Hawk Tea Flavonoids as Natural Hepatoprotective Agents Alleviate Acute Liver Damage by Reshaping the Intestinal Microbiota and Modulating the Nrf2 and NF-κB Signaling Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
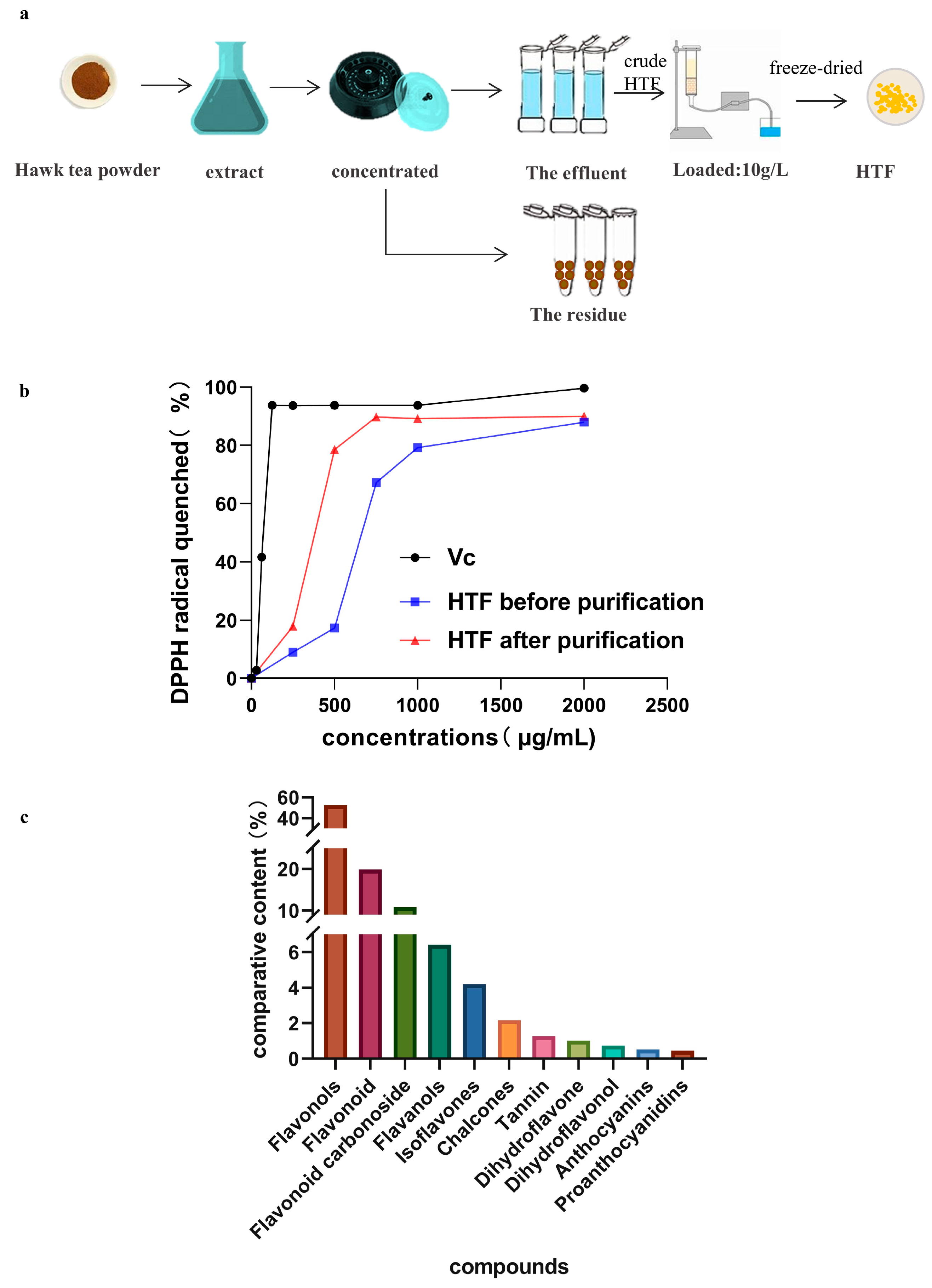
2.2. Preparations of HTF
2.3. Total Flavonoids Quantification
2.4. Qualitation of HTF Composition
2.5. In Vitro Antioxidant Activity
2.6. Animal Experimental Design
2.7. Biochemical Analysis
2.8. Histopathological Analysis and Lipid Droplets Quantification
2.9. ELISA Kit Analysis
2.10. Western Blot Analysis
2.11. Gut Microbiota Analysis
2.12. Statistical Analysis
3. Results
3.1. In Vitro Antioxidant Activity and Identification of HTF
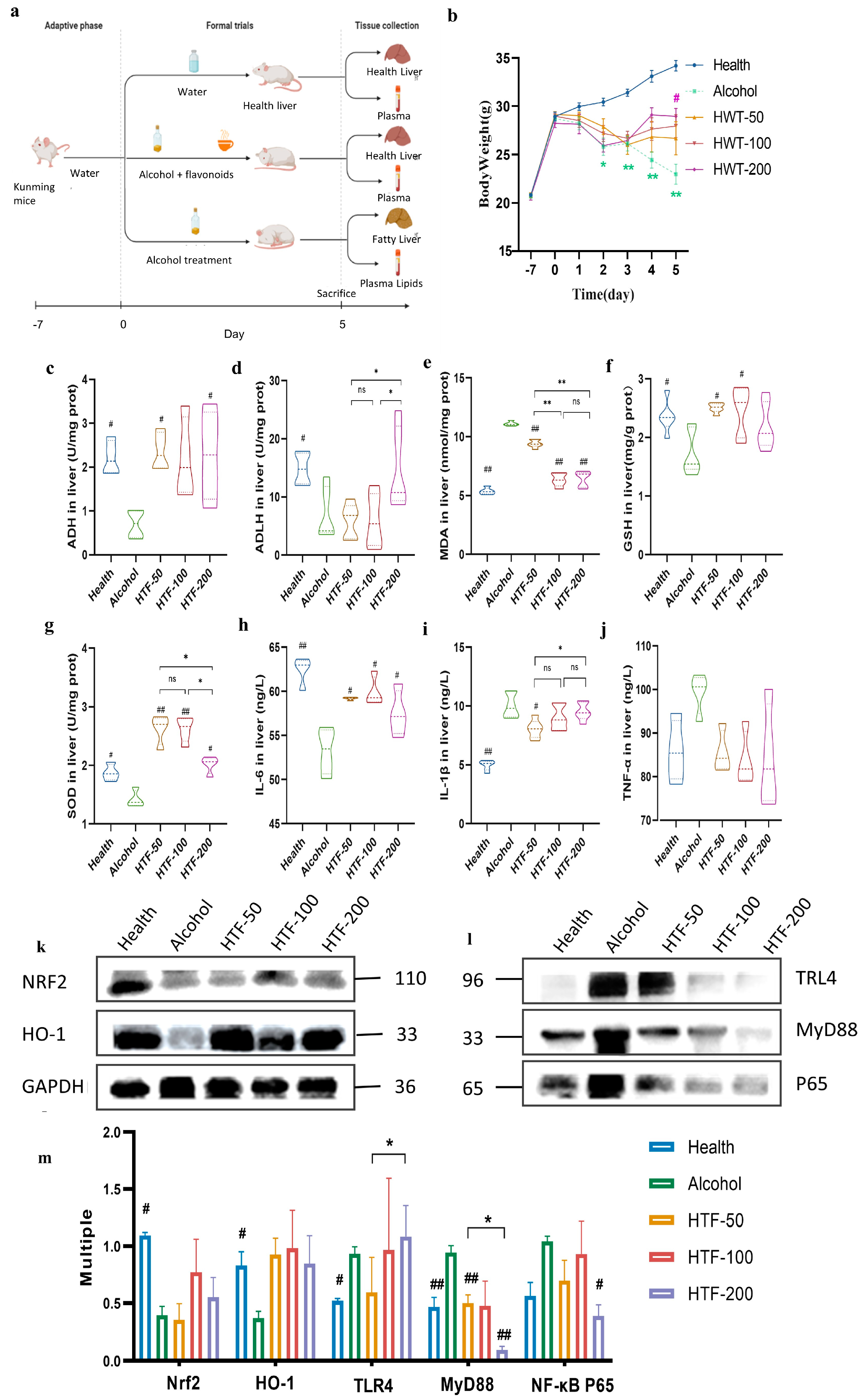
3.2. HTF Reduced Alcohol-Induced Body Weight Loss
3.3. HTF Reduced Alcohol-Induced Oxidative Stress
3.4. HTF Inhibited Hepatic Inflammation in Acute ALD-Model Mice
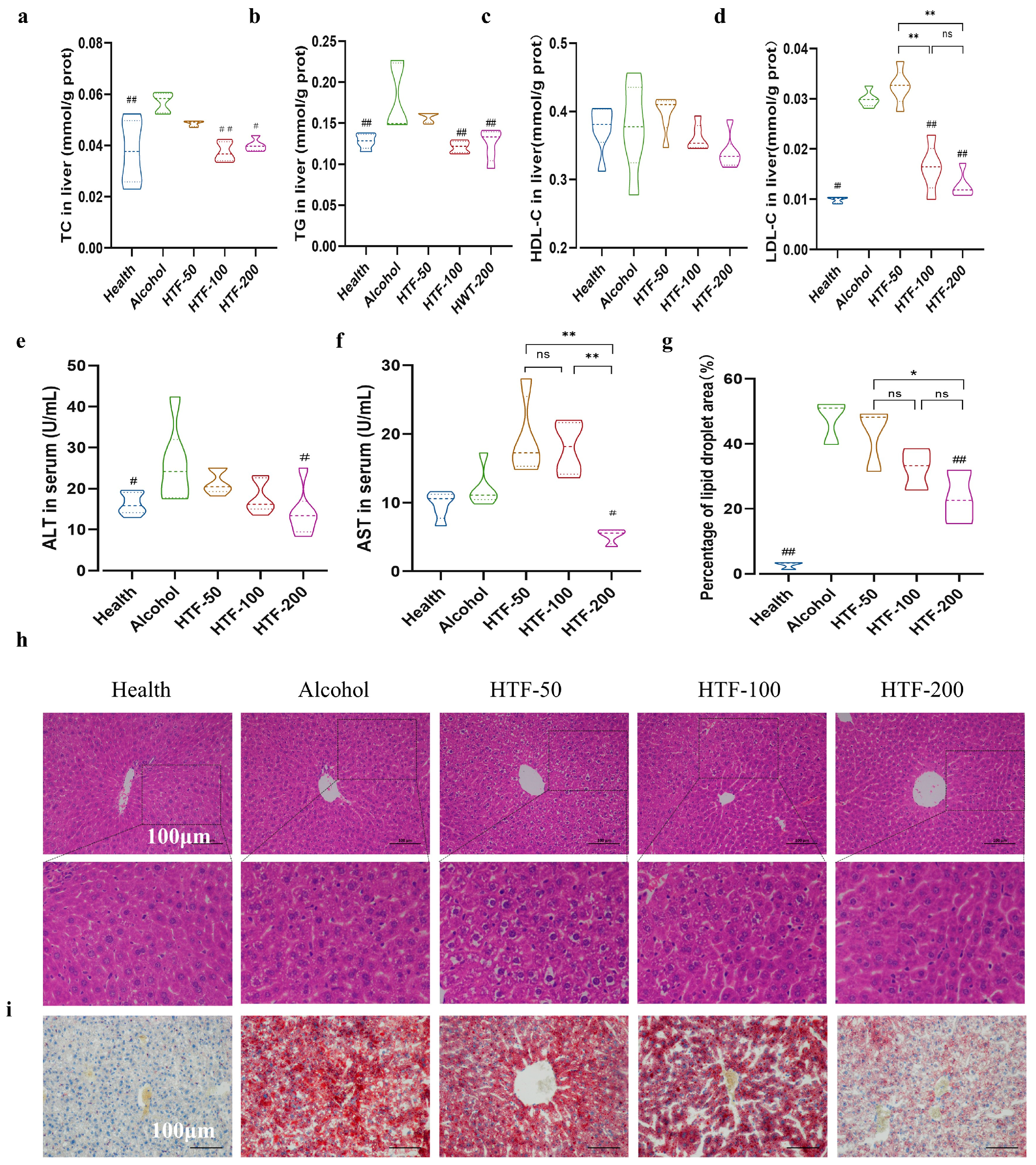
3.5. HTF Reduced Alcohol-Induced Lipid Deposition in the Liver
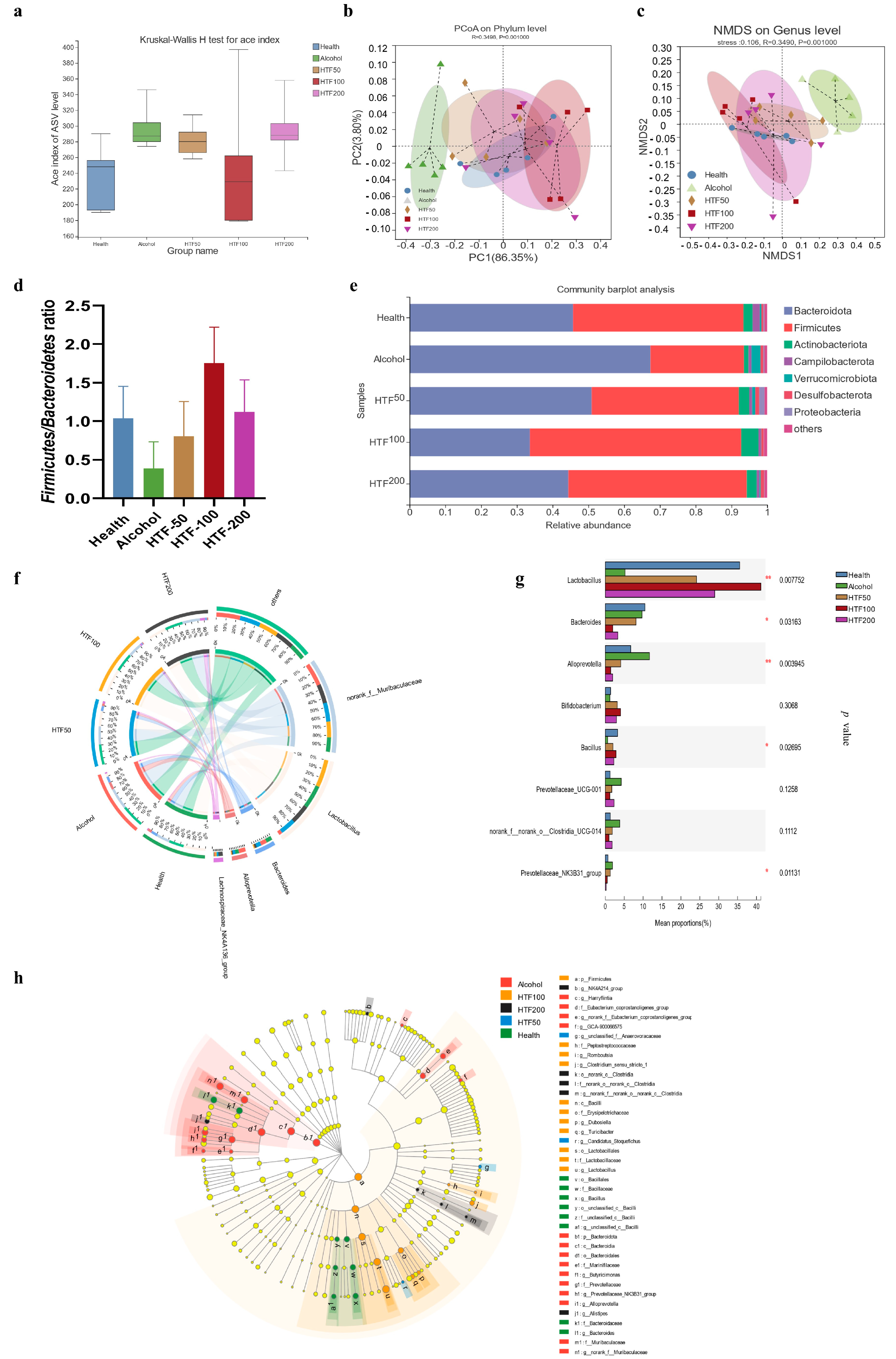
3.6. HTF Reshaped the Gut Microbiota in Acute ALD-Model Mice
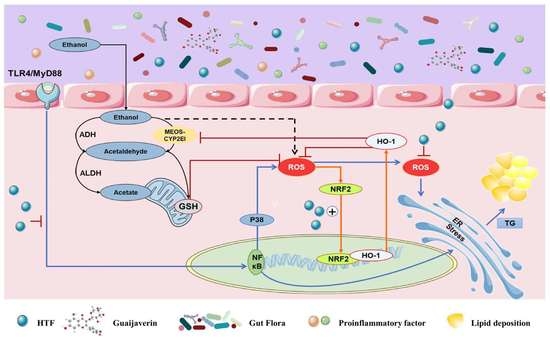
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akbar, M.; Egli, M.; Cho, Y.E.; Song, B.J.; Noronha, A. Medications for alcohol use disorders: An overview. Pharmacol. Ther. 2018, 185, 64–85. [Google Scholar] [CrossRef]
- Szabo, G.; Kamath, P.S.; Shah, V.H.; Thursz, M.; Mathurin, P.; Meeting, E.-A.J. Alcohol-Related Liver Disease: Areas of Consensus, Unmet Needs and Opportunities for Further Study. Hepatology 2019, 69, 2271–2283. [Google Scholar] [CrossRef]
- Louvet, A.; Mathurin, P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 231–242. [Google Scholar] [CrossRef]
- Miller, A.M.; Horiguchi, N.; Jeong, W.I.; Radaeva, S.; Gao, B. Molecular mechanisms of alcoholic liver disease: Innate immunity and cytokines. Alcohol. Clin. Exp. Res. 2011, 35, 787–793. [Google Scholar] [CrossRef]
- Kong, L.Z.; Chandimali, N.; Han, Y.H.; Lee, D.H.; Kim, J.S.; Kim, S.U.; Kim, T.D.; Jeong, D.K.; Sun, H.N.; Lee, D.S.; et al. Pathogenesis, Early Diagnosis, and Therapeutic Management of Alcoholic Liver Disease. Int. J. Mol. Sci. 2019, 20, 107752. [Google Scholar] [CrossRef]
- Wen, B.; Zhang, C.; Zhou, J.; Zhang, Z.; Che, Q.; Cao, H.; Bai, Y.; Guo, J.; Su, Z. Targeted treatment of alcoholic liver disease based on inflammatory signalling pathways. Pharmacol. Ther. 2021, 222, 107752. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Q.; Wen, Z.; Feng, H.; Deng, X.; Ci, X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3beta-Nrf2 signal axis. Redox Biol. 2017, 12, 311–324. [Google Scholar] [CrossRef]
- Tossetta, G.; Marzioni, D. Natural and synthetic compounds in Ovarian Cancer: A focus on NRF2/KEAP1 pathway. Pharmacol. Res. 2022, 183, 106365. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Pourbagher-Shahri, A.M.; Azimi-Nezhad, M.; Forouzanfar, F.; Brockmueller, A.; Ashrafizadeh, M.; Talebi, M.; Shakibaei, M.; Samarghandian, S. Roles of Nrf2 in Gastric Cancer: Targeting for Therapeutic Strategies. Molecules 2021, 26, 3157. [Google Scholar] [CrossRef]
