Lesser-Consumed Tropical Fruits and Their by-Products: Phytochemical Content and Their Antioxidant and Anti-Inflammatory Potential
Abstract
1. Introduction
2. Methods and Data Collection
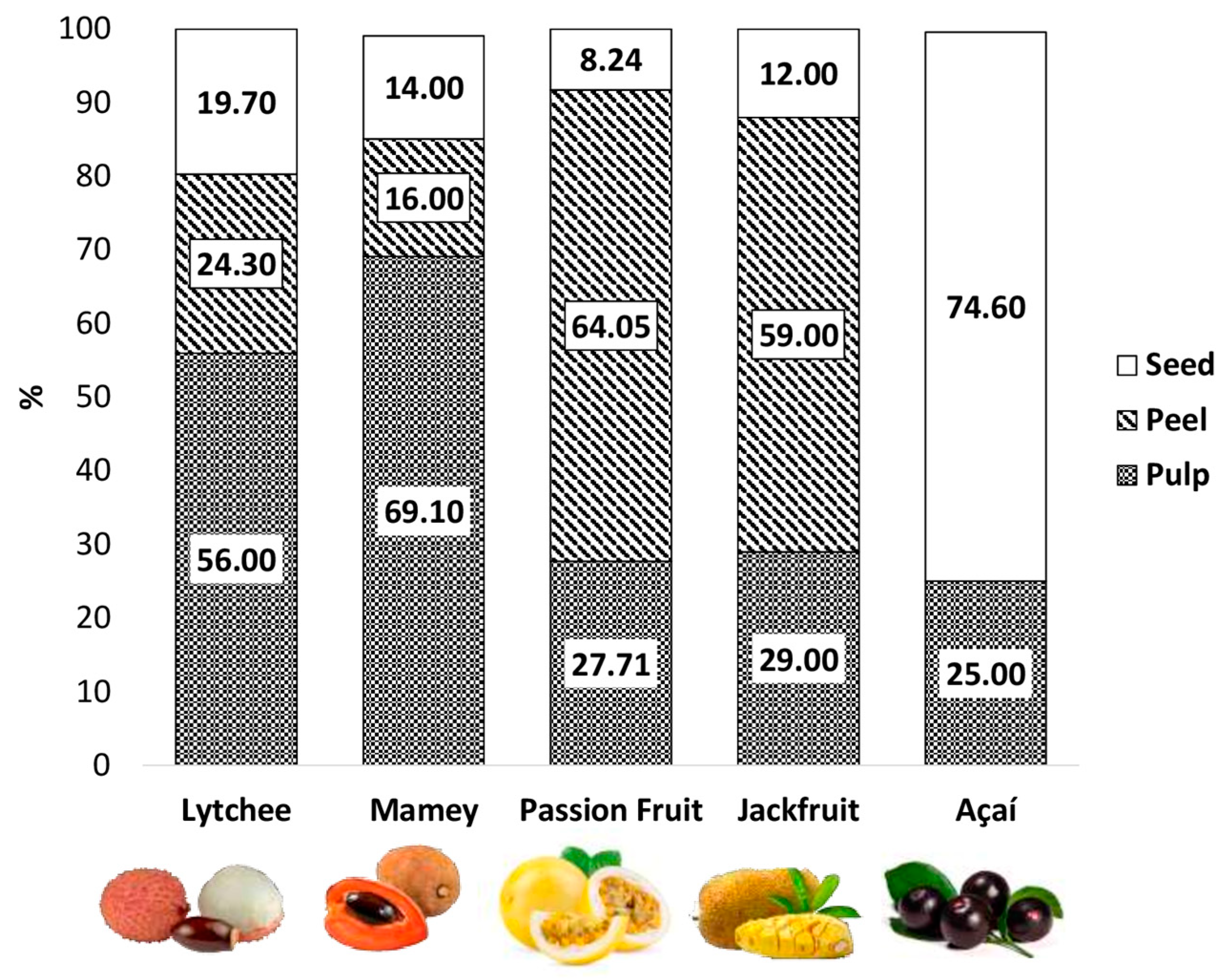
3. Chemical Composition of Peel, Seed, and Pulp of Lesser-Consumed Tropical Fruits
| Component | Mamey | Açaí | Passion Fruit | Lychee | Jackfruit |
|---|---|---|---|---|---|
| Water | PP: 61.53 ± 0.42% [43] | PP: 3.4 g/100 g dw [44] | SD: 57.09 g/100 g fw PL: 87.14 g/100 g fw PP: 90.06 g/100 g fw [36] | PL: 68.93 g/100 g PP: 83.91 g/100 g SD: 47.11 g/100 g [45] PP: 80.7% [39] | PP: 72-94 g/100 g fw [46] SD: 51.0-64.5 g/100 g fw [46] |
| Energy | PP: 1287 ± 26 KJ/100 g dw [31] PL:632 ± 18 KJ/100 g dw [31] | PP: 77 kcal/100 g fw [47] | NR | PP: 70.2 kcal/100 g [39] SD: 397.4 g/100 g [45] PL: 343.04 Kcal/100 g [45] | PP: 88-410 KJ/100 g fw [46] SD: 133–139 KJ/100 g fw [46] |
| Protein | PP: 4.84 ± 0.07 g/100 g dw [31] | PP: 8.1-21 g/100 g dw [44] | SD:13.07 g/100 g dw PL: 3.40 g/100 g dw PP: 8.57 g/100 g dw [36] | PP: 6.68 g/100 g dw; 0.7% [39,45] SD: 4.83 g/100 g dw [45] PL: 10.86 g/100 g dw [45] | PP: 1.2–1.9 g/100 g fw [46] SD: 20.19% dw [21] |
| Lipids | PP: 2.82 ± 0.66 g/100 g dw [31] | PP: 32.5-48 g/100 g dw [44] | SD: 12.31 g/100 g dw PL: 4.20 g/100 g dw PP: 1.11 g/100 g dw [36] | PP: 3.80 g/100 g dw; 0.8% [39,45] SD: 2.77 g/100 g dw [45] PL: 6.97 g/100 g dw [45] | PP: 0.1-0.4 g/100 g fw [46] SD: 11.39% dw [21] |
| Carbohydrates | PL: 65.7 ± 0.4 g/100 g dw [31] | PP: 36 ± 4 g/100 g dw [35] | SD: 71.07 g/100 g dw PL: 85.78 g/100 g dw PL: 83.37 g/100 g dw [36] | PP: 85.38 g/100 g dw; 15.3% [39,45] SD: 86.63 g/100 g dw [45] PL: 85.38 g/100 g dw [45] | PP: 16-25.4 g/100 g fw [46] SD: 25.8-38.4 g/100 g fw [46] SD: 51.82% dw [21] |
| Total dietary fiber | PP: 21.50 ± 1.13 dw [48] PP: 22.29 g/100 g dw [31] PL: 61.43 g/100 g dw [31] | PP: 44.2 g/100 g dw [44] | SD: 65.60 g/100 g dw PL: 61.16 g/100 g dw PP: 7.15 g/100 g dw [36] | PP: 2.47 g/100 g; 2.2% [39,45] SD: 4.07 g/100 g dw [45] PL: 18.21 g/100 g dw [45] | SD: 7.10% dw [21] |
| Total sugars | PP: 55.81 ± 0.39 [43] | NR | NR | NR | NR |
| Calcium | NR | PP: 260 mg/100 g dw [44] | SD:0.030 mg/100 g PL: 0.25 mg/100 g PP: 0.05 mg/100 g [36] | PP: 1.80 mg/100 g dw [39] | SD: 190 ppm dw [21] |
| Iron | PP: 0.0052–0.0262 g/kg [49] | PP: 49.8 mg/kg dw [50] | SD:0.0052 mg/100 g PL: 3.20 mg/100 g PP: 0.0055 mg/100 g [36] | PP: 0.8 mg/100 g [39] | SD: 148.5 ppm dw [21] |
| Magnesium | PP: 0.28–1.21 g/kg [49] | PP: 286 mg/kg dw [50] | SD: 0.094 mg/100 g PL: 0.12 mg/100 g PP: 0.02 mg/100 g [36] | PP: 12.90 mg/100 g [39] | SD: 240 ppm dw [21] |
| Phosphorus | PP: 0.28–0.30 g/kg [49] | PP: 186 ± 1.5 mg/100 g dw [35] | PL: 0.310 mg/100 g [36] | NR | |
| Potasium | PP: 2.26 g/kg [49] | PP: 930 ± 9.9 mg dw [35] | SD: 0.760 mg/100 g PL: 2.60 mg/100 g PP: 3.8 mg/100 g [36] | PP: 1067.33 mg/100 g [39] | SD: 2470.00 ppm dw [21] |
| Sodium | PP: 0.06–0.10 g/kg [49] | PP: 6.8 ± 0.7 mg/100 g dw [35] | SD: 0.0041 mg/100 g PL: 0.0022 mg/100 g PP: 0.0014 mg/100 g [36] | PP: 5.9 mg/100 g [39] | SD: 398.50 ppm dw [21] |
| Zinc | NR | PP: 2.1 mg/100 g dw [35] | SD: 0.0041 g/100 g PL: 1.00 mg/100 g [36] PP: 1.9 mg/100 g [23] | PP: 0.22 mg/100 g [39] | SD: 40.85 ppm dw [21] |
| Vitamin C | PP: 29.37 ± 3.58 mg of vitamin C/100 g fw [48] | PP: <0.1 mg/100 g dw [44] | NR | PP: 26.9 mg/100 g [39] | PP: 7.0-10.0 mg/100 g fw SD: 11 mg/100 g fw [46] |
| Total saturated fatty acids | SD: 39.91 g/100 g [43] | NR | SD: 14.69 g/100 g [23] | NR | NR |
| Total mono unsaturated fatty acids | SD: 48.62 g/100 g [43] | NR | SD: 17.18 g/100 g [23] | NR | NR |
| Total polyunsaturated fatty acids | SD: 11.35 g/100 g [43] | NR | SD: 68.12 g/100 g [23] | NR | NR |
4. Phytochemical Content
4.1. Phenolic Compounds
4.1.1. Phenolic Acids
4.1.2. Flavonoids
Flavanols
Flavonols
Flavones
Anthocyanins
4.2. Carotenoids
| Phenolic Acids | |
|---|---|
| Phytochemical | Content |
| Gallic acid | Mamey PP: 0.47 mg/100 g fw [56]; 1.92 mg/100 g dw [57]; 170.91 ± 0.53 ppm fw [63] Açaí PP:6.87 ± 0.28 mg/100 g dw [60] Lychee: cv Qingke: 0.1055, cv Baila: 0.063, cv Jizui: 0.048 mg/100 g fw [58] |
| p-hydroxybenzoic acid | Mamey PP: 484 mg/100 g dw [63] Açaí PP: 1.0 ± 0.8 mg/100 g dw [10] Passion fruit: 0.0124 ± 0.0011 mg/100 g fw [11] Jackfruit PP: 19.978 ± 1.66 mg/g dw [75] |
| Protocatechuic acid | Açaí PP: 0.717 ± 0.054 mg/100 g [59]; PP: 1.7 ± 0.4 mg/100 g dw [10] |
| Protocatechuic acid hexoside | PP: 0.9 ± 0.6 mg/100 g dw [10] |
| Chlorogenic acid | Açaí PP: 0.909 ± 0.102 mg/100 g [59]; PP: 5.01 ± 0.78 mg/100 g dw [60] Passion fruit: 0.0183 ± 0.002 mg/100 g fw [11] Lychee: cv Qingke:0.008, cv Baila: 0.0219, cv Jizui: 0.064 mg/100 g fw [58] |
| Caffeic acid | Açaí PP: 0.238 ± 0.018 mg/100 g [59]; PP: 0.61 ± 0.22 mg/100 g dw [60]; PP: 1.9 ± 0.8 mg/100 g dw [10] Passion fruit: 0.0056 ± 0.0005 mg/100 g fw [11] Lychee: cv Qingke: 0.0621, cv Baila: 0.0576, cv Jizui: 0.114 mg/100 g fw [58] |
| Vanillic acid | Açaí PP: 4.655 ± 0.233 mg/100 g [59]; PP: 11.0 ± 5.8 mg/100 g dw [10] Passion fruit: 0.0426 ± 0.0029 mg/100 g fw [11] |
| Syringic acid | Açaí PP: 1.903 ± 0.120 mg/100 g [59]; PP: 1.62 ± 0.37 mg/100 g dw [60]; PP: 4.8 ± 1.1 mg/100 g dw [10] Lychee PP: 3.96 ± 0.95 μg/g fw [76] |
| Synapic acid | Açaí PP: 0.082 ± 0.010 mg/100 g [59] |
| p-coumaric acid | Açaí PP: 0.22 ± 0.015 mg/100 g [59]; PP: 1.74 ± 0.33 mg/100 g dw [60] Passion fruit: 0.024 ± 0.0015 mg/100 g fw [11] Lychee PP: 0.894 ± 0.119 mg/g dw [76] |
| Ferulic acid | Açaí PP: 0.322 ± 0.020 mg/100 g [59] Passion fruit: 0.0015 ± 0.0003 mg/100 g fw [11] Lychee PP: 6.26 ± 1.01 μg/g fw [76] |
| Kaftaric acid | Açaí PP: 0.86 ± 0.10 mg/100 g dw [60] |
| 5-caffeoylquinic acid | Açaí PP: 4.3 mg/100 g dw [10] Passion fruit PP: 0.0104 mg/100 g [61] Jackfruit PP: 3.42 ± 0.04 mg/100 g [20] |
