Haskap Berry Leaves (Lonicera caerulea L.)—The Favorable Potential of Medical Use
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Materials
2.3. Extract Preparation
2.4. Microbiological Purity of the Haskap Berry Leaves
2.5. Determination of the Water Content
2.6. Determination of Total Phenolic Content (TPC)
2.7. Chlorophyll and Carotenoids Content-Spectroscopic Determination
- Absx—absorbance at x nm;
- v—volume of solvent (mL);
- w—the weight of the sample (mg);
- DL—dry leaves.
2.8. Phenolic Acids Content-Chromatophical Determination
2.9. Inhibition of In Vitro Activity of Enzymes
2.9.1. Inhibition of α-Glucosidase
- AC—the absorbance of the control (100% enzyme activity);
- AS—the absorbance of the tested sample (haskap berries leaves water extract or acarbose).
2.9.2. Inhibition of Lipase
- ABS test—absorbance of sample;
- ABS blank—absorbance of blank sample;
- ABS negative control—absorbance of the negative control sample.
2.9.3. Inhibition of Hyaluronidase
- TS—absorbance of sample;
- TEblank—absorbance of the enzyme + examined substance;
- THblank—absorbance of the HA + examined substance.
2.10. Antioxidant Action
2.10.1. DPPH Assay
- A0—the absorbance of the control
- A1—the absorbance of the sample.
2.10.2. FRAP Assay
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smolik, M.; Ochmian, I.; Grajkowski, J. Genetic Variability of Polish and Russian Accessions of Cultivated Blue Honeysuckle (Lonicera caerulea). Russ. J. Genet. 2010, 46, 960–966. [Google Scholar] [CrossRef]
- Liu, X.; Lv, Y.; Zheng, M.; Yin, L.; Wang, X.; Fu, Y.; Yu, B.; Li, J. Polyphenols from Blue Honeysuckle (Lonicera caerulea Var. edulis) Berry Inhibit Lipid Accumulation in Adipocytes by Suppressing Lipogenesis. J. Ethnopharmacol. 2021, 279, 114403. [Google Scholar] [CrossRef] [PubMed]
- Perova, I.B.; Rylina, E.V.; Eller, K.I.; Akimov, M.Y. The study of the polyphenolic complex and iridoid glycosides in various cultivars of edible honeysuckle fruits Lonicera edulis Turcz. ex Freyn. Vopr. Pitan. 2019, 88, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, H.; Xu, R.; Zhang, X.; Zhang, W.; Du, M.; Liu, X.; Fan, L. Simultaneous Optimization of the Acidified Water Extraction for Total Anthocyanin Content, Total Phenolic Content, and Antioxidant Activity of Blue Honeysuckle Berries (Lonicera caerulea L.) Using Response Surface Methodology. Food Sci. Nutr. 2019, 7, 2968–2976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, L.; Dong, Y.; Fang, Z.; Nisar, T.; Zhao, T.; Wang, Z.-C.; Guo, Y. Chemical Compositions and α-Glucosidase Inhibitory Effects of Anthocyanidins from Blueberry, Blackcurrant and Blue Honeysuckle Fruits. Food Chem. 2019, 299, 125102. [Google Scholar] [CrossRef]
- Senica, M.; Bavec, M.; Stampar, F.; Mikulic-Petkovsek, M. Blue Honeysuckle (Lonicera caerulea Subsp. edulis (Turcz. Ex Herder) Hultén.) Berries and Changes in Their Ingredients across Different Locations. J. Sci. Food Agric. 2018, 98, 3333–3342. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kim, J.W.; Ku, S.-K.; Choi, J.-S.; Lee, H.-J. Anti-Diabetic Effects of Blue Honeyberry on High-Fed-Diet-Induced Type II Diabetic Mouse. Nutr. Res. Pract. 2019, 13, 367–376. [Google Scholar] [CrossRef]
- Podsędek, A.; Majewska, I.; Redzynia, M.; Sosnowska, D.; Koziołkiewicz, M. In Vitro Inhibitory Effect on Digestive Enzymes and Antioxidant Potential of Commonly Consumed Fruits. J. Agric. Food Chem. 2014, 62, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, J.; Valentová, K.; Vacek, J.; Palíková, I.; Zatloukalová, M.; Kosina, P.; Ulrichová, J.; Vrbková, J.; Šimánek, V. Metabolic Profiling of Phenolic Acids and Oxidative Stress Markers after Consumption of Lonicera caerulea L. Fruit. J. Agric. Food Chem. 2013, 61, 4526–4532. [Google Scholar] [CrossRef] [PubMed]
- Jurgoński, A.; Juśkiewicz, J.; Zduńczyk, Z. An Anthocyanin-Rich Extract from Kamchatka Honeysuckle Increases Enzymatic Activity within the Gut and Ameliorates Abnormal Lipid and Glucose Metabolism in Rats. Nutrition 2013, 29, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Yang, H.; Ma, C.; Li, X.; Li, D.; Yang, Y.; Xu, Y.; Wang, L. Characterization and Inhibitory Activities on α-Amylase and α-Glucosidase of the Polysaccharide from Blue Honeysuckle Berries. Int. J. Biol. Macromol. 2020, 163, 414–422. [Google Scholar] [CrossRef]
- Tanentzap, A.J.; Lamb, A.; Walker, S.; Farmer, A. Resolving Conflicts between Agriculture and the Natural Environment. PLoS Biol. 2015, 13, e1002242. [Google Scholar] [CrossRef]
- Kanianska, R. Agriculture and Its Impact on Land-Use, Environment, and Ecosystem Services. In Landscape Ecology; IntechOpen: London, UK, 2016; ISBN 978-953-51-2513-6. [Google Scholar]
- Zhang, C.; Yang, D.; Liang, Z.; Liu, J.; Yan, K.; Zhu, Y.; Yang, S. Climatic Factors Control the Geospatial Distribution of Active Ingredients in Salvia Miltiorrhiza Bunge in China. Sci. Rep. 2019, 9, 904. [Google Scholar] [CrossRef]
- Liu, W.; Yin, D.; Li, N.; Hou, X.; Wang, D.; Li, D.; Liu, J. Influence of Environmental Factors on the Active Substance Production and Antioxidant Activity in Potentilla fruticosa L. and Its Quality Assessment. Sci. Rep. 2016, 6, 28591. [Google Scholar] [CrossRef]
- Lauritzen, E.; Maughan, T.; Black, B. Haskap (Blue Honeysuckle) in the Garden; Utah State University: Logan, UT, USA, 2016. [Google Scholar]
- Studzińska-Sroka, E.; Galanty, A.; Gościniak, A.; Wieczorek, M.; Kłaput, M.; Dudek-Makuch, M.; Cielecka-Piontek, J. Herbal Infusions as a Valuable Functional Food. Nutrients 2021, 13, 4051. [Google Scholar] [CrossRef]