- Tascioglu Aliyev, A.; Panieri, E.; Stepanić, V.; Gurer-Orhan, H.; Saso, L. Involvement of NRF2 in Breast Cancer and Possible Therapeutical Role of Polyphenols and Melatonin. Molecules 2021, 26, 1853. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal. Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Schupack, D.A.; Mars, R.A.T.; Voelker, D.H.; Abeykoon, J.P.; Kashyap, P.C. The promise of the gut microbiome as part of individualized treatment strategies. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 7–25. [Google Scholar] [CrossRef]
- Jia, X.; Li, P.; Wan, J.; He, C. A review on phytochemical and pharmacological properties of Litsea coreana. Pharm. Biol. 2017, 55, 1368–1374. [Google Scholar] [CrossRef]
- Feng, J.; Yang, J.; Chang, Y.; Qiao, L.; Dang, H.; Luo, K.; Guo, H.; An, Y.; Ma, C.; Shao, H.; et al. Caffeine-free hawk tea lowers cholesterol by reducing free cholesterol uptake and the production of very-low-density lipoprotein. Commun. Biol. 2019, 2, 173. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Chen, Q.; Luo, L.; Ma, M.; Xiao, B.; Zeng, L. Camellia sinensis and Litsea coreana Ameliorate Intestinal Inflammation and Modulate Gut Microbiota in Dextran Sulfate Sodium-Induced Colitis Mice. Mol. Nutr. Food Res. 2020, 64, 1900943. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, Y.; Zhang, L.; Luo, L.; Xu, T.; Wang, J.; Ma, M.; Zeng, L. Chemical composition, sensory qualities, and pharmacological properties of primary leaf hawk tea as affected using different processing methods. Food Biosci. 2020, 36, 100618. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, D.; Guo, J.J.; Tao, W.; Gong, R.X.; Yao, L.; Zhang, X.L.; Cao, W.G. Active Components, Antioxidant, Inhibition on Metabolic Syndrome Related Enzymes, and Monthly Variations in Mature Leaf Hawk Tea. Molecules 2019, 24, 657. [Google Scholar] [CrossRef]
- Huang, C.; Ma, T.; Meng, X.; Lv, X.; Zhang, L.; Wang, J.; Li, J. Potential protective effects of a traditional Chinese herb, Litsea coreana Levl., on liver fibrosis in rats. J. Pharm. Pharmacol. 2010, 62, 223–230. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, L.Y.; Liao, C.X.; Chen, L.; Wang, J.; Zeng, L. Effects of brewing conditions on the phytochemical composition, sensory qualities and antioxidant activity of green tea infusion: A study using response surface methodology. Food Chem. 2018, 269, 24–34. [Google Scholar] [CrossRef]
- Fantin, M.; Garelli, F.; Napoli, B.; Forgiarini, A.; Gumeni, S.; De Martin, S.; Montopoli, M.; Vantaggiato, C.; Orso, G. Flavonoids Regulate Lipid Droplets Biogenesis in Drosophila melanogaster. Nat. Prod. Commun. 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Hu, S.S.; Li, S.; Liu, Y.; Sun, K.; Luo, L.Y.; Zeng, L. Aged Ripe Pu-erh Tea Reduced Oxidative Stress-Mediated Inflammation in Dextran Sulfate Sodium-Induced Colitis Mice by Regulating Intestinal Microbes. J. Agric. Food Chem. 2021, 69, 10592–10605. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Chen, S.; Wu, X.; Zhu, J.; Li, J. Resveratrol Relieves Gouty Arthritis by Promoting Mitophagy to Inhibit Activation of NLRP3 Inflammasomes. J. Inflamm. Res. 2021, 14, 3523–3536. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.T.; Lin, L.C.; Liu, Y.L.; Ho, S.T.; Lin, C.Y.; Chuang, H.L.; Chiu, C.C.; Huang, C.C.; Wu, J.H. Antioxidative phytochemicals from Rhododendron oldhamii Maxim. leaf extracts reduce serum uric acid levels in potassium oxonate-induced hyperuricemic mice. BMC Complement. Altern Med. 2015, 15, 423. [Google Scholar] [CrossRef] [PubMed]
