Evaluation of the New Individual Fatty Acid Dataset for UK Biobank: Analysis of Intakes and Sources in 207,997 Participants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
2.3. Dietary Assessment Using the Oxford WebQ Questionnaire
2.4. Individual Fatty Acid Calculation for the Oxford WebQ Questionnaire
2.4.1. Food Code Matching
- 1
- Food codes were replaced with similar food codes from MCW (e.g., English cheddar cheese was used instead of Caerphilly cheese) or a different form of the same food code from MCW (e.g., raw herring with flesh was used instead of grilled herring no bones). Food codes were considered similar if they had similar descriptions and similar macronutrient (including SFA, PUFA and MUFA) content per 100 g of food to the MCW food code being replaced. In total, 103 food codes were replaced with similar MCW food codes.
- 2
- If step 1 above could not be applied because there was no closely similar food code with FA data, we replaced the food code with a composite of several MCW food codes in a recipe based on ingredients from industry and online recipes. For example, we replaced Waldorf salad (food code: 15-878) using 45% raw apples (14-326), 30% raw celery (13-636), 15% mayonnaise (17-654), and 10% walnuts (14-879). In total, 42 food codes were replaced with recipes using MCW food codes.
- 3
- If neither step 1 nor step 2 could be applied, we used similar food codes from USDA, and if we were unable to replace the food code with a similar USDA food code, we replaced the food code with a composite of MCW and USDA food codes in a recipe. In total, 40 food codes were replaced with similar USDA food codes, and 5 food codes were replaced with recipes using both MCW and USDA food codes.
2.4.2. Quality Assessment
2.5. Statistical Analyses
3. Results
3.1. Intakes of Individual Fatty Acids
3.2. Comparison of Major Nutrient Intakes in the Individual Fatty Acid Dataset and Main Dataset
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
IARC Disclaimer
References
- De Carvalho, C.; Caramujo, M.J. The various roles of fatty acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Krauss, R.M.; Taubes, G.; Willett, W. Dietary fat and cardiometabolic health: Evidence, controversies, and consensus for guidance. BMJ 2018, 361, k2139. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505s–1519s. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.; Thorpe, G.; Winstanley, L.; Abdelhamid, A.S.; Hooper, L. Omega-3, omega-6 and total dietary polyunsaturated fat on cancer incidence: Systematic review and meta-analysis of randomised trials. Br. J. Cancer 2020, 122, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Radzikowska, U.; Rinaldi, A.O.; Çelebi Sözener, Z.; Karaguzel, D.; Wojcik, M.; Cypryk, K.; Akdis, M.; Akdis, C.A.; Sokolowska, M. The influence of dietary datty acids on immune responses. Nutrients 2019, 11, 2990. [Google Scholar] [CrossRef] [PubMed]
- Zong, G.; Li, Y.; Wanders, A.J.; Alssema, M.; Zock, P.L.; Willett, W.C.; Hu, F.B.; Sun, Q. Intake of individual saturated fatty acids and risk of coronary heart disease in US men and women: Two prospective longitudinal cohort studies. BMJ 2016, 355, i5796. [Google Scholar] [CrossRef]
- Praagman, J.; de Jonge, E.A.; Kiefte-de Jong, J.C.; Beulens, J.W.; Sluijs, I.; Schoufour, J.D.; Hofman, A.; van der Schouw, Y.T.; Franco, O.H. Dietary saturated fatty acids and coronary heart disease risk in a Dutch middle-aged and elderly population. Arter. Thromb. Vasc. Biol. 2016, 36, 2011–2018. [Google Scholar] [CrossRef]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Ascherio, A.; Colditz, G.A.; Speizer, F.E.; Hennekens, C.H.; Willett, W.C. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am. J. Clin. Nutr. 1999, 70, 1001–1008. [Google Scholar] [CrossRef]
