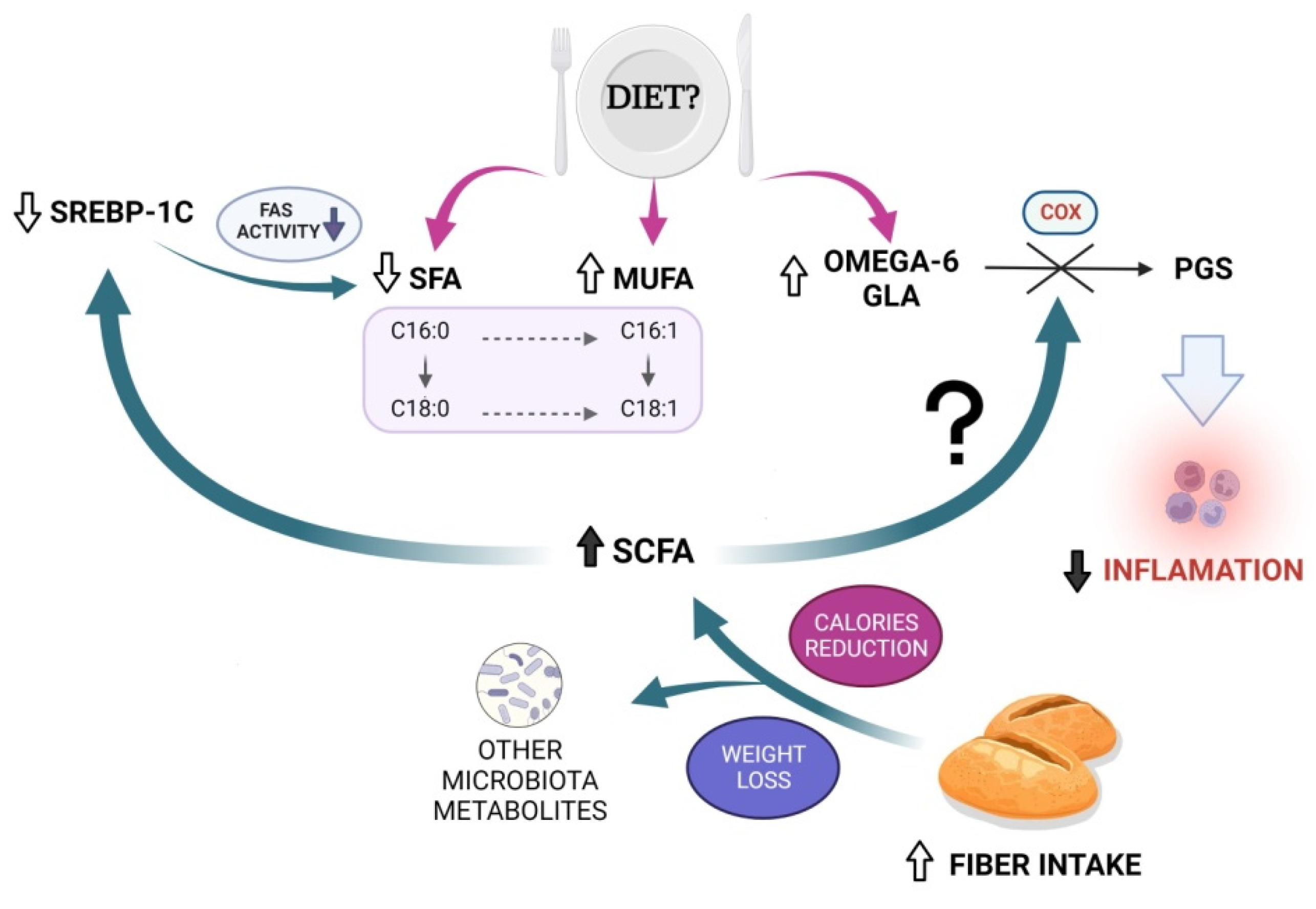
1. Introduction
2. Materials and Methods
2.1. Design of the Study
2.2. Dietary Guidelines for Patients
2.3. Fatty Acids (FAs) Analysis
2.4. Eicosanoids Analysis
2.5. Statistical Analysis
3. Results
3.1. NAFLD and Stiffness Status Evaluation
3.2. Biochemical Parameters
3.3. Fatty Acids and Eicosanoids Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Cholongitas, E.; Pavlopoulou, I.; Papatheodoridi, M.; Markakis, G.E.; Bouras, E.; Haidich, A.-B.; Papatheodoridis, G. Epidemiology of Nonalcoholic Fatty Liver Disease in Europe: A Systematic Review and Meta-Analysis. Ann. Gastroenterol. 2021, 34, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Bellentani, S. The Epidemiology of Non-Alcoholic Fatty Liver Disease. Liver Int. 2017, 37 (Suppl. 1), 81–84. [Google Scholar] [CrossRef] [PubMed]
- Le, M.H.; Devaki, P.; Ha, N.B.; Jun, D.W.; Te, H.S.; Cheung, R.C.; Nguyen, M.H. Prevalence of Non-Alcoholic Fatty Liver Disease and Risk Factors for Advanced Fibrosis and Mortality in the United States. PLoS ONE 2017, 12, e0173499. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yang, A.; Mao, L.; Quan, Y.; Cui, J.; Sun, Y. Association Between Dietary Fiber Intake and Non-Alcoholic Fatty Liver Disease in Adults. Front. Nutr. 2020, 7, 593735. [Google Scholar] [CrossRef] [PubMed]
- Hannah, W.N.; Harrison, S.A. Effect of Weight Loss, Diet, Exercise, and Bariatric Surgery on Nonalcoholic Fatty Liver Disease. Clin. Liver Dis. 2016, 20, 339–350. [Google Scholar] [CrossRef]
- St-Pierre, D.H.; Rabasa-Lhoret, R.; Lavoie, M.-E.; Karelis, A.D.; Strychar, I.; Doucet, E.; Coderre, L. Fiber Intake Predicts Ghrelin Levels in Overweight and Obese Postmenopausal Women. Eur. J. Endocrinol. 2009, 161, 65–72. [Google Scholar] [CrossRef]
- Stachowska, E.; Portincasa, P.; Jamioł-Milc, D.; Maciejewska-Markiewicz, D.; Skonieczna-Żydecka, K. The Relationship between Prebiotic Supplementation and Anthropometric and Biochemical Parameters in Patients with NAFLD—A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 3460. [Google Scholar] [CrossRef]
- Loomba, R.; Quehenberger, O.; Armando, A.; Dennis, E.A. Polyunsaturated Fatty Acid Metabolites as Novel Lipidomic Biomarkers for Noninvasive Diagnosis of Nonalcoholic Steatohepatitis. J. Lipid Res. 2015, 56, 185–192. [Google Scholar] [CrossRef]