- Krasteva, I.; Bratkov, V.; Bucar, F.; Kunert, O.; Kollroser, M.; Kondeva-Burdina, M.; Ionkova, I. Flavoalkaloids and Flavonoids from Astragalus monspessulanus. J. Nat. Prod. 2015, 78, 2565–2571. [Google Scholar] [CrossRef]
- Borges, G.; Ottaviani, J.I.; van der Hooft, J.J.J.; Schroeter, H.; Crozier, A. Absorption, metabolism, distribution and excretion of (-)-epicatechin: A review of recent findings. Mol. Asp. Med. 2018, 61, 18–30. [Google Scholar] [CrossRef]
- Tapas, A.R.; Sakarkar, D.M.; Kakde, R.B. Flavonoids as Nutraceuticals: A Review. Trop. J. Pharm. Res. 2008, 7, 1089–1099. [Google Scholar] [CrossRef]
- Feng, J.; Wang, C.; Liu, T.; Li, J.; Wu, L.; Yu, Q.; Li, S.; Zhou, Y.; Zhang, J.; Chen, J.; et al. Procyanidin B2 inhibits the activation of hepatic stellate cells and angiogenesis via the Hedgehog pathway during liver fibrosis. J. Cell Mol. Med. 2019, 23, 6479–6493. [Google Scholar] [CrossRef]
- Zhan, Z.Y.; Wu, M.; Shang, Y.; Jiang, M.; Liu, J.; Qiao, C.Y.; Ye, H.; Lin, Y.C.; Piao, M.H.; Sun, R.H.; et al. Taxifolin ameliorate high-fat-diet feeding plus acute ethanol binge-induced steatohepatitis through inhibiting inflammatory caspase-1-dependent pyroptosis. Food Funct. 2021, 12, 362–372. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Mei, S.; Jie, X.; Luo, Q.; Corke, H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef]
- Yang, W.; Tu, H.; Tang, K.; Huang, H.; Ou, S.; Wu, J. Reynoutrin Improves Ischemic Heart Failure in Rats Via Targeting S100A1. Front. Pharmacol. 2021, 12, 703962. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, S.; You, S.; Liu, T.; Xu, F.; Ji, T.; Gu, Z. Hepatoprotective Effects of Nicotiflorin from Nymphaea candida against Concanavalin A-Induced and D-Galactosamine-Induced Liver Injury in Mice. Int J. Mol. Sci 2017, 18, 587. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, K.; Jho, E.H.; Jung, Y.J.; Nho, C.W.; Um, B.H.; Pan, C.H. Hepatoprotective effect of flavonoid glycosides from Lespedeza cuneata against oxidative stress induced by tert-butyl hyperoxide. Phytother. Res. 2011, 25, 1011–1017. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, Q.; Deng, X.; Shi, K.; Zhang, W.; Jiang, Y.; Ma, X.; Zeng, J.; Wang, X. Old wine in new bottles: Kaempferol is a promising agent for treating the trilogy of liver diseases. Pharmacol. Res. 2022, 175, 106005. [Google Scholar] [CrossRef]
- Jayachitra, J.; Nalini, N. Effect of Naringenin (Citrus Flavanone) on Lipid Profile in Ethanol-Induced Toxicity in Rats. J. Food Biochem. 2012, 36, 502–511. [Google Scholar] [CrossRef]
- Hernandez-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef]
- Liang, X.; Hu, C.; Liu, C.; Yu, K.; Zhang, J.; Jia, Y. Dihydrokaempferol (DHK) ameliorates severe acute pancreatitis (SAP) via Keap1/Nrf2 pathway. Life Sci. 2020, 261, 118340. [Google Scholar] [CrossRef]
- Singh, C.; Prakash, C.; Mishra, P.; Tiwari, K.N.; Mishra, S.K.; More, R.S.; Kumar, V.; Singh, J. Hepatoprotective efficacy of Premna integrifolia L. leaves against aflatoxin B1-induced toxicity in mice. Toxicon 2019, 166, 88–100. [Google Scholar] [CrossRef]
- Abdallah, H.M.; Mohamed, M.A.; Abdou, A.M.; Hamed, M.M.; Abdel-Naim, A.B.; Ashour, O.M. Protective effect of Centaurea pallescens Del. against CCl4-induced injury on a human hepatoma cell line (Huh7). Med. Chem. Res. 2013, 22, 5700–5706. [Google Scholar] [CrossRef]