- Steur, M.; Johnson, L.; Sharp, S.J.; Imamura, F.; Sluijs, I.; Key, T.J.; Wood, A.; Chowdhury, R.; Guevara, M.; Jakobsen, M.U.; et al. Dietary fatty acids, macronutrient substitutions, food sources and incidence of coronary heart disease: Findings from the EPIC-CVD case-cohort study across nine European countries. J. Am. Heart Assoc. 2021, 10, e019814. [Google Scholar] [CrossRef]
- Praagman, J.; Beulens, J.W.; Alssema, M.; Zock, P.L.; Wanders, A.J.; Sluijs, I.; van der Schouw, Y.T. The association between dietary saturated fatty acids and ischemic heart disease depends on the type and source of fatty acid in the European Prospective Investigation into Cancer and Nutrition-Netherlands cohort. Am. J. Clin. Nutr. 2016, 103, 356–365. [Google Scholar] [CrossRef] [Green Version]
- Praagman, J.; Vissers, L.E.T.; Mulligan, A.A.; Laursen, A.S.D.; Beulens, J.W.J.; van der Schouw, Y.T.; Wareham, N.J.; Hansen, C.P.; Khaw, K.T.; Jakobsen, M.U.; et al. Consumption of individual saturated fatty acids and the risk of myocardial infarction in a UK and a Danish cohort. Int. J. Cardiol. 2019, 279, 18–26. [Google Scholar] [CrossRef]
- Mensink, R. Effects of Saturated Fatty Acids on Serum Lipids and Lipoproteins: A Systematic Review and Regression Analysis. Available online: https://apps.who.int/iris/handle/10665/246104 (accessed on 17 September 2021).
- Clarke, R.; Frost, C.; Collins, R.; Appleby, P.; Peto, R. Dietary lipids and blood cholesterol: Quantitative meta-analysis of metabolic ward studies. BMJ 1997, 314, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Young, H.; Crowe, F.L.; Benson, V.S.; Spencer, E.A.; Key, T.J.; Appleby, P.N.; Beral, V. Development and evaluation of the Oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies. Public Health Nutr. 2011, 14, 1998–2005. [Google Scholar] [CrossRef]
- Fry, A.; Littlejohns, T.J.; Sudlow, C.; Doherty, N.; Adamska, L.; Sprosen, T.; Collins, R.; Allen, N.E. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am. J. Epidemiol. 2017, 186, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Reeves, G.K.; Floud, S.; Barnes, I.; Cairns, B.J.; Gathani, T.; Pirie, K.; Sweetland, S.; Yang, T.O.; Beral, V. Cohort profile: The Million Women Study. Int. J. Epidemiol. 2019, 48, 28–29e. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cornago, A.; Pollard, Z.; Young, H.; van Uden, M.; Andrews, C.; Piernas, C.; Key, T.J.; Mulligan, A.; Lentjes, M. Description of the updated nutrition calculation of the Oxford WebQ questionnaire and comparison with the previous version among 207,144 participants in UK Biobank. Eur. J. Nutr. 2021, 60, 4019–4030. [Google Scholar] [CrossRef]
- Whitton, C.; Nicholson, S.K.; Roberts, C.; Prynne, C.J.; Pot, G.K.; Olson, A.; Fitt, E.; Cole, D.; Teucher, B.; Bates, B.; et al. National Diet and Nutrition Survey: UK food consumption and nutrient intakes from the first year of the rolling programme and comparisons with previous surveys. Br. J. Nutr. 2011, 106, 1899–1914. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. Composition of Foods Integrated Dataset (CoFID). Available online: https://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid (accessed on 2 January 2022).
- US Department of Agriculture. USDA National Nutrient Database for Standard Reference, Release 26: Documentation and User Guide. Available online: http://www.ars.usda.gov/ba/bhnrc/ndl (accessed on 2 January 2022).