| 4-caffeoylquinic acid | Passion fruit PP: 0.012 mg/100 g [61] Jackfruit 0.144 ± 0.004 mg/100 g [20] |
| 3,5-dicaffeoylquinic acid | Passion fruit PP: 0.0576 mg/100 g [61] Jackfruit PP: 0.131 ± 0.01 mg/100 g [20] |
| 4,5-dicaffeoylquinic Acid | Passion fruit PP: 0.0587 mg/100 g [61] Jackfruit PP: 0.050 ± 0.004 mg/100 g [20] |
| p-coumaric acid, hexoside | Açaí PP: 1.0 ± 0.5 mg/100 g dw [10] |
| Isomer 1 of feruloyl sinapic acid | Açaí PP: 1.3 ± 0.6 mg/100 g dw [10] |
| Feruroylhydroxypyruvic acid | Açaí PP: 1.4 ± 0.5 mg/100 g dw [10] |
| Isomer 1 of caffeoyl shikimic acid | Açaí PP: 1.7 ± 1.5 mg/100 g dw [10] |
| Isomer 2 of feruloyl sinapic acid | Açaí PP: 0.8 ± 0.3 mg/100 g dw [10] |
| Isomer 2 of caffeoyl shikimic acid | Açaí PP: 5.4 mg/100 g dw [10] |
| Sinapoyl hexose | Açaí PP: 1.0 ± 0.8 mg/100 g dw [10] |
| Feruloylquinic hydroxy acid | Açaí PP: 0.7 ± 0.4 mg/100 g dw [10] |
| Sinapoyl rhamnose | Açaí PP: 1.4 ± 0.9 mg/100 g dw [10] |
| Feruloyl derivative | Açaí PP: 2.3 ± 0.7 mg/100 g dw [10] |
| Flavanols | |
| Catechin | Mamey PP: 0.99 -11.31 mg/100 g fw [56]; 75.01 ± 2.67 ppm fw [63] Açaí PP: 5.07 ± 0.48 mg/100 g dw [60] Lychee cv Qingke: 0.486, cv Baila: 0.246, cv Jizui: 0.215 mg/100 g fw [58] |
| Galocatechin-3-gallate | Mamey PP: 1.19 mg/100 g fw [56] Açaí PP: 25.00 ± 0.64 mg/100 g dw [60] |
| Gallocatechin | Mamey PP: 172.85 ± 2.21 ppm fw [63] Lychee PP: 2307.91 ± 66.76 μg/g fw [76] |
| Catechin-3-O-gallate | Mamey PP: 80.50 ± 0.81 ppm fw [63] |
| Epicatechin | Mamey PP: 0.58 mg/100 g fw [56];0.78 mg/100 g dw [57]; 24.42 ± 0.97 ppm fw [63] Açaí PP: 2.03 ± 0.09 mg/100 g dw [60] Lychee cv Qingke: 0.498, cv Baila: 0.393, cv Jizui: 0.249 mg/100 g fw [58]; PP: cv Hemaoil: 0.0425, cv Feizixiao: 0.0196, cv Lanzuhu: 0.008 mg/100 g dw [64] |
| Flavonols | |
| Flavonoids | Mamey PP: 65.24 ± 4.49 mg quercetin/100 g fw [48] Passion fruit PP: 158.037 ± 0.602 mg/L fw [62] Jackfruit PL: 279 ± 4; PP: 227 ± 31; SD: 162 ± 10 mg quercetin/100 g dw [22]; PL: 87,140 mg QE/100 g dw [28] |
| Rutin | Açaí PP: 3.89 ± 0.15 mg/100 g dw [60]; PP: 3.4 ± 0.7 mg/100 g dw [10] Passion fruit PP: 0.0227 ± 0.0027 mg/100 g fw [11] Lychee cv Qingke: 0.591, cv Baila: 0.563, cv Jizui: 1.888 mg/100 g [58]; PP: cv Hemaoil: 0.009, cv Feizixiao: 0.065, cv Lanzuhu: 0.023 mg/100 g dw [20] |
| Isorhamnetin rutinoside | Açaí PP: 1.7 ± 0.3 mg/100 g dw [10] |
| Dihydromyricetin | Mamey PP: 200.77 ± 11.73 ppm fw [63] |
| Myricitrin | Mamey PP: 25.48 ± 3.70 ppm fw [63] |
| Quercetin | Açaí PP: 13.566 ± 0.098 mg/100 g dw [59] Passion fruit PP: 0.0416 ± 0.0006 mg/100 g fw [11] Lychee PP: 1.325 ± 0.007 mg/g dw [76] |
| Quercetin-3-glucoside | Açaí PP: 1.54 ± 0.34 mg/100 g dw [60] |
| Kaempferol | Açaí PP: 0.521 ± 0.036 mg/100 g dw [59] |
| Flavanones | |
| Naringenin | Açaí PP: 1.64 ± 0.48 mg/100 g dw [60] |
| Hesperidin | Açaí PP: 1.96 ± 0.51 mg/100 g dw [60] |
| Flavones | |
| Isovitexin | Açaí PP: 12.0 ± 4.8 mg/100 g dw [10] Passion fruit PP: 2.76 mg/100 g dw [20] |
| Homoorientine | Açaí PP: 9.9 ± 4.9 mg/100 g dw [10] |
| Vitexin | Açaí PP: 9.8 ± 5.2 mg/100 g dw [10] |
| Escoparina | Açaí PP: 0.6 ± 0.2 mg/100 g dw [10] |
| Chrysoeriol | Açaí PP: 0.5 ± 0.3 mg/100 g dw [10] |
| Orientin | Açaí PP: 15.0 ± 6.3 mg/100 g dw [10] Passion fruit PL: 0.970 mg/100 g dw [20] |
| Isoorientin | Passion fruit PL: 19.63 mg/100 g dw [62]; PP: 16.226 ± 0.050 mg/L fw [11] |
| Luteolin | Açaí PP: 2.161 ± 0.216 mg/100 g [59]; PP: 0.9 ± 0.3 mg/100 g dw [10] |
| Apigenin | Açaí PP: 1.257 ± 0.134 mg/100 g [59] |
| Flavonones | |
| Taxifolin deoxyhexose isomer 1 | Açaí PP: 2.8 ± 1.7 mg/100 g dw [10] |
| Taxifolin deoxyhexose isomer 2 | Açaí PP: 1.3 ± 0.7 mg/100 g dw [10] |
| Taxifolin | Açaí PP: 1.2 ± 0.4 mg/100 g dw [10] |
| Anthocyanins | |
| Malvidin-3-glucoside | Açaí PP: 6.9 ± 0.82 mg/100 g dw [60] |
| Malvidin-3.5-diglucoside | Açaí PP: 11.51 ± 1.37 mg/100 g dw [60] |
| Cyanidin-3-glucoside | Açaí PP: 67.33 ± 1.06 mg/100 g dw [60]; PP: 0.13–541.5 mg/100 g fw [44] |
| Cyanidin-3-rutinoside | Açaí PP: 2.57–1395.3 mg RAE/100 g fw [44] |
| Pelargonidin-3-glucoside | Açaí PP: 111.92 ± 3.04 mg/100 g dw [60] |
| Peonidin-3-glucoside | Açaí PP: 1.32 ± 0.29 mg/100 g dw [60] |
| Total anthocyanins | Mamey: PP: 5.57 ± 0.07 mg TA/100 g fw [48] Açaí PP: 35.41 mg of cianidine-3-glucoside equivalent/100 g fw [68]; PP: 587 ± 53 mg cyanidin-3-glucoside equivalents/100 g of dw [67] Jackfruit PP: 0.46 mg TA/100 g fw [69] |
| Proanthocyanidins | |
| Procyanidin B1 | Açaí PP: 1.99 ± 0.36 mg/100 g dw [60] |
| Procyanidin B2 | Açaí PP: 5.03 ± 0.4 mg/100 g dw [60] Lychee PP: cv Hemaoil: 39.93, cv Feizixiao: 0.032, cv Lanzuhu: 0.017 mg/100 g dw [20] |
| Procyanidin A2 | Açaí PP: 11.53 ± 1.53 mg/100 g dw [60] Lychee PP: cv Hemaoi: 0.018, cv Feizixiao: 0.001 mg/100 g dw [20] |
| Stilbenes | |
| trans-resveratrol | Açaí PP: 0.38±0.14 mg/100 g dw [60] |
| Carotenoids | |
| Neoxanthin | Mamey PP: Genotype 8747: 1.024 ± 0.263, Genotype 11,129: 0.370 ± 0.099 mg/100 g dw [19] Jackfruit PP: All-trans-neoxanthin: 8.85 μg/100 g wm; 9-cis-neoxanthin: 6.87 μg/100 g [73] |
| Lycopene | Lychee SD: 0.0043 mg/100 g [77] |
| Violaxanthin | Mamey PP: Genotype 8747: 0.360 ± 0.119, Genotype 11,129: 0.164 ± 0.057 mg/100 g dw [19] |
| Luteoxanthin | Mamey PP: Genotype 8747: 0.569 ± 0.163, Genotype: 11,129: 0.180 ± 0.0 80 mg/100 g dw [19] |
| Lutein and zeaxanthin | Açaí PP: 0.367 ± 0.142 mg/100 g dw [17]; PP: 0.717 mg/100 g dw [44] Passion fruit Lutein; PL: 0.504 mg/100 g dw [36]; Zeaxanthin; PL: 0.065 mg/100 g dw [36]; PP: 0.044 mg/100 g dw [36] Passion fruit PP: All-trans-lutein: 37.02 μg/100 g fw; All-trans-zeaxanthin: 0.96 μg/100 g fw [75] |
| Capsoneoxanthin | Mamey PP: Genotype: 8747: 1.428 ± 0.402, Genotype: 11,129: 0.454 ± 0.170 mg/100 g dw [19] |
| α-Carotene | Açaí PP: 0.450 ± 0.002 mg/100 g dw [17] Jackfruit PP: All trans-αcarotene: 1.24 μg/100 g fw [73] |