- Etheridge, C.J.; Derbyshire, E. Herbal Infusions and Health: A Review of Findings from Human Studies, Mechanisms and Future Research Directions. Nutr. Food Sci. 2020, 50, 969–985. [Google Scholar] [CrossRef]
- Nie, J.; Yu, C.; Guo, Y.; Pei, P.; Chen, L.; Pang, Y.; Du, H.; Yang, L.; Chen, Y.; Yan, S.; et al. Tea Consumption and Long-Term Risk of Type 2 Diabetes and Diabetic Complications: A Cohort Study of 0.5 Million Chinese Adults. Am. J. Clin. Nutr. 2021, 114, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S. Cognitive Behavioural Therapy and Glycaemic Control in Diabetes Mellitus. Pract. Diabetes 2012, 29, 67–71. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Current Issues in the Treatment of Type 2 Diabetes. Overview of Newer Agents: Where Treatment Is Going. Am. J. Med. 2010, 123, S38–S48. [Google Scholar] [CrossRef]
- Willcox, M.L.; Elugbaju, C.; Al-Anbaki, M.; Lown, M.; Graz, B. Effectiveness of Medicinal Plants for Glycaemic Control in Type 2 Diabetes: An Overview of Meta-Analyses of Clinical Trials. Front. Pharmacol. 2021, 12, 3402. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.M.; Tewari, S.; Mendes, R.H. The Role of Oxidative Stress in the Development of Diabetes Mellitus and Its Complications. J. Diabetes Res. 2019, 2019, e4189813. [Google Scholar] [CrossRef] [PubMed]
- Réus, G.Z.; Carlessi, A.S.; Silva, R.H.; Ceretta, L.B.; Quevedo, J. Relationship of Oxidative Stress as a Link between Diabetes Mellitus and Major Depressive Disorder. Oxidative Med. Cell. Longev. 2019, 2019, e8637970. [Google Scholar] [CrossRef]
- Gorjanović, S.; Komes, D.; Pastor, F.T.; Belščak-Cvitanović, A.; Pezo, L.; Hečimović, I.; Sužnjević, D. Antioxidant Capacity of Teas and Herbal Infusions: Polarographic Assessment. J. Agric. Food Chem. 2012, 60, 9573–9580. [Google Scholar] [CrossRef]
- Jan, B.; Parveen, R.; Zahiruddin, S.; Khan, M.U.; Mohapatra, S.; Ahmad, S. Nutritional Constituents of Mulberry and Their Potential Applications in Food and Pharmaceuticals: A Review. Saudi J. Biol. Sci. 2021, 28, 3909–3921. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Z.F.; Luo, X.; Li, X. Effects of Mulberry Fruit (Morus alba L.) Consumption on Health Outcomes: A Mini-Review. Antioxidants 2018, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.; Buschmann, C. Chlorophylls and Carotenoids: Measurements and Characterization by UV-Vis Spectroscopy. Food Anal. Chem. Pigment. Colorants Flavors Texture Bioact. Food Compon. 2005, 2, 171–178. [Google Scholar] [CrossRef]
- Aono, Y.; Asikin, Y.; Wang, N.; Tieman, D.; Klee, H.; Kusano, M. High-Throughput Chlorophyll and Carotenoid Profiling Reveals Positive Associations with Sugar and Apocarotenoid Volatile Content in Fruits of Tomato Varieties in Modern and Wild Accessions. Metabolites 2021, 11, 398. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. Nippon Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Ito, H.; Horie, H. Proper Solvent Selection for Lycopene Extraction in Tomatoes and Application to a Rapid Determination. Bull. Natl. Inst. Veg. Tea Sci. 2009, 8, 165–173. [Google Scholar]
- Telagari, M.; Hullatti, K. In-Vitro α-Amylase and α-Glucosidase Inhibitory Activity of Adiantum caudatum Linn. and Celosia Argentea Linn. Extracts and Fractions. Indian J. Pharm. 2015, 47, 425–429. [Google Scholar] [CrossRef]
- Lewis, D.; Liu, D. Direct Measurement of Lipase Inhibition by Orlistat Using a Dissolution Linked In Vitro Assay. Clin. Pharmacol. Biopharm. 2012, 1, 1000103. [Google Scholar] [CrossRef]
- Grabowska, K.; Podolak, I.; Galanty, A.; Załuski, D.; Makowska-Wąs, J.; Sobolewska, D.; Janeczko, Z.; Żmudzki, P. In Vitro Anti-Denaturation and Anti-Hyaluronidase Activities of Extracts and Galactolipids from Leaves of Impatiens Parviflora DC. Nat. Prod. Res. 2016, 30, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Piotrowska, H.; Kucińska, M.; Murias, M.; Bylka, W. Cytotoxic Activity of Physodic Acid and Acetone Extract from Hypogymnia Physodes against Breast Cancer Cell Lines. Pharm. Biol. 2016, 54, 2480–2485. [Google Scholar] [CrossRef]
- Tiveron, A.P.; Melo, P.S.; Bergamaschi, K.B.; Vieira, T.M.F.S.; Regitano-d’Arce, M.A.B.; Alencar, S.M. Antioxidant Activity of Brazilian Vegetables and Its Relation with Phenolic Composition. Int. J. Mol. Sci. 2012, 13, 8943–8957. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.V.; Boehm, M.M.A.; Sekhon-Loodu, S.; Parmar, I.; Bors, B.; Jamieson, A.R. Anti-Inflammatory Activity of Haskap Cultivars Is Polyphenols-Dependent. Biomolecules 2015, 5, 1079–1098. [Google Scholar] [CrossRef]
- Zehfus, L.R.; Gillespie, Z.E.; Almendáriz-Palacios, C.; Low, N.H.; Eskiw, C.H. Haskap Berry Phenolic Subclasses Differentially Impact Cellular Stress Sensing in Primary and Immortalized Dermal Fibroblasts. Cells 2021, 10, 2643. [Google Scholar] [CrossRef]
- De Silva, A.B.K.H.; Rupasinghe, H.P.V. Polyphenols Composition and Anti-Diabetic Properties in Vitro of Haskap (Lonicera caerulea L.) Berries in Relation to Cultivar and Harvesting Date. J. Food Compos. Anal. 2020, 88, 103402. [Google Scholar] [CrossRef]
- Müller, J.; Heindl, A. Drying of Medicinal Plants. In Medicinal and Aromatic Plants; Murphy & Moore Publishing: New York, NY, USA, 2006; pp. 237–252. ISBN 978-1-4020-5448-8. [Google Scholar]
- Ratajczak, M.; Kubicka, M.M.; Kamińska, D.; Sawicka, P.; Długaszewska, J. Microbiological Quality of Non-Sterile Pharmaceutical Products. Saudi Pharm. J. 2015, 23, 303–307. [Google Scholar] [CrossRef]
- Reflection Paper on Microbiological Aspects of Herbal Medicinal Products and Traditional Herbal Medicinal Products. 2015. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-microbiological-aspects-herbal-medicinal-products-traditional-herbal-medicinal_en.pdf (accessed on 28 September 2021).