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Rhee, J.J.; Sampson, L.; Cho, E.; Hughes, M.D.; Hu, F.B.; Willett, W.C. Comparison of methods to account for implausible reporting of energy intake in epidemiologic studies. Am. J. Epidemiol. 2015, 181, 225–233. [Google Scholar] [CrossRef]
- Greenwood, D.C.; Hardie, L.J.; Frost, G.S.; Alwan, N.A.; Bradbury, K.E.; Carter, M.; Elliott, P.; Evans, C.E.L.; Ford, H.E.; Hancock, N.; et al. Validation of the Oxford WebQ online 24-hour dietary questionnaire using biomarkers. Am. J. Epidemiol. 2019, 188, 1858–1867. [Google Scholar] [CrossRef] [Green Version]
- Greenfield, H.; Southgate, D.A.T. Food Composition Data: Production, Management and Use, 2nd ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; pp. 5–20. [Google Scholar]
- Nettleton, J.A. Omega-3 fatty acids: Comparison of plant and seafood sources in human nutrition. J. Am. Diet Assoc. 1991, 91, 331–337. [Google Scholar] [CrossRef]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Rimm, E.B.; Wolk, A.; Colditz, G.A.; Hennekens, C.H.; Willett, W.C. Dietary intake of alpha-linolenic acid and risk of fatal ischemic heart disease among women. Am. J. Clin. Nutr. 1999, 69, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.K.; Watling, C.Z.; Tong, T.Y.N.; Piernas, C.; Carter, J.L.; Papier, K.; Key, T.J.; Perez-Cornago, A. Associations between macronutrients from different dietary sources and serum lipids in 24 639 UK Biobank study participants. Arter. Thromb. Vasc. Biol. 2021, 41, 2190–2200. [Google Scholar] [CrossRef]
- Piernas, C.; Perez-Cornago, A.; Gao, M.; Young, H.; Pollard, Z.; Mulligan, A.; Lentjes, M.; Carter, J.; Bradbury, K.; Key, T.J.; et al. Describing a new food group classification system for UK biobank: Analysis of food groups and sources of macro- and micronutrients in 208,200 participants. Eur. J. Nutr. 2021, 60, 2879–2890. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Ministry of Agriculture, Fisheries and Food. Fatty Acids: Seventh Supplement to 5th Edition of McCance and Widdowson’s the Composition of Foods, 2nd ed.; The Royal Society of Chemistry: Cambridge, UK, 1998. [Google Scholar]
- US Department of Agriculture. What We Eat In America Data Tables 2011–2012. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables/ (accessed on 10 January 2022).
- Imamura, F.; Micha, R.; Khatibzadeh, S.; Fahimi, S.; Shi, P.; Powles, J.; Mozaffarian, D. Dietary quality among men and women in 187 countries in 1990 and 2010: A systematic assessment. Lancet Glob. Health 2015, 3, e132–e142. [Google Scholar] [CrossRef]
- US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans 2010. Available online: https://health.gov/sites/default/files/2020-01/DietaryGuidelines2010.pdf (accessed on 4 March 2022).
- Public Health England. NDNS: Results from Years 1–4 (Combined). Available online: https://www.gov.uk/government/statistics/national-diet-and-nutrition-survey-results-from-years-1-to-4-combined-of-the-rolling-programme-for-2008-and-2009-to-2011-and-2012 (accessed on 12 September 2021).
- Batty, G.D.; Gale, C.R.; Kivimäki, M.; Deary, I.J.; Bell, S. Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: Prospective cohort study and individual participant meta-analysis. BMJ 2020, 368, m131. [Google Scholar] [CrossRef]
- Linseisen, J.; Welch, A.A.; Ocké, M.; Amiano, P.; Agnoli, C.; Ferrari, P.; Sonestedt, E.; Chajès, V.; Bueno-de-Mesquita, H.B.; Kaaks, R.; et al. Dietary fat intake in the European Prospective Investigation into Cancer and Nutrition: Results from the 24-h dietary recalls. Eur. J. Clin. Nutr. 2009, 63 (Suppl. S4), S61–S80. [Google Scholar] [CrossRef]
- US Department of Agriculture. USDA Coexistence Fact Sheets: Soybeans. Available online: https://www.usda.gov/sites/default/files/documents/coexistence-soybeans-factsheet.pdf (accessed on 20 February 2022).
- Tennant, D.; Gosling, J.P. Modelling consumer intakes of vegetable oils and fats. Food Addit. Contam. Part A 2015, 32, 1397–1405. [Google Scholar] [CrossRef]
- US Department of Agriculture. United Kingdom—Fish and Seafood Market Update 2021. Available online: https://www.fas.usda.gov/data/united-kingdom-united-kingdom-fish-and-seafood-market-update (accessed on 4 June 2022).