- Maciejewska, D.; Drozd, A.; Ossowski, P.; Ryterska, K.; Jamioł-Milc, D.; Banaszczak, M.; Raszeja-Wyszomirska, J.; Kaczorowska, M.; Sabinicz, A.; Stachowska, E. Fatty Acid Changes Help to Better Understand Regression of Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2015, 21, 301–310. [Google Scholar] [CrossRef]
- Maciejewska, D.; Palma, J.; Dec, K.; Skonieczna-Żydecka, K.; Gutowska, I.; Szczuko, M.; Jakubczyk, K.; Stachowska, E. Is the Fatty Acids Profile in Blood a Good Predictor of Liver Changes? Correlation of Fatty Acids Profile with Fatty Acids Content in the Liver. Diagnostics 2019, 9, 197. [Google Scholar] [CrossRef]
- Caussy, C.; Chuang, J.-C.; Billin, A.; Hu, T.; Wang, Y.; Subramanian, G.M.; Djedjos, C.S.; Myers, R.P.; Dennis, E.A.; Loomba, R. Plasma Eicosanoids as Noninvasive Biomarkers of Liver Fibrosis in Patients with Nonalcoholic Steatohepatitis. Ther. Adv. Gastroenterol. 2020, 13, 1756284820923904. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, D.; Drozd, A.; Skonieczna-Żydecka, K.; Skórka-Majewicz, M.; Dec, K.; Jakubczyk, K.; Pilutin, A.; Stachowska, E. Eicosanoids in Nonalcoholic Fatty Liver Disease (NAFLD) Progression. Do Serum Eicosanoids Profile Correspond with Liver Eicosanoids Content during NAFLD Development and Progression? Molecules 2020, 25, 2026. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.-S. Advances in Lipid Extraction Methods—A Review. Int. J. Mol. Sci. 2021, 22, 13643. [Google Scholar] [CrossRef] [PubMed]
- Szczuko, M.; Kotlęga, D.; Palma, J.; Zembroń-Łacny, A.; Tylutka, A.; Gołąb-Janowska, M.; Drozd, A. Lipoxins, RevD1 and 9, 13 HODE as the Most Important Derivatives after an Early Incident of Ischemic Stroke. Sci. Rep. 2020, 10, 12849. [Google Scholar] [CrossRef]
- Kaczmarczyk, M.M.; Miller, M.J.; Freund, G.G. The Health Benefits of Dietary Fiber: Beyond the Usual Suspects of Type 2 Diabetes Mellitus, Cardiovascular Disease and Colon Cancer. Metabolism 2012, 61, 1058–1066. [Google Scholar] [CrossRef]
- Dorosti, M.; Jafary Heidarloo, A.; Bakhshimoghaddam, F.; Alizadeh, M. Whole-Grain Consumption and Its Effects on Hepatic Steatosis and Liver Enzymes in Patients with Non-Alcoholic Fatty Liver Disease: A Randomised Controlled Clinical Trial. Br. J. Nutr. 2020, 123, 328–336. [Google Scholar] [CrossRef]