- Mrozek-Szetela, A.; Rejda, P.; Wińska, K. A Review of Hygienization Methods of Herbal Raw Materials. Appl. Sci. 2020, 10, 8268. [Google Scholar] [CrossRef]
- Khodadad, C.L.M.; Hummerick, M.E.; Spencer, L.E.; Dixit, A.R.; Richards, J.T.; Romeyn, M.W.; Smith, T.M.; Wheeler, R.M.; Massa, G.D. Microbiological and Nutritional Analysis of Lettuce Crops Grown on the International Space Station. Front. Plant Sci. 2020, 11, 199. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, P.; Cegielska-Radziejewska, R.; Mroczek, E.; Dziedzic, J. Analysis of air quality in selected areas of a poultry processing plant with the use of a microbiological air sampler. Braz. J. Poult. Sci. 2016, 18, 401–406. [Google Scholar] [CrossRef]
- Kumar, A.; Pathak, H.; Bhadauria, S.; Sudan, J. Aflatoxin Contamination in Food Crops: Causes, Detection, and Management: A Review. Food Prod. Processing Nutr. 2021, 3, 17. [Google Scholar] [CrossRef]
- Loi, M.; Paciolla, C.; Logrieco, A.F.; Mulè, G. Plant Bioactive Compounds in Pre- and Postharvest Management for Aflatoxins Reduction. Front. Microbiol. 2020, 11, 243. [Google Scholar] [CrossRef]
- Saleem, F.; Sadia, B.; Awan, F.S. Control of Aflatoxin Production Using Herbal Plant Extract; IntechOpen: London, UK, 2017; ISBN 978-953-51-3458-9. [Google Scholar]
- Assessing Chemical Risks. Available online: https://www.who.int/activities/improving-treatment-for-snakebite-patients (accessed on 28 September 2021).
- Urbańska, B.; Kowalska, J. Comparison of the Total Polyphenol Content and Antioxidant Activity of Chocolate Obtained from Roasted and Unroasted Cocoa Beans from Different Regions of the World. Antioxidants 2019, 8, 283. [Google Scholar] [CrossRef]
- Makori, S.I.; Mu, T.-H.; Sun, H.-N. Total Polyphenol Content, Antioxidant Activity, and Individual Phenolic Composition of Different Edible Parts of 4 Sweet Potato Cultivars. Nat. Prod. Commun. 2020, 15, 1934578X20936931. [Google Scholar] [CrossRef]
- Lee, J.; Park, G.; Chang, Y. Nutraceuticals and Antioxidant Properties of Lonicera Japonica Thunb. as Affected by Heating Time. Int. J. Food Prop. 2019, 22, 630–645. [Google Scholar] [CrossRef]
