Effect of Casein Hydrolysate on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection and Selection Criteria
2.3. Data Extraction
2.4. Risk of Bias
2.5. Statistical Analysis
3. Results
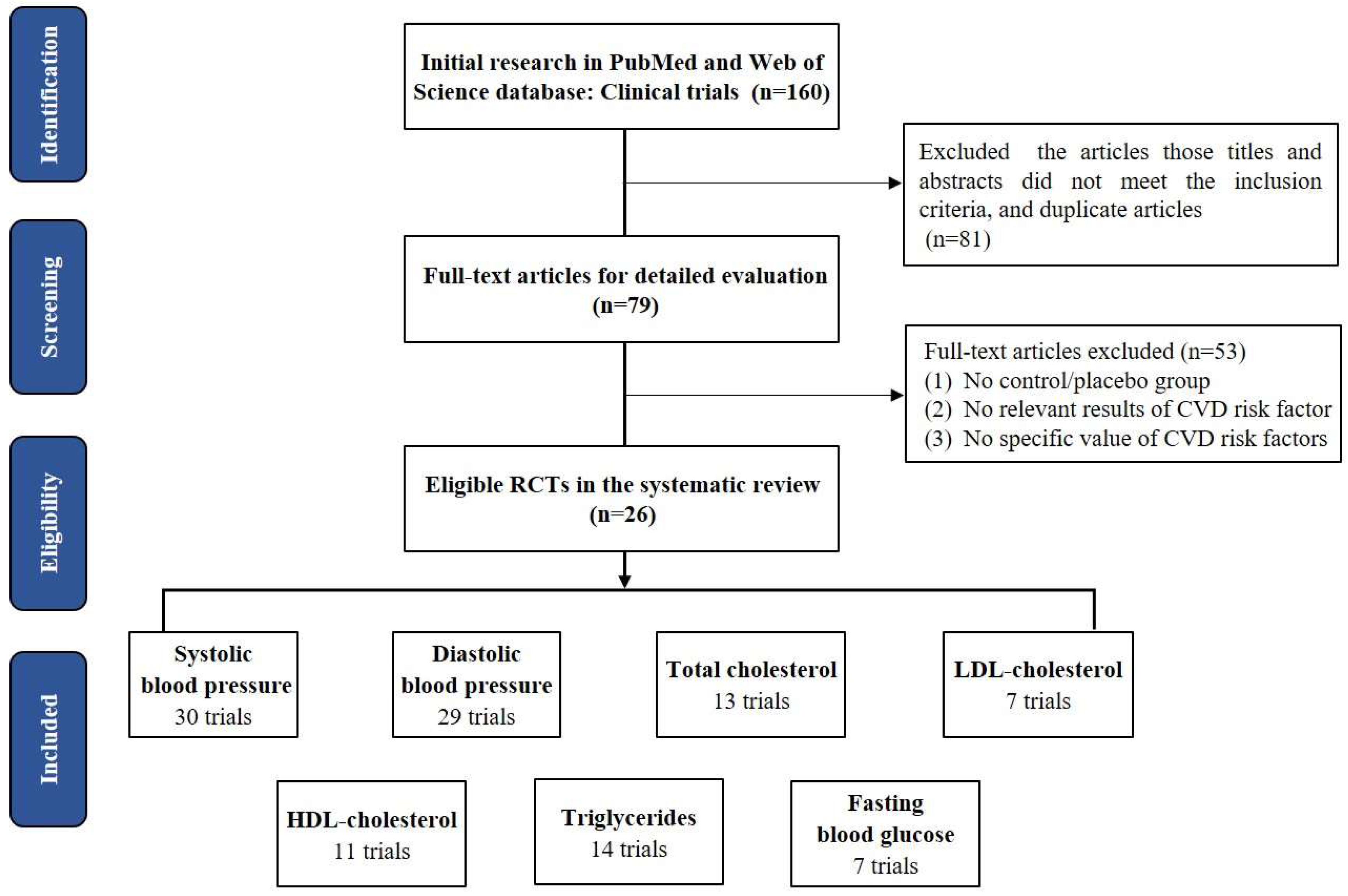
3.1. Study Selection and Study Characteristics
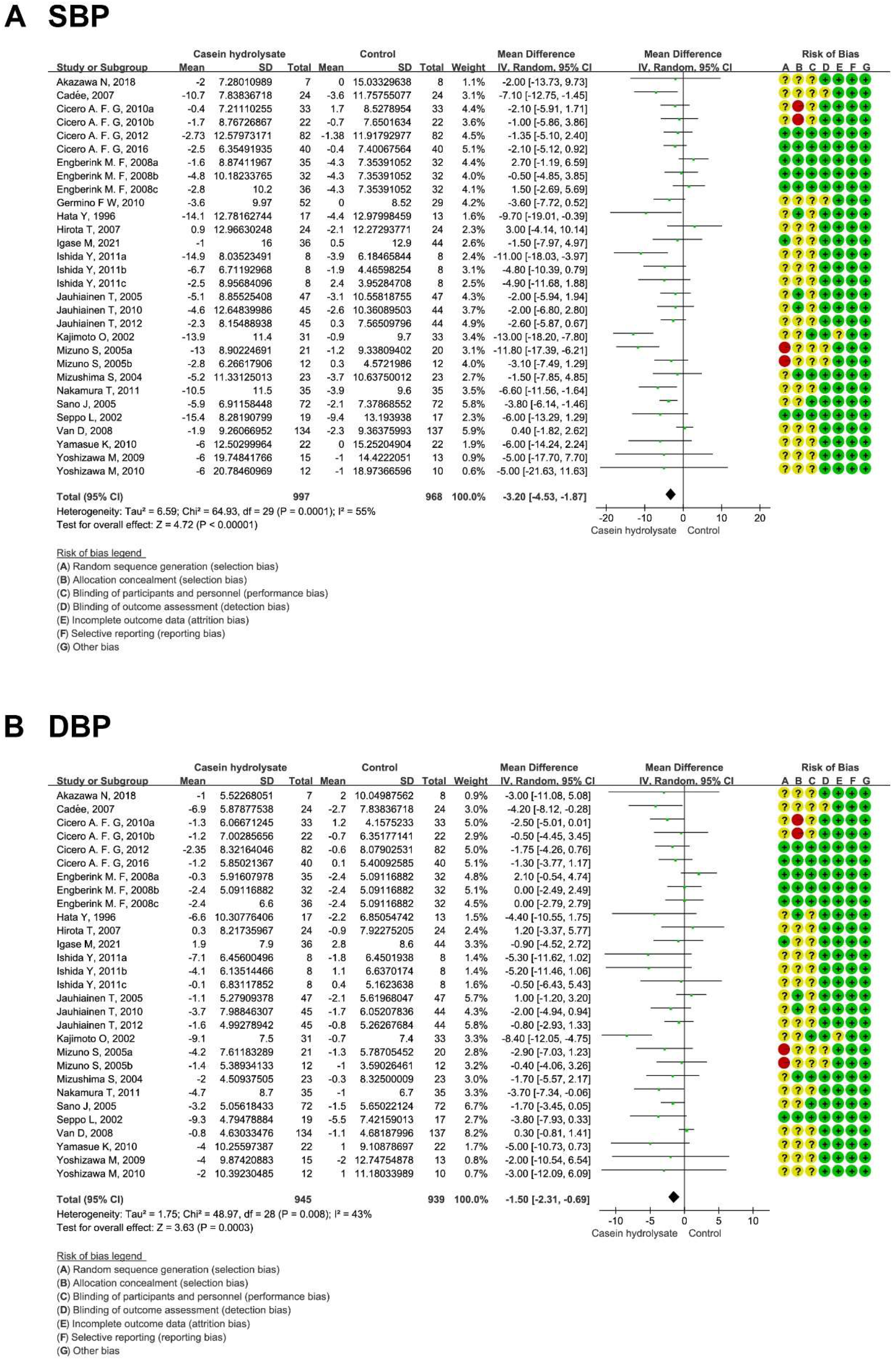
3.2. Effects of Casein Hydrolysate on BP
3.3. Effects of Casein Hydrolysate on Blood Lipids
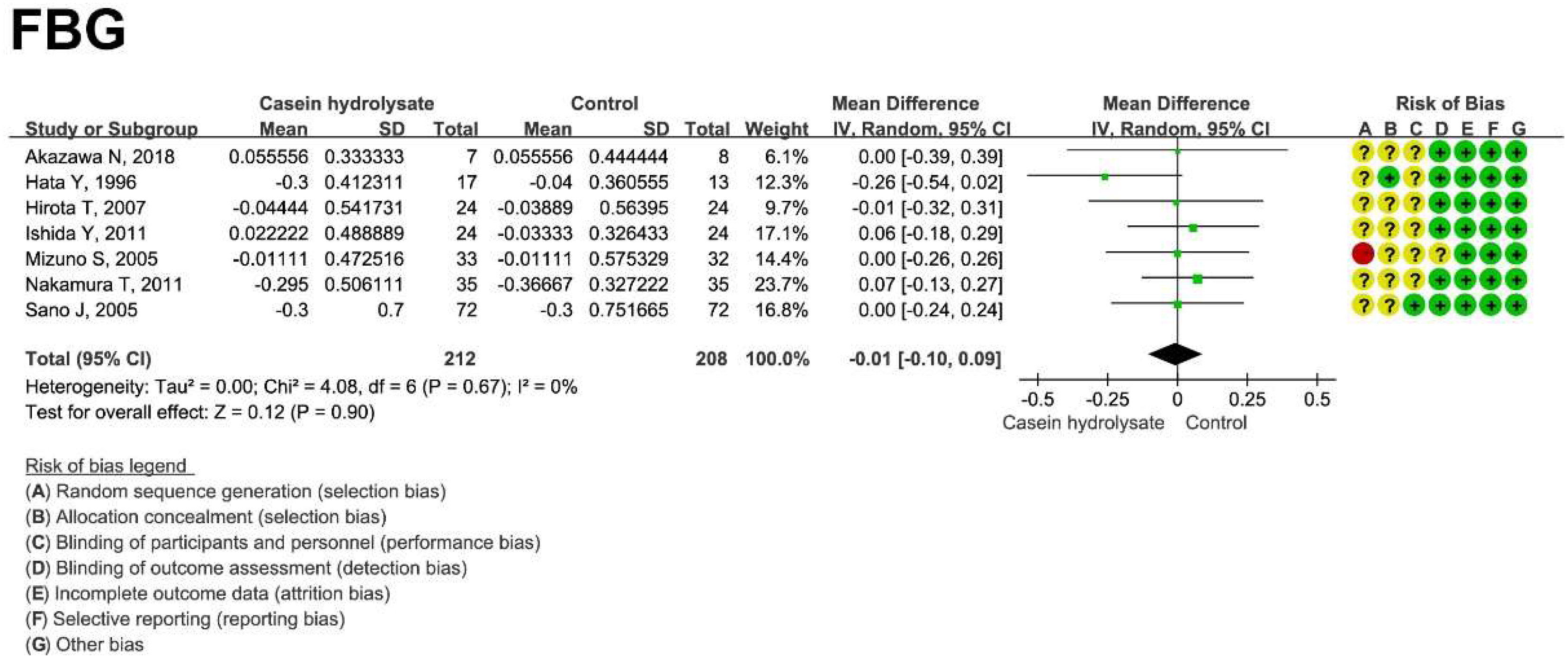
3.4. Effect of Casein Hydrolysate on FBG
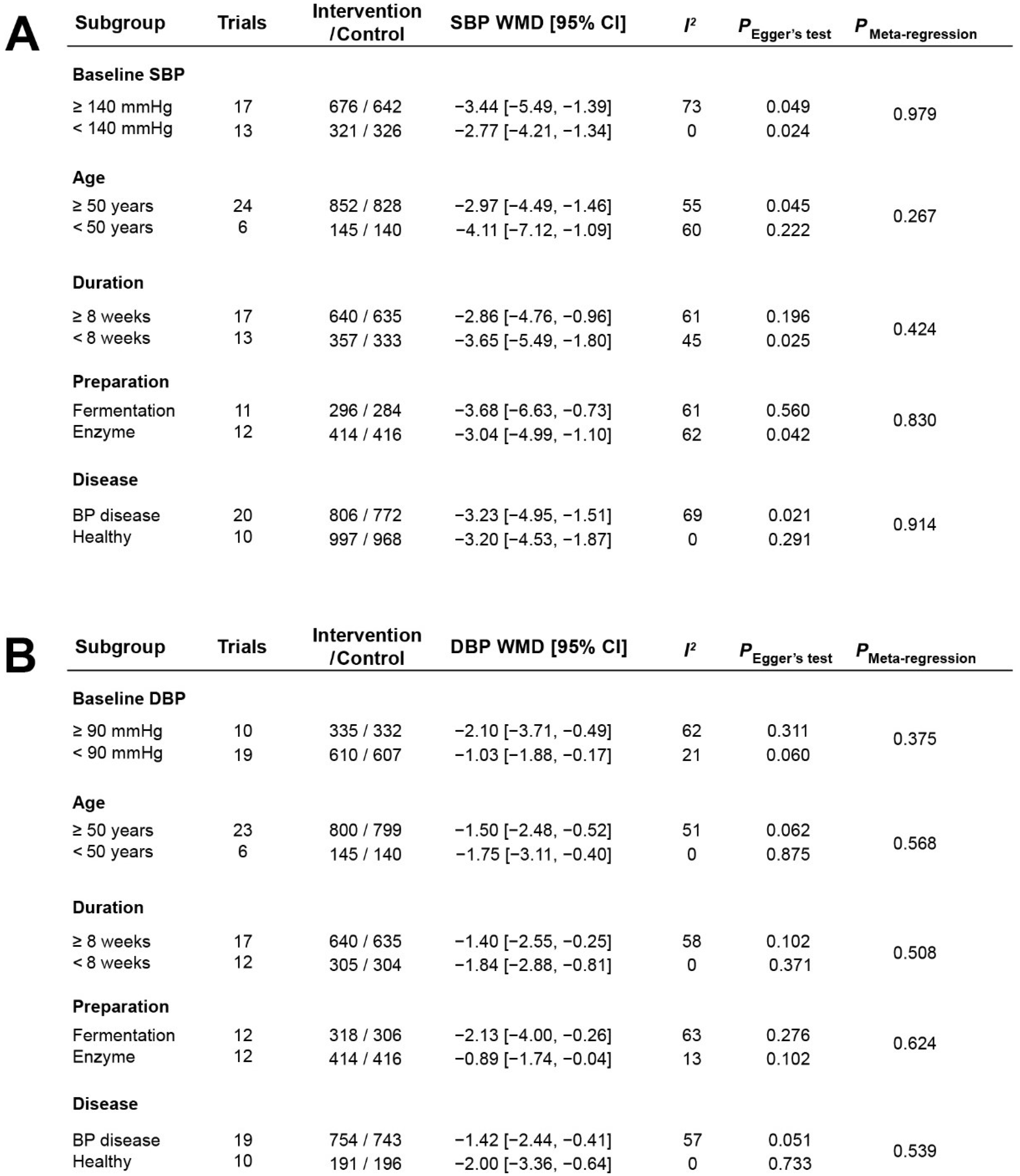
3.5. Stratified Analysis of the Effects of Casein Hydrolysate on BP
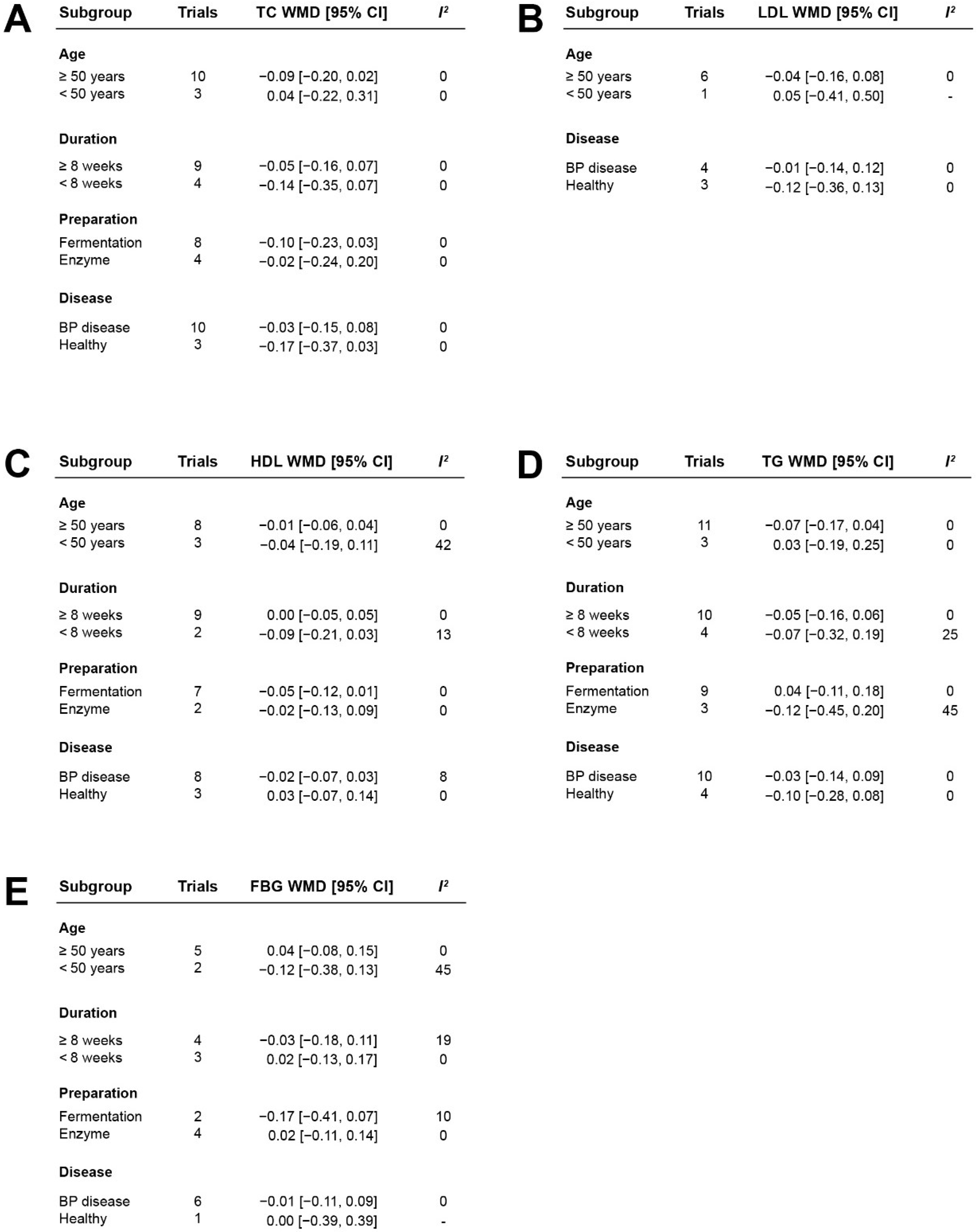
3.6. Stratified Analysis of the Effects of Casein Hydrolysate on Blood Lipids and Blood Glucose
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- World Health Statistics 2021: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2021.
- Tu, M.; Qiao, X.; Wang, C.; Liu, H.; Cheng, S.; Xu, Z.; Du, M. In Vitro and in Silico Analysis of Dual-Function Peptides Derived from Casein Hydrolysate. Food Sci. Hum. Wellness 2021, 10, 32–37. [Google Scholar] [CrossRef]
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.-D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 1: The Epidemiology and Risk Factors. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; van Horn, L.; Wylie-Rosett, J.; et al. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e472–e487. [Google Scholar] [CrossRef]
- Asgary, S.; Rastqar, A.; Keshvari, M. Functional Food and Cardiovascular Disease Prevention and Treatment: A Review. J. Am. Coll. Nutr. 2018, 37, 429–455. [Google Scholar] [CrossRef] [PubMed]
- Naghshi, S.; Sadeghi, O.; Willett, W.C.; Esmaillzadeh, A. Dietary Intake of Total, Animal, and Plant Proteins and Risk of All Cause, Cardiovascular, and Cancer Mortality: Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. BMJ 2020, 370, m2412. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wofford, M.R.; Reynolds, K.; Chen, J.; Chen, C.-S.; Myers, L.; Minor, D.L.; Elmer, P.J.; Jones, D.W.; Whelton, P.K. Effect of Dietary Protein Supplementation on Blood Pressure: A Randomized, Controlled Trial. Circulation 2011, 124, 589–595. [Google Scholar] [CrossRef]
- Fekete, Á.A.; Giromini, C.; Chatzidiakou, Y.; Givens, D.I.; Lovegrove, J.A. Whey Protein Lowers Blood Pressure and Improves Endothelial Function and Lipid Biomarkers in Adults with Prehypertension and Mild Hypertension: Results from the Chronic Whey2Go Randomized Controlled Trial. Am. J. Clin. Nutr. 2016, 104, 1534–1544. [Google Scholar] [CrossRef]
- Tu, M.; Wang, C.; Chen, C.; Zhang, R.; Liu, H.; Lu, W.; Jiang, L.; Du, M. Identification of a Novel ACE-Inhibitory Peptide from Casein and Evaluation of the Inhibitory Mechanisms. Food Chem. 2018, 256, 98–104. [Google Scholar] [CrossRef]
- Koury, O.H.; Scheede-Bergdahl, C.; Bergdahl, A. The Role of Casein in the Development of Hypercholesterolemia. J. Physiol. Biochem. 2014, 70, 1021–1028. [Google Scholar] [CrossRef]
- Hu, F.B. Protein, Body Weight, and Cardiovascular Health. Am. J. Clin. Nutr. 2005, 82 (Suppl. S1), 242S–247S. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Bioactive Peptides: Production and Functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Sowmya, K.; Bhat, M.I.; Bajaj, R.K.; Kapila, S.; Kapila, R. Buffalo Milk Casein Derived Decapeptide (YQEPVLGPVR) Having Bifunctional Anti-inflammatory and Antioxidative Features Under Cellular Milieu. Int. J. Pept. Res. Ther. 2019, 25, 623–633. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Ahmed, A.S.; Miyata, T. Novel Angiotensin-Converting Enzyme Inhibitory Peptides from Caseins and Whey Proteins of Goat Milk. J. Adv. Res. 2017, 8, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Liu, H.; Cheng, S.; Xu, Z.; Wang, L.-S.; Du, M. Identification and Analysis of Transepithelial Transport Properties of Casein Peptides with Anticoagulant and ACE Inhibitory Activities. Food Res. Int. 2020, 138 Pt A, 109764. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic Inflammation in Ageing, Cardiovascular Disease, and Frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Wen, Z.-J.; Wang, Z.-Y.; Zhang, Y.-F. Adverse Cardiovascular Effects and Potential Molecular Mechanisms of DEHP and Its Metabolites-A Review. Sci. Total Environ. 2022, 847, 157443. [Google Scholar] [CrossRef]
- Sánchez, D.; Kassan, M.; Contreras, M.D.M.; Carrón, R.; Recio, I.; Montero, M.-J.; Sevilla, M.-Á. Long-Term Intake of a Milk Casein Hydrolysate Attenuates the Development of Hypertension and Involves Cardiovascular Benefits. Pharmacol. Res. 2011, 63, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, J.; Klempt, M.; Clawin-Rädecker, I.; Lorenzen, P.C.; Meisel, H. Identification of a NFκB Inhibitory Peptide from Tryptic β-Casein Hydrolysate. Food Chem. 2014, 165, 129–133. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Lorenzo, O.; Suzuki, Y.; Rupérez, M.; Egido, J. Proinflammatory Actions of Angiotensins. Curr. Opin. Nephrol. Hypertens. 2001, 10, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Hayden, M.S. Celebrating 25 Years of NF-ΚB Research. Immunol. Rev. 2012, 246, 5–13. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, L.; Su, G.; Zeng, X.-A.; Sun, B.; Zhao, M. Evaluation and Exploration of Potentially Bioactive Peptides in Casein Hydrolysates against Liver Oxidative Damage in STZ/HFD-Induced Diabetic Rats. J. Agric. Food Chem. 2020, 68, 2393–2405. [Google Scholar] [CrossRef]
- Rao, P.S.; Bajaj, R.; Mann, B. Impact of Sequential Enzymatic Hydrolysis on Antioxidant Activity and Peptide Profile of Casein Hydrolysate. J. Food Sci. Technol. 2020, 57, 4562–4575. [Google Scholar] [CrossRef]
- Shazly, A.B.; Mu, H.; Liu, Z.; El-Aziz, M.A.; Zeng, M.; Qin, F.; Zhang, S.; He, Z.; Chen, J. Release of Antioxidant Peptides from Buffalo and Bovine Caseins: Influence of Proteases on Antioxidant Capacities. Food Chem. 2019, 274, 261–267. [Google Scholar] [CrossRef]
- Van der Zander, K.; Bots, M.L.; Bak, A.A.A.; Koning, M.M.G.; de Leeuw, P.W. Enzymatically Hydrolyzed Lactotripeptides Do Not Lower Blood Pressure in Mildly Hypertensive Subjects. Am. J. Clin. Nutr. 2008, 88, 1697–1702. [Google Scholar] [CrossRef]
- Ishida, Y.; Shibata, Y.; Fukuhara, I.; Yano, Y.; Takehara, I.; Kaneko, K. Effect of an Excess Intake of Casein Hydrolysate Containing Val-Pro-Pro and Ile-Pro-Pro in Subjects with Normal Blood Pressure, High-Normal Blood Pressure, or Mild Hypertension. Biosci. Biotechnol. Biochem. 2011, 75, 427–433. [Google Scholar] [CrossRef]
- Fekete, Á.A.; Givens, D.I.; Lovegrove, J.A. Casein-Derived Lactotripeptides Reduce Systolic and Diastolic Blood Pressure in a Meta-Analysis of Randomised Clinical Trials. Nutrients 2015, 7, 659–681. [Google Scholar] [CrossRef]
- Turpeinen, A.M.; Järvenpää, S.; Kautiainen, H.; Korpela, R.; Vapaatalo, H. Antihypertensive Effects of Bioactive Tripeptides-a Random Effects Meta-Analysis. Ann. Med. 2013, 45, 51–56. [Google Scholar] [CrossRef]
- Qin, L.-Q.; Xu, J.-Y.; Dong, J.-Y.; Zhao, Y.; van Bladeren, P.; Zhang, W. Lactotripeptides Intake and Blood Pressure Management: A Meta-Analysis of Randomised Controlled Clinical Trials. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 395–402. [Google Scholar] [CrossRef]
- Chanson-Rolle, A.; Aubin, F.; Braesco, V.; Hamasaki, T.; Kitakaze, M. Influence of the Lactotripeptides Isoleucine-Proline-Proline and Valine-Proline-Proline on Systolic Blood Pressure in Japanese Subjects: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2015, 10, e0142235. [Google Scholar] [CrossRef]
- Stewart, L.A.; Tierney, J.F.; Clarke, M. Reviews of Individual Patient Data. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley and Sons, Ltd.: Chichester, UK, 2008; pp. 547–558. [Google Scholar]
- Jackson, D.; White, I.R.; Riley, R.D. Quantifying the Impact of Between-Study Heterogeneity in Multivariate Meta-Analyses. Stat. Med. 2012, 31, 3805–3820. [Google Scholar] [CrossRef] [PubMed]
- Engberink, M.F.; Schouten, E.G.; Kok, F.J.; van Mierlo, L.A.J.; Brouwer, I.A.; Geleijnse, J.M. Lactotripeptides Show No Effect on Human Blood Pressure: Results from a Double-Blind Randomized Controlled Trial. Hypertension 2008, 51, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, M.T.; Nakamura, Y.; Yamamoto, N.; Yamada, K.; Iketani, T. Effects of Val-Pro-Pro and Ile-Pro-Pro on Nondipper Patients: A Preliminary Study. J. Med. Food 2011, 14, 538–542. [Google Scholar] [CrossRef]
- Igase, M.; Okada, Y.; Igase, K.; Matsumoto, S.; Senzaki, K.; Ochi, M.; Ohyagi, Y.; Yamagishi, S.-I. Casein Hydrolysate Containing Milk-Derived Peptides Reduces Facial Pigmentation Partly by Decreasing Advanced Glycation End Products in the Skin: A Randomized Double-Blind Placebo-Controlled Trial. Rejuvenation Res. 2021, 24, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, N.; Hamasaki, A.; Tanahashi, K.; Kosaki, K.; Yoshikawa, T.; Myoenzono, K.; Maeda, S. Lactotripeptide Ingestion Increases Cerebral Blood Flow Velocity in Middle-Aged and Older Adults. Nutr. Res. 2018, 53, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Hove, K.D.; Brøns, C.; Færch, K.; Lund, S.S.; Rossing, P.; Vaag, A. Effects of 12 Weeks of Treatment with Fermented Milk on Blood Pressure, Glucose Metabolism and Markers of Cardiovascular Risk in Patients with Type 2 Diabetes: A Randomised Double-Blind Placebo-Controlled Study. Eur. J. Endocrinol. 2015, 172, 11–20. [Google Scholar] [CrossRef]
- Usinger, L.; Ibsen, H.; Linneberg, A.; Azizi, M.; Flambard, B.; Jensen, L.T. Human in Vivo Study of the Renin-Angiotensin-Aldosterone System and the Sympathetic Activity after 8 Weeks Daily Intake of Fermented Milk. Clin. Physiol. Funct. Imaging 2010, 30, 162–168. [Google Scholar] [CrossRef]
- Sánchez-Rivera, L.; Ferreira Santos, P.; Sevilla, M.A.; Montero, M.J.; Recio, I.; Miralles, B. Implication of Opioid Receptors in the Antihypertensive Effect of a Bovine Casein Hydrolysate and As1-Casein-Derived Peptides. J. Agric. Food Chem. 2020, 68, 1877–1883. [Google Scholar] [CrossRef]
- Schättin, A.; Baur, K.; Stutz, J.; Wolf, P.; de Bruin, E.D. Effects of Physical Exercise Combined with Nutritional Supplements on Aging Brain Related Structures and Functions: A Systematic Review. Front. Aging Neurosci. 2016, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Fekete, Á.A.; Giromini, C.; Chatzidiakou, Y.; Givens, D.I.; Lovegrove, J.A. Whey Protein Lowers Systolic Blood Pressure and Ca-Caseinate Reduces Serum TAG after a High-Fat Meal in Mildly Hypertensive Adults. Sci. Rep. 2018, 8, 5026. [Google Scholar] [CrossRef]
- Chin-Dusting, J.; Shennan, J.; Jones, E.; Williams, C.; Kingwell, B.; Dart, A. Effect of Dietary Supplementation with Beta-Casein A1 or A2 on Markers of Disease Development in Individuals at High Risk of Cardiovascular Disease. Br. J. Nutr. 2006, 95, 136–144. [Google Scholar] [CrossRef]
- Mariotti, F.; Valette, M.; Lopez, C.; Fouillet, H.; Famelart, M.-H.; Mathé, V.; Airinei, G.; Benamouzig, R.; Gaudichon, C.; Tomé, D.; et al. Casein Compared with Whey Proteins Affects the Organization of Dietary Fat during Digestion and Attenuates the Postprandial Triglyceride Response to a Mixed High-Fat Meal in Healthy, Overweight Men. J. Nutr. 2015, 145, 2657–2664. [Google Scholar] [CrossRef]
- Pal, S.; Ellis, V. The Chronic Effects of Whey Proteins on Blood Pressure, Vascular Function, and Inflammatory Markers in Overweight Individuals. Obesity 2010, 18, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Pan, D.; Zhen, X.; Cao, J. Angiotensin I-Converting Enzyme Inhibitory Peptides Derived from Bovine Casein and Identified by MALDI-TOF-MS/MS. J. Sci. Food Agric. 2013, 93, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Zhang, F.; Ma, Z.; Chen, S.; Ding, G.; Tian, X.; Feng, R. Isolation and Identification of the Angiotensin-I Converting Enzyme (ACE) Inhibitory Peptides Derived from Cottonseed Protein: Optimization of Hydrolysis Conditions. Int. J. Food Prop. 2019, 22, 1296–1309. [Google Scholar] [CrossRef]
- Riordan, J.F. Angiotensin-I-Converting Enzyme and Its Relatives. Genome Biol. 2003, 4, 225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- López-Fandiño, R.; Otte, J.; van Camp, J. Physiological, Chemical and Technological Aspects of Milk-Protein-Derived Peptides with Antihypertensive and ACE-Inhibitory Activity. Int. Dairy J. 2006, 16, 1277–1293. [Google Scholar] [CrossRef]
- Contreras, M.D.M.; Carrón, R.; Montero, M.J.; Ramos, M.; Recio, I. Novel Casein-Derived Peptides with Antihypertensive Activity. Int. Dairy J. 2009, 19, 566–573. [Google Scholar] [CrossRef]
- Ponstein-Simarro Doorten, A.Y.; vd Wiel, J.a.G.; Jonker, D. Safety Evaluation of an IPP Tripeptide-Containing Milk Protein Hydrolysate. Food Chem. Toxicol. 2009, 47, 55–61. [Google Scholar] [CrossRef]
- Dent, M.P.; O’Hagan, S.; Braun, W.H.; Schaetti, P.; Marburger, A.; Vogel, O. A 90-Day Subchronic Toxicity Study and Reproductive Toxicity Studies on ACE-Inhibiting Lactotripeptide. Food Chem. Toxicol. 2007, 45, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Mennear, J.H.; Matsuura, K.; Bernard, B.K. Studies of the Toxicological Potential of Tripeptides (L-Valyl-L-Prolyl-L-Proline and L-Isoleucyl-L-Prolyl-L-Proline): V. A 13-Week Toxicity Study of Tripeptides-Containing Casein Hydrolysate in Male and Female Rats. Int. J. Toxicol. 2005, 24 (Suppl. S4), 41–59. [Google Scholar] [CrossRef]
- Sano, J.; Ohki, K.; Higuchi, T.; Aihara, K.; Mizuno, S.; Kajimoto, O.; Nakagawa, S.; Kajimoto, Y.; Nakamura, Y. Effect of Casein Hydrolysate, Prepared with Protease Derived from Aspergillus Oryzae, on Subjects with High-Normal Blood Pressure or Mild Hypertension. J. Med. Food 2005, 8, 423–430. [Google Scholar] [CrossRef]
- Duffuler, P.; Bhullar, K.S.; de Campos Zani, S.C.; Wu, J. Bioactive Peptides: From Basic Research to Clinical Trials and Commercialization. J. Agric. Food Chem. 2022, 70, 3585–3595. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Gerocarni, B.; Laghi, L.; Borghi, C. Blood Pressure Lowering Effect of Lactotripeptides Assumed as Functional Foods: A Meta-Analysis of Current Available Clinical Trials. J. Hum. Hypertens. 2011, 25, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Aubin, F.; Azais-Braesco, V.; Borghi, C. Do the Lactotripeptides Isoleucine-Proline-Proline and Valine-Proline-Proline Reduce Systolic Blood Pressure in European Subjects? A Meta-Analysis of Randomized Controlled Trials. Am. J. Hypertens. 2013, 26, 442–449. [Google Scholar] [CrossRef]
- Jauhiainen, T.; Niittynen, L.; Orešič, M.; Järvenpää, S.; Hiltunen, T.P.; Rönnback, M.; Vapaatalo, H.; Korpela, R. Effects of Long-Term Intake of Lactotripeptides on Cardiovascular Risk Factors in Hypertensive Subjects. Eur. J. Clin. Nutr. 2012, 66, 843–849. [Google Scholar] [CrossRef]
- Lillefosse, H.H.; Tastesen, H.S.; Du, Z.-Y.; Ditlev, D.B.; Thorsen, F.A.; Madsen, L.; Kristiansen, K.; Liaset, B. Hydrolyzed Casein Reduces Diet-Induced Obesity in Male C57BL/6J Mice. J. Nutr. 2013, 143, 1367–1375. [Google Scholar] [CrossRef]
- O’Halloran, F.; Bruen, C.; McGrath, B.; Schellekens, H.; Murray, B.; Cryan, J.F.; Kelly, A.L.; McSweeney, P.L.H.; Giblin, L. A Casein Hydrolysate Increases GLP-1 Secretion and Reduces Food Intake. Food Chem. 2018, 252, 303–310. [Google Scholar] [CrossRef]
- Cadée, J.A.; Chang, C.-Y.; Chen, C.-W.; Huang, C.-N.; Chen, S.-L.; Wang, C.-K. Bovine Casein Hydrolysate (C12 Peptide) Reduces Blood Pressure in Prehypertensive Subjects. Am. J. Hypertens. 2007, 20, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Rosticci, M.; Ferroni, A.; Bacchelli, S.; Veronesi, M.; Strocchi, E.; Borghi, C. Predictors of the Short-Term Effect of Isoleu-cine-Proline-Proline/Valine-Proline-Proline Lactotripeptides from Casein on Office and Ambulatory Blood Pressure in Subjects with Pharmacologically Untreated High-Normal Blood Pressure or First-Degree Hypertension. Clin. Exp. Hypertens. 2012, 34, 601–605. [Google Scholar] [CrossRef]
- Germino, F.W.; Neutel, J.; Nonaka, M.; Hendler, S.S. The Impact of Lactotripeptides on Blood Pressure Response in Stage 1 and Stage 2 Hypertensives. J. Clin. Hypertens. 2010, 12, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Matsuura, K.; Gotou, T.; Nishimura, S.; Kajimoto, O.; Yabune, M.; Kajimoto, Y.; Yamamoto, N. Antihypertensive Effect of Casein Hydrolysate in a Placebo-Controlled Study in Subjects with High-Normal Blood Pressure and Mild Hypertension. Br. J. Nutr. 2005, 94, 84–91. [Google Scholar] [CrossRef]
- Nakamura, T.; Mizutani, J.; Ohki, K.; Yamada, K.; Yamamoto, N.; Takeshi, M.; Takazawa, K. Casein Hydrolysate Containing Val-Pro-Pro and Ile-Pro-Pro Improves Central Blood Pressure and Arterial Stiffness in Hypertensive Subjects: A Randomized, Double-Blind, Placebo-Controlled Trial. Atherosclerosis 2011, 219, 298–303. [Google Scholar] [CrossRef]
- Usinger, L.; Jensen, L.T.; Flambard, B.; Linneberg, A.; Ibsen, H. The Antihypertensive Effect of Fermented Milk in Individuals with Prehypertension or Borderline Hypertension. J. Hum. Hypertens. 2010, 24, 678–683. [Google Scholar] [CrossRef][Green Version]
- Yoshizawa, M.; Maeda, S.; Miyaki, A.; Misono, M.; Choi, Y.; Shimojo, N.; Ajisaka, R.; Tanaka, H. Additive Beneficial Effects of Lactotripeptides and Aerobic Exercise on Arterial Compliance in Postmenopausal Women. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1899–H1903. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Maeda, S.; Miyaki, A.; Misono, M.; Choi, Y.; Shimojo, N.; Ajisaka, R.; Tanaka, H. Additive Beneficial Effects of Lactotripeptides In-take with Regular Exercise on Endothelium-Dependent Dilatation in Postmenopausal Women. Am. J. Hypertens. 2010, 23, 368–372. [Google Scholar] [CrossRef]
- Kajimoto, O.; Kurosaki, T.; Mizutani, J.; Ikeda, N.; Kaneko, K.; Aihara, K.; Yabune, M.; Nakamura, Y. Antihypertensive Effects of Liquid Yogurts Containing “Lactotripeptides (VPP, IPP)” in Mild Hypertensive Subjects. J. Nutr. Food 2002, 5, 55–66. [Google Scholar]
- Hata, Y.; Yamamoto, M.; Ohni, M.; Nakajima, K.; Nakamura, Y.; Takano, T. A Placebo-Controlled Study of the Effect of Sour Milk on Blood Pressure in Hypertensive Subjects. Am. J. Clin. Nutr. 1996, 64, 767–771. [Google Scholar] [CrossRef]
- Jauhiainen, T.; Vapaatalo, H.; Poussa, T.; Kyrönpalo, S.; Rasmussen, M.; Korpela, R. Lactobacillus Helveticus Fermented Milk Lowers Blood Pressure in Hypertensive Subjects in 24-h Ambulatory Blood Pressure Measurement. Am. J. Hypertens. 2005, 18, 1600–1605. [Google Scholar] [CrossRef]
- Jauhiainen, T.; Rönnback, M.; Vapaatalo, H.; Wuolle, K.; Kautiainen, H.; Groop, P.-H.; Korpela, R. Long-Term Intervention with Lactobacillus Helveticus Fermented Milk Reduces Augmentation Index in Hypertensive Subjects. Eur. J. Clin. Nutr. 2010, 64, 424–431. [Google Scholar] [CrossRef]
- Mizushima, S.; Ohshige, K.; Watanabe, J.; Kimura, M.; Kadowaki, T.; Nakamura, Y.; Tochikubo, O.; Ueshima, H. Randomized Controlled Trial of Sour Milk on Blood Pressure in Borderline Hypertensive Men. Am. J. Hypertens. 2004, 17, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Seppo, L.; Jauhiainen, T.; Poussa, T.; Korpela, R. A Fermented Milk High in Bioactive Peptides Has a Blood Pressure-Lowering Effect in Hypertensive Subjects. Am. J. Clin. Nutr. 2003, 77, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chiu, H.; Lu, Y.; Han, Y.-C.; Shen, Y.; Kamesh, V.; Golovinskaia, O.; Wang, C.-K. Efficacy of Fermented Goat Milk on Blood Pressure in Pre-hypertensive Adults: A Randomized, Placebo-controlled, Clinical Trial. J. Food Biochem. 2017, 42, e12474. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A.; Rosticci, M.; Cagnati, M.; Urso, R.; Giovannini, M.; Borghi, C.; D’Addato, S. Effect of Lactotripeptides (Isoleu-cine-Proline-Proline/Valine-Proline-Proline) on Blood Pressure and Arterial Stiffness Changes in Subjects with Suboptimal Blood Pressure Control and Metabolic Syndrome: A Double-Blind, Randomized, Crossover Clinical Trial. Metab. Syndr. Relat. Disord. 2016, 14, 161–166. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Rosticci, M.; Veronesi, M.; Bacchelli, S.; Strocchi, E.; Melegari, C.; Grandi, E.; Borghi, C. Hemodynamic Effects of Lactotripeptides from Casein Hydrolysate in Mediterranean Normotensive Subjects and Patients with High-Normal Blood Pressure: A Randomized, Double-Blind, Crossover Clinical Trial. J. Med. Food. 2010, 13, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Hirota, T.; Ohki, K.; Kawagishi, R.; Kajimoto, Y.; Mizuno, S.; Nakamura, Y.; Kitakaze, M. Casein Hydrolysate Containing the Antihypertensive Tripep-tides Val-Pro-Pro and Ile-Pro-Pro Improves Vascular Endothelial Function Independent of Blood Pressure-Lowering Effects: Contribution of the Inhibi-tory Action of Angiotensin-Converting Enzyme. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2007, 30, 489–496. [Google Scholar] [CrossRef]
- Yamasue, K.; Morikawa, N.; Mizushima, S.; Tochikubo, O. The Blood Pressure Lowering Effect of Lactotripeptides and Salt Intake in 24-h Ambulatory Blood Pressure Measurements. Clin. Exp. Hypertens. 2010, 32, 214–220. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Xu, T.; Zhang, X.; Luo, J.; An, P.; Luo, Y. Effect of Casein Hydrolysate on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 4207. https://doi.org/10.3390/nu14194207
Zhou S, Xu T, Zhang X, Luo J, An P, Luo Y. Effect of Casein Hydrolysate on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2022; 14(19):4207. https://doi.org/10.3390/nu14194207
Chicago/Turabian StyleZhou, Shuaishuai, Teng Xu, Xu Zhang, Junjie Luo, Peng An, and Yongting Luo. 2022. "Effect of Casein Hydrolysate on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 14, no. 19: 4207. https://doi.org/10.3390/nu14194207
APA StyleZhou, S., Xu, T., Zhang, X., Luo, J., An, P., & Luo, Y. (2022). Effect of Casein Hydrolysate on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 14(19), 4207. https://doi.org/10.3390/nu14194207