- Love, D.C.; Asche, F.; Conrad, Z.; Young, R.; Harding, J.; Nussbaumer, E.M.; Thorne-Lyman, A.L.; Neff, R. Food sources and expenditures for seafood in the United States. Nutrients 2020, 12, 1810. [Google Scholar] [CrossRef]
- Coombes, R. Trans fats: Chasing a global ban. BMJ 2011, 343, d5567. [Google Scholar] [CrossRef] [PubMed]
- Slimani, N.; Deharveng, G.; Unwin, I.; Southgate, D.A.; Vignat, J.; Skeie, G.; Salvini, S.; Parpinel, M.; Moller, A.; Ireland, J.; et al. The EPIC nutrient database project (ENDB): A first attempt to standardize nutrient databases across the 10 European countries participating in the EPIC study. Eur. J. Clin. Nutr. 2007, 61, 1037–1056. [Google Scholar] [CrossRef] [PubMed]
- Schakel, S.; Buzzard, M.; Gebhardt, S. Procedures for estimating nutrient values for food composition databases. J. Food Compos. Anal. 1997, 10, 102–114. [Google Scholar] [CrossRef]
- Cowin, I.; Emmett, P. The effect of missing data in the supplements to McCance and Widdowson’s food tables on calculated nutrient intakes. Eur. J. Clin. Nutr. 1999, 53, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Seppänen-Laakso, T.; Laakso, I.; Hiltunen, R. Analysis of fatty acids by gas chromatography, and its relevance to research on health and nutrition. Anal. Chim. Acta 2002, 465, 39–62. [Google Scholar] [CrossRef]
- Schwendel, B.H.; Wester, T.J.; Morel, P.C.; Tavendale, M.H.; Deadman, C.; Shadbolt, N.M.; Otter, D.E. Invited review: Organic and conventionally produced milk-an evaluation of factors influencing milk composition. J. Dairy Sci. 2015, 98, 721–746. [Google Scholar] [CrossRef]
- Poulsen, N.A.; Gustavsson, F.; Glantz, M.; Paulsson, M.; Larsen, L.B.; Larsen, M.K. The influence of feed and herd on fatty acid composition in 3 dairy breeds (Danish Holstein, Danish Jersey, and Swedish Red). J. Dairy Sci. 2012, 95, 6362–6371. [Google Scholar] [CrossRef]
- Larsen, M.K.; Nielsen, J.H.; Butler, G.; Leifert, C.; Slots, T.; Kristiansen, G.H.; Gustafsson, A.H. Milk quality as affected by feeding regimens in a country with climatic variation. J. Dairy Sci. 2010, 93, 2863–2873. [Google Scholar] [CrossRef]
- Van Puyvelde, H.; Perez-Cornago, A.; Casagrande, C.; Nicolas, G.; Versele, V.; Skeie, G.; Schulze, M.B.; Johansson, I.; María Huerta, J.; Oliverio, A.; et al. Comparing calculated nutrient intakes using different food composition databases: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Nutrients 2020, 12, 2906. [Google Scholar] [CrossRef]
- Wang, D.D.; Li, Y.; Chiuve, S.E.; Stampfer, M.J.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Association of specific dietary fats with total and cause-specific mortality. JAMA Intern. Med. 2016, 176, 1134–1145. [Google Scholar] [CrossRef]
- Leitzmann, M.F.; Stampfer, M.J.; Michaud, D.S.; Augustsson, K.; Colditz, G.C.; Willett, W.C.; Giovannucci, E.L. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am. J. Clin. Nutr. 2004, 80, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wilson, K.M.; Stampfer, M.J.; Willett, W.C.; Giovannucci, E.L. A 24-year prospective study of dietary α-linolenic acid and lethal prostate cancer. Int. J. Cancer 2018, 142, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cornago, A.; Huybrechts, I.; Appleby, P.N.; Schmidt, J.A.; Crowe, F.L.; Overvad, K.; Tjønneland, A.; Kühn, T.; Katzke, V.; Trichopoulou, A.; et al. Intake of individual fatty acids and risk of prostate cancer in the European prospective investigation into cancer and nutrition. Int. J. Cancer 2020, 146, 44–57. [Google Scholar] [CrossRef]
- Md Noh, M.F.; Gunasegavan, R.D.; Mustafa Khalid, N.; Balasubramaniam, V.; Mustar, S.; Abd Rashed, A. Recent techniques in nutrient analysis for food composition database. Molecules 2020, 25, 4567. [Google Scholar] [CrossRef]
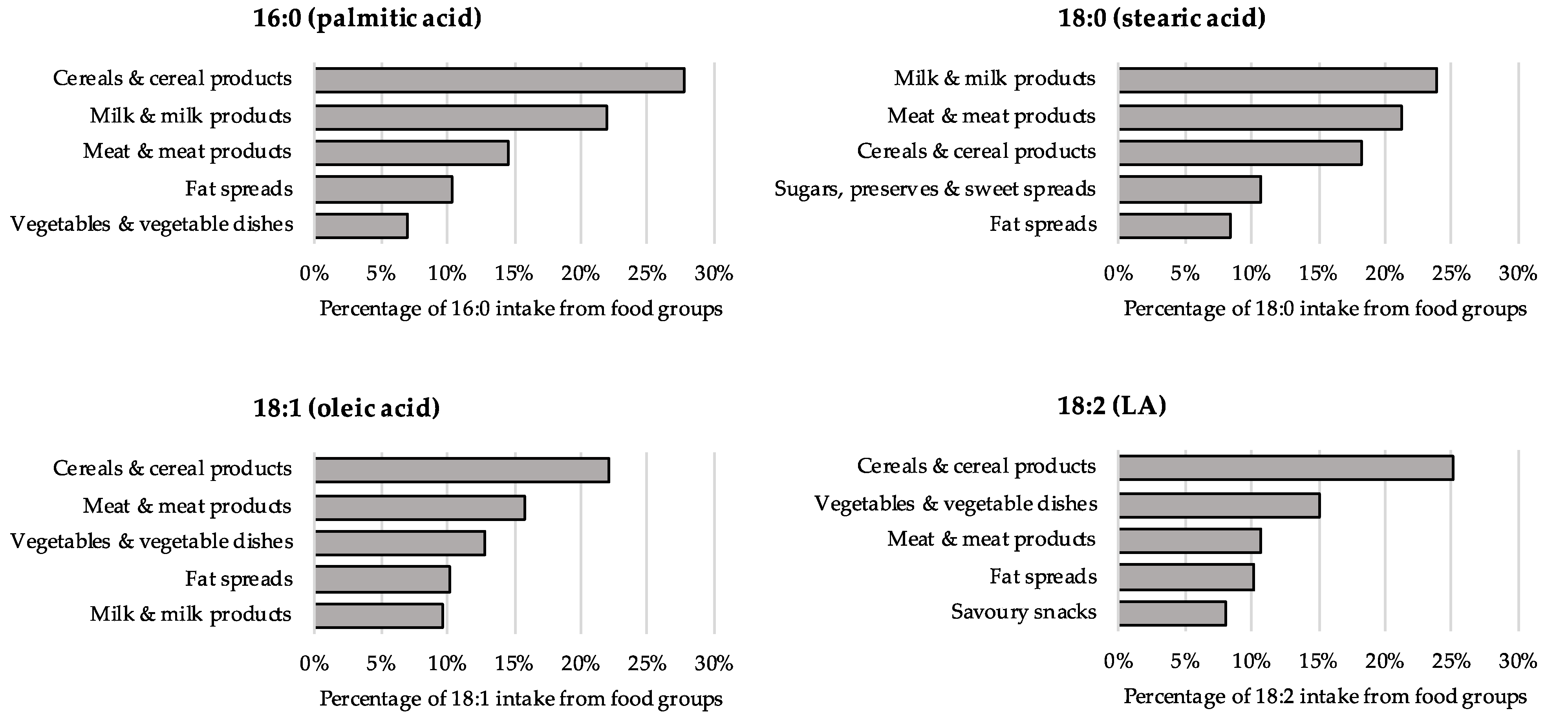
| Individual FAs (g/d), Mean (SD) | Total n = 207,997 | Women n = 114,406 | Men n = 93,591 |
|---|---|---|---|
| SFAs | data | ||
| 4:0 (butyric acid) | 0.67 (0.45) | 0.64 (0.41) | 0.71 (0.48) |
| 6:0 (caproic acid) | 0.42 (0.28) | 0.40 (0.26) | 0.44 (0.30) |
| 8:0 (caprylic acid) | 0.34 (0.19) | 0.32 (0.18) | 0.36 (0.21) |
| 10:0 (capric acid) | 0.59 (0.36) | 0.56 (0.34) | 0.62 (0.39) |
| 12:0 (lauric acid) | 1.20 (0.69) | 1.13 (0.64) | 1.30 (0.74) |
| 14:0 (myristic acid) | 2.41 (1.27) | 2.27 (1.16) | 2.58 (1.38) |
| 15:0 (pentadecanoic acid) | 0.27 (0.16) | 0.26 (0.14) | 0.30 (0.17) |
| 16:0 (palmitic acid) | 13.45 (5.66) | 12.44 (5.01) | 14.70 (6.15) |