- Li, J.; Lian, X.; Ye, C.; Wang, L. Analysis of Flower Color Variations at Different Developmental Stages in Two Honeysuckle (Lonicera japonica Thunb.) Cultivars. HortScience 2019, 54, 779–782. [Google Scholar] [CrossRef]
- Royer, J.; Shanklin, J.; Balch-Kenney, N.; Mayorga, M.; Houston, P.; de Jong, R.M.; McMahon, J.; Laprade, L.; Blomquist, P.; Berry, T.; et al. Rhodoxanthin Synthase from Honeysuckle; a Membrane Diiron Enzyme Catalyzes the Multistep Conversion of β-Carotene to Rhodoxanthin. Sci. Adv. 2020, 6, eaay9226. [Google Scholar] [CrossRef]
- Pu, X.; Li, Z.; Tian, Y.; Gao, R.; Hao, L.; Hu, Y.; He, C.; Sun, W.; Xu, M.; Peters, R.J.; et al. The Honeysuckle Genome Provides Insight into the Molecular Mechanism of Carotenoid Metabolism Underlying Dynamic Flower Coloration. New Phytol. 2020, 227, 930–943. [Google Scholar] [CrossRef] [PubMed]
- Czerwińska, E.; Piotrowski, W. Microbiological Purity and Selected Physicochemical Properties of Cereal Products Stored in Different Packages. 12. Annu. Set Environ. Prot. 2014, 16, 161–172. [Google Scholar]
- Rezaei, F.; Vandergheynst, J.S. Critical Moisture Content for Microbial Growth in Dried Food-Processing Residues. J. Sci. Food Agric. 2010, 90, 2000–2005. [Google Scholar] [CrossRef]
- Chandra, D.; Lee, J.-S.; Choi, H.J.; Kim, J.G. Effects of Packaging on Shelf Life and Postharvest Qualities of Radish Roots during Storage at Low Temperature for an Extended Period. J. Food Qual. 2018, 2018, e3942071. [Google Scholar] [CrossRef]
- Kunle, O.F.; Egharevba, H.O.; Ahmadu, P.O. Standardization of Herbal Medicines—A Review. Int. J. Biodvers. Conserv. 2012, 4, 101–112. [Google Scholar] [CrossRef]
- Djordjevic, S.M. From Medicinal Plant Raw Material to Herbal Remedies; IntechOpen: London, UK, 2017; ISBN 978-953-51-2978-3. [Google Scholar]
- Yu, Y.; Li, H.; Zhang, B.; Wang, J.; Shi, X.; Huang, J.; Yang, J.; Zhang, Y.; Deng, Z. Nutritional and Functional Components of Mulberry Leaves from Different Varieties: Evaluation of Their Potential as Food Materials. Int. J. Food Prop. 2018, 21, 1495–1507. [Google Scholar] [CrossRef]