- Javadi, L.; Ghavami, M.; Khoshbaten, M.; Safaiyan, A.; Barzegari, A.; Gargari, B.P. The Potential Role of Probiotics or/and Prebiotic on Serum Lipid Profile and Insulin Resistance in Alcoholic Fatty Liver Disease: A Double Blind Randomized Clinical Trial. Crescent J. Med. Biol. Sci. 2017, 4, 8. [Google Scholar]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Han, S.; Jiao, J.; Zhang, W.; Xu, J.; Wan, Z.; Zhang, W.; Gao, X.; Qin, L. Dietary Fiber Prevents Obesity-Related Liver Lipotoxicity by Modulating Sterol-Regulatory Element Binding Protein Pathway in C57BL/6J Mice Fed a High-Fat/Cholesterol Diet. Sci. Rep. 2015, 5, 15256. [Google Scholar] [CrossRef]
- DeBose-Boyd, R.A.; Ye, J. SREBPs in Lipid Metabolism, Insulin Signaling, and Beyond. Trends Biochem. Sci. 2018, 43, 358–368. [Google Scholar] [CrossRef]
- Yoon, M. The Role of PPARalpha in Lipid Metabolism and Obesity: Focusing on the Effects of Estrogen on PPARalpha Actions. Pharmacol. Res. 2009, 60, 151–159. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of Fatty Acids Stored in Liver and Secreted via Lipoproteins in Patients with Nonalcoholic Fatty Liver Disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Brighenti, F.; Royall, D.; Jenkins, A.L.; Jenkins, D.J. Effect of Rectal Infusion of Short Chain Fatty Acids in Human Subjects. Am. J. Gastroenterol. 1989, 84, 1027–1033. [Google Scholar] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Norambuena, F.; Mackenzie, S.; Bell, J.G.; Callol, A.; Estévez, A.; Duncan, N. Prostaglandin (F and E, 2- and 3-Series) Production and Cyclooxygenase (COX-2) Gene Expression of Wild and Cultured Broodstock of Senegalese Sole (Solea Senegalensis). Gen. Comp. Endocrinol. 2012, 177, 256–262. [Google Scholar] [CrossRef]
- Wang, W.; Zhong, X.; Guo, J. Role of 2-Series Prostaglandins in the Pathogenesis of Type 2 Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease (Review). Int. J. Mol. Med. 2021, 47, 114. [Google Scholar] [CrossRef]
- Hsieh, P.-S.; Jin, J.-S.; Chiang, C.-F.; Chan, P.-C.; Chen, C.-H.; Shih, K.-C. COX-2-Mediated Inflammation in Fat Is Crucial for Obesity-Linked Insulin Resistance and Fatty Liver. Obesity (Silver Spring) 2009, 17, 1150–1157. [Google Scholar] [CrossRef]
- Henkel, J.; Frede, K.; Schanze, N.; Vogel, H.; Schürmann, A.; Spruss, A.; Bergheim, I.; Püschel, G.P. Stimulation of Fat Accumulation in Hepatocytes by PGE2-Dependent Repression of Hepatic Lipolysis, β-Oxidation and VLDL-Synthesis. Lab. Invest. 2012, 92, 1597–1606. [Google Scholar] [CrossRef]
- Brea, R.; Motiño, O.; Francés, D.; García-Monzón, C.; Vargas, J.; Fernández-Velasco, M.; Boscá, L.; Casado, M.; Martín-Sanz, P.; Agra, N. PGE2 Induces Apoptosis of Hepatic Stellate Cells and Attenuates Liver Fibrosis in Mice by Downregulating MiR-23a-5p and MiR-28a-5p. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2018, 1864, 325–337. [Google Scholar] [CrossRef]
- Pérez, S.; Aspichueta, P.; Ochoa, B.; Chico, Y. The 2-Series Prostaglandins Suppress VLDL Secretion in an Inflammatory Condition-Dependent Manner in Primary Rat Hepatocytes. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2006, 1761, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.-Y.; Mah, E.; Masterjohn, C.; Noh, S.K.; Park, H.J.; Clark, R.M.; Park, Y.-K.; Lee, J.-Y.; Bruno, R.S. Green Tea Lowers Hepatic COX-2 and Prostaglandin E2 in Rats with Dietary Fat-Induced Nonalcoholic Steatohepatitis. J. Med. Food. 2015, 18, 648–655. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Intervention with 12 g | p | Intervention with 24 g | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |||
| Fasting glucose (mg/dL) | 95 | 14.1 | 90 | 33.5 | 0.66 | 91.6 | 15.4 | 97.9 | 10.6 | 0.94 |
| Total cholesterol (mg/dL) | 195.2 | 53.2 | 178.2 | 27.5 | 0.18 | 210 | 51.6 | 197.3 | 47.7 | 0.03 ↓ |
| HDL (mg/dL) | 45.2 | 12 | 42.8 | 16.1 | 0.58 | 47.1 | 6.4 | 48.7 | 6.5 | 0.07 |
| LDL (mg/dL) | 127.9 | 57.7 | 110.4 | 41.8 | 0.33 | 138.9 | 31.9 | 129.7 | 46 | 0.09 |
| TG (mg/dL) | 164.1 | 95.3 | 119.7 | 165 | 0.14 | 169.6 | 152.3 | 161.6 | 113.2 | 0.11 |
| ALT (U/L) | 38 | 19 | 38 | 19 | 0.94 | 41 | 21 | 31 | 18 | 0.06 |
| AST (U/L) | 26 | 12 | 30 | 11 | 0.68 | 26 | 8 | 23 | 6 | 0.03 ↓ |
| GGTP (U/L) | 32 | 10 | 35 | 16 | 0.23 | 28 | 12 | 24 | 14 | 0.12 |
| Fasting insulin (uU/mL) | 19.3 | 20.7 | 16.9 | 13.6 | 0.66 | 33.7 | 94.9 | 37.6 | 31 | 0.09 |
| Age (years) | 47 | 12.8 | - | - | - | 47.5 | 14.7 | - | - | - |
| BMI (kg/m2) | 29.1 | 3.8 | 28.7 | 5.2 | 0.04 ↓ | 28.2 | 10.5 | 28.9 | 9.7 | 0.54 |
| Fatty Acid (%) | Intervention with 12 g | p | Intervention with 24 g | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |||
| C13:0 Tridecanoic acid | 0.20 | 0.05 | 0.20 | 0.08 | 0.53 | 0.22 | 0.06 | 0.23 | 0.09 | 0.45 |
| C14:0 Myristic acid | 1.24 | 0.67 | 1.30 | 0.30 | 0.35 | 1.27 | 0.30 | 1.11 | 0.43 | 0.76 |
| C14:1 Myristolenic acid | 0.05 | 0.06 | 0.06 | 0.04 | 0.0001 ↑ | 0.05 | 0.03 | 0.07 | 0.04 | 0.001 ↑ |
| C15:0 Pentadecanoic acid | 0.16 | 0.07 | 0.19 | 0.03 | 0.15 | 0.14 | 0.05 | 0.15 | 0.05 | 0.58 |
| C16:0 Palmitic acid | 3.46 | 2.08 | 29.27 | 1.89 | 0.001 ↓ | 31.45 | 2.56 | 29.98 | 1.86 | 0.02 ↓ |
| C16:1 Palmitoleic acid | 1.03 | 0.43 | 1.64 | 0.99 | 0.001 ↑ | 1.15 | 0.49 | 1.20 | 0.90 | 0.001 ↑ |
| C17:0 Heptadecanoic acid | 0.25 | 0.04 | 0.26 | 0.05 | 0.3 | 0.24 | 0.05 | 0.25 | 0.07 | 0.07 |
| C17:1 Heptadecanoic acid | 0.06 | 0.02 | 0.08 | 0.03 | 0.0001 ↑ | 0.06 | 0.02 | 0.07 | 0.06 | 0.0001 ↑ |
| C18:0 Stearic acid | 21.39 | 3.98 | 19.12 | 3.63 | 0.03 ↓ | 21.76 | 5.53 | 19.78 | 5.61 | 0.019 ↓ |
| C18:1n9 Oleic acid | 12.44 | 4.57 | 14.50 | 4.48 | 0.07 | 12.04 | 4.45 | 14.37 | 3.67 | 0.07 |
| C18:1n7 Vaccinic acid | 1.34 | 0.49 | 1.47 | 0.41 | 0.71 | 1.30 | 0.42 | 1.59 | 0.53 | 0.17 |
| C18:2n6 Linoleic acid | 12.25 | 5.12 | 14.07 | 4.87 | 0.02 ↑ | 12.12 | 5.26 | 14.67 | 3.75 | 0.016 ↑ |
| C18:3n6 Gamma linoleic acid (GLA) | 0.17 | 0.10 | 0.26 | 0.16 | 0.003 ↑ | 0.14 | 0.06 | 0.24 | 0.10 | 0.021 ↑ |
| C18:3n3 Linolenic acid | 0.18 | 0.07 | 0.13 | 0.06 | 0.02 ↑ | 0.17 | 0.05 | 0.12 | 0.04 | 0.09 |
| C20:4n6 Arachidonic acid (AA) | 6.03 | 1.46 | 5.76 | 1.73 | 0.47 | 5.75 | 1.10 | 6.10 | 1.44 | 0.76 |
| C20:5n3 EPA | 0.48 | 0.11 | 0.51 | 0.21 | 0.14 | 0.58 | 0.40 | 0.51 | 0.55 | 0.46 |
| C22:4n6 Docosatetraenoic acid | 0.49 | 0.24 | 0.39 | 0.29 | 0.46 | 0.50 | 0,19 | 0.46 | 0.16 | 0.32 |
| C22:5n3 Docosapentaenoic acid | 0.56 | 0.18 | 0.63 | 0.19 | 0.05 ↑ | 0.52 | 0.18 | 0.62 | 0.33 | 0.008 ↑ |
| C22:6n3 DHA | 1.55 | 0.51 | 1.50 | 0.44 | 0.46 | 1.60 | 0.59 | 1.85 | 0.90 | 0.005 ↑ |
| Eicosanoids (ng/mL) | Intervention with 12 g | p | Intervention with 24 g | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |||
| Resolvine E1 | 0.52 | 0.26 | 0.43 | 0.15 | 0.1 | 0.51 | 0.17 | 0.33 | 0.27 | 0.3 |
| Prostaglandin E2 | 7.42 | 2.84 | 5.25 | 3.78 | 0.017 ↓ | 9.89 | 6.95 | 5.61 | 9.51 | 0.008 ↓ |
| LTX A4 | 3.72 | 8.93 | 4.04 | 6.58 | 0.89 | 5.74 | 3.20 | 7.92 | 11.52 | 0.42 |
| DiHDHA Protectin DX | 0.58 | 0.39 | 0.13 | 0.06 | 0.58 | 0.33 | 0.12 | 0.14 | 0.05 | 0.01 |
| Leucotriene B4 | 0.03 | 0.04 | 0.03 | 0.02 | 0.41 | 0.04 | 0.03 | 0.04 | 0.01 | 0.52 |
| 18 HEPE | 0.11 | 0.08 | 0.09 | 0.05 | 0.54 | 0.16 | 0.07 | 0.09 | 0.05 | 0.3 |
| 13 HODE | 0.08 | 0.06 | 0.10 | 0.04 | 0.78 | 0.09 | 0.07 | 0.13 | 0.11 | 0.36 |
| 9 HODE | 0.06 | 0.03 | 0.09 | 0.07 | 0.27 | 0.10 | 0.05 | 0.12 | 0.08 | 0.23 |
| 15 HETE | 0.63 | 0.30 | 0.73 | 0.62 | 0.41 | 0.75 | 0.47 | 0.70 | 0.41 | 0.63 |
| 17 HDHA | 0.15 | 0.09 | 0.12 | 0.05 | 0.68 | 0.19 | 0.29 | 0.12 | 0.04 | 0.19 |
| 12 HETE | 1.45 | 1.19 | 1.74 | 4.98 | 0.43 | 1.94 | 0.62 | 2.65 | 1.72 | 0.11 |
| 5 HETE | 0.52 | 0.49 | 0.79 | 0.86 | 0.54 | 0.67 | 0.40 | 0.68 | 0.70 | 0.78 |
| 5 oxo ETE | 0.66 | 0.67 | 0.72 | 0.37 | 0.21 | 0.43 | 0.65 | 0.62 | 0.50 | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciejewska-Markiewicz, D.; Drozd, A.; Palma, J.; Ryterska, K.; Hawryłkowicz, V.; Załęska, P.; Wunsh, E.; Kozłowska-Petriczko, K.; Stachowska, E. Fatty Acids and Eicosanoids Change during High-Fiber Diet in NAFLD Patients—Randomized Control Trials (RCT). Nutrients 2022, 14, 4310. https://doi.org/10.3390/nu14204310
Maciejewska-Markiewicz D, Drozd A, Palma J, Ryterska K, Hawryłkowicz V, Załęska P, Wunsh E, Kozłowska-Petriczko K, Stachowska E. Fatty Acids and Eicosanoids Change during High-Fiber Diet in NAFLD Patients—Randomized Control Trials (RCT). Nutrients. 2022; 14(20):4310. https://doi.org/10.3390/nu14204310
Chicago/Turabian StyleMaciejewska-Markiewicz, Dominika, Arleta Drozd, Joanna Palma, Karina Ryterska, Viktoria Hawryłkowicz, Patrycja Załęska, Ewa Wunsh, Katarzyna Kozłowska-Petriczko, and Ewa Stachowska. 2022. "Fatty Acids and Eicosanoids Change during High-Fiber Diet in NAFLD Patients—Randomized Control Trials (RCT)" Nutrients 14, no. 20: 4310. https://doi.org/10.3390/nu14204310
APA StyleMaciejewska-Markiewicz, D., Drozd, A., Palma, J., Ryterska, K., Hawryłkowicz, V., Załęska, P., Wunsh, E., Kozłowska-Petriczko, K., & Stachowska, E. (2022). Fatty Acids and Eicosanoids Change during High-Fiber Diet in NAFLD Patients—Randomized Control Trials (RCT). Nutrients, 14(20), 4310. https://doi.org/10.3390/nu14204310