- Muller, C.J.F.; Malherbe, C.J.; Chellan, N.; Yagasaki, K.; Miura, Y.; Joubert, E. Potential of rooibos, its major C-glucosyl flavonoids, and Z-2-(beta-D-glucopyranosyloxy)-3-phenylpropenoic acid in prevention of metabolic syndrome. Crit Rev. Food Sci. Nutr. 2018, 58, 227–246. [Google Scholar] [CrossRef]
- Taher, R.F.; Raslan, M.A.; Masoud, M.A.; Nassar, M.I.; Aboutabl, M.E. HPLC-ESI/MS profiling, phytoconstituent isolation and evaluation of renal function, oxidative stress and inflammation in gentamicin-induced nephrotoxicity in rats of Ficus spragueana Mildbr. & Burret. Biomed. Chromatogr. 2021, 35, e5135. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Wozniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, R.; Ding, L.; Zhang, F.; Hu, L.; Ding, B.; Zhu, L.; Xia, Y.; Dou, X. Rutin exhibits hepatoprotective effects in a mouse model of non-alcoholic fatty liver disease by reducing hepatic lipid levels and mitigating lipid-induced oxidative injuries. Int. Immunopharmacol. 2017, 49, 132–141. [Google Scholar] [CrossRef]
- Yue, S.; Xue, N.; Li, H.; Huang, B.; Chen, Z.; Wang, X. Hepatoprotective Effect of Apigenin Against Liver Injury via the Non-canonical NF-κB Pathway In Vivo and In Vitro. Inflammation 2020, 254, 112714. [Google Scholar] [CrossRef]
- Song, X.; Zhang, J.; Li, J.; Jia, L. Acetylated Polysaccharides From Pleurotus geesteranus Alleviate Lung Injury Via Regulating NF-kappaB Signal Pathway. Int. J. Mol. Sci. 2020, 21, 2810. [Google Scholar] [CrossRef]
- Ali, H.; Kabir, N.; Shah, M.R.; Muhammad, A.; Ali, S.; Mehmood, S.; Ali, A.; Jahan, A. Hepatoprotective activity of viscosine is mediated by attenuation of hepatic macrophages and iNOS expression in CCl(4)-intoxicated rats. Toxicol. Res. 2016, 5, 1688–1698. [Google Scholar] [CrossRef]
- Winter, A.N.; Ross, E.K.; Khatter, S.; Miller, K.; Linseman, D.A. Chemical basis for the disparate neuroprotective effects of the anthocyanins, callistephin and kuromanin, against nitrosative stress. Free Radic. Biol. Med. 2017, 103, 23–34. [Google Scholar] [CrossRef]
- Serra, D.; Almeida, L.M.; Dinis, T.C. Anti-inflammatory protection afforded by cyanidin-3-glucoside and resveratrol in human intestinal cells via Nrf2 and PPAR-gamma: Comparison with 5-aminosalicylic acid. Chem. Biol. Interact. 2016, 260, 102–109. [Google Scholar] [CrossRef]
- Dubinkina, V.B.; Tyakht, A.V.; Odintsova, V.Y.; Yarygin, K.S.; Kovarsky, B.A.; Pavlenko, A.V.; Ischenko, D.S.; Popenko, A.S.; Alexeev, D.G.; Taraskina, A.Y.; et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome 2017, 5, 141. [Google Scholar] [CrossRef] [PubMed]
- Seeff, L.B.; Bonkovsky, H.L.; Navarro, V.J.; Wang, G. Herbal products and the liver: A review of adverse effects and mechanisms. Gastroenterology 2015, 148, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Weng, J.H.; Mitchison, T.J. Immunomodulatory drug discovery from herbal medicines: Insights from organ-specific activity and xenobiotic defenses. eLife 2021, 10, e73673. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, L.; Luo, Y.; Zhang, J.; Wang, X.; Sun, K.; Zeng, L. Prebiotic Properties of Green and Dark Tea Contribute to Protective Effects in Chemical-Induced Colitis in Mice: A Fecal Microbiota Transplantation Study. J. Agric. Food Chem. 2020, 68, 6368–6380. [Google Scholar] [CrossRef]
- Li, F.S.; Weng, J.K. Demystifying traditional herbal medicine with modern approach. Nat. Plants 2017, 3, 17109. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Wachtel-Galor, S. Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Berndt, N.; Bulik, S.; Wallach, I.; Wunsch, T.; Konig, M.; Stockmann, M.; Meierhofer, D.; Holzhutter, H.G. HEPATOKIN1 is a biochemistry-based model of liver metabolism for applications in medicine and pharmacology. Nat. Commun. 2018, 9, 2386. [Google Scholar] [CrossRef] [PubMed]
- Plapp, B.V.; Leidal, K.G.; Murch, B.P.; Green, D.W. Contribution of liver alcohol dehydrogenase to metabolism of alcohols in rats. Chem. Biol. Interact. 2015, 234, 85–95. [Google Scholar] [CrossRef]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Primers 2018, 4, 16. [Google Scholar] [CrossRef]
- Lei, P.; Zhao, W.; Pang, B.; Yang, X.; Li, B.-L.; Ren, M.; Shan, Y.-J. Broccoli Sprout Extract Alleviates Alcohol-Induced Oxidative Stress and Endoplasmic Reticulum Stress in C57BL/6 Mice. J. Agric. Food Chem. 2018, 66, 5574–5580. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Liu, L.; Sha, X.-Y.; Wu, Y.-N.; Chen, M.-T.; Zhong, J.-X. Lycium barbarum polysaccharides protects retinal ganglion cells against oxidative stress injury. Neural Regen. Res. 2020, 15, 1526–1531. [Google Scholar] [CrossRef]
- Li, C.; Schluesener, H. Health-promoting effects of the citrus flavanone hesperidin. Crit. Rev. Food Sci. Nutr. 2017, 57, 613–631. [Google Scholar] [CrossRef]
- Li, S.; Hong, M.; Tan, H.Y.; Wang, N.; Feng, Y. Insights into the Role and Interdependence of Oxidative Stress and Inflammation in Liver Diseases. Oxid. Med. Cell Longev. 2016, 2016, 4234061. [Google Scholar] [CrossRef]
- Li, X.; Jin, Q.; Yao, Q.; Xu, B.; Li, Z.; Tu, C. Quercetin attenuates the activation of hepatic stellate cells and liver fibrosis in mice through modulation of HMGB1-TLR2/4-NF-kappaB signaling pathways. Toxicol. Lett. 2016, 261, 1–12. [Google Scholar] [CrossRef]
- Comalada, M.; Camuesco, D.; Sierra, S.; Ballester, I.; Xaus, J.; Galvez, J.; Zarzuelo, A. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur. J. Immunol. 2005, 35, 584–592. [Google Scholar] [CrossRef]
- Glaser, T.; Baiocchi, L.; Zhou, T.; Francis, H.; Lenci, I.; Grassi, G.; Kennedy, L.; Liangpunsakul, S.; Glaser, S.; Alpini, G.; et al. Pro-inflammatory signalling and gut-liver axis in non-alcoholic and alcoholic steatohepatitis: Differences and similarities along the path. J. Cell Mol. Med. 2020, 24, 5955–5965. [Google Scholar] [CrossRef]
- Chen, L.; Teng, H.; Jia, Z.; Battino, M.; Miron, A.; Yu, Z.; Cao, H.; Xiao, J. Intracellular signaling pathways of inflammation modulated by dietary flavonoids: The most recent evidence. Crit. Rev. Food Sci. Nutr. 2018, 58, 2908–2924. [Google Scholar] [CrossRef]
- Xiao, P.T.; Xie, Z.S.; Kuang, Y.J.; Liu, S.Y.; Zeng, C.; Li, P.; Liu, E.H. Discovery of a potent FKBP38 agonist that ameliorates HFD-induced hyperlipidemia via mTOR/P70S6K/SREBPs pathway. Acta Pharm. Sin. B 2021, 11, 3542–3552. [Google Scholar] [CrossRef]
- Morrow, N.M.; Trzaskalski, N.A.; Hanson, A.A.; Fadzeyeva, E.; Telford, D.E.; Chhoker, S.S.; Sutherland, B.G.; Edwards, J.Y.; Huff, M.W.; Mulvihill, E.E. Nobiletin Prevents High-Fat Diet-Induced Dysregulation of Intestinal Lipid Metabolism and Attenuates Postprandial Lipemia. Arter. Thromb. Vasc. Biol. 2022, 42, 127–144. [Google Scholar] [CrossRef]