- Burin, V.M.; Ferreira-Lima, N.E.; Panceri, C.P.; Bordignon-Luiz, M.T. Bioactive Compounds and Antioxidant Activity of Vitis Vinifera and Vitis Labrusca Grapes: Evaluation of Different Extraction Methods. Microchem. J. 2014, 114, 155–163. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Huang, D.; Chen, S.; Xia, Y.; Zhu, S. The Inhibitory Mechanism of Chlorogenic Acid and Its Acylated Derivatives on α-Amylase and α-Glucosidase. Food Chem. 2022, 372, 131334. [Google Scholar] [CrossRef]
- Sozański, T.; Kucharska, A.Z.; Rapak, A.; Szumny, D.; Trocha, M.; Merwid-Ląd, A.; Dzimira, S.; Piasecki, T.; Piórecki, N.; Magdalan, J.; et al. Iridoid-Loganic Acid versus Anthocyanins from the Cornus mas Fruits (Cornelian Cherry): Common and Different Effects on Diet-Induced Atherosclerosis, PPARs Expression and Inflammation. Atherosclerosis 2016, 254, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and Chlorogenic Acids Inhibit Key Enzymes Linked to Type 2 Diabetes (in Vitro): A Comparative Study. J. Basic Clin. Physiol. Pharm. 2015, 26, 165–170. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid. Med. Cell Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef] [PubMed]
- Van de Laar, F.A.; Lucassen, P.L.; Akkermans, R.P.; van de Lisdonk, E.H.; Rutten, G.E.; Weel, C. van α-Glucosidase Inhibitors for Patients with Type 2 Diabetes: Results from a Cochrane Systematic Review and Meta-Analysis. Diabetes Care 2005, 28, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Van de Laar, F.A. Alpha-Glucosidase Inhibitors in the Early Treatment of Type 2 Diabetes. Vasc. Health Risk Manag. 2008, 4, 1189–1195. [Google Scholar] [CrossRef]
- Liu, T.-T.; Liu, X.-T.; Chen, Q.-X.; Shi, Y. Lipase Inhibitors for Obesity: A Review. Biomed. Pharmacother. 2020, 128, 110314. [Google Scholar] [CrossRef]
- Seyedan, A.; Alshawsh, M.A.; Alshagga, M.A.; Koosha, S.; Mohamed, Z. Medicinal Plants and Their Inhibitory Activities against Pancreatic Lipase: A Review. Evid.-Based Complementary Altern. Med. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Suksungworn, R.; Andrade, P.B.; Oliveira, A.P.; Valentão, P.; Duangsrisai, S.; Gomes, N.G.M. Inhibition of Proinflammatory Enzymes and Attenuation of IL-6 in LPS-Challenged RAW 264.7 Macrophages Substantiates the Ethnomedicinal Use of the Herbal Drug Homalium Bhamoense Cubitt & W.W.Sm. Int. J. Mol. Sci. 2020, 21, 2421. [Google Scholar] [CrossRef]