| β-cryptoxanthin epoxide | Mamey PP: Genotype 8747: 0.208 ± 0.058, Genotype 11,129: 0.042 ± 0.020 mg/100 g dw [19] Passion fruit PL: 0.075 mg/100 g dw [36]; PP: 0.254 μm/100 g dw [36] Jackfruit PP: 1.21 μg/100 g fw [73] |
| 13-cis-β-Carotene | Açaí PP: 0.055 ± 0.037 mg/100 g dw [17] Jackfruit PP: 2.45 μg/100 g fw [73] |
| 9-cis-β-Carotene | Açaí PP: 0.365 ± 0.002 mg/100 g dw [17] Jackfruit PP: 0.79 μg/100 g fw [73] |
| β-Carotene | Mamey 1.2–1.5 mg β-carotene/100 g [78] Açaí PP: 0.010–0.149 mg/100 g dw [44] Passion fruit PL: 0.272; PP: 1.334 mg of β-carotene equivalents mg/100 g dw [36] Lychee SD: 2.77 mg/mL [77]; PL: 195.09 mg/mL [39]; PP: 0.291 mg of β-carotene equivalents/100 g fw [39] Jackfruit PP: All trans-β-carotene 29.55 μg/100 g fw [73] |
| Total carotenoids | Mamey PP: Genotype 8747: 8.076, Genotype 11,129: 3.786 mg/100 g fw [19]; PP: 36.12 ± 1.24 mg β-carotene/100 g fw [48]; PP: 1.127 ± 0.005 mg β-carotene/100 g fw [57] Açaí PP: 4.15 ± 0.41 mg/100 g dw [67]; PP: 4.2345 ± 0.007 mg/100 g dw [17] Passion fruit PP: 25.10 mg/100 g fw [72] Jackfruit PP: 0.592 mg/100 g fw [41]; PP: 107.98 μg/100 g fw [73] |
| Tocopherols | |
| Total tocopherols | Passion fruit PP: 0.52 mg/100 g fw [72] |
| δ- Tocopherol | Mamey PP: 0.360 ± 0.030 mg/100 g dw [57] |
| Ascorbic acid | |
| Vitamin C | Mamey PP: 29.37 ± 3.58 mg vitamin C/100 g fw [48] Lychee PP: 34.7 ± 7.8 mg vitamin C/100 g fw [39] |
5. Bioactivities
5.1. Antioxidant Activity (AOXA)
| Sample | Extraction Solvent | Solid: Liquid Ratio | Method | Total Phenolic Compounds (mg GAE/100 g dw) | Antioxidant Activity | Reference |
|---|---|---|---|---|---|---|
| Jackfruit (peel, pulp, and seed) | 90% methanol | 1:30 | 6 h stir at 100 rpm | PL: 4804 ± 457; PP:1034 ± 16; Flake: 1157 ± 6 SD: 971 ± 6 | IC50 mg dw/mL DPPH: PL: 1.25 ± 0.14; PP > 10; SD > 10 ABTS PL: 0.23 ± 0.02; PP: 5.70 ± 0.37; SD: 7.62 ± 0.13 | [22] |
| Jackfruit pulp | 60% methanol 0.1% HCl | 5:10 (w/v) | Water bath for 2 h at 85 °C | 29.0 ± 6.3 fw | ABTS 0.63 ± 0.0; DPPH 0.16 ± 0.03 μM TE/g fw | [69] |
| Passion fruit seed | Ethanol | 1:4 (w/v) | Homogenized by exhaustive extraction | 346.69 | DPPH: IC50 = 1.18 ± 0.03 g/100 mL ABTS: IC50 = 3.84 ± 0.08 g/100 mL | [36] |
| Passion fruit seed | Ethanol:water | 1/10 (w/v) | Thermostatic bath under constant agitation | 3.11 | DPPH IC50 = 26.96 ± 0.34 μg/mL FRAP: 3.6 ± 0.29 μg AAE/g ORAC: 6.2 ± 0.53 μmol TE/g | [23] |
| Lychee seed | Methanol: water (50:50 v/v) | NR | 3 consecutive refluxes at 80 °C | 11.45 wm 34.72 | NR | [40] |
| Lychee seed | Ethanol:water (50:50 v/v) | 1:30 (w/v) | Heating to 50 °C, in a water bath with intermittent mixing at 200 rpm for 50 min | 12.90 wm | TEAC: 21.40 ± 1.98 μmol Trolox/g | [26] |
| Açaí seed extract | Ethanol | 1:2 (w/v) | Boiled in 400 mL of water. 400 mL of ethanol was added. Stirred 2 h a day for 10 days | 26,500 | NR | [83] |
| Açaí seed | Ethanol/water | 57/43 (v/v) 1:10 (w/v) | 10 g mixed with 100 mL of ethanol/water (57/43, v/v), sonicated for 15 min and centrifuged at 5000× g | 49,099 ± 8 | NR | [54] |
| Passion fruit peel | Ethanol | 1:4 (w/v) | Homogenized by exhaustive extraction | 1061.87 | DPPH: IC50 = 1.69 ± 0.03 g/100 mL ABTS: IC50 = 2.22 ± 0.01 g/100 mL | [36] |
| Passion fruit peel | Water/ethanol/formic acid (94/5/1; v/v/v) | 1:4 (w/v) | Extraction with pressurized hot water. 2.5 g sample, 99 °C (at 50 bar), 7 min extraction | 2496 | DPPH: 718.91 ± 40.55 μg/mL TEAC: 0.08 ± 0.01 mmol Trolox/g | [84] |
| Lychee peel | Methanol:water (50:50 v/v) | NR | 3 consecutive refluxes at 80 °C | 22.04 fw 71.71 | NR | [40] |
| Lychee peel | Ethanol:water (50:50 v/v) | 1:30 (w/v) | Heating to 50 °C, in a water bath with intermittent mixing at 200 rpm for 50 min. | 25.10 | TEAC: 43.80 ± 2.02 μmol Trolox/g | [26] |
| Passion fruit pulp | Ethanol | 1:4 (w/v) | Homogenized by exhaustive extraction | 1297.31 | DPPH: IC50 = 0.20 ± 0.03 g/100 mL ABTS: IC50 = 0.82 ± 0.03 g/100 mL | [36] |
| Lychee pulp | Methanol: water (50:50 v/v) | NR | 3 consecutive refluxes at 80 °C | 21.20 fw | NR | [40] |
| Lychee pulp | Ethanol:water (50:50 v/v) | 1:30 (w/v) | Heating to 50 °C with intermittent mixing at 200 rpm for 50 min | 20.30 | TEAC: 13.20 ± 1.52 μmol Trolox/g | [26] |
| Açaí pulp | CO2 | 5 g pulp | 50 °C/350 bar, 60 °C/420 bar, and 70 °C/490 bar. Solvent mass flow rate of 8.85 ×10−5 kg/s and 0.005 kg of dry matter | 1542.82 | TEAC: 5795 μM Trolox/100 g dw DPPH: 93,682 μM Trolox/100 g dw | [85] |
| Açaí pulp | Methanol | 1:2 w/v | Sonicated with methanol for 20 min/16 °C, centrifuged at 2800× g/10 min. Pellet re-extracted with methanol/water (80:20, v/v) until discoloration. | 4786 ± 1880 | ABTS: 24.7 ± 10.6 μmol TE/100 g dw DPPH: 21,049 ± 3071 μmol TE/100 g dw | [10] |
| Açaí pulp | Not mentioned | 50 g pulp | High pressure 600 MPa/5 min/25ºC | 235.70 | FRAP: 31.3 μmol TE/g ORAC: 42.7 μmol TE/g | [68] |
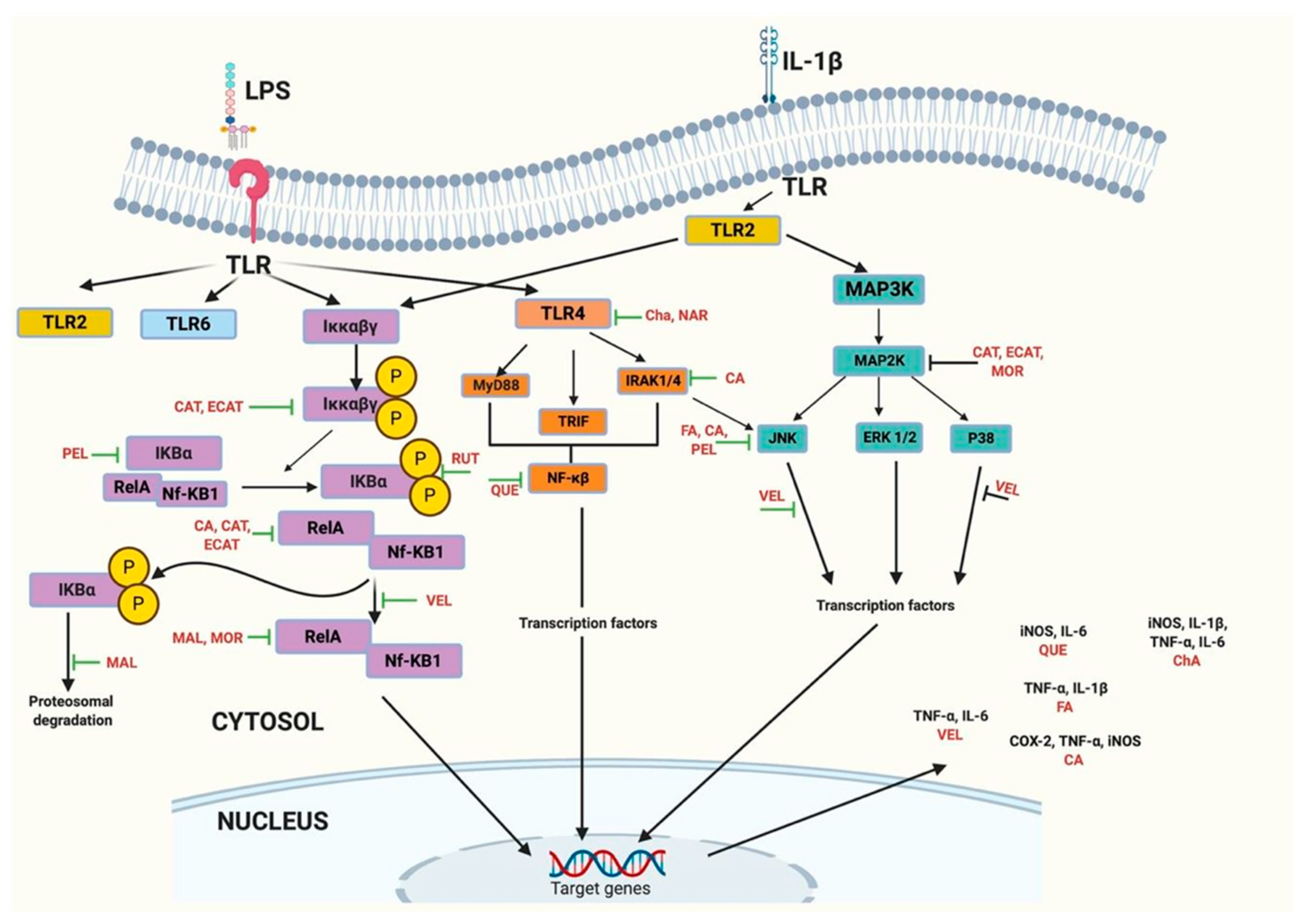
5.2. Anti-Inflammatory Activity
5.2.1. Changes Exerted by Altering Gene Expression
5.2.2. Changes Exerted by Targeting Different Metabolites
| Source | Compound | Classification | Activity | Reference |
|---|---|---|---|---|
| Açaí | Velutin | Flavone | Inhibit SEAP secretion Inhibited the expression of TNF-α and IL-6 | [91,96] |
| Açaí pulp | Anthocyanins cyanidin-3-rutinoside and cyanidin-3-glucoside | Anthocyanins | ↓ IL-6 and IFN-γ | [47] |
| Açaí seed extract | Catechin, epicatechin, and polymeric procyanidins | Polyphenols | ↓ NF-κB and IL-6 | [25] |
| Lychee | Catechin-type monomers and oligomers of proanthocyanidins | Flavanols and proanthocyanidins | Suppression of NF-κB activation and ↓ IL-6 and TNF-α | [97] |
| Lychee seed extract | 21 compounds, including 3,5-dihydroxybenzoic acid, 3,4-dihydroxybenzaldehyde, procyanidin D, cianidanol, cinnamtannin B1, procyanidin A1, scopoletin, rutin, phlorizin and epicatechin–epicatechin– catechin | Polyphenols | ↓ mRNA levels of NF-κB | [94] |