- Pan, M.H.; Lai, C.S.; Tsai, M.L.; Ho, C.T. Chemoprevention of nonalcoholic fatty liver disease by dietary natural compounds. Mol. Nutr. Food Res. 2014, 58, 147–171. [Google Scholar] [CrossRef]
- Wu, W.Y.; Ding, X.Q.; Gu, T.T.; Guo, W.J.; Jiao, R.Q.; Song, L.; Sun, Y.; Pan, Y.; Kong, L.D. Pterostilbene Improves Hepatic Lipid Accumulation via the MiR-34a/Sirt1/SREBP-1 Pathway in Fructose-Fed Rats. J. Agric. Food Chem. 2020, 68, 1436–1446. [Google Scholar] [CrossRef]
- Liu, P.; Jin, X.; Lv, H.; Li, J.; Xu, W.; Qian, H.H.; Yin, Z. Icaritin ameliorates carbon tetrachloride-induced acute liver injury mainly because of the antioxidative function through estrogen-like effects. In Vitro Cell Dev. Biol. Anim. 2014, 50, 899–908. [Google Scholar] [CrossRef]
- Rufino, A.T.; Costa, V.M.; Carvalho, F.; Fernandes, E. Flavonoids as antiobesity agents: A review. Med. Res. Rev. 2021, 41, 556–585. [Google Scholar] [CrossRef]
- Malaguarnera, G.; Giordano, M.; Nunnari, G.; Bertino, G.; Malaguarnera, M. Gut microbiota in alcoholic liver disease: Pathogenetic role and therapeutic perspectives. World J. Gastroenterol. 2014, 20, 16639–16648. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Cui, B.; Jiang, A.; Tao, H.; Cheng, S.; Liu, Y. Combination of Chronic Alcohol Consumption and High-Salt Intake Elicits Gut Microbial Alterations and Liver Steatosis in Mice. J. Agric. Food Chem. 2020, 68, 1750–1759. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, K.; Yang, X.; Zhao, Y. Gut Microbiota and Metabolome Response of Decaisnea insignis Seed Oil on Metabolism Disorder Induced by Excess Alcohol Consumption. J. Agric. Food Chem. 2019, 67, 10667–10677. [Google Scholar] [CrossRef]
- Vassallo, G.; Mirijello, A.; Ferrulli, A.; Antonelli, M.; Landolfi, R.; Gasbarrini, A.; Addolorato, G. Review article: Alcohol and gut microbiota—The possible role of gut microbiota modulation in the treatment of alcoholic liver disease. Aliment. Pharmacol. Ther. 2015, 41, 917–927. [Google Scholar] [CrossRef]
- Ding, Q.; Cao, F.; Lai, S.; Zhuge, H.; Chang, K.; Valencak, T.G.; Liu, J.; Li, S.; Ren, D. Lactobacillus plantarum ZY08 relieves chronic alcohol-induced hepatic steatosis and liver injury in mice via restoring intestinal flora homeostasis. Food Res. Int. 2022, 157, 111259. [Google Scholar] [CrossRef]
- Russell, D.A.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Metabolic activities and probiotic potential of bifidobacteria. Int. J. Food Microbiol. 2011, 149, 88–105. [Google Scholar] [CrossRef]
- Duan, Y.; Llorente, C.; Lang, S.; Brandl, K.; Chu, H.; Jiang, L.; White, R.C.; Clarke, T.H.; Nguyen, K.; Torralba, M.; et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019, 575, 505–511. [Google Scholar] [CrossRef]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef]
- Hu, S.; Chen, Y.; Zhao, S.; Sun, K.; Luo, L.; Zeng, L. Ripened Pu-Erh Tea Improved the Enterohepatic Circulation in a Circadian Rhythm Disorder Mice Model. J. Agric. Food Chem. 2021, 69, 13533–13545. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [Green Version]