- Bralley, E.; Greenspan, P.; Hargrove, J.L.; Hartle, D.K. Inhibition of Hyaluronidase Activity by Vitis Rotundifolia. (Muscadine) Berry Seeds and Skins. Pharm. Biol. 2007, 45, 667–673. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of Antioxidant Potential of Plants and Its Relevance to Therapeutic Applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Lee, Y.-S.; Seol, D.J.; Cho, I.J.; Ku, S.K.; Choi, J.-S.; Lee, H.-J. Anti-Obesity and Fatty Liver-Preventing Activities of Lonicera caerulea in High-Fat Diet-Fed Mice. Int. J. Mol. Med. 2018, 42, 3047–3064. [Google Scholar] [CrossRef]
- Han, M.H.; Lee, W.S.; Nagappan, A.; Hong, S.H.; Jung, J.H.; Park, C.; Kim, H.J.; Kim, G.-Y.; Kim, G.; Jung, J.-M.; et al. Flavonoids Isolated from Flowers of Lonicera japonica Thunb. Inhibit Inflammatory Responses in BV2 Microglial Cells by Suppressing TNF-α and IL-β Through PI3K/Akt/NF-Kb Signaling Pathways. Phytother. Res. 2016, 30, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Cho, I.J.; Kim, J.W.; Lee, M.-K.; Ku, S.K.; Choi, J.-S.; Lee, H.-J. Hepatoprotective Effects of Blue Honeysuckle on CCl4-Induced Acute Liver Damaged Mice. Food Sci. Nutr. 2019, 7, 322–338. [Google Scholar] [CrossRef]
- Chun, Y.-S.; Ku, S.-K.; Kim, J.-K.; Park, S.; Cho, I.-H.; Lee, N.-J. Hepatoprotective and Anti-Obesity Effects of Korean Blue Honeysuckle Extracts in High Fat Diet-Fed Mice. J. Exerc. Nutr. Biochem. 2018, 22, 39–54. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In Vitro and in Vivo Antioxidant Properties of Chlorogenic Acid and Caffeic Acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes Mellitus and Oxidative Stress—A Concise Review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef]
- Williamson, G. Possible Effects of Dietary Polyphenols on Sugar Absorption and Digestion. Mol. Nutr. Food Res. 2013, 57, 48–57. [Google Scholar] [CrossRef] [PubMed]
| Samples | Counts of Microorganisms (CFU/g DL **) | |||||
|---|---|---|---|---|---|---|
| AB * | ANB * | MY * | En * | E.coli | Sal * | |
| Atut | 5.1 × 103 | 5.5 × 103 | 2.1 × 102 | n/f | n/f | n/f |
| Duet | 9.2 × 102 | 9.9 × 102 | 3.0 × 101 | n/f | n/f | n/f |
| Wojtek | 3.5 × 102 | 3.8 × 102 | 2.0 × 101 | n/f | n/f | n/f |
| Zojka | 2.0 × 102 | 2.9 × 102 | 5.0 × 102 | n/f | n/f | n/f |
| Jugana | 6.4 × 103 | 5.2 × 103 | 3.2 × 102 | n/f | n/f | n/f |
| Variety | TPC (mg/g DL *) | Chlorophyll A (mg/g DL *) | Chlorophyll B (mg/g DL *) | Carotenoids (mg/g DL *) | Water Content (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| T = 0 Months | T = 6 Months | T = 0 Months | T = 6 Months | T = 0 Months | T = 6 Months | T = 0 Months | T = 6 Months | T = 0 Months | T = 6 Months | |
| Atut | 49.618 ± 1.352 | 47.494 ± 1.471 | 11.659 ± 0.511 | 11.401 ± 0.131 | 4.540 ± 0.121 | 4.398 ± 0.141 | 1.956 ± 0.084 | 1.901 ± 0.061 | 5.43 ± 0.32 | 5.66 ± 0.15 |
| Duet | 44.893 ± 2.344 | 43.146 ± 0.782 | 12.753 ± 0.449 | 12.483 ± 0.097 | 5.484 ± 0.096 | 5.331 ± 0.135 | 2.163 ± 0.095 | 2.096 ± 0.023 | 5.75 ± 0.41 | 5.97 ± 0.18 |
| Wojtek | 28.179 ± 0.983 | 27.072 ± 0.761 | 10.642 ± 0.387 | 10.293 ± 0.190 | 4.463 ± 0.174 | 4.309 ± 0.136 | 1.848 ± 0.117 | 1.807 ± 0.021 | 6.23 ± 0.37 | 6.48 ± 0.33 |
| Zojka | 32.127 ± 1.085 | 31.223 ± 0.752 | 13.382 ± 0.533 | 12.902 ± 0.122 | 5.632 ± 0.153 | 5.474 ± 0.152 | 2.217 ± 0.126 | 2.150 ± 0.041 | 6.56 ± 0.49 | 6.84 ± 0.40 |
| Jugana | 52.399 ± 1.730 | 50.956 ± 0.971 | 14.003 ± 0.312 | 13.673 ± 0.097 | 5.575 ± 0.113 | 5.409 ± 0.243 | 2.876 ± 0.081 | 2.806 ± 0.097 | 6.38 ± 0.37 | 6.59 ± 0.22 |
| Variety | Loganic Acid (mg/g DL *) | Chlorogenic Acid (mg/g DL *) | Caffeic Acid (mg/g DL *) | Rutin (mg/g DL *) | ||||
|---|---|---|---|---|---|---|---|---|
| T = 0 Months | T = 6 Months | T = 0 Months | T = 6 Months | T = 0 Months | T = 6 Months | T = 0 Months | T = 6 Months | |
| Atut | 2.511 ± 0.121 * | 2.498 ± 0.103 * | 0.879 ± 0.112 * | 0.804 ± 0.107 * | 0.039 ± 0.003 * | 0.035 ± 0.002 * | 0.730 ± 0.243 * | 0.708 ± 0.222 * |
| Duet | 1.870 ± 0.151 * | 1.801 ± 0.125 * | 0.618 ± 0.157 * | 0.578 ± 0.121 * | 0.019 ± 0.002 * | 0.017 ± 0.001 * | 0.519 ± 0.353 * | 0.498 ± 0.334 * |
| Wojtek | 1.764 ± 0.183 * | 1.705 ± 0.091 * | 0.345 ± 0.104 * | 0.309 ± 0.070 * | 0.045 ± 0.003 * | 0.041 ± 0.003 * | 0.125 ± 0.274 * | 0.111 ± 0.207 * |
