Studies on the Reaction of Dietary Methylglyoxal and Creatine during Simulated Gastrointestinal Digestion and in Human Volunteers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Digestion Experiments
2.3. Human Intervention Study
2.4. High-Pressure Liquid Chromatography with UV Detection (HPLC–UV) of MGO after Derivatization to Quinoxalines
2.5. High-Pressure Liquid Chromatography with Tandem Mass Spectrometric (HPLC–MS/MS) Detection
2.6. Statistical Treatment
3. Results
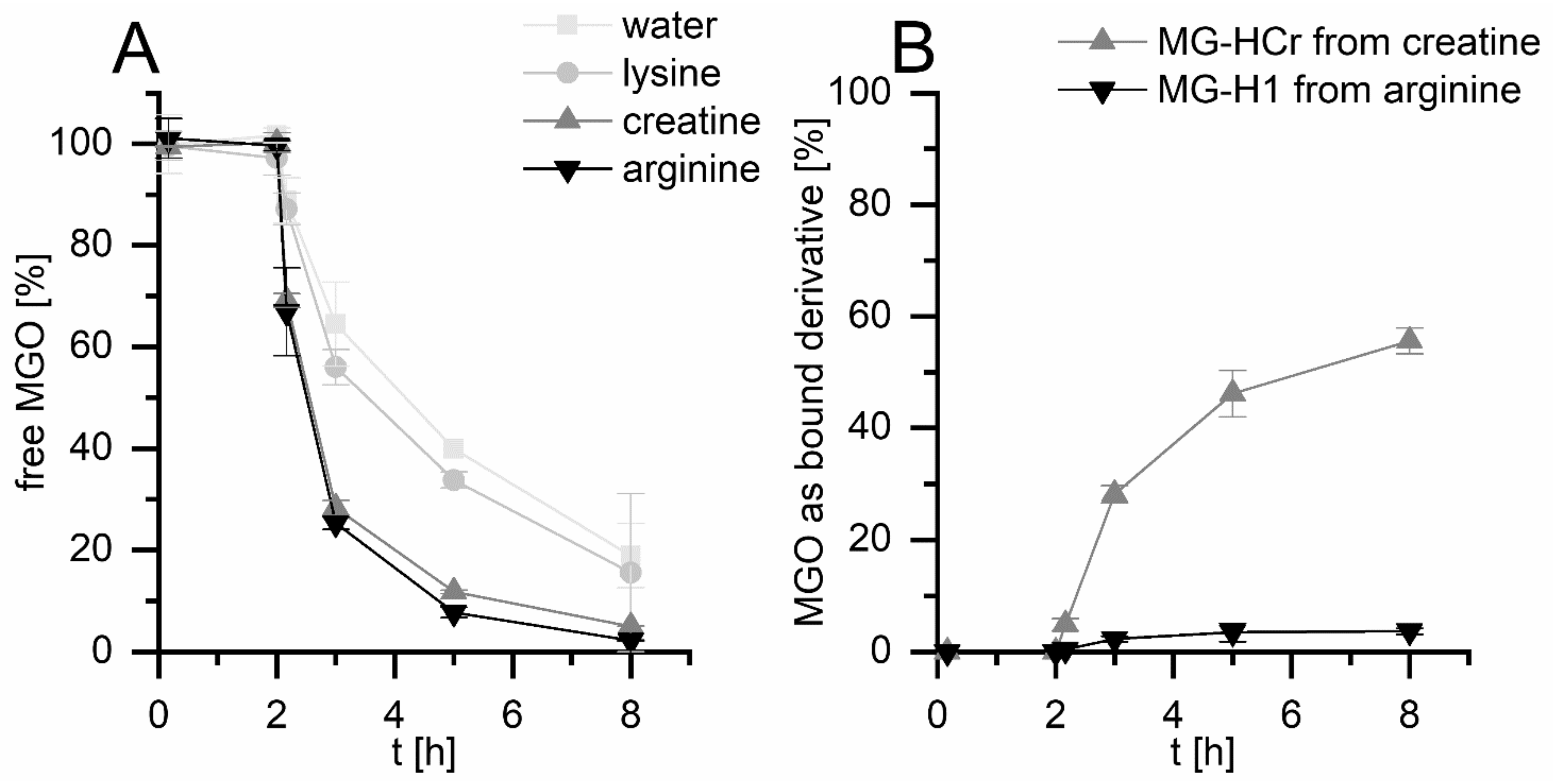
3.1. Methylglyoxal (MGO) Reacts with Lysine, Arginine, and Creatine during Simulated Digestion
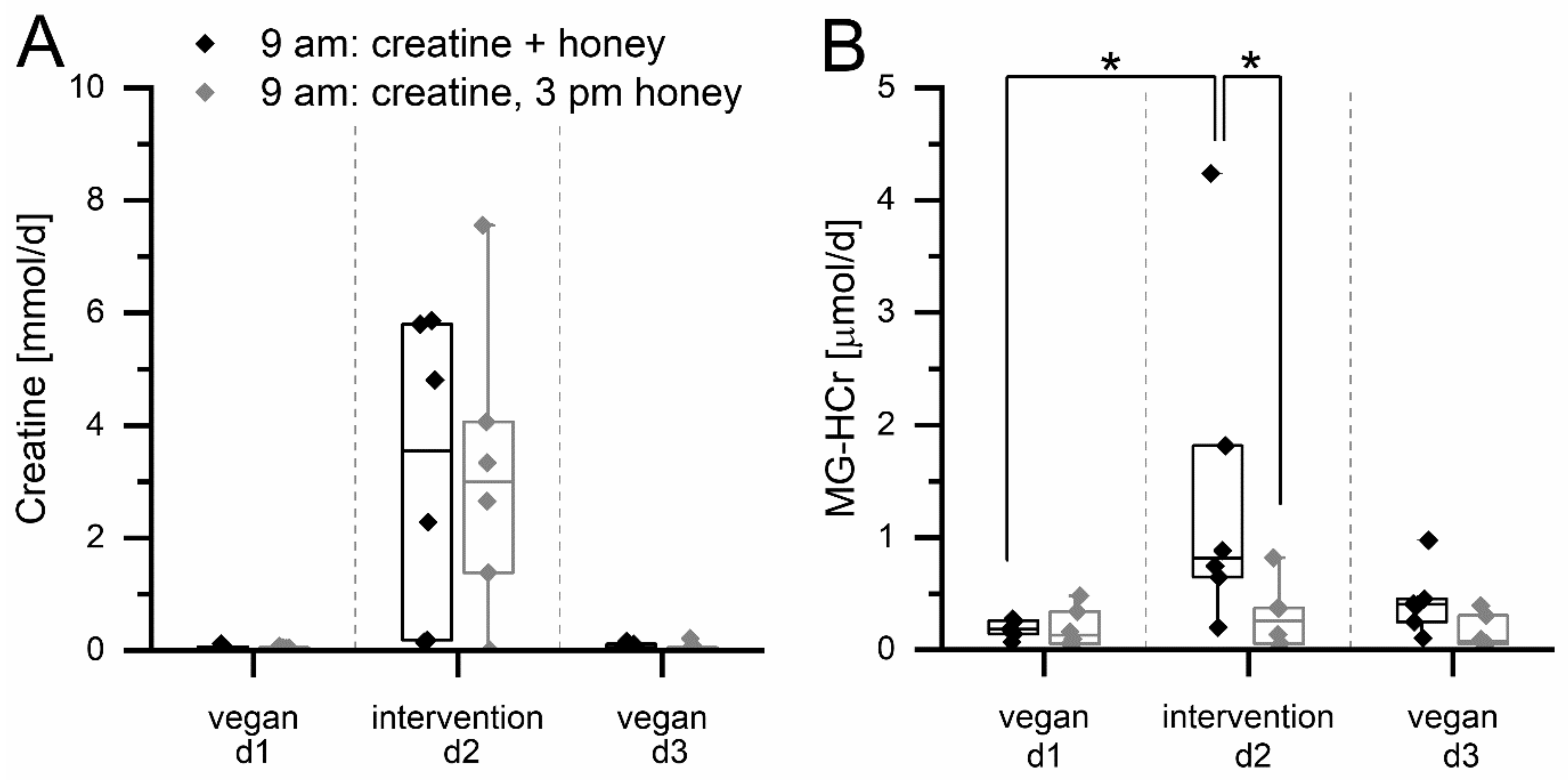
3.2. Reaction of MGO and Creatine during Simultaneous Digestion in Human Volunteers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phillips, S.A.; Thornalley, P.J. The formation of methylglyoxal from triose phosphates: Investigation using a specific assay for methylglyoxal. Eur. J. Biochem. 1993, 212, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Kroh, L.W. Caramelisation in food and beverages. Food Chem. 1994, 51, 373–379. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Langborg, A.; Minhas, H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999, 344, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Methylglyoxal, glyoxalase 1 and the dicarbonyl proteome. Amino Acids. 2012, 42, 1133–1142. [Google Scholar] [CrossRef]