| 17:0 (heptadecanoic acid) | 0.28 (0.15) | 0.26 (0.13) | 0.31 (0.16) |
| 18:0 (stearic acid) | 5.20 (2.46) | 4.81 (2.20) | 5.67 (2.67) |
| MUFAs | |||
| 16:1 (palmitoleic acid) | 0.81 (0.39) | 0.76 (0.36) | 0.87 (0.42) |
| 18:1 (oleic acid) | 24.20 (9.82) | 22.65 (8.95) | 26.09 (10.49) |
| 20:1 (eicosenoic acid) | 0.50 (0.51) | 0.48 (0.49) | 0.52 (0.52) |
| 22:1 (docosenoic acid) | 0.21 (0.39) | 0.21 (0.39) | 0.21 (0.40) |
| PUFAs | |||
| n-3 PUFAs | |||
| 18:3 (ALA) | 1.58 (0.84) | 1.54 (0.83) | 1.64 (0.85) |
| 18:4 (stearidonic acid) | 0.04 (0.09) | 0.04 (0.09) | 0.04 (0.09) |
| 20:5 (EPA) | 0.03 (0.02) | 0.02 (0.02) | 0.03 (0.02) |
| 22:5 (DPA) | 0.06 (0.07) | 0.06 (0.07) | 0.06 (0.07) |
| 22:6 (DHA) | 0.18 (0.33) | 0.19 (0.33) | 0.18 (0.34) |
| n-6 PUFAs | |||
| 18:2 (LA) | 9.67 (4.30) | 9.08 (3.97) | 10.40 (4.57) |
| 20:4 (arachidonic acid) | 0.11 (0.09) | 0.10 (0.08) | 0.12 (0.09) |
| Nutrients | Main Dataset (UKNDB) | Individual FA Dataset (MCW + USDA) | Mean Difference 1 | Percentage Difference 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | 5th Percentile | 95th Percentile | Mean (SD) | Median (IQR) | 5th Percentile | 95th Percentile | |||
| Total energy intake, kJ/d | 8573 (2220) | 8369 (2871) | 5264 | 12587 | 8406 (2189) | 8207 (2834) | 5139 | 12371 | −166.7 | −1.94 |
| Fat, g/d | 72.4 (26.4) | 69.5 (33.9) | 34.4 | 120.1 | 70.6 (26.0) | 67.8 (33.5) | 33.2 | 117.7 | −1.75 | −2.42 |
| SFAs, g/d | 26.8 (11.3) | 25.3 (14.4) | 11.1 | 47.4 | 25.7 (10.9) | 24.3 (14.0) | 10.4 | 45.7 | −1.09 | −4.07 |
| MUFAs, g/d | 26.2 (10.2) | 25.0 (12.9) | 11.8 | 44.8 | 26.8 (10.6) | 25.6 (13.5) | 11.7 | 45.9 | 0.57 | 2.17 |
| PUFAs 3, g/d | 12.8 (5.5) | 12.0 (6.7) | 5.5 | 22.9 | 12.4 (5.1) | 11.7 (6.3) | 5.3 | 21.6 | −0.43 | −3.38 |
| n-3 PUFAs 4, g/d | 1.97 (0.97) | 1.79 (1.12) | 0.77 | 3.76 | 1.90 (1.01) | 1.72 (1.24) | 0.61 | 3.76 | −0.08 | −3.82 |
| n-6 PUFAs 5, g/d | 10.8 (4.9) | 10.1 (5.8) | 4.4 | 19.8 | 9.8 (4.3) | 9.2 (5.3) | 3.9 | 17.6 | −1.06 | −9.77 |
| TFAs, g/d | 1.18 (0.65) | 1.08 (0.78) | 0.34 | 2.37 | 1.04 (0.63) | 0.93 (0.75) | 0.26 | 2.20 | −0.14 | −11.96 |
| Carbohydrates, g/d | 252.7 (73.2) | 246.8 (92.8) | 143.7 | 382.4 | 247.9 (71.8) | 242.3 (91.1) | 140.6 | 374.3 | −4.81 | −1.90 |