| Zojka | 0.803 ± 0.156 * | 0.801 ± 0.103 * | 0.389 ± 0.156 * | 0.366 ± 0.123 * | 0.056 ± 0.001 * | 0.055 ± 0.002 * | 0.143 ± 0.196 * | 0.140 ± 0.204 |
| Jugana | 2.981 ± 0.172 * | 2905 ± 0.150 * | 0.987 ± 0.073 * | 0.924 ± 0.097 * | 0.078 ± 0.002 * | 0.070 ± 0.003 * | 0.879 ± 0.124 * | 0.877 ± 0.113 |
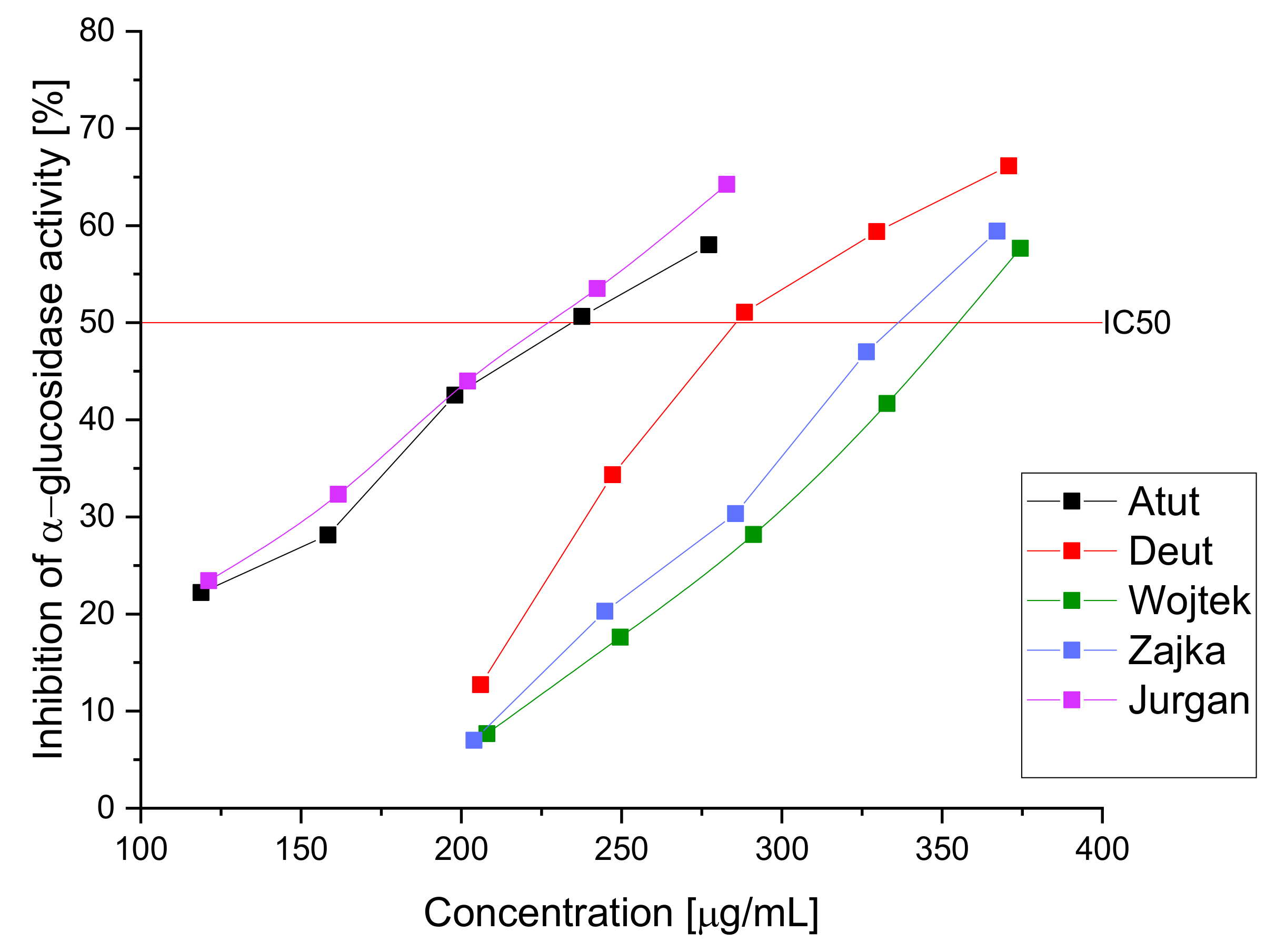
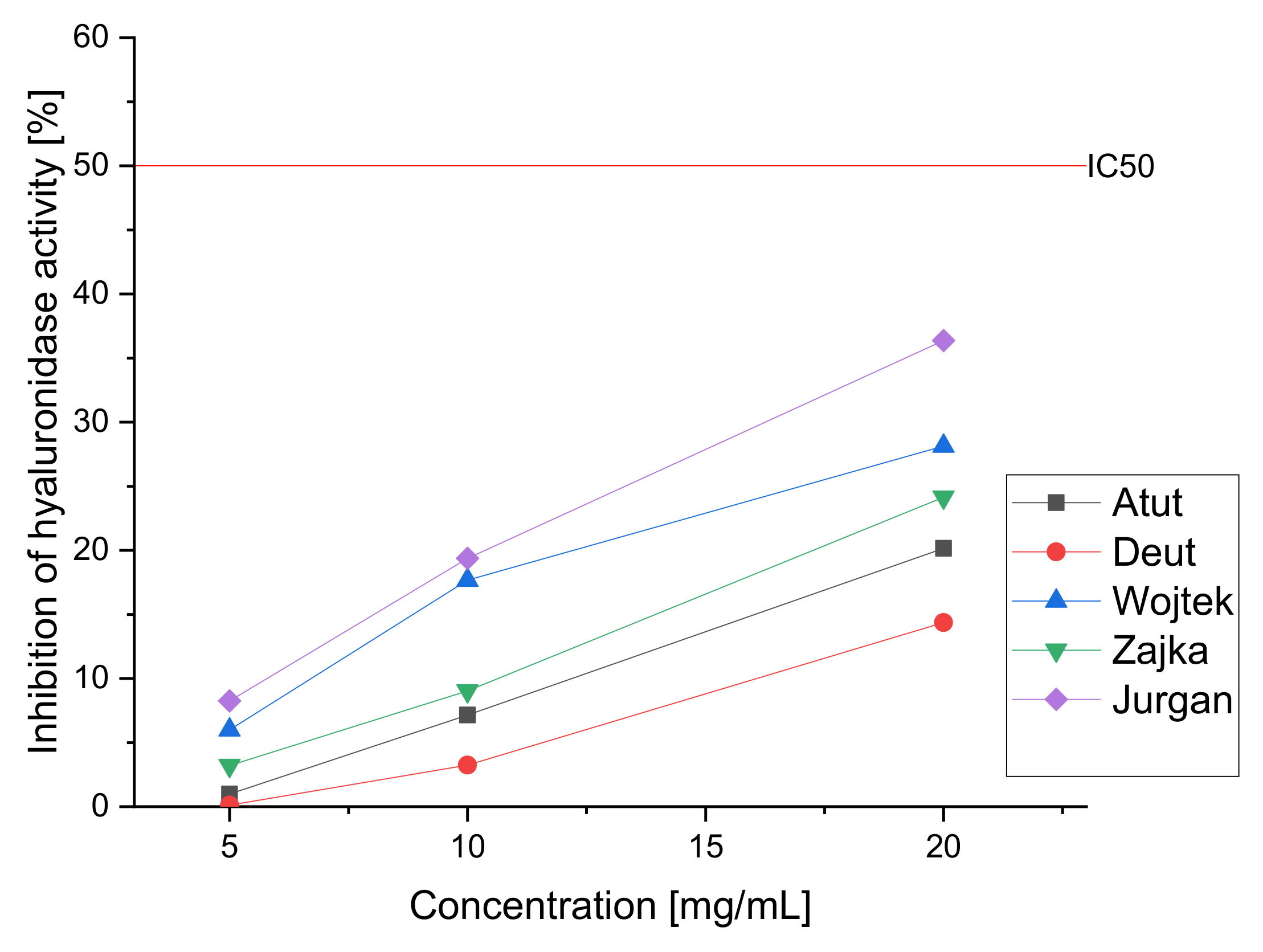
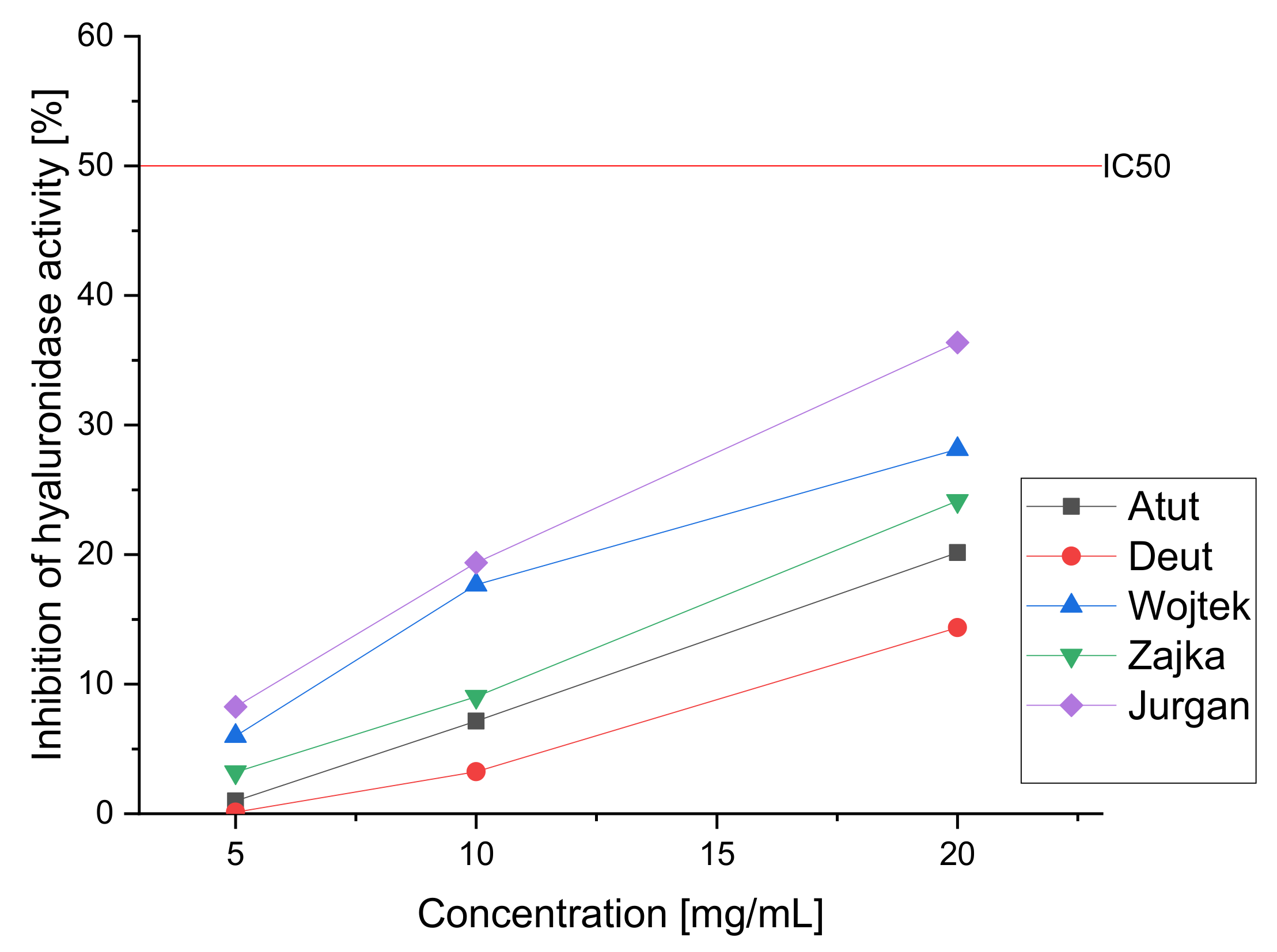
| Cultivar | IC50 (µg/mL) | For an Extract with a Concentration of 20 mg/mL | |
|---|---|---|---|
| Inhibition of α-Glucosidase | % of Inhibition of Lipase | % of Inhibition of Hyaluronidase | |
| Atut | 234.53 ± 3.35 * | 21.26 ± 1.32 * | 20.16 ± 1.77 * |
| Duet | 285.76 ± 2.84 * | 15.21 ± 1.11 * | 14.37 ± 1.65 * |
| Wojtek | 354.45 ± 4.01 * | 26.26 ± 2.01 * | 28.14 ± 1.43 * |
| Zojka | 336.23 ± 3.07 * | 22.78 ± 1.87 * | 24.14 ± 2.03 * |
| Jugana | 227.43 ± 2.98 * | 32.87 ± 1.56 * | 36.37 ± 1.67 * |
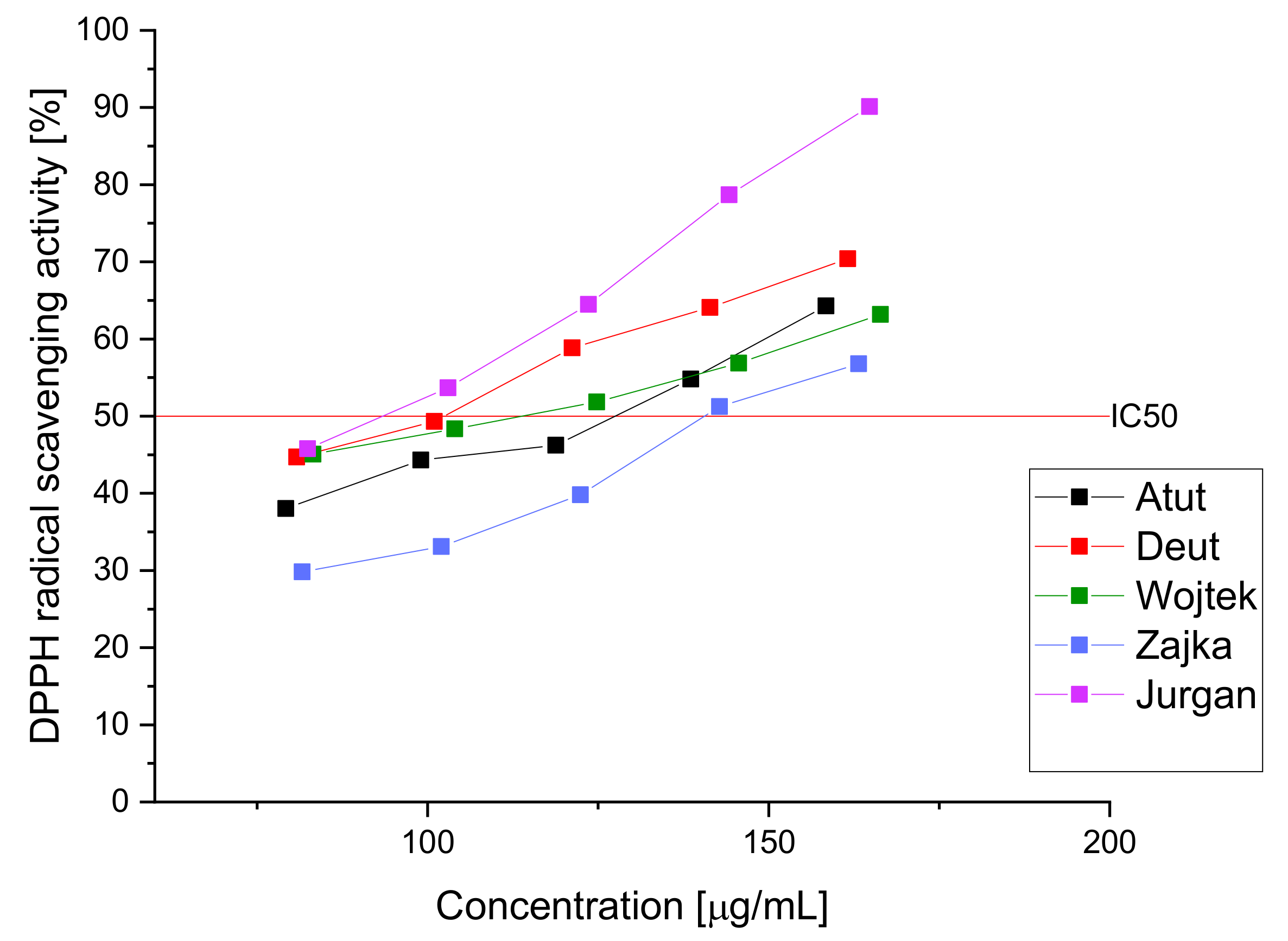
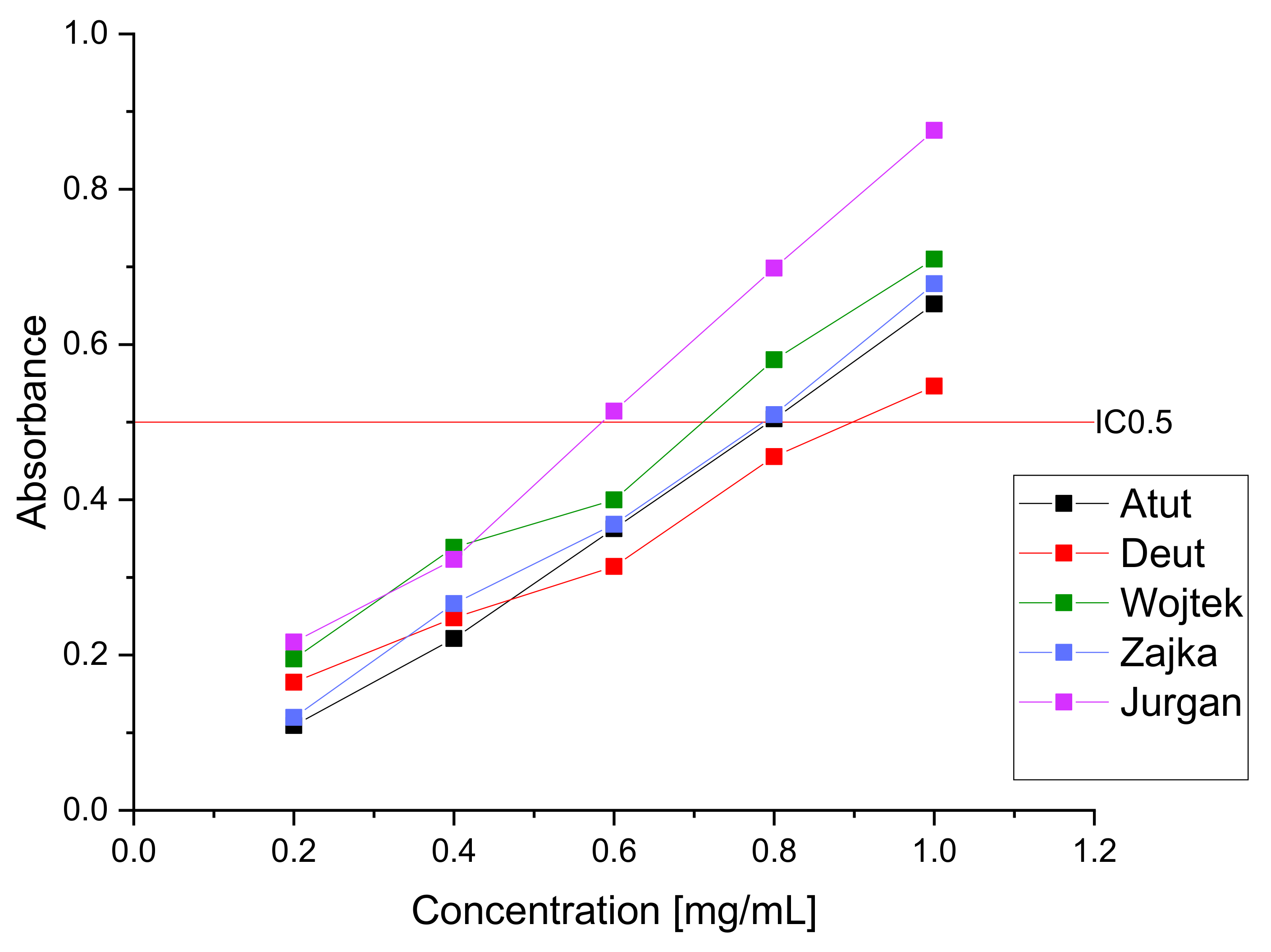
| IC50 (µg/mL) | IC0.5 (mg/mL) | ||
| DPPH | FRAP | ||
| Atut | 121.04 ± 2.11 * | 0.79 ± 0.08 * | |
| Duet | 102.42 ± 4.33 * | 0.89 ± 0.04 * | |
| Wojtek | 113.77 ± 1.79 * | 0.71 ± 0.05 * | |
| Zojka | 140.57 ± 1.24 * | 0.77 ± 0.07 * | |
| Jugana | 93.36 ± 2.18 * | 0.59 ± 0.02 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sip, S.; Sip, A.; Szulc, P.; Cielecka-Piontek, J. Haskap Berry Leaves (Lonicera caerulea L.)—The Favorable Potential of Medical Use. Nutrients 2022, 14, 3898. https://doi.org/10.3390/nu14193898
Sip S, Sip A, Szulc P, Cielecka-Piontek J. Haskap Berry Leaves (Lonicera caerulea L.)—The Favorable Potential of Medical Use. Nutrients. 2022; 14(19):3898. https://doi.org/10.3390/nu14193898
Chicago/Turabian StyleSip, Szymon, Anna Sip, Piotr Szulc, and Judyta Cielecka-Piontek. 2022. "Haskap Berry Leaves (Lonicera caerulea L.)—The Favorable Potential of Medical Use" Nutrients 14, no. 19: 3898. https://doi.org/10.3390/nu14193898
APA StyleSip, S., Sip, A., Szulc, P., & Cielecka-Piontek, J. (2022). Haskap Berry Leaves (Lonicera caerulea L.)—The Favorable Potential of Medical Use. Nutrients, 14(19), 3898. https://doi.org/10.3390/nu14193898