- Miyata, T.; Ueda, Y.; Yamada, Y.; Izuhara, Y.; Wada, T.; Jadoul, M.; Saito, A.; Kurokawa, K.; De Strihou, C.V.Y. Accumulation of carbonyls accelerates the formation of pentosidine, an advanced glycation end product: Carbonyl stress in uremia. J. Am. Soc. Nephrol. 1998, 9, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Löbner, J.; Degen, J.; Henle, T. Creatine Is a scavenger for methylglyoxal under physiological conditions via formation of N-(4-methyl-5-oxo-1-imidazolin-2-yl)sarcosine (MG-HCr). J. Agric. Food Chem. 2015, 63, 2249–2256. [Google Scholar] [CrossRef] [PubMed]
- Cömert, E.D.; Gökmen, V. Kinetic evaluation of the reaction between methylglyoxal and certain scavenging compounds and determination of their in vitro dicarbonyl scavenging activity. Food Res. Int. 2019, 121, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhou, W. Role of quercetin in the physicochemical properties, antioxidant and antiglycation activities of bread. J. Funct. Foods. 2018, 40, 299–306. [Google Scholar] [CrossRef]
- Treibmann, S.; Spengler, F.; Degen, J.; Löbner, J.; Henle, T. Studies on the formation of 3-deoxyglucosone- and methylglyoxal-derived hydroimidazolones of creatine during heat treatment of meat. J. Agric. Food Chem. 2019, 67, 5874–5881. [Google Scholar] [CrossRef]
- Thornalley, P.J. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch. Biochem. Biophys. 2003, 419, 31–40. [Google Scholar] [CrossRef]
- Van den Eynde, M.D.; Geleijnