Geraniol Treatment for Irritable Bowel Syndrome: A Double-Blind Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Treatment and Randomization
2.3. Study Outcomes
2.4. Study Visits
2.5. Gut Microbiota and Inflammatory Evaluations
2.6. Statistical Analyses
3. Results
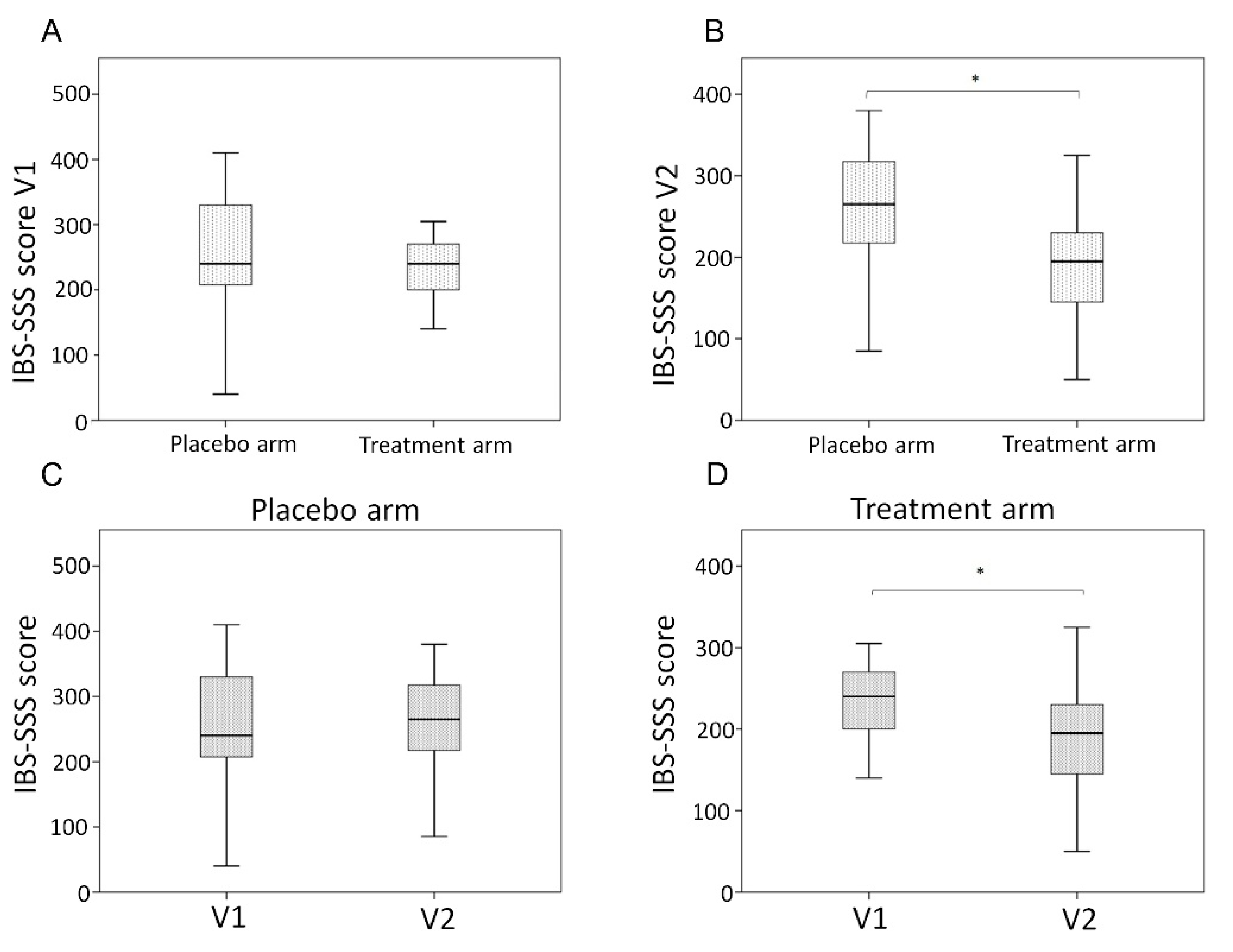
3.1. Clinical Characteristics and Outcomes
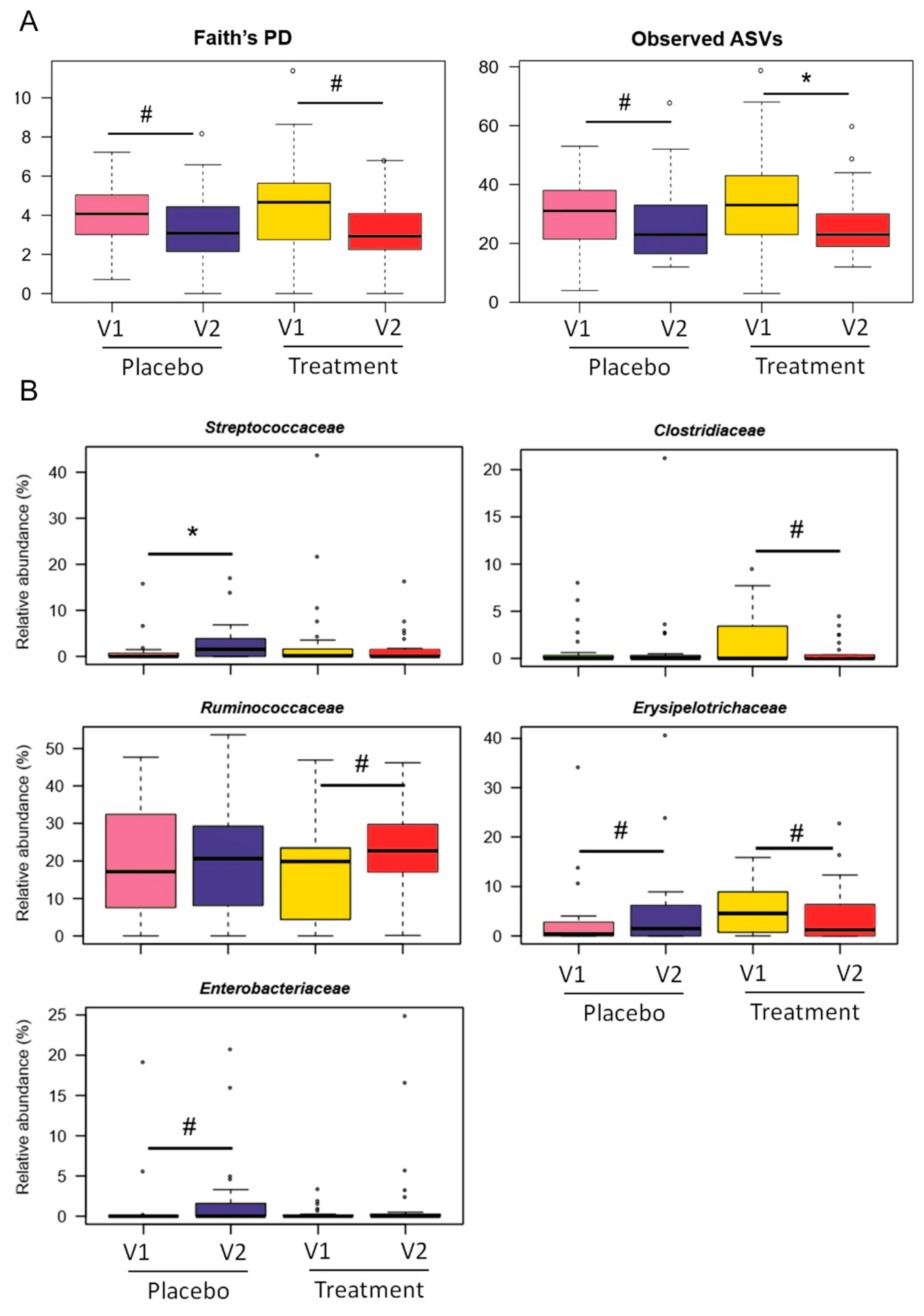
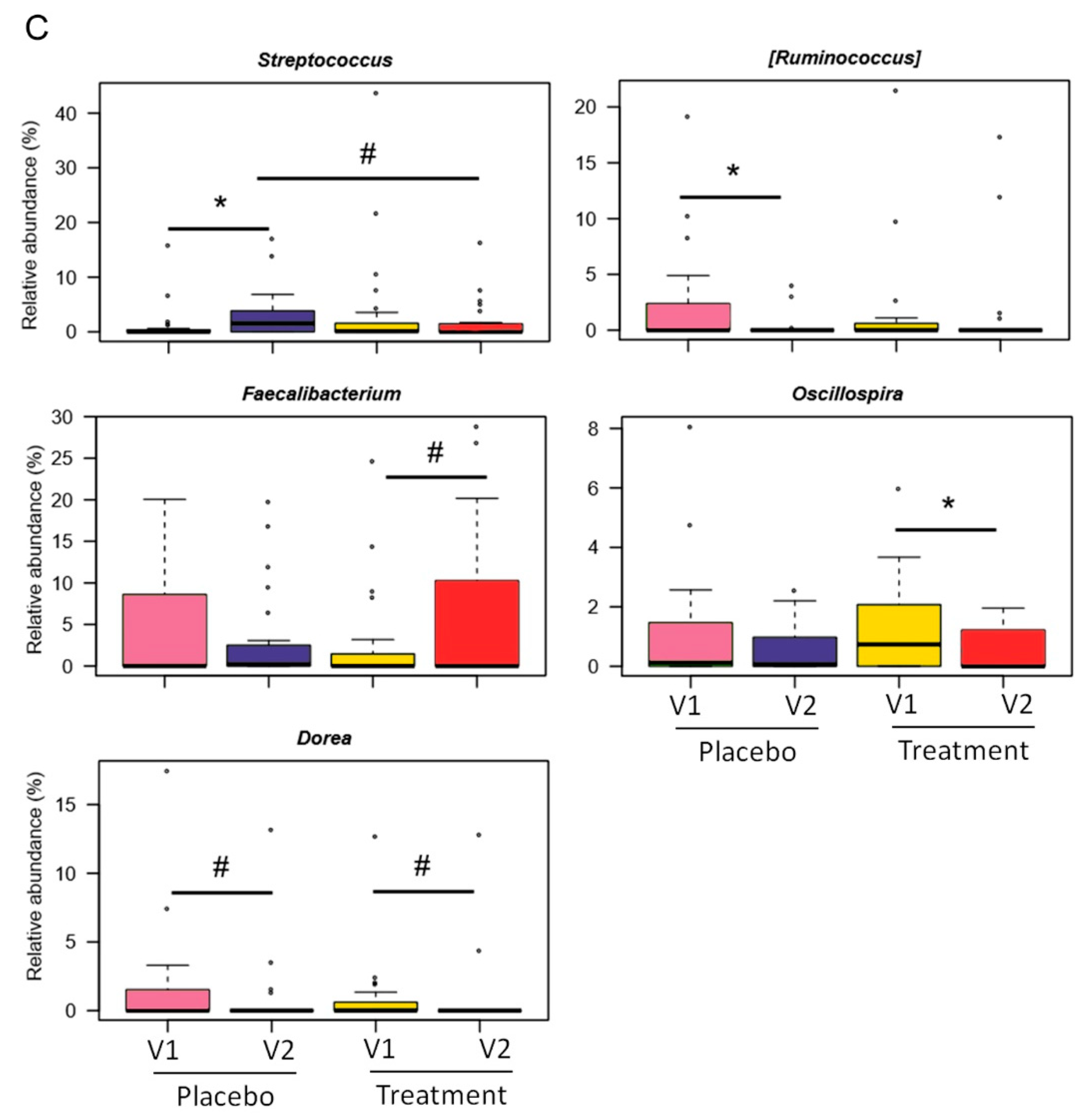
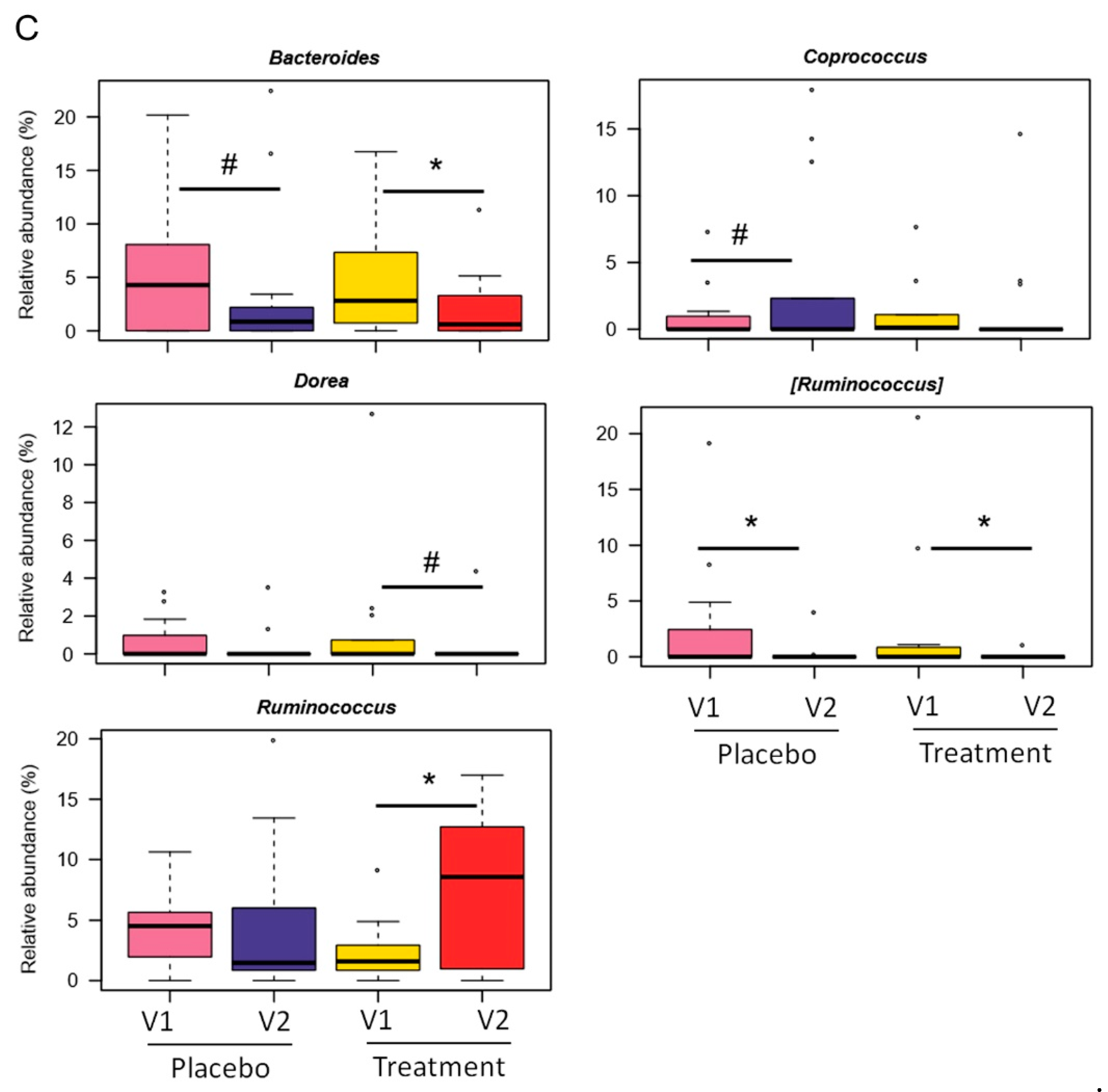
3.2. Gut Microbiota Modulation
3.3. Inflammation and Intestinal Permeability Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef] [PubMed]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar]
- Radovanovic-Dinic, B.; Tesic-Rajkovic, S.; Grgov, S.; Petrovic, G.; Zivkovic, V. Irritable bowel syndrome—From etiopathogenesis to therapy. Biomed Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2018, 162, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.R.; Melkus, G.D.; Henderson, W.A. Irritable bowel syndrome. Am. J. Nurs. 2017, 117, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Feinle-Bisset, C.; Ghoshal, U.C.; Quigley, E.M.; Santos, J.; Vanner, S.; Vergnolle, N.; Zoetendal, E.G. The intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology 2016, 150, 1305–1318.e8. [Google Scholar] [CrossRef]
- Bhattarai, Y.; Muniz Pedrogo, D.A.; Kashyap, P.C. Irritable bowel syndrome: A gut microbiota-related disorder? Am. J. Physiol. Gastrointest Liver Physiol. 2017, 312, G52–G62. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M. A role for the gut microbiota in IBS. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 497–505. [Google Scholar] [CrossRef]
- Jeffery, I.B.; O’Toole, P.W.; Öhman, L.; Claesson, M.J.; Deane, J.; Quigley, E.M.; Simrén, M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2012, 61, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; Biagi, E.; Heilig, H.G.; Kajander, K.; Kekkonen, R.A.; Tims, S.; de Vos, W.M. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 2011, 141, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Hyland, N.P.; Quigley, E.M.; Brint, E. Microbiota-host interactions in irritable bowel syndrome: Epithelial barrier, immune regulation and brain-gut interactions. World J. Gastroenterol. 2014, 20, 8859–8866. [Google Scholar]