| Passion fruit peel flour | Vicenin, isoorientin, orientin, vitexin and isovitexin | C-glycosyl flavonoids | ↓ IL-1β, Il-6 and IL-17 | [20] |
| Purple passion fruit peel | Quercetin, luteolin, cyanidin 3-O-glucoside | Flavonoid | ↓ NO levels | [98] |
| Yellow passion fruit peel flour | Ferulic acid | Hydroxycinnamic acid | ↓ Lipid peroxidation ↑ GPx and GR in liver ↓ TNF-α and IL-1β Inactivation of JNK | [88] |
| Jackfruit | Artocarpesin | Flavone | Suppressed LPS-induced production of NO and PGE2, by downregulating inducible iNOS and COX-2 protein expressions | [86] |
| Jackfruit | Moracin C | Arylbenzofurane | Inhibited LPS-activated ROS and NO release, ↓ mRNA and protein expression of iNOS, COX-2, IL-1β, IL-6 and TNF-α | [87] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonçalves, R.F.S.; Martins, J.T.; Duarte, C.M.M.; Vicente, A.A.; Pinheiro, A.C. Advances in Nutraceutical Delivery Systems: From Formulation Design for Bioavailability Enhancement to Efficacy and Safety Evaluation. Trends Food Sci. Technol. 2018, 78, 270–291. [Google Scholar] [CrossRef]
- Boeira, L.S.; Bastos, P.H.; Uchôa, N.R.; Bezerra, J.A.; Cád, S.V.; Duvoisin, S.; Albuquerque, P.M.; Mar, J.M.; Ramos, A.S.; Machado, M.B.; et al. Chemical and Sensorial Characterization of a Novel Alcoholic Beverage Produced with Native Acai (Euterpe Precatoria) from Di Ff Erent Regions of the Amazonas State. LWT 2020, 117, 108632. [Google Scholar] [CrossRef]
- Choy, K.W.; Murugan, D.; Leong, X.F.; Abas, R.; Alias, A.; Mustafa, M.R. Flavonoids as Natural Anti-Inflammatory Agents Targeting Nuclear Factor-Kappa B (NFκB) Signaling in Cardiovascular Diseases: A Mini Review. Front. Pharmacol. 2019, 10, 1295. [Google Scholar] [CrossRef] [PubMed]
- Feio, C.A.; Izar, M.C.; Ihara, S.S.; Kasmas, S.H.; Martins, C.M.; Feio, M.N.; Maués, L.A.; Borges, N.C.; Moreno, R.A.; Póvoa, R.M.; et al. Euterpe oleracea (Açai) Modifies Sterol Metabolism and Attenuates Experimentally-Induced Atherosclerosis. J. Atheroscler. Thromb. 2012, 19, 237–245. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grace, M.H.; Esposito, D.; Dunlap, K.L.; Lila, M.A. Comparative Analysis of Phenolic Content and Profile, Antioxidant Capacity, and Anti-Inflammatory Bioactivity in Wild Alaskan and Commercial Vaccinium Berries. J. Agric. Food Chem. 2014, 62, 4007–4017. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Montañez, G.; Burgos-Hernández, A.; Calderón-Santoyo, M.; López-Saiz, C.M.; Velázquez-Contreras, C.A.; Navarro-Ocaña, A.; Ragazzo-Sánchez, J.A. Screening Antimutagenic and Antiproliferative Properties of Extracts Isolated from Jackfruit Pulp (Artocarpus heterophyllus Lam). Food Chem. 2015, 175, 409–416. [Google Scholar] [CrossRef]
- Sun, J.; Li, L.; You, X.; Li, C.; Zhang, E.; Li, Z.; Chen, G.; Peng, H. Phenolics and Polysaccharides in Major Tropical Fruits: Chemical Compositions, Analytical Methods and Bioactivities. Anal. Methods 2011, 3, 2212–2220. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Poovarodom, S.; Leontowicz, H.; Leontowicz, M.; Vearasilp, S.; Trakhtenberg, S.; Gorinstein, S. The Multiple Nutrition Properties of Some Exotic Fruits: Biological Activity and Active Metabolites. Food Res. Int. 2011, 44, 1671–1701. [Google Scholar] [CrossRef]
- Sarkar, T.; Salauddin, M.; Roy, A.; Sharma, N.; Sharma, A.; Yadav, S.; Jha, V.; Rebezov, M.; Khayrullin, M.; Thiruvengadam, M.; et al. Minor Tropical Fruits as a Potential Source of Bioactive and Functional Foods. Crit. Rev. Food Sci. Nutr. 2022, 1–45. [Google Scholar] [CrossRef]
- Garzón, G.A.; Narváez-Cuenca, C.E.; Vincken, J.P.; Gruppen, H. Polyphenolic Composition and Antioxidant Activity of Açai (Euterpe oleracea Mart.) from Colombia. Food Chem. 2017, 217, 364–372. [Google Scholar] [CrossRef]
- Rotta, E.M.; Rodrigues, C.A.; Jardim, I.C.S.F.; Maldaner, L.; Visentainer, J.V. Determination of Phenolic Compounds and Antioxidant Activity in Passion Fruit Pulp (Passiflora Spp.) Using a Modified QuEChERS Method and UHPLC-MS/MS. LWT 2019, 100, 397–403. [Google Scholar] [CrossRef]
- Torres-becerril, M.; Arvizu-barrón, E.; Ojeda-enciso, L.A.; Zaldívar-cruz, J.M. Current Status of Litchi Cultivation in Producing Municipalities of Veracruz and Oaxaca, Mexico. Revista Mexicana de Ciencias Agrícolas 2019, 10, 563–574. [Google Scholar] [CrossRef]
- FAO. Las Principales Frutas Tropicales Análisis Del Mercado 2018. Roma 2020. Available online: https://www.fao.org/economic/est/est-commodities/frutas-tropicales/es/ (accessed on 14 August 2022).
- Altendorf, S. Minor Tropical Fruits. Food Outlook 2018, 1, 67–75. [Google Scholar]
- Servicio de Información Agroalimentario y Pesquera Anuario Estadístico de La Producción Agrícola. Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 14 August 2022).
- Instituto Brasilieiro de Geografía e Estatística Produção de Açaí (Cultivo). Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/acai-cultivo/br (accessed on 14 August 2022).
- Fragoso, M.F.; Romualdo, G.R.; Vanderveer, L.A.; Franco-Barraza, J.; Cukierman, E.; Clapper, M.L.; Carvalho, R.F.; Barbisan, L.F. Lyophilized Açaí Pulp (Euterpe oleracea Mart) Attenuates Colitis-Associated Colon Carcinogenesis While Its Main Anthocyanin Has the Potential to Affect the Motility of Colon Cancer Cells. Food Chem. Toxicol. 2018, 121, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Mohamed, G.A. Litchi Chinensis: Medicinal Uses, Phytochemistry, and Pharmacology. J. Ethnopharmacol. 2015, 174, 492–513. [Google Scholar] [CrossRef]
- Chacón-Ordóñez, T.; Schweiggert, R.M.; Bosy-Westphal, A.; Jiménez, V.M.; Carle, R.; Esquivel, P. Carotenoids and Carotenoid Esters of Orange- and Yellow-Fleshed Mamey Sapote (Pouteria sapota (Jacq.) H.E. Moore & Stearn) Fruit and Their Post-Prandial Absorption in Humans. Food Chem. 2017, 221, 673–682. [Google Scholar] [CrossRef]
