Intestinal Epithelial Toll-like Receptor 4 Deficiency Modifies the Response to the Activity-Based Anorexia Model in a Sex-Dependent Manner: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Activity-Based Anorexia Model
2.3. Open Filed Test
2.4. Body Composition Assessment
2.5. Euthanasia and Sample Collection
2.6. Evaluation of Plasma Leptin, Adiponectin and Corticosterone
2.7. RNA Extraction and RT-qPCR
2.8. Statistical Analysis
3. Results
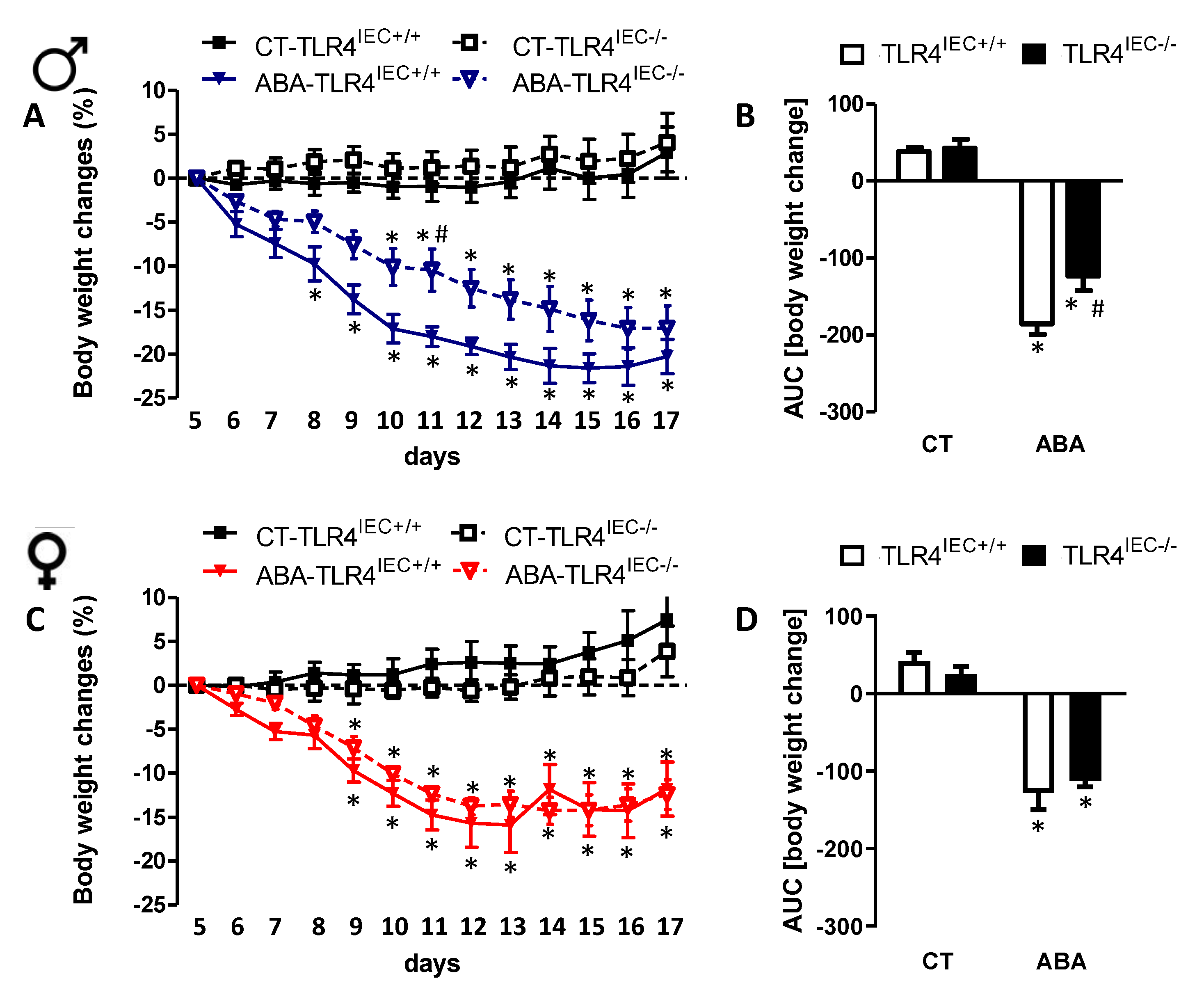
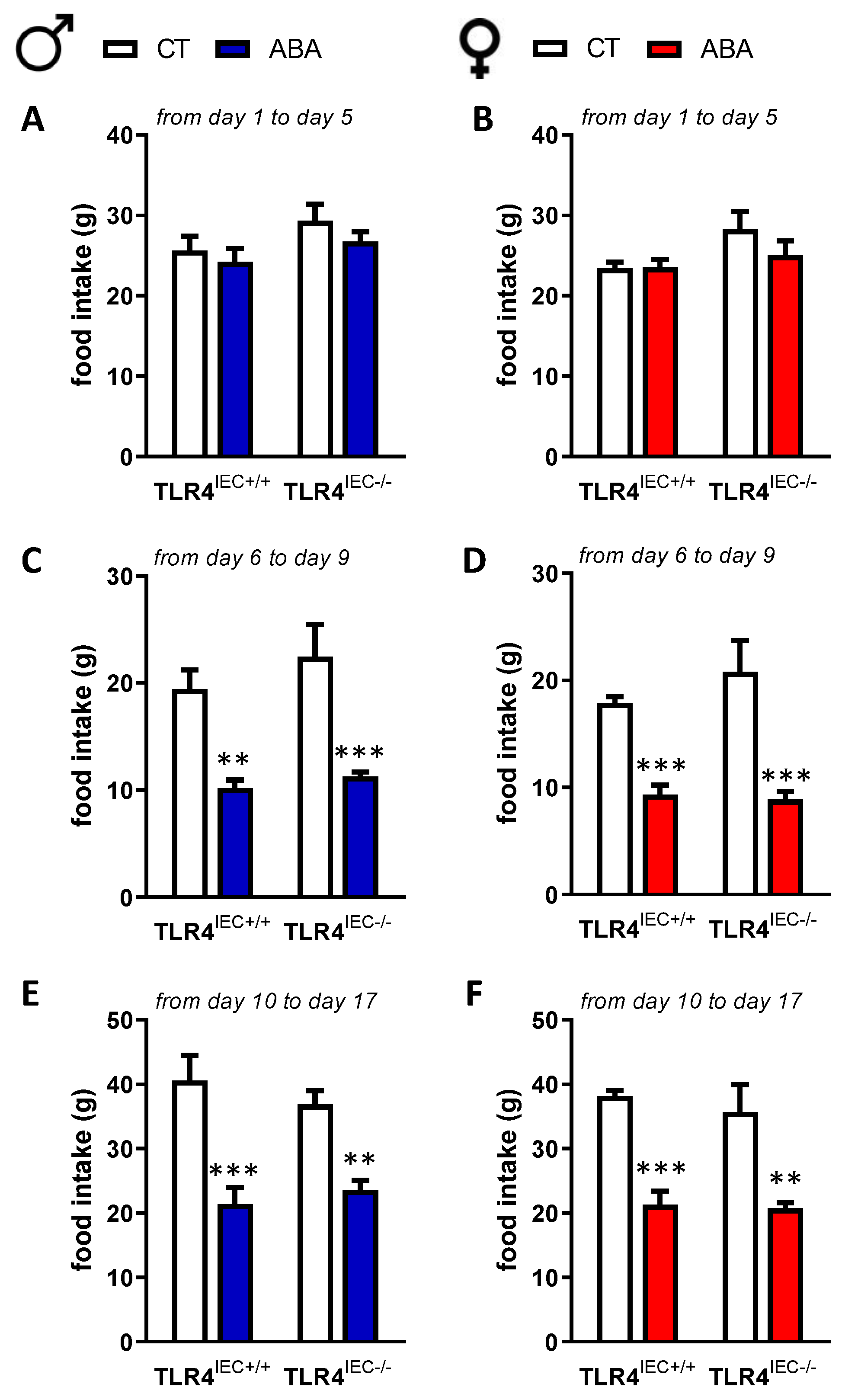
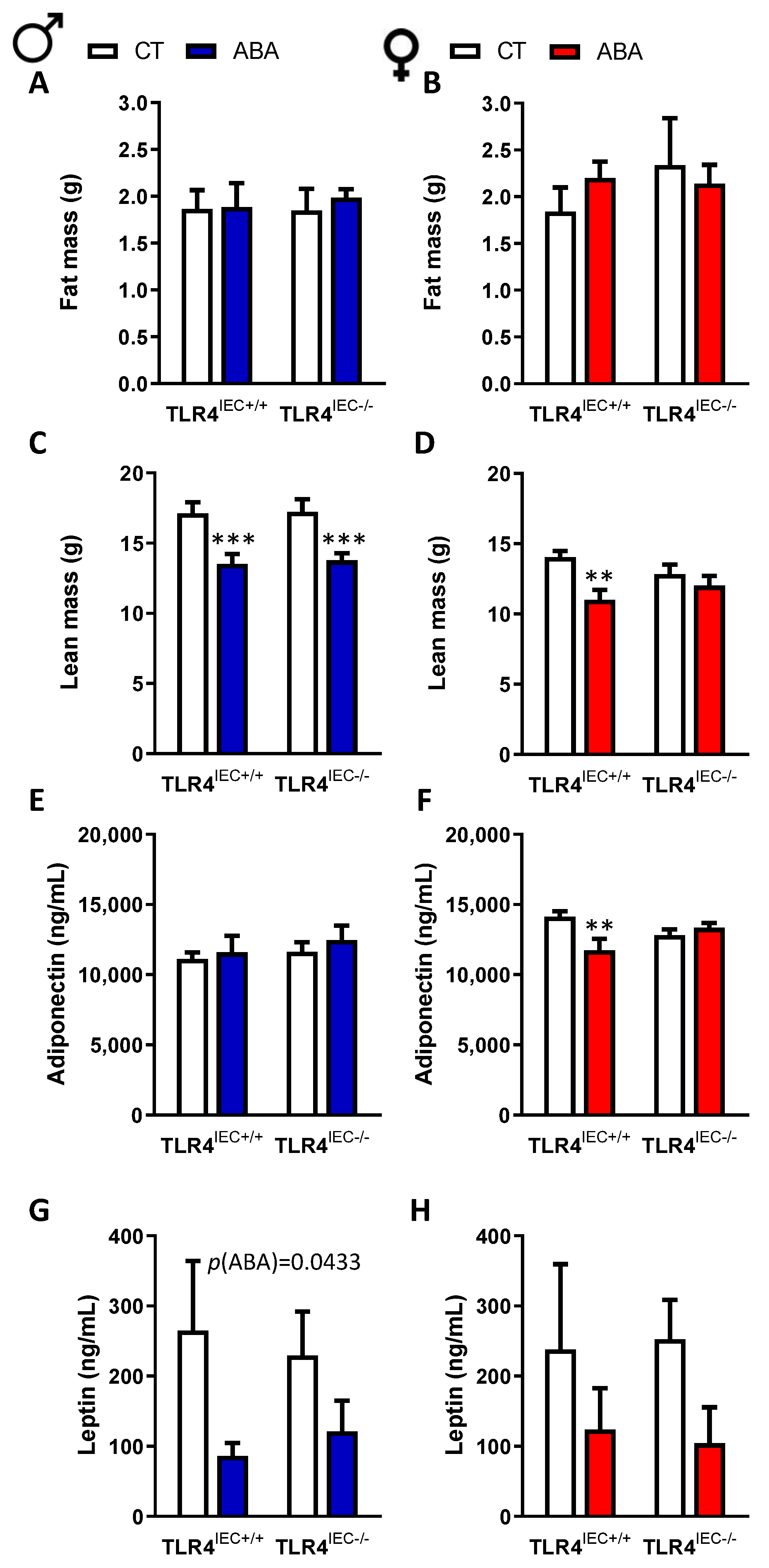
3.1. Effects of Intestinal Epithelial TLR4 Knockout on Body Weight, Body Composition and Food Intake in Response to ABA Model
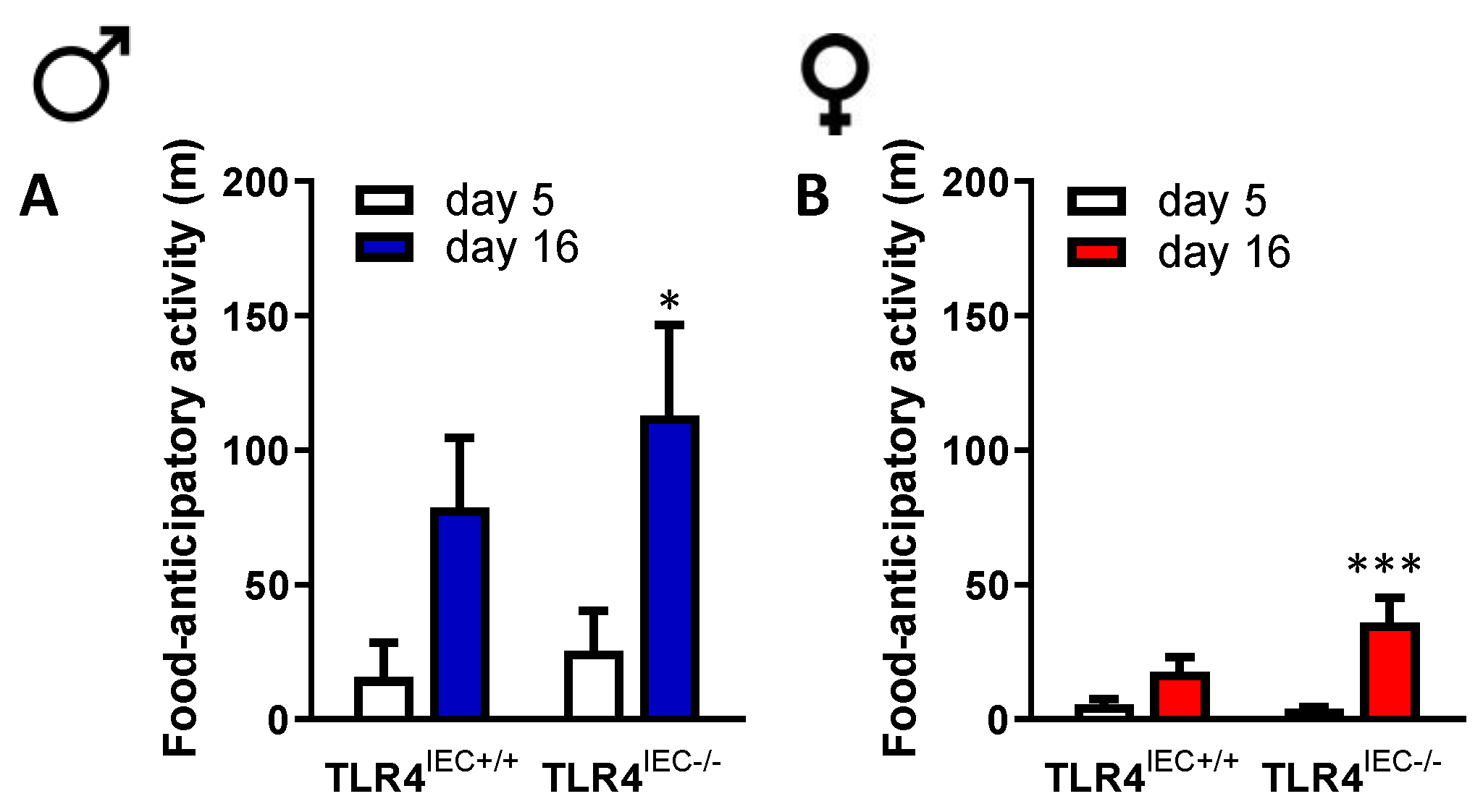
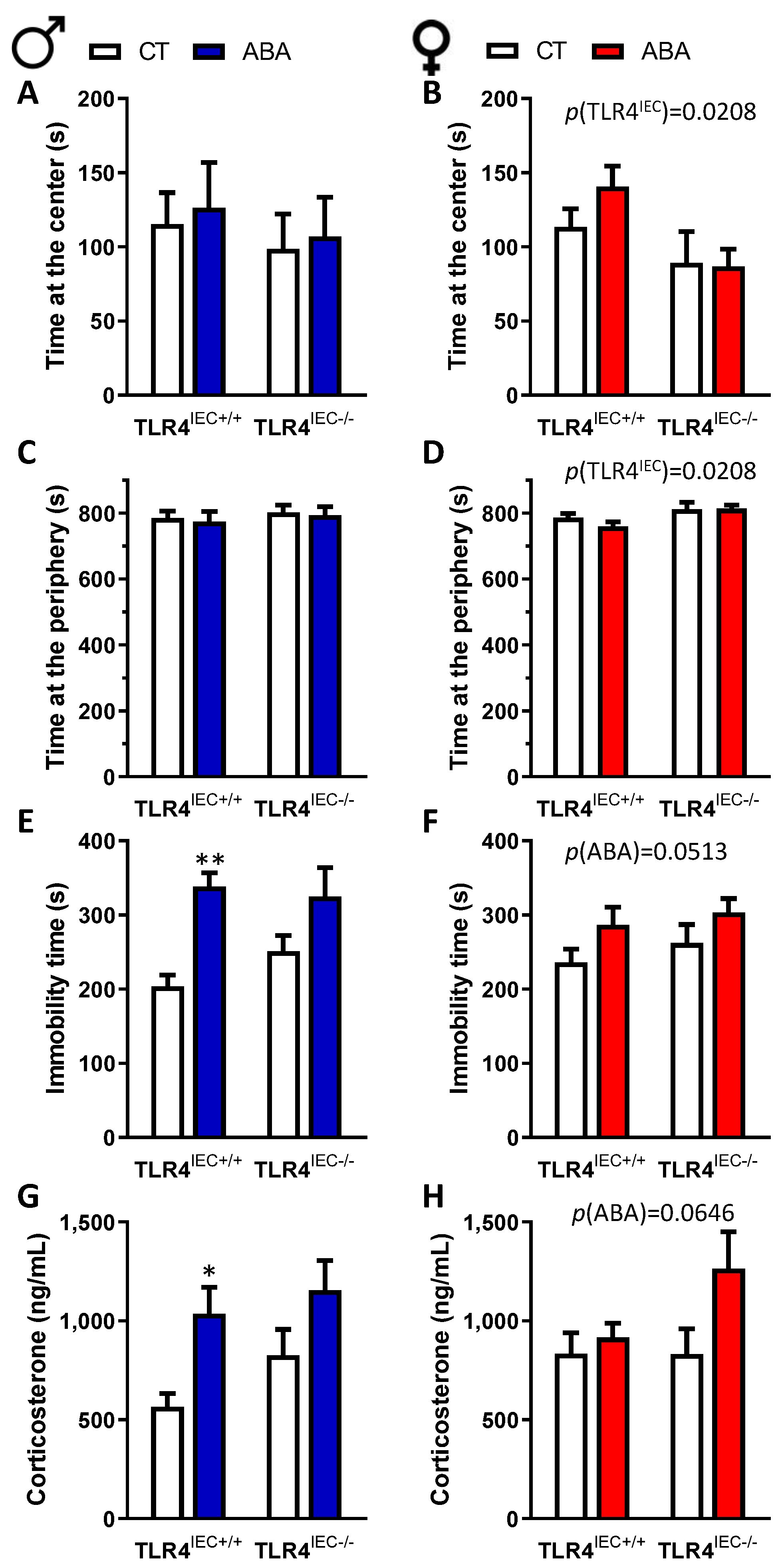
3.2. Effects of Intestinal Epithelial TLR4 Knockout on Behavioural Response
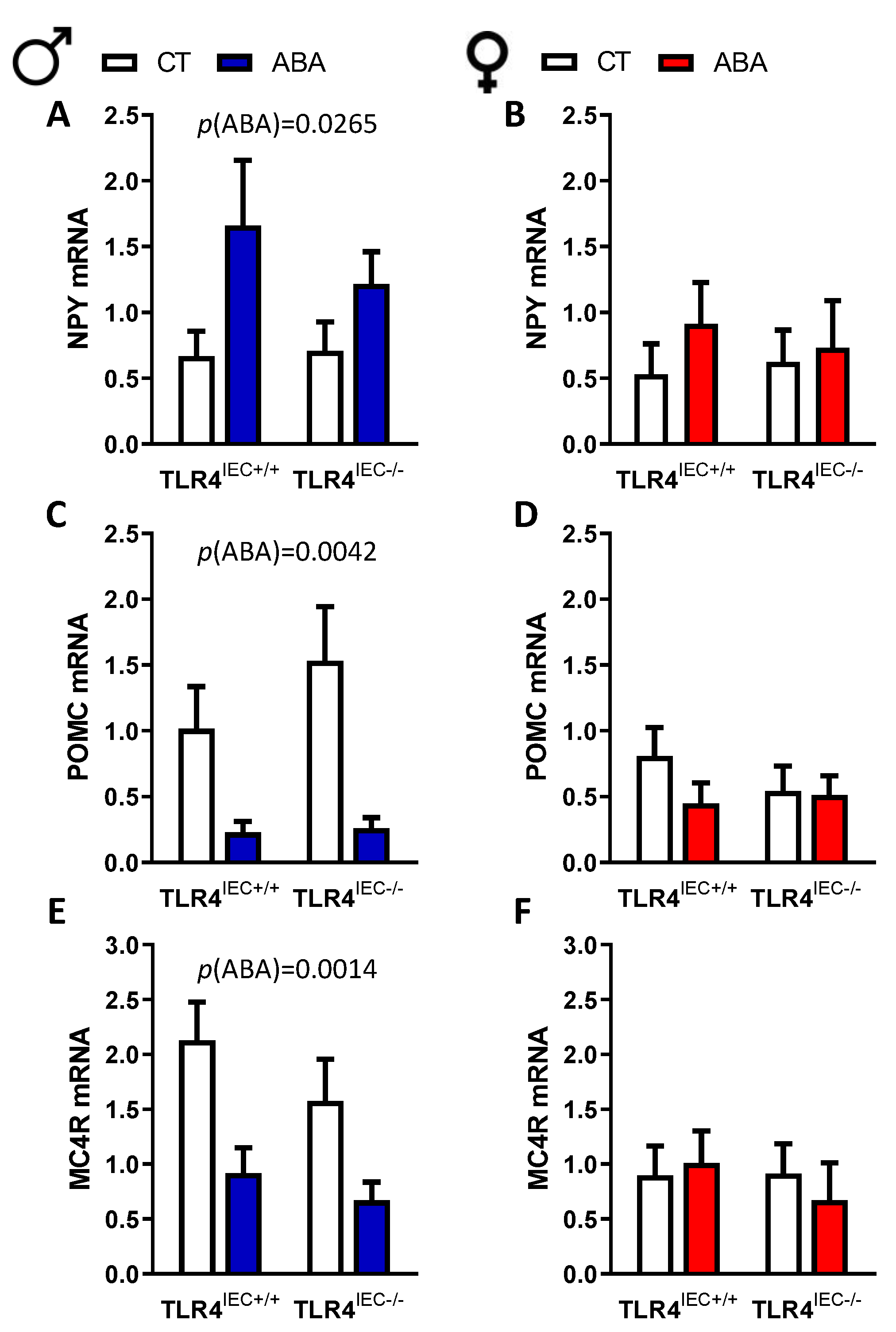
3.3. Effects of Intestinal Epithelial TLR4 Knockout on the Hypothalamic Response to ABA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steinhausen, H.-C.; Jensen, C.M. Time trends in lifetime incidence rates of first-time diagnosed anorexia nervosa and bulimia nervosa across 16 years in a danish nationwide psychiatric registry study. Int. J. Eat. Disord. 2015, 48, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Galmiche, M.; Déchelotte, P.; Lambert, G.; Tavolacci, M.P. Prevalence of eating disorders over the 2000–2018 period: A systematic literature review. Am. J. Clin. Nutr. 2019, 109, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Gravina, G.; Milano, W.; Nebbiai, G.; Piccione, C.; Capasso, A. Medical Complications in Anorexia and Bulimia Nervosa. Endocr. Metab. Immune Disord.