- Barbara, G.; Cremon, C.; Azpiroz, F. Probiotics in irritable bowel syndrome: Where are we? Neurogastroenterol. Motil. 2018, 30, e13513. [Google Scholar] [CrossRef] [PubMed]
- Cremon, C.; Barbaro, M.R.; Ventura, M.; Barbara, G. Pre- and probiotic overview. Curr. Opin. Pharmacol. 2018, 43, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Barbara, G.; DuPont, H.L.; Mearin, F.; Gasbarrini, A.; Tack, J. New concepts on intestinal microbiota and the role of the non-absorbable antibiotics with special reference to rifaximin in digestive diseases. Dig. Liver Dis. 2018, 50, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Barbaro, M.R.; Fuschi, D.; Palombo, M.; Falangone, F.; Cremon, C.; Marasco, G.; Stanghellini, V. Inflammatory and microbiota-related regulation of the intestinal epithelial barrier. Front. Nut. 2021, 8, 718356. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Ford, A.C.; Talley, N.J.; Cremonini, F.; Foxx-Orenstein, A.E.; Brandt, L.J.; Quigley, E.M. The efficacy of probiotics in the treatment of irritable bowel syndrome: A systematic review. Gut 2010, 59, 325–332. [Google Scholar] [CrossRef]
- Shanahan, F.; Quigley, E.M. Manipulation of the microbiota for treatment of IBS and IBD-challenges and controversies. Gastroenterology 2014, 146, 1554–1563. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. Altered gastrointestinal microbiota in irritable bowel syndrome and its modification by diet: Probiotics, prebiotics and the low FODMAP diet. Proc. Nutr. Soc. 2016, 75, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Currò, D.; Ianiro, G.; Pecere, S.; Bibbò, S.; Cammarota, G. Probiotics, fibre and herbal medicinal products for functional and inflammatory bowel disorders. Br. J. Pharmacol. 2016, 174, 1426–1449. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Ianiro, G. Faecal microbial transplantation in IBS: Ready for prime time? Gut 2020, 69, 795–796. [Google Scholar] [CrossRef] [PubMed]
- Pinn, D.M.; Aroniadis, O.C.; Brandt, L.J. Is fecal microbiota transplantation the answer for irritable bowel syndrome? A single-center experience. Am. J. Gastroenterol. 2014, 109, 1831–1832. [Google Scholar] [CrossRef]
- Oviedo-Rondón, E.O.; Hume, M.E.; Hernández, C.; Clemente-Hernández, S. Intestinal microbial ecology of broilers vaccinated and challenged with mixed Eimeria species, and supplemented with essential oil blends. Poult. Sci. 2006, 85, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Meah, D.; Ahmed, N.; Conniff-Jenkins, R.; Chileshe, E.; Phillips, C.O.; Claypole, T.C.; Forman, D.W.; Row, P.E. Comparison of the antibacterial activity of essential oils and extracts of medicinal and culinary herbs to investigate potential new treatments for irritable bowel syndrome. BMC Complement. Altern. Med. 2013, 13, 338. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Khan, R.; Qamar, W.; Lateef, A.; Rehman, M.U.; Tahir, M.; Ali, F.; Hamiza, O.O.; Hasan, S.K.; Sultana, S. Geraniol attenuates 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced oxidative stress and inflammation in mouse skin: Possible role of p38 MAP Kinase and NF-κB. Exp. Mol. Pathol. 2013, 94, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Losa, R.; Zweifel, B.; Wallace, R.J. Sensitivity of pathogenic and commensal bacteria from the human colon to essential oils. Microbiology 2012, 158, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in membrane fatty acids composition of microbial cells induced by addition of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef] [PubMed]
- Turina, A.V.; Nolan, M.V.; Zygadlo, J.A.; Perillo, M.A. Natural terpenes: Self-assembly and membrane partitioning. Biophys. Chem. 2006, 122, 101–113. [Google Scholar] [CrossRef]
- De Fazio, L.; Spisni, E.; Cavazza, E.; Strillacci, A.; Candela, M.; Centanni, M.; Ricci, C.; Rizzello, F.; Campieri, M.; Valerii, M.C. Dietary geraniol by oral or enema administration strongly reduces dysbiosis and systemic inflammation in dextran sulfate sodium-treated mice. Front. Pharmacol. 2016, 7, 38. [Google Scholar] [CrossRef]
- Medicherla, K.; Sahu, B.D.; Kuncha, M.; Kumar, J.M.; Sudhakar, G.; Sistla, R. Oral administration of geraniol ameliorates acute experimental murine colitis by inhibiting pro-inflammatory cytokines and NF-κB signaling. Food Funct. 2015, 6, 2984–2995. [Google Scholar] [CrossRef]
- Rizzello, F.; Ricci, C.; Scandella, M.; Cavazza, E.; Giovanardi, E.; Valerii, M.C.; Campieri, M.; Comparone, A.; De Fazio, L.; Candela, M.; et al. Dietary geraniol ameliorates intestinal dysbiosis and relieves symptoms in irritable bowel syndrome patients: A pilot study. BMC Complement. Altern. Med. 2018, 18, 338. [Google Scholar] [CrossRef]
- Pavan, B.; Dalpiaz, A.; Marani, L.; Beggiato, S.; Ferraro, L.; Canistro, D.; Paolini, M.; Vivarelli, F.; Valerii, M.C.; Comparone, A.; et al. Geraniol pharmacokinetics, bioavailability and its multiple effects on the liver antioxidant and xenobiotic-metabolizing enzymes. Front. Pharmacol. 2018, 9, 18. [Google Scholar] [CrossRef]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitor-ing irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Morello, W.; D’Amico, F.; Serafinelli, J.; Turroni, S.; Abati, I.; Fiori, J.; Baskin, E.; Yalcinkaya, F.; Jankauskiene, A.; Pennesi, M.; et al. Low-dose antibiotic prophylaxis induces rapid modifications of the gut microbiota in infants with vesicoureteral reflux. Front. Pediatr. 2021, 9, 674716. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Chase, J.; Pitman, T.A.; Shiffer, A.; Mercurio, W.; Dillon, M.R.; Caporaso, J.G. An introduction to applied bioinformatics: A free, open, and interactive text. J. Open Source Educ. 2018, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Brigotti, M.; Carnicelli, D.; Arfilli, V.; Porcellini, E.; Galassi, E.; Valerii, M.C.; Spisni, E. Human monocytes stimulated by Shiga toxin 1a via globotriaosylceramide release proinflammatory molecules associated with hemolytic uremic syndrome. Int. J. Med. Microbiol. 2018, 308, 940–946. [Google Scholar] [CrossRef]
- Barbaro, M.R.; Cremon, C.; Morselli-Labate, A.M.; Di Sabatino, A.; Giuffrida, P.; Corazza, G.R.; Di Stefano, M.; Caio, G.; Latella, G.; Ciacci, C.; et al. Serum zonulin and its diagnostic performance in non-coeliac gluten sensitivity. Gut 2020, 69, 1966–1974. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, H.M.H.; Wagner, H.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. Available online: https://cran.r-project.org/pack-age=vegan (accessed on 25 May 2021).
- Cremon, C.; Guglielmetti, S.; Gargari, G.; Taverniti, V.; Castellazzi, A.M.; Valsecchi, C.; Tagliacarne, C.; Fiore, W.; Bellini, M.; Bertani, L.; et al. Effect of Lactobacillus paracasei CNCM I-1572 on symptoms, gut microbiota, short chain fatty acids, and immune activation in patients with irritable bowel syndrome: A pilot randomized clinical trial. United Eur. Gastroenterol. J. 2018, 6, 604–613. [Google Scholar] [CrossRef]
- Drossman, D.A.; Chang, L.; Bellamy, N.; Gallo-Torres, H.E.; Lembo, A.; Mearin, F.; Norton, N.J.; Whorwell, P. Severity in irritable bowel syndrome: A Rome foundation working team report. Am. J. Gastroenterol. 2011, 106, 1749–1760. [Google Scholar] [CrossRef]
- Camilleri, M. Diagnosis and treatment of irritable bowel syndrome: A review. JAMA 2021, 325, 865–877. [Google Scholar] [CrossRef]
- Vasant, D.H.; Paine, P.A.; Black, C.J.; Houghton, L.A.; Everitt, H.A.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British society of gastroenterology guidelines on the management of irritable bowel syndrome. Gut 2021, 70, 1214–1240. [Google Scholar] [CrossRef] [PubMed]
- Chumpitazi, B.P.; Kearns, G.L.; Shulman, R.J. The physiological effects and safety of peppermint oil and its efficacy in irritable bowel syndrome and other functional disorders. Aliment. Pharmacol. Ther. 2018, 47, 738–752. [Google Scholar] [CrossRef] [PubMed]
- Weerts, Z.; Masclee, A.; Witteman, B.; Clemens, C.; Winkens, B.; Brouwers, J.; Frijlink, H.W.; Muris, J.; De Wit, N.J.; Essers, B.; et al. Efficacy and safety of peppermint oil in a randomized, double-blind trial of patients with irritable bowel syndrome. Gastroenterology 2020, 158, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Nee, J.; Ballou, S.; Kelley, J.M.; Kaptchuk, T.J.; Hirsch, W.; Katon, J.; Cheng, V.; Rangan, V.; Lembo, A.; Iturrino, J. Peppermint oil treatment for irritable bowel syndrome: A randomized placebo-controlled trial. Am. J. Gastroenterol. 2021, 116, 2279–2285. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Ma, X.; Geng, S.; Jiang, X.; Huang, Q.; Hu, C.; Han, X. Intestinal microbiome-metabolome responses to essential oils in piglets. Front. Microbiol. 2018, 9, 1988. [Google Scholar] [CrossRef]
- Spisni, E.; Turroni, S.; Alvisi, P.; Spigarelli, R.; Azzinnari, D.; Ayala, D.; Imbesi, V.; Valerii, M.C. Nutraceuticals in the modulation of the intestinal microbiota: Current status and future directions. Front. Pharmacol. 2022, 13, 841782. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, J.; Lei, Y.; Bai, Y.; Jia, L.; Li, Z.; Liu, T.; Xu, Y.; Sun, J.; Wang, Y.; et al. Oregano essential oils promote rumen digestive ability by modulating epithelial development and microbiota composition in beef cattle. Front. Nutr. 2021, 8, 722557. [Google Scholar] [CrossRef]
- Qneibi, M.; Nidal, J.; Nour, E. Effect of geraniol and citronellol essential oils on the biophysical gating properties of AMPA receptors. Appl. Sci. 2019, 21, 4693. [Google Scholar] [CrossRef]
- Mars, R.; Frith, M.; Kashyap, P.C. Functional gastrointestinal disorders and the microbiome-what is the best strategy for moving microbiome-based therapies for functional gastrointestinal disorders into the clinic? Gastroenterology 2021, 160, 538–555. [Google Scholar] [CrossRef]
- Ford, A.C.; Harris, L.A.; Lacy, B.E.; Quigley, E.; Moayyedi, P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 48, 1044–1060. [Google Scholar] [CrossRef]
- Ianiro, G.; Eusebi, L.H.; Black, C.J.; Gasbarrini, A.; Cammarota, G.; Ford, A.C. Systematic review with meta-analysis: Efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment. Pharmacol. Ther. 2019, 50, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Myneedu, K.; Deoker, A.; Schmulson, M.J.; Bashashati, M. Fecal microbiota transplantation in irritable bowel syndrome: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2019, 7, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Martín, R.; Bridonneau, C.; Robert, V.; Sokol, H.; Bermúdez-Humarán, L.G.; Thomas, M.; Langella, P. Ecology and metabolism of the beneficial intestinal commensal bacterium Faecalibacterium prausnitzii. Gut Microbes 2014, 5, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Turroni, S.; Brigidi, P.; Cavalli, A.; Candela, M. Microbiota-host transgenomic metabolism, bioactive molecules from the inside. J. Med. Chem. 2018, 61, 47–61. [Google Scholar] [CrossRef]
- Tigchelaar, E.F.; Bonder, M.J.; Jankipersadsing, S.A.; Fu, J.; Wijmenga, C.; Zhernakova, A. Gut microbiota composition associated with stool consistency. Gut 2016, 65, 540–542. [Google Scholar] [CrossRef]
- Lo Presti, A.; Zorzi, F.; Del Chierico, F.; Altomare, A.; Cocca, S.; Avola, A.; De Biasio, F.; Russo, A.; Cella, E.; Reddel, S.; et al. Fecal and mucosal microbiota profiling in irritable bowel syndrome and inflammatory bowel disease. Front. Microbiol. 2019, 10, 1655. [Google Scholar] [CrossRef]
- Chen, Y.R.; Zheng, H.M.; Zhang, G.X.; Chen, F.L.; Chen, L.D.; Yang, Z.C. High oscillospira abundance indicates constipation and low BMI in the Guangdong Gut Microbiome Project. Sci. Rep. 2020, 10, 9364. [Google Scholar] [CrossRef]
- Kaakoush, N.O. Insights into the role of Erysipelotrichaceae in the human host. Front. Cell. Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef]