- Cazarin, C.B.B.; Rodriguez-Nogales, A.; Algieri, F.; Utrilla, M.P.; Rodríguez-Cabezas, M.E.; Garrido-Mesa, J.; Guerra-Hernández, E.; de Campos Braga, P.A.; Reyes, F.G.R.; Maróstica, M.R.; et al. Intestinal Anti-Inflammatory Effects of Passiflora edulis Peel in the Dextran Sodium Sulphate Model of Mouse Colitis. J. Funct. Foods 2016, 26, 565–576. [Google Scholar] [CrossRef]
- Ajayi, I.A. Comparative Study of the Chemical Composition and Mineral Element Content of Artocarpus heterophyllus and Treculia Africana Seeds and Seed Oils. Bioresour. Technol. 2008, 99, 5125–5129. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, Z.; Xie, X.; Wang, H.; Wang, H.; Wang, Z.; Sha, X.; Lu, Y. Jackfruit (Artocarpus heterophyllus Lam.) Peel: A Better Source of Antioxidants and a -Glucosidase Inhibitors than Pulp, Flake and Seed, and Phytochemical Profile by HPLC-QTOF-MS/MS. Food Chem. 2017, 234, 303–313. [Google Scholar] [CrossRef]
- de Santana, F.C.; de Oliveira Torres, L.R.; Shinagawa, F.B.; de Oliveira e Silva, A.M.; Yoshime, L.T.; de Melo, I.L.P.; Marcellini, P.S.; Mancini-Filho, J. Optimization of the Antioxidant Polyphenolic Compounds Extraction of Yellow Passion Fruit Seeds (Passiflora Edulis Sims) by Response Surface Methodology. J. Food Sci. Technol. 2017, 54, 3552–3561. [Google Scholar] [CrossRef]
- Mamun, F.; Rahman, M.M.; Zamila, M.; Subhan, N.; Hossain, H.; Raquibul Hasan, S.M.; Alam, M.A.; Haque, M.A. Polyphenolic Compounds of Litchi Leaf Augment Kidney and Heart Functions in 2K1C Rats. J. Funct. Foods 2020, 64, 103662. [Google Scholar] [CrossRef]
- Santos, I.B.; de Bem, G.F.; da Costa, C.A.; de Carvalho, L.C.R.M.; de Medeiros, A.F.; Silva, D.L.B.; Romão, M.H.; de Andrade Soares, R.; Ognibene, D.T.; de Moura, R.S.; et al. Açaí Seed Extract Prevents the Renin-Angiotensin System Activation, Oxidative Stress and Inflammation in White Adipose Tissue of High-Fat Diet–Fed Mice. Nutr. Res. 2020, 79, 35–49. [Google Scholar] [CrossRef]
- de Almeida, M.E.F.; Ferreira, J.T.; Augusto-Obara, T.R.; da Cruz, R.G.; Arruda, H.S.; Santos, V.S.; de Souza Cruz Ramos Ramos, J.A.; Botrel, D.A.; Botrel, R.V. de B.F. Can Lychee Reducing the Adipose Tissue Mass in Rats? Braz. Arch. Biol. Technol. 2018, 61, 1–12. [Google Scholar] [CrossRef]
- Bhat, R.S.; Al-daihan, S. Antimicrobial Activity of Litchi chinensis and Nephelium lappaceum Aqueous Seed Extracts against Some Pathogenic Bacterial Strains. J. King Saud Univ.-Sci. 2014, 26, 79–82. [Google Scholar] [CrossRef]
- Daud, M.N.H.; Fatanah, D.N.; Abdullah, N.; Ahmad, R. Evaluation of Antioxidant Potential of Artocarpus heterophyllus L. J33 Variety Fruit Waste from Different Extraction Methods and Identification of Phenolic Constituents by LCMS. Food Chem. 2017, 232, 621–632. [Google Scholar] [CrossRef]
- Abboud, K.Y.; da Luz, B.B.; Dallazen, J.L.; de PaulaWerner, M.F.; Cazarin, C.B.B.; Maróstica Junior, M.R.; Iacomini, M.; Cordeiro, L.M.C. Gastroprotective Effect of Soluble Dietary Fibres from Yellow Passion Fruit (Passiflora edulis f. flavicarpa) Peel against Ethanol-Induced Ulcer in Rats. J. Funct. Foods 2019, 54, 552–558. [Google Scholar] [CrossRef]
- Chacón-Ordóñez, T.; Esquivel, P.; Quesada, S.; Jiménez, R.R.; Cordero, A.; Carle, R.; Schweiggert, R. Mamey Sapote Fruit and Carotenoid Formulations Derived Thereof Are Dietary Sources of Vitamin A—A Comparative Randomized Cross-over Study. Food Res. Int. 2019, 122, 340–347. [Google Scholar] [CrossRef]
- Garcia-Amezquita, L.E.; Tejada-Ortigoza, V.; Heredia-Olea, E.; Serna-Saldívar, S.O.; Welti-Chanes, J. Differences in the Dietary Fiber Content of Fruits and Their By-Products Quantified by Conventional and Integrated AOAC Official Methodologies. J. Food Compos. Anal. 2018, 67, 77–85. [Google Scholar] [CrossRef]
- Ferreira, D.S.; Gomes, A.L.; Gomes da Silva, M.; Alves, A.B.; Dall Agnol, W.H.; Ferrari, R.A.; Nogueira Carvalho, P.R.; Bertoldo Pacheco, M.T. Antioxidant Capacity and Chemical Characterization of Açaí (Euterpe oleracea Mart.) Fruit Fractions. Food Sci. Technol. 2016, 4, 95–102. [Google Scholar] [CrossRef]
- Pessoa, J.D.C.; Arduin, M.; Martins, M.A.; de Carvalho, J.E.U. Characterization of Açaí (E. oleracea) Fruits and Its Processing Residues. Braz. Arch. Biol. Technol. 2010, 53, 1451–1460. [Google Scholar] [CrossRef]
- Heinrich, M.; Dhanji, T.; Casselman, I. Açai (Euterpe oleracea Mart.)—A Phytochemical and Pharmacological Assessment of the Species’ Health Claims. Phytochem. Lett. 2011, 4, 10–21. [Google Scholar] [CrossRef]
- Gordon, A.; Cruz, A.P.G.; Cabral, L.M.C.; De Freitas, S.C.; Taxi, C.M.A.D.; Donangelo, C.M.; De Andrade Mattietto, R.; Friedrich, M.; Da Matta, V.M.; Marx, F. Chemical Characterization and Evaluation of Antioxidant Properties of Açaí Fruits (Euterpe Oleraceae Mart.) during Ripening. Food Chem. 2012, 133, 256–263. [Google Scholar] [CrossRef] [PubMed]
- dos Reis, L.C.R.; Facco, E.M.P.; Salvador, M.; Flôres, S.H.; de Oliveira Rios, A. Antioxidant Potential and Physicochemical Characterization of Yellow, Purple and Orange Passion Fruit. J. Food Sci. Technol. 2018, 55, 2679–2691. [Google Scholar] [CrossRef]
- Pinheiro, E.R.; Silva, I.M.D.A.; Gonzaga, L.V.; Amante, E.R.; Teófilo, R.F.; Ferreira, M.M.C.; Amboni, R.D.M.C. Optimization of Extraction of High-Ester Pectin from Passion Fruit Peel (Passiflora edulis Flavicarpa) with Citric Acid by Using Response Surface Methodology. Bioresour. Technol. 2008, 99, 5561–5566. [Google Scholar] [CrossRef]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Bonafe, E.G.; Suzuki, R.M.; Souza, N.E.; Matsushita, M.; Visentainer, J.V. Proximate Composition, Mineral Contents and Fatty Acid Composition of the Different Parts and Dried Peels of Tropical Fruits Cultivated in Brazil. J. Braz. Chem. Soc. 2017, 28, 308–318. [Google Scholar] [CrossRef]