-Drug Targets 2018, 18, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.E.; Cook-Myers, T.; Wonderlich, S.A. Diagnostic criteria for anorexia nervosa: Looking ahead to DSM-V. Int. J. Eat. Disord. 2005, 37, S95–S97. [Google Scholar] [CrossRef] [PubMed]
- Swinbourne, J.M.; Touyz, S.W. The co-morbidity of eating disorders and anxiety disorders: A review. Eur. Eat. Disord. Rev. 2007, 15, 253–274. [Google Scholar] [CrossRef] [PubMed]
- Gorwood, P.; Blanchet-Collet, C.; Chartrel, N.; Duclos, J.; Dechelotte, P.; Hanachi, M.; Fetissov, S.; Godart, N.; Melchior, J.C.; Ramoz, N.; et al. New Insights in Anorexia Nervosa. Front. Neurosci. 2016, 10, 256. [Google Scholar] [CrossRef]
- van de Wouw, M.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: Modulator of Host Metabolism and Appetite. J. Nutr. 2017, 147, 727–745. [Google Scholar] [CrossRef]
- Breton, J.; Déchelotte, P.; Ribet, D. Intestinal microbiota and Anorexia Nervosa. Clin. Nutr. Exp. 2019, 28, 11–21. [Google Scholar] [CrossRef]
- Million, Á.; Angelakis, E.; Maraninchi, M.; Henry, M.; Giorgi, R.; Valero, R.; Vialettes, B.; Raoult, D. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int. J. Obes. 2013, 37, 1460–1466. [Google Scholar] [CrossRef]
- Faggioni, R.; Fuller, J.; Moser, A.; Feingold, K.R.; Grunfeld, C. LPS-induced anorexia in leptin-deficient (ob/ob) and leptin receptor-deficient (db/db) mice. Am. J. Physiol. 1997, 273, R181–R186. [Google Scholar] [CrossRef]
- Sulakhiya, K.; Keshavlal, G.P.; Bezbaruah, B.B.; Dwivedi, S.; Gurjar, S.S.; Munde, N.; Jangra, A.; Lahkar, M.; Gogoi, R. Lipopolysaccharide induced anxiety- and depressive-like behaviour in mice are prevented by chronic pre-treatment of esculetin. Neurosci. Lett. 2016, 611, 106–111. [Google Scholar] [CrossRef]
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef]
- Schalla, M.A.; Stengel, A. Activity Based Anorexia as an Animal Model for Anorexia Nervosa-A Systematic Review. Front. Nutr. 2019, 6, 69. [Google Scholar] [CrossRef]
- Belmonte, L.; Achamrah, N.; Nobis, S.; Guérin, C.; Riou, G.; Bôle-Feysot, C.; Boyer, O.; Richard, V.; Rego, J.C.D.; Déchelotte, P.; et al. A role for intestinal TLR4-driven inflammatory response during activity-based anorexia. Sci. Rep. 2016, 6, 35813. [Google Scholar] [CrossRef]
- Achamrah, N.; Nobis, S.; Goichon, A.; Breton, J.; Legrand, R.; Do Rego, J.L.; Do Rego, J.C.; Déchelotte, P.; Fetissov, S.O.; Belmonte, L.; et al. Sex differences in response to activity-based anorexia model in C57Bl/6 mice. Physiol. Behav. 2017, 170, 1–5. [Google Scholar] [CrossRef]
- el Marjou, F.; Janssen, K.-P.; Chang, B.; Li, M.; Hindie, V.; Chan, L.; Louvard, D.; Chambon, P.; Metzger, D.; Robine, S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genes 2004, 39, 186–193. [Google Scholar] [CrossRef]
- Jésus, P.; Ouelaa, W.; Francois, M.; Riachy, L.; Guérin, C.; Aziz, M.; Do Rego, J.-C.; Déchelotte, P.; Fetissov, S.O.; Coeffier, M. Alteration of intestinal barrier function during activity-based anorexia in mice. Clin. Nutr. 2014, 33, 1046–1053. [Google Scholar] [CrossRef]
- Ghouzali, I.; Lemaitre, C.; Bahlouli, W.; Azhar, S.; Bôle-Feysot, C.; Meleine, M.; Ducrotté, P.; Déchelotte, P.; Coëffier, M. Targeting immunoproteasome and glutamine supplementation prevent intestinal hyperpermeability. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 3278–3288. [Google Scholar] [CrossRef]
- Morita, C.; Tsuji, H.; Hata, T.; Gondo, M.; Takakura, S.; Kawai, K.; Yoshihara, K.; Ogata, K.; Nomoto, K.; Miyazaki, K.; et al. Gut Dysbiosis in Patients with Anorexia Nervosa. PLoS ONE 2015, 10, e0145274. [Google Scholar] [CrossRef]
- Breton, J.; Tirelle, P.; Hasanat, S.; Pernot, A.; l’Huillier, C.; Do Rego, J.C.; Déchelotte, P.; Coëffier, M.; Bindels, L.B.; Ribet, D. Gut microbiota alteration in a mouse model of Anorexia Nervosa. Clin. Nutr. 2021, 40, 181–189. [Google Scholar] [CrossRef]
- Kim, K.K.; Jin, S.H.; Lee, B.J. Herpes Virus Entry Mediator Signaling in the Brain Is Imperative in Acute Inflammation-Induced Anorexia and Body Weight Loss. Endocrinol. Metab. 2013, 28, 214–220. [Google Scholar] [CrossRef]
- Pålsson-McDermott, E.M.; O’Neill LA, J. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 2004, 113, 153–162. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Tirelle, P.; Breton, J.; Kauffmann, A.; Bahlouli, W.; l’Huillier, C.; Salameh, E.; Amamou, A.; Jarbeau, M.; Guérin, C.; Goichon, A.; et al. Gut microbiota depletion affects nutritional and behavioral responses to activity-based anorexia model in a sex-dependent manner. Clin. Nutr. 2021, 40, 2734–2744. [Google Scholar] [CrossRef]
- Tirelle, P.; Breton, J.; Riou, G.; Déchelotte, P.; Coëffier, M.; Ribet, D. Comparison of different modes of antibiotic delivery on gut microbiota depletion efficiency and body composition in mouse. BMC Microbiol. 2020, 20, 340. [Google Scholar] [CrossRef]
- Everard, A.; Geurts, L.; Caesar, R.; Van Hul, M.; Matamoros, S.; Duparc, T.; Denis, R.G.; Cochez, P.; Pierard, F.; Castel, J.; et al. Intestinal epithelial MyD88 is a sensor switching host metabolism towards obesity according to nutritional status. Nat. Commun. 2014, 5, 5648. [Google Scholar] [CrossRef]
- Queipo-Ortuño, M.I.; Seoane, L.M.; Murri, M.; Pardo, M.; Gomez-Zumaquero, J.M.; Cardona, F.; Casanueva, F.; Tinahones, F.J. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS ONE 2013, 8, e65465. [Google Scholar] [CrossRef]
- Lian, Q.; Zuo, X.; Mao, Y.; Luo, S.; Zhang, S.; Tu, X.; Lou, C.; Zhou, W. Anorexia nervosa, depression and suicidal thoughts among Chinese adolescents: A national school-based cross-sectional study. Environ. Health Prev. Med. 2017, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Lynch, M.; Lu, J.; Tang, V.; Deng, Y.; Jury, J.; Umeh, G.; Miranda, P.; Pastor, M.P.; Sidani, S.; et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci. Transl. Med. 2017, 9, eaaf6397. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Buisman-Pijlman, F.; Hutchinson, M.R. Toll-like receptor 4: Innate immune regulator of neuroimmune and neuroendocrine interactions in stress and major depressive disorder. Front. Neurosci. 2014, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Femenia, T.; Qian, Y.; Arentsen, T.; Forssberg, H.; Diaz Heijtz, R. Toll-like receptor-4 regulates anxiety-like behavior and DARPP-32 phosphorylation. Brain Behav. Immun. 2018, 69, 273–282. [Google Scholar] [CrossRef]
- Lim, J.E.; Song, M.; Jin, J.; Kou, J.; Pattanayak, A.; Lalonde, R.; Fukuchi, K.I. The effects of MyD88 deficiency on exploratory activity, anxiety, motor coordination and spatial learning in C57BL/6 and APPswe/PS1dE9 mice. Behav. Brain Res. 2012, 227, 36–42. [Google Scholar] [CrossRef][Green Version]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef]
- Fields, C.T.; Chassaing, B.; Castillo-Ruiz, A.; Osan, R.; Gewirtz, A.T.; de Vries, G.J. Effects of gut-derived endotoxin on anxiety-like and repetitive behaviors in male and female mice. Biol. Sex Differ. 2018, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Santos-Galindo, M.; Acaz-Fonseca, E.; Bellini, M.J.; Garcia-Segura, L.M. Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biol. Sex Differ. 2011, 2, 7. [Google Scholar] [CrossRef]
- Nobis, S.; Goichon, A.; Achamrah, N.; Guérin, C.; Azhar, S.; Chan, P.; Morin, A.; Bôle-Feysot, C.; do Rego, J.C.; Vaudry, D.; et al. Alterations of proteome, mitochondrial dynamic and autophagy in the hypothalamus during activity-based anorexia. Sci Rep. 2018, 8, 7233. [Google Scholar] [CrossRef]
- Breton, J.; Giallourou, N.; Nobis, S.; Morin, A.; Achamrah, N.; Goichon, A.; Belmonte, L.; Dechelotte, P.; Do Rego, J.L.; Coëffier, M.; et al. Characterizing the metabolic perturbations induced by activity-based anorexia in the C57Bl/6 mouse using (1)H NMR spectroscopy. Clin. Nutr. 2020, 39, 2428–2434. [Google Scholar] [CrossRef]
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| RPS18 | TGCGAGTACTCAACACCAACA | TTCCTCAACACCACATGAGC |
| GAPDH | ATCACTGCCACTCAGAAGA | TCACTGCCACTCAGAAGA |
| NPY | CTGCGACACTACATCAATCT | CTTCAAGCCTTGTTCTGG |
| POMC | CCTCCTGCTTCAGACCTCCA | GGCTGTTCATCTCCGTTGC |
| MC4R | TCTCTATGTCCACATGTTCCTG | GGGGCCCAGCAGACAACAAAG |
| BDNF | TGTGACAGTATTAGCGAGTGG | TACGATTGGGTAGTTCGGCATT |
| TLR4 | AGATCTGAGCTTCAACCCCTTG | AGAGGTGGTGTAAGCCATGC |
| IL-6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| For genotyping | ||
| Villin-CreERT2 | CAAGCCTGGCTCGACGGCC | CGCGAACATCTTCAGGTTCT |
| TLR4LoxP | TGACCACCATATTGCCTATAC | TGATGGTGTGAGCAGGAGAG |
| To control DNA recombination | ||
| TLR4 | GAACCTAGTACATGTGGATCTTTCTTATAACT | GTCTTGAATGAAGTCAATTGGGTTCA |
| Cre Activity | TGACCACCCATATTGCCTATAC | CCTCTTCTGTGCTATCTGGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirelle, P.; Salaün, C.; Kauffmann, A.; Bôle-Feysot, C.; Guérin, C.; Huré, M.; Goichon, A.; Amamou, A.; Breton, J.; do Rego, J.-L.; et al. Intestinal Epithelial Toll-like Receptor 4 Deficiency Modifies the Response to the Activity-Based Anorexia Model in a Sex-Dependent Manner: A Preliminary Study. Nutrients 2022, 14, 3607. https://doi.org/10.3390/nu14173607
Tirelle P, Salaün C, Kauffmann A, Bôle-Feysot C, Guérin C, Huré M, Goichon A, Amamou A, Breton J, do Rego J-L, et al. Intestinal Epithelial Toll-like Receptor 4 Deficiency Modifies the Response to the Activity-Based Anorexia Model in a Sex-Dependent Manner: A Preliminary Study. Nutrients. 2022; 14(17):3607. https://doi.org/10.3390/nu14173607
Chicago/Turabian StyleTirelle, Pauline, Colin Salaün, Alexandre Kauffmann, Christine Bôle-Feysot, Charlène Guérin, Marion Huré, Alexis Goichon, Asma Amamou, Jonathan Breton, Jean-Luc do Rego, and et al. 2022. "Intestinal Epithelial Toll-like Receptor 4 Deficiency Modifies the Response to the Activity-Based Anorexia Model in a Sex-Dependent Manner: A Preliminary Study" Nutrients 14, no. 17: 3607. https://doi.org/10.3390/nu14173607
APA StyleTirelle, P., Salaün, C., Kauffmann, A., Bôle-Feysot, C., Guérin, C., Huré, M., Goichon, A., Amamou, A., Breton, J., do Rego, J.-L., Déchelotte, P., Achamrah, N., & Coëffier, M. (2022). Intestinal Epithelial Toll-like Receptor 4 Deficiency Modifies the Response to the Activity-Based Anorexia Model in a Sex-Dependent Manner: A Preliminary Study. Nutrients, 14(17), 3607. https://doi.org/10.3390/nu14173607