- Barandouzi, Z.A.; Lee, J.; Maas, K.; Starkweather, A.R.; Cong, X.S. Altered gut microbiota in irritable bowel syndrome and its association with food components. J. Pers. Med. 2021, 11, 35. [Google Scholar] [CrossRef]
- Malinen, E.; Krogius-Kurikka, L.; Lyra, A.; Nikkilä, J.; Jääskeläinen, A.; Rinttilä, T.; Vilpponen-Salmela, T.; von Wright, A.J.; Palva, A. Association of symptoms with gastrointestinal microbiota in irritable bowel syndrome. World J. Gastroenterol. 2010, 16, 4532–4540. [Google Scholar] [CrossRef]
- Williams, B.B.; Van Benschoten, A.H.; Cimermancic, P.; Donia, M.S.; Zimmermann, M.; Taketani, M.; Ishihara, A.; Kashyap, P.C.; Fraser, J.S.; Fischbach, M.A. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe 2014, 16, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Mars, R.; Yang, Y.; Ward, T.; Houtti, M.; Priya, S.; Lekatz, H.R.; Tang, X.; Sun, Z.; Kalari, K.R.; Korem, T.; et al. Longitudinal multi-omics reveals subset-specific mechanisms underlying irritable bowel syndrome. Cell 2020, 182, 1460–1473.e17. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J.; Yiannakou, Y.; Houghton, L.A.; Ford, A.C. Epidemiological, clinical, and psychological characteristics of individuals with self-reported irritable bowel syndrome based on the Rome IV vs Rome III criteria. Clin. Gastroenterol. Hepatol. 2020, 18, 392–398.e2. [Google Scholar] [CrossRef] [PubMed]

| Patient Weight (kg) | Capsules (n.) |
|---|---|
| 45–67 | 2 |
| 68–89 | 3 |
| 90–111 | 4 |
| Placebo N (%) or Median (IQR) n = 24 | Treatment N (%) or Median (IQR) n =25 | p | |
|---|---|---|---|
| Age | 40 (20–58) | 30 (19–52) | 0.05982 |
| Gender (Female) | 50% | 72% | / |
| BMI | 22.4 (18–26) | 22.6 (18–27) | 0.9597 |
| Placebo N (%) or Median (IQR) n = 24 | Treatment N (%) or Median (IQR) n =25 | p | |
|---|---|---|---|
| IBS-SSS Items | |||
| Abdominal Pain V1 | 35 (27.5–65) | 35 (25–55) | 0.695 |
| Abdominal Pain V2 | 50 (42.5–70) | 20 (5–35) | 0.001 |
| Days with abdominal Pain in the last 10 V1 | 55 (35–80) | 40 (20–70) | 0.315 |
| Days with abdominal Pain in the last 10 V2 | 55 (30–90) | 30 (20–60) | 0.032 |
| Bloating V1 | 65 (40–82.5) | 55 (50–75) | 0.567 |
| Bloating V2 | 62.5 (37.5–80) | 40 (25–65) | 0.021 |
| Satisfaction bowel habits V1 | 22.5 (10–50) | 25 (15–40) | 0.951 |
| Satisfaction bowel habits V2 | 30 (15–50) | 45 (30–70) | 0.035 |