- Cabral, T.A.; Cardoso, L.D.M.; Pinheiro-Sant’Ana, H.M. Chemical Composition, Vitamins and Minerals of a New Cultivar of Lychee (Litchi chinensis Cv. Tailandes) Grown in Brazil. Fruits 2014, 69, 425–434. [Google Scholar] [CrossRef]
- de Rezende Queiroz, E.; de Abreu, C.M.P.; Denise, A.R.; Mariana, A.B.; Queiroz, E.D.R.; De Abreu, C.M.P.; Rocha, D.A.; Simo, A.A.; Bastos, V.A.A.; Botelho, L.N.S.; et al. Anti-Nutritional Compounds in Fresh and Dried Lychee Fractions (Litchi chinensis Sonn.). Afr. J. Agric. Res. 2015, 10, 499–504. [Google Scholar] [CrossRef]
- Jagadeesh, S.L.; Reddy, B.S.; Swamy, G.S.K.; Gorbal, K.; Hegde, L.; Raghavan, G.S.V. Chemical Composition of Jackfruit (Artocarpus heterophyllus Lam.) Selections of Western Ghats of India. Food Chem. 2007, 102, 361–365. [Google Scholar] [CrossRef]
- López-Vargas, J.H.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, Physico-Chemical, Technological, Antibacterial and Antioxidant Properties of Dietary Fiber Powder Obtained from Yellow Passion Fruit (Passiflora edulis Var. Flavicarpa) Co-Products. Food Res. Int. 2013, 51, 756–763. [Google Scholar] [CrossRef]
- Solís-Fuentes, J.A.; Ayala-Tirado, R.C.; Fernández-Suárez, A.D.; Durán-De-Bazúa, M.C. Mamey Sapote Seed Oil (Pouteria sapota). Potential, Composition, Fractionation and Thermal Behavior. Grasas y Aceites 2015, 66, e056. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Soriano Sancho, R.A.; Pereira, A.P.A.; Pastore, G.M. Small Brazilian Wild Fruits: Nutrients, Bioactive Compounds, Health-Promotion Properties and Commercial Interest. Food Res. Int. 2018, 103, 345–360. [Google Scholar] [CrossRef]
- de Rezende Queiroz, E.; de Abreu, C.M.P.; da Silva Oliveira, K. Constituintes Químicos Das Frações de Lichia in Natura e Submetidas à Secagem: Potencial Nutricional Dos Subprodutos. Rev. Bras. Frutic. 2012, 34, 1174–1179. [Google Scholar] [CrossRef]
- Baliga, M.S.; Shivashankara, A.R.; Haniadka, R.; Dsouza, J.; Bhat, H.P. Phytochemistry, Nutritional and Pharmacological Properties of Artocarpus heterophyllus Lam (Jackfruit): A Review. Food Res. Int. 2011, 44, 1800–1811. [Google Scholar] [CrossRef]
- Aranha, L.N.; Silva, M.G.; Uehara, S.K.; Luiz, R.R.; Nogueira Neto, J.F.; Rosa, G.; Moraes de Oliveira, G.M. Effects of a Hypoenergetic Diet Associated with Açaí (Euterpe oleracea Mart.) Pulp Consumption on Antioxidant Status, Oxidative Stress and Inflammatory Biomarkers in Overweight, Dyslipidemic Individuals. Clin. Nutr. 2020, 39, 1464–1469. [Google Scholar] [CrossRef]
- Moo-Huchin, V.M.; Estrada-Mota, I.; Estrada-León, R.; Cuevas-Glory, L.; Ortiz-Vázquez, E.; De Lourdes Vargas, Y.; Vargas, M.; Betancur-Ancona, D.; Sauri-Duch, E. Determination of Some Physicochemical Characteristics, Bioactive Compounds and Antioxidant Activity of Tropical Fruits from Yucatan, Mexico. Food Chem. 2014, 152, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Alia-Tejacal, I.; Villanueva-Arce, R.; Pelayo-Zaldívar, C.; Colinas-León, M.T.; López-Martínez, V.; Bautista-Baños, S. Postharvest Physiology and Technology of Sapote Mamey Fruit (Pouteria sapota (Jacq.) H.E. Moore & Stearn). Postharvest Biol. Technol. 2007, 45, 285–297. [Google Scholar] [CrossRef]
- Oliveira, S.R.; Chacón-Madrid, K.; Arruda, M.A.Z.; Barbosa Júnior, F. In Vitro Gastrointestinal Digestion to Evaluate the Total, Bioaccessible and Bioavailable Concentrations of Iron and Manganese in Açaí (Euterpe oleracea Mart.) Pulps. J. Trace Elem. Med. Biol. 2019, 53, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.K.; Kivimäenpää, M.; Julkunen-Tiitto, R. New Light for Phytochemicals. Trends Biotechnol. 2018, 36, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Rajbhar, K.; Dawda, H.; Mukundan, U. Polyphenols: Methods of Extraction. Sci. Revs. Chem. Commun 2015, 5, 1–6. [Google Scholar]
- Melo, P.S.; Arrivetti, L.d.O.R.; de Alencar, S.M.; Skibsted, L.H. Antioxidative and Prooxidative Effects in Food Lipids and Synergism with α-Tocopherol of Açaí Seed Extracts and Grape Rachis Extracts. Food Chem. 2016, 213, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Teresa, M.; Marcos, R.; Hernández, S. Daños Por Frío En Zapote Mamey (Pouteria sapota (Jacq.) H. E. Moore and Stearn). I. Cambios En Volátiles, Firmeza y Azúcares Totales. Rev. Fitotec. Mex. 2005, 28, 17–24. [Google Scholar]
- Torres-Rodríguez, A.; Salinas-Moreno, Y.; Valle-Guadarrama, S.; Alia-Tejacal, I. Soluble Phenols and Antioxidant Activity in Mamey Sapote (Pouteria sapota) Fruits in Postharvest. Food Res. Int. 2011, 44, 1956–1961. [Google Scholar] [CrossRef]
- Yahia, E.M.; Gutiérrez-Orozco, F.; Arvizu-de Leon, C. Phytochemical and Antioxidant Characterization of Mamey (Pouteria sapota Jacq. H.E. Moore & Stearn) Fruit. Food Res. Int. 2011, 44, 2175–2181. [Google Scholar] [CrossRef]
- Zhang, R.; Zeng, Q.; Deng, Y.; Zhang, M.; Wei, Z.; Zhang, Y.; Tang, X. Phenolic Profiles and Antioxidant Activity of Litchi Pulp of Different Cultivars Cultivated in Southern China. Food Chem. 2013, 136, 1169–1176. [Google Scholar] [CrossRef]
- Bataglion, G.A.; Da Silva, F.M.A.; Eberlin, M.N.; Koolen, H.H.F. Determination of the Phenolic Composition from Brazilian Tropical Fruits by UHPLC-MS/MS. Food Chem. 2015, 180, 280–287. [Google Scholar] [CrossRef]
- Dantas, A.M.; Mafaldo, I.M.; de Lima Oliveira, P.M.; dos Santos Lima, M.; Magnani, M.; Borges, G.D.S.C. Bioaccessibility of Phenolic Compounds in Native and Exotic Frozen Pulps Explored in Brazil Using a Digestion Model Coupled with a Simulated Intestinal Barrier. Food Chem. 2019, 274, 202–214. [Google Scholar] [CrossRef]
- Meinhart, A.D.; Damin, F.M.; Caldeirão, L.; de Jesus Filho, M.; da Silva, L.C.; da Silva Constant, L.; Filho, J.T.; Wagner, R.; Godoy, H.T. Chlorogenic and Caffeic Acids in 64 Fruits Consumed in Brazil. Food Chem. 2019, 286, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Zeraik, M.L.; Yariwake, J.H. Quantification of Isoorientin and Total Flavonoids in Passiflora edulis Fruit Pulp by HPLC-UV/DAD. Microchem. J. 2010, 96, 86–91. [Google Scholar] [CrossRef]
- Ma, J.; Yang, H.; Basile, M.J.; Kennelly, E.J. Analysis of Polyphenolic Antioxidants from the Fruits of Three Pouteria Species by Selected Ion Monitoring Liquid Chromatography-Mass Spectrometry. J. Agric. Food Chem. 2004, 52, 5873–5878. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Si, M.; Yan, Y.; Luo, F.; Hu, G.; Wu, H.; Sun, C.; Li, X.; Chen, K. Effects of Phenolic-Rich Litchi (Litchi chinensis Sonn.) Pulp Extracts on Glucose Consumption in Human HepG2 Cells. J. Funct. Foods 2014, 7, 621–629. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food sources, bioavailability, metabolism, and bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Cai, D.; Li, X.; Chen, J.; Jiang, X.; Ma, X.; Sun, J.; Tian, L.; Vidyarthi, S.K.; Xu, J.; Pan, Z.; et al. A Comprehensive Review on Innovative and Advanced Stabilization Approaches of Anthocyanin by Modifying Structure and Controlling Environmental Factors. Food Chem. 2022, 366, 130611. [Google Scholar] [CrossRef]
- Lucas, B.F.; Zambiazi, R.C.; Costa, J.A.V. Biocompounds and Physical Properties of Açaí Pulp Dried by Different Methods. Lwt 2018, 98, 335–340. [Google Scholar] [CrossRef]
- de Jesus, A.L.T.; Cristianini, M.; dos Santos, N.M.; Maróstica Júnior, M.R. Effects of High Hydrostatic Pressure on the Microbial Inactivation and Extraction of Bioactive Compounds from Açaí (Euterpe oleracea Martius) Pulp. Food Res. Int. 2020, 130, 108856. [Google Scholar] [CrossRef]
- Almeida, M.M.B.; de Sousa, P.H.M.; Arriaga, Â.M.C.; do Prado, G.M.; de Carvalho Magalhães, C.E.; Maia, G.A.; de Lemos, T.L.G. Bioactive Compounds and Antioxidant Activity of Fresh Exotic Fruits from Northeastern Brazil. Food Res. Int. 2011, 44, 2155–2159. [Google Scholar] [CrossRef]
- Cacciola, F.; Giuffrida, D.; Utczas, M.; Mangraviti, D.; Dugo, P.; Menchaca, D.; Murillo, E.; Mondello, L. Application of Comprehensive Two-Dimensional Liquid Chromatography for Carotenoid Analysis in Red Mamey (Pouteria sapote) Fruit. Food Anal. Methods 2016, 9, 2335–2341. [Google Scholar] [CrossRef]
- Agócs, A.; Murillo, E.; Turcsi, E.; Béni, S.; Darcsi, A.; Szappanos, Á.; Kurtán, T.; Deli, J. Isolation of Allene Carotenoids from Mamey. J. Food Compos. Anal. 2018, 65, 1–5. [Google Scholar] [CrossRef]
- Pertuzatti, P.B.; Sganzerla, M.; Jacques, A.C.; Barcia, M.T.; Zambiazi, R.C. Carotenoids, Tocopherols and Ascorbic Acid Content in Yellow Passion Fruit (Passiflora edulis) Grown under Different Cultivation Systems. LWT-Food Sci. Technol. 2015, 64, 259–263. [Google Scholar] [CrossRef]
- Ranasinghe, R.A.S.N.; Maduwanthi, S.D.T.; Marapana, R.A.U.J. Nutritional and Health Benefits of Jackfruit (Artocarpus heterophyllus Lam.): A Review. Int. J. Food Sci. 2019, 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Murillo, E.; McLean, R.; Britton, G.; Agócs, A.; Nagy, V.; Deli, J. Sapotexanthin, an A-Provitamin Carotenoid from Red Mamey (Pouteria sapota). J. Nat. Prod. 2011, 74, 283–285. [Google Scholar] [CrossRef]
- Tan, S.; Tang, J.; Shi, W.; Wang, Z.; Xiang, Y.; Deng, T.; Gao, X.; Li, W.; Shi, S. Effects of Three Drying Methods on Polyphenol Composition and Antioxidant Activities of Litchi chinensis Sonn. Food Sci. Biotechnol. 2020, 29, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Wang, Z.; Dong, L.; Huang, F.; Zhang, R.; Jia, X.; Wu, G.; Zhang, M. Impact of Thermal Processing and Storage Temperature on the Phenolic Profile and Antioxidant Activity of Different Varieties of Lychee Juice. Lwt 2019, 116, 108578. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Stanislas, G.; Douraguia, E.; Gonthier, M.P. Evaluation of Nutritional and Antioxidant Properties of the Tropical Fruits Banana, Litchi, Mango, Papaya, Passion Fruit and Pineapple Cultivated in Réunion French Island. Food Chem. 2016, 212, 225–233. [Google Scholar] [CrossRef]
- Sauri, E.; Tamayo, E.; Díaz, J.; García, K.; Vargas, L.; González, S.; Centurión, A. Calidad y Vida Útil de Dos Cultivares de Mamey (Pouteria sapota) Cosechados en Yucatán, México. V Congreso Iberoamericano de Tecnología Postcosecha y Agroexportaciones 2007, 2007, 463–470. [Google Scholar]
- Basu, P.; Maier, C. In Vitro Antioxidant Activities and Polyphenol Contents of Seven Commercially Available Fruits. Pharmacog. Res. 2016, 8, 258–264. [Google Scholar] [CrossRef]
- Ambriz-Perez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic Compounds: Natural Alternative in Inflammation Treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar] [CrossRef]
- de Albuquerque, M.A.C.; Levit, R.; Beres, C.; Bedani, R.; de Moreno de LeBlanc, A.; Saad, S.M.I.; LeBlanc, J.G. Tropical Fruit By-Products Water Extracts of Tropical Fruit by-Products as Sources of Soluble Fibres and Phenolic Compounds with Potential Antioxidant, Anti-Inflammatory, and Functional Properties. J. Funct. Foods 2019, 52, 724–733. [Google Scholar] [CrossRef]
- Derouich, M.; Bouhlali, E.D.T.; Hmidani, A.; Bammou, M.; Bourkhis, B.; Sellam, K.; Alem, C. Assessment of Total Polyphenols, Flavonoids and Anti-Inflammatory Potential of Three Apiaceae Species Grown in the Southeast of Morocco. Sci. Afr. 2020, 9, e00507. [Google Scholar] [CrossRef]
- De Oliveira, P.R.B.; Da Costa, C.A.; De Bem, G.F.; Cordeiro, V.S.C.; Santos, I.B.; De Carvalho, L.C.R.M.; Da Conceição, E.P.S.; Lisboa, P.C.; Ognibene, D.T.; Sousa, P.J.C.; et al. Euterpe oleracea Mart.-Derived Polyphenols Protect Mice from Diet-Induced Obesity and Fatty Liver by Regulating Hepatic Lipogenesis and Cholesterol Excretion. PLoS ONE 2015, 10, e0143721. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Rodríguez, G.; García, M.C.; Plaza, M.; Marina, M.L. Revalorization of Passiflora Species Peels as a Sustainable Source of Antioxidant Phenolic Compounds. Sci. Total Environ. 2019, 696, 134030. [Google Scholar] [CrossRef]
- Silva, M.P.; Cunha, V.M.B.; Sousa, S.H.B.; Menezes, E.G.O.; Bezerra, P.D.N.; De Farias Neto, J.T.; Filho, G.N.R.; Araújo, M.E.; De Carvalho, R.N. Supercritical CO2 Extraction of Lyophilized Açaí (Euterpe oleracea Mart.) Pulp Oil from Three Municipalities in the State of Pará, Brazil. J. CO2 Util. 2019, 31, 226–234. [Google Scholar] [CrossRef]
- Fang, S.C.; Hsu, C.L.; Yen, G.C. Anti-Inflammatory Effects of Phenolic Compounds Isolated from the Fruits of Artocarpus heterophyllus. J. Agric. Food Chem. 2008, 56, 4463–4468. [Google Scholar] [CrossRef]
- Yao, X.; Wu, D.; Dong, N.; Ouyang, P.; Pu, J.; Hu, Q.; Wang, J.; Lu, W.; Huang, J. Moracin C, A Phenolic Compound Isolated from Artocarpus heterophyllus, Suppresses Lipopolysaccharide-Activated Inflammatory Responses in Murine Raw264.7 Macrophages. Int. J. Mol. Sci. 2016, 17, 1199. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, G.C.; Batista, Â.G.; Carazin, C.B.B.; Cintra, D.E.; Prado, M.A.; Júnior, M.R.M. Passion Fruit Peel Intake Decreases Inflammatory Response and Reverts Lipid Peroxidation and Adiposity in Diet-Induced Obese Rats. Nutr. Res. 2020, 76, 106–117. [Google Scholar] [CrossRef]
- Schauss, A.G.; Wu, X.; Prior, R.L.; Ou, B.; Huang, D.; Owens, J.; Agarwal, A.; Jensen, G.S.; Hart, A.N.; Shanbrom, E. Antioxidant Capacity and Other Bioactivities of the Freeze-Dried Amazonian Palm Berry, Euterpe Oleraceae Mart. (Acai). J. Agric. Food Chem. 2006, 54, 8604–8610. [Google Scholar] [CrossRef]
- Noratto, G.D.; Angel-Morales, G.; Talcott, S.T.; Mertens-Talcott, S.U. Polyphenolics from Açaí (Euterpe oleracea Mart.) and Red Muscadine Grape (Vitis rotundifolia) Protect Human Umbilical Vascular Endothelial Cells (HUVEC) from Glucose- and Lipopolysaccharide (LPS)-Induced Inflammation and Target MicroRNA-126. J. Agric. Food Chem. 2011, 59, 7999–8012. [Google Scholar] [CrossRef]
- Kang, J.; Xie, C.; Li, Z.; Nagarajan, S.; Schauss, A.G.; Wu, T.; Wu, X. Flavonoids from Acai (Euterpe oleracea Mart.) Pulp and Their Antioxidant and Anti-Inflammatory Activities. Food Chem. 2011, 128, 152–157. [Google Scholar] [CrossRef]
- Nishizawa, M.; Hara, T.; Miura, T.; Fujita, S.; Yoshigai, E.; Ue, H.; Hayashi, Y.; Kwon, A.H.; Okumura, T.; Isaka, T. Supplementation with a Flavanol-Rich Lychee Fruit Extract Influences the Inflammatory Status of Young Athletes. Phyther. Res. 2011, 25, 1486–1493. [Google Scholar] [CrossRef]
- Yamanishi, R.; Yoshigai, E.; Okuyama, T.; Mori, M.; Murase, H.; Machida, T.; Okumura, T.; Nishizawa, M. The Anti-Inflammatory Effects of Flavanol-Rich Lychee Fruit Extract in Rat Hepatocytes. PLoS ONE 2014, 9, e93818. [Google Scholar] [CrossRef] [PubMed]
- Man, S.; Ma, J.; Wang, C.; Li, Y.; Gao, W.; Lu, F. Chemical Composition and Hypoglycaemic Effect of Polyphenol Extracts from Litchi chinensis Seeds. J. Funct. Foods 2016, 22, 313–324. [Google Scholar] [CrossRef]
- Wei, B.L.; Weng, J.R.; Chiu, P.H.; Hung, C.F.; Wang, J.P.; Lin, C.N. Antiinflammatory Flavonoids from Artocarpus heterophyllus and Artocarpus communis. J. Agric. Food Chem. 2005, 53, 3867–3871. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Kang, J.; Li, Z.; Schauss, A.G.; Badger, T.M.; Nagarajan, S.; Wu, T.; Wu, X. The Açaí Flavonoid Velutin Is a Potent Anti-Inflammatory Agent: Blockade of LPS-Mediated TNF-α and IL-6 Production through Inhibiting NF-ΚB Activation and MAPK Pathway. J. Nutr. Biochem. 2012, 23, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Shin, M.S.; Kang, Y.; Park, K.; Maeda, T.; Nishioka, H.; Fujii, H.; Kang, I. Oligonol, a Lychee Fruit-Derived Low-Molecular Form of Polyphenol Mixture, Suppresses Inflammatory Cytokine Production from Human Monocytes. Hum. Immunol. 2016, 77, 512–515. [Google Scholar] [CrossRef]
- Zibadi, S.; Farid, R.; Moriguchi, S.; Lu, Y.; Foo, L.Y.; Tehrani, P.M.; Ulreich, J.B.; Watson, R.R. Oral Administration of Purple Passion Fruit Peel Extract Attenuates Blood Pressure in Female Spontaneously Hypertensive Rats and Humans. Nutr. Res. 2007, 27, 408–416. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belmonte-Herrera, B.H.; Domínguez-Avila, J.A.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Preciado-Saldaña, A.M.; Salazar-López, N.J.; López-Martínez, L.X.; Yahia, E.M.; Robles-Sánchez, R.M.; González-Aguilar, G.A. Lesser-Consumed Tropical Fruits and Their by-Products: Phytochemical Content and Their Antioxidant and Anti-Inflammatory Potential. Nutrients 2022, 14, 3663. https://doi.org/10.3390/nu14173663
Belmonte-Herrera BH, Domínguez-Avila JA, Wall-Medrano A, Ayala-Zavala JF, Preciado-Saldaña AM, Salazar-López NJ, López-Martínez LX, Yahia EM, Robles-Sánchez RM, González-Aguilar GA. Lesser-Consumed Tropical Fruits and Their by-Products: Phytochemical Content and Their Antioxidant and Anti-Inflammatory Potential. Nutrients. 2022; 14(17):3663. https://doi.org/10.3390/nu14173663
Chicago/Turabian StyleBelmonte-Herrera, Beatriz Haydee, J. Abraham Domínguez-Avila, Abraham Wall-Medrano, J. Fernando Ayala-Zavala, Alejandra M. Preciado-Saldaña, Norma J. Salazar-López, Leticia X. López-Martínez, Elhadi M. Yahia, R. Maribel Robles-Sánchez, and Gustavo A. González-Aguilar. 2022. "Lesser-Consumed Tropical Fruits and Their by-Products: Phytochemical Content and Their Antioxidant and Anti-Inflammatory Potential" Nutrients 14, no. 17: 3663. https://doi.org/10.3390/nu14173663
APA StyleBelmonte-Herrera, B. H., Domínguez-Avila, J. A., Wall-Medrano, A., Ayala-Zavala, J. F., Preciado-Saldaña, A. M., Salazar-López, N. J., López-Martínez, L. X., Yahia, E. M., Robles-Sánchez, R. M., & González-Aguilar, G. A. (2022). Lesser-Consumed Tropical Fruits and Their by-Products: Phytochemical Content and Their Antioxidant and Anti-Inflammatory Potential. Nutrients, 14(17), 3663. https://doi.org/10.3390/nu14173663