| Protein, g/d | 80.3 (23.0) | 78.5 (27.7) | 46.2 | 120.2 | 79.6 (22.8) | 77.8 (27.5) | 45.8 | 119.2 | −0.70 | −0.88 |
| Nutrients | Spearman’s r | Percentage in the Same Fifth | Percentage in the Same or Adjacent Fifth | Weighted κ |
|---|---|---|---|---|
| Total energy intake, kJ/d | 0.996 | 91.2 | 100.0 | 0.945 |
| Fat, g/d | 0.987 | 85.3 | 99.9 | 0.907 |
| SFAs, g/d | 0.985 | 84.0 | 99.9 | 0.899 |
| MUFAs, g/d | 0.981 | 81.4 | 99.8 | 0.883 |
| PUFAs 1, g/d | 0.952 | 72.2 | 98.6 | 0.817 |
| n-3 PUFAs 2, g/d | 0.867 | 58.5 | 93.8 | 0.694 |
| n-6 PUFAs 3, g/d | 0.930 | 66.7 | 97.4 | 0.775 |
| TFAs, g/d | 0.892 | 62.6 | 94.9 | 0.729 |
| Carbohydrates, g/d | 0.993 | 88.5 | 100.0 | 0.928 |
| Protein, g/d | 0.984 | 83.3 | 99.9 | 0.895 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelly, R.K.; Pollard, Z.; Young, H.; Piernas, C.; Lentjes, M.; Mulligan, A.; Huybrechts, I.; Carter, J.L.; Key, T.J.; Perez-Cornago, A. Evaluation of the New Individual Fatty Acid Dataset for UK Biobank: Analysis of Intakes and Sources in 207,997 Participants. Nutrients 2022, 14, 3603. https://doi.org/10.3390/nu14173603
Kelly RK, Pollard Z, Young H, Piernas C, Lentjes M, Mulligan A, Huybrechts I, Carter JL, Key TJ, Perez-Cornago A. Evaluation of the New Individual Fatty Acid Dataset for UK Biobank: Analysis of Intakes and Sources in 207,997 Participants. Nutrients. 2022; 14(17):3603. https://doi.org/10.3390/nu14173603
Chicago/Turabian StyleKelly, Rebecca K., Zoe Pollard, Heather Young, Carmen Piernas, Marleen Lentjes, Angela Mulligan, Inge Huybrechts, Jennifer L. Carter, Timothy J. Key, and Aurora Perez-Cornago. 2022. "Evaluation of the New Individual Fatty Acid Dataset for UK Biobank: Analysis of Intakes and Sources in 207,997 Participants" Nutrients 14, no. 17: 3603. https://doi.org/10.3390/nu14173603
APA StyleKelly, R. K., Pollard, Z., Young, H., Piernas, C., Lentjes, M., Mulligan, A., Huybrechts, I., Carter, J. L., Key, T. J., & Perez-Cornago, A. (2022). Evaluation of the New Individual Fatty Acid Dataset for UK Biobank: Analysis of Intakes and Sources in 207,997 Participants. Nutrients, 14(17), 3603. https://doi.org/10.3390/nu14173603