| Interference with daily activities V1 | 70 (47.5–90) | 55 (40–90) | 0.421 |
| Interference with daily activities V2 | 55 (40–75) | 40 (30–65) | 0.118 |
| Clinical outcomes | |||
| IBS-SSS Score V1 | 240 (207.5–330) | 240 (200–270) | 0.250 |
| IBS-SSS Score V2 | 265 (217.5–317.5) | 195 (145–230) | 0.007 |
| IBSS-SSS Score variations (Delta V1–V2) | 1.5 (−37.5–35) | 50 (−5–75) | 0.032 |
| Responders (reduction 50 points IBS-SSS) | 4 (16.7%) | 13 (52%) | 0.009 |
| IBS Subtypes | |||
| IBS-C | n = 2 | n = 4 | |
| IBS-SSS Score V2 | 155 (60–250) | 180 (160–192.5) | 1 |
| Responders (reduction 50 points IBS-SSS) | 1 (50%) | 3 (75%) | 0.540 |
| IBS-D | n = 9 | n = 8 | |
| IBS-SSS Score V2 | 245 (220–305) | 218.5 (185–260) | 0.470 |
| Responders (reduction 50 points IBS-SSS) | 2 (22.2%) | 3 (37.5%) | 0.490 |
| IBS-M | n = 13 | n = 13 | |
| IBS-SSS Score V2 | 310 (255–320) | 190 (130–230) | 0.005 |
| Responders (reduction 50 points IBS-SSS) | 1 (7.7%) | 7 (53.9%) | 0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, C.; Rizzello, F.; Valerii, M.C.; Spisni, E.; Gionchetti, P.; Turroni, S.; Candela, M.; D’Amico, F.; Spigarelli, R.; Bellocchio, I.; et al. Geraniol Treatment for Irritable Bowel Syndrome: A Double-Blind Randomized Clinical Trial. Nutrients 2022, 14, 4208. https://doi.org/10.3390/nu14194208
Ricci C, Rizzello F, Valerii MC, Spisni E, Gionchetti P, Turroni S, Candela M, D’Amico F, Spigarelli R, Bellocchio I, et al. Geraniol Treatment for Irritable Bowel Syndrome: A Double-Blind Randomized Clinical Trial. Nutrients. 2022; 14(19):4208. https://doi.org/10.3390/nu14194208
Chicago/Turabian StyleRicci, Chiara, Fernando Rizzello, Maria Chiara Valerii, Enzo Spisni, Paolo Gionchetti, Silvia Turroni, Marco Candela, Federica D’Amico, Renato Spigarelli, Irene Bellocchio, and et al. 2022. "Geraniol Treatment for Irritable Bowel Syndrome: A Double-Blind Randomized Clinical Trial" Nutrients 14, no. 19: 4208. https://doi.org/10.3390/nu14194208
APA StyleRicci, C., Rizzello, F., Valerii, M. C., Spisni, E., Gionchetti, P., Turroni, S., Candela, M., D’Amico, F., Spigarelli, R., Bellocchio, I., Marasco, G., & Barbara, G. (2022). Geraniol Treatment for Irritable Bowel Syndrome: A Double-Blind Randomized Clinical Trial. Nutrients, 14(19), 4208. https://doi.org/10.3390/nu14194208