| CAS | Components | Content (%) | Outcomes and Potential Molecular Mechanisms | Biological Activities | OB (%) | DL | Contribution (mg) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 22255-13-6 | Quercetin-3-O-arabinoside (Guaijaverin) * | 2.72 | Reduction in the levels of IL-1β, IL-18, and Caspase-1 inhibits the expression of P62 and Pink1; inhibition of MAPK and PI3K/Akt signaling pathways | Antioxidant and anti-inflammatory | 29.65 | 0.7 | 0.016 | [24,25] |
| 23627-87-4 | Kaempferol-3-O-galactoside (Trifolin) | 2.05 | Activation of aldehyde dehydrogenase; radical-scavenging activity | Antioxidant and hepatoprotective activity | 19.61 | 0.74 | 0.008 | [26] |
| 490-46-0 | Epicatechin | 1.16 | Amelioration of high circulating levels of lipids and endotoxins, and mitigates systemic inflammation; ease Hepatic dysregulation of lipid metabolism; inhibition of SCAP and prevents the activation of SREBP-1c | Ease hepatic dysregulation of lipid metabolism | 28.93 | 0.24 | 0.007 | [27] |
| 117-39-5 | Quercetin | 0.51 | Reduction in the levels of TNF-α; inhibition of the lipoxygenase and cyclooxygenase pathways | Anti-inflammatory | 46.43 | 0.28 | 0.005 | [28] |
| 20315-25-7 | Procyanidin B2 | 0.31 | Proliferation inhibited and apoptosis induced in HSCs; down-regulate the expressions of VEGF-A, HIF-1α, α-SMA, Col-1 and TGF-β1 of HSCs | Hepatoprotective effect and anti-inflammatory | 67.87 | 0.66 | 0.004 | [29] |
| 480-18-2 | Dihydroquercetin (Taxifolin) | 0.34 | Inhibit the expression of P2X7R, IL-1β, and caspase-1; exhibit an inhibitory effect on lipid accumulation | Hepatoprotective effect and anti-inflammatory | 57.84 | 0.27 | 0.004 | [30] |
| 5373-11-5 | Luteolin-7-O-glucoside (Cynaroside) | 2.60 | Inhibited HMGB1/TLR4/NF-κB/MAPKs signaling pathways | Antioxidant | 7.29 | 0.78 | 0.004 | [31] |
| 154-23-4 | Catechin | 0.46 | Superoxide anion and superoxide-scavenging activity; suppress inflammation-related signal expression, including TNFA, COX-2, and iNOS | Antioxidant and anti-inflammatory | 29.86 | 0.02 | 0.003 | [28] |
| 549-32-6 | Quercetin-3-O-xyloside (Reynoutrin) | 5.06 | Inhibit the transcriptional activity of nuclear factor kappa-B | Antioxidant | 1.68 | 0.7 | 0.002 | [32] |
| 17650-84-9 | Kaempferol-3-O-rutinoside (Nicotiflorin) | 2.08 | Reduce the levels of IL-1β, IL-6, TNF-α, IFN-γ; decreased the MDA levels; increase GSH and the SOD activity; decrease the AST, ALT level | Hepatoprotective effect | 3.64 | 0.73 | 0.002 | [33] |
| 572-30-5 | Avicularin (Quercetin-3-O-α-L-arabinofuranoside) | 3.47 | A high radical-scavenging activity | Hepatoprotective effect | 2.06 | 0.7 | 0.001 | [34] |
| 16290-07-6 | Kaempferol-7-O-glucoside | 2.21 | NF-κB inhibitor | Antioxidant, anti-inflammatory and hepatoprotective activity | 41.88 | 0.24 | 0.001 | [35] |
| 520-18-3 | Kaempferol (3,5,7,4′-Tetrahydroxyflavone) | 0.14 | NF-κB inhibitor | Antioxidant, anti-inflammatory and hepatoprotective activity | 41.88 | 0.24 | 0.001 | [35] |
| 480-41-1 | Naringenin (5,7,4′-Trihydroxyflavanone) | 0.10 | Decreased levels of plasma and tissue total cholesterol; inhibition of oxidative stress through TGF-β pathway and prevention of the trans-differentiation of hepatic stellate cells (HSC). Pro-apoptotic effect, inhibition of MAPK, TLR, VEGF, and TGF-β, modulation of lipids and cholesterol synthesis, triglycerides, free fatty acids, HMG CoA reductase and collagen content | Hepatoprotective effect and anti-inflammatory | 42.36 | 0.21 | 0.001 | [36,37,38] |
| 491-50-9 | Quercetin-7-O-glucoside | 1.31 | Reduction in the levels of TNF-α, inhibition of COX2 and iNOS protein expression, inhibition of cow milk xanthine oxidase | Hepatoprotective effect | 2.85 | 0.79 | 0.001 | [28] |
| 480-20-6 | Aromadendrin (Dihydrokaempferol) | 0.11 | Regulation of the Keap1/Nrf2 pathway and regulate oxidative stress | Ameliorates severe acute pancreatitis | 24.15 | 0.24 | 0.001 | [39] |
| 99882-10-7 | Kaempferol-3-O-arabinoside | 1.14 | Antioxidant and anti-apoptotic properties, increase GSH and the SOD activity, decrease the AST, ALT level | Hepatoprotective effect | 2.73 | 0.65 | 0.001 | [40] |
| 19833-12-6 | Myricetin-3-O-glucoside | 1.38 | increase GSH and the SOD activity, decrease the AST, ALT level | Hepatoprotective effect | 1.43 | 0.79 | 0.000 | [41] |
| 28608-75-5 | Luteolin-8-C-glucoside (Orientin) | 0.87 | Inhibition of LPS-induced hyperpermeability in HUVEC cells | Anti-inflammatory | 1.79 | 0.75 | 0.000 | [42] |
| 571-74-4 | Sexangularetin | 0.02 | Decrease in the inflammatory markers IL-1β and myeloperoxidase | Anti-inflammatory | 62.86 | 0.3 | 0.000 | [43] |
| 153-18-4 | Rutin | 0.11 | Lower triglyceride content and abundance of lipid droplets; reduce cellular malondialdehyde level and restore superoxide dismutase activity in hepatocytes; suppress TGF-β/Smad signaling pathway | Hepatoprotective effect; dysfunctions of lipid metabolism | 3.2 | 0.68 | 0.000 | [44,45] |
| 520-36-5 | Apigenin | 0.01 | Inhibition of PI3K/Akt/mTOR pathway; activate the SIRT1 pathway; inhibit hepatic stellate cell activation and autophagy via TGF-β 1/Smad3 and p38/PPAR α Pathways | Hepatoprotective effect | 23.06 | 0.21 | 0.000 | [46] |
| 124027-51-6 | Quercetin-3-O-(6″-acetyl)galactoside | 1.89 | NI | NI | NI | NI | - | [47] |
| 52525-35-6 | Quercetin-3-O-robinobioside | 1.00 | Reactive oxygen species scavenging activity | Hepatoprotective effect | NI | NI | - | [48] |
| 47705-70-4 | Cyanidin-3-O-glucoside (Kuromanin) | 0.52 | Reduction in the levels of IL-1β, IL-6; activate mitophagy via the PINK1-PARKIN signaling pathway | Anti-inflammatory | NI | NI | - | [49,50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Hu, S.; Liu, Y.; Sun, K.; Luo, L.; Zeng, L. Hawk Tea Flavonoids as Natural Hepatoprotective Agents Alleviate Acute Liver Damage by Reshaping the Intestinal Microbiota and Modulating the Nrf2 and NF-κB Signaling Pathways. Nutrients 2022, 14, 3662. https://doi.org/10.3390/nu14173662
Xu T, Hu S, Liu Y, Sun K, Luo L, Zeng L. Hawk Tea Flavonoids as Natural Hepatoprotective Agents Alleviate Acute Liver Damage by Reshaping the Intestinal Microbiota and Modulating the Nrf2 and NF-κB Signaling Pathways. Nutrients. 2022; 14(17):3662. https://doi.org/10.3390/nu14173662
Chicago/Turabian StyleXu, Ting, Shanshan Hu, Yan Liu, Kang Sun, Liyong Luo, and Liang Zeng. 2022. "Hawk Tea Flavonoids as Natural Hepatoprotective Agents Alleviate Acute Liver Damage by Reshaping the Intestinal Microbiota and Modulating the Nrf2 and NF-κB Signaling Pathways" Nutrients 14, no. 17: 3662. https://doi.org/10.3390/nu14173662
APA StyleXu, T., Hu, S., Liu, Y., Sun, K., Luo, L., & Zeng, L. (2022). Hawk Tea Flavonoids as Natural Hepatoprotective Agents Alleviate Acute Liver Damage by Reshaping the Intestinal Microbiota and Modulating the Nrf2 and NF-κB Signaling Pathways. Nutrients, 14(17), 3662. https://doi.org/10.3390/nu14173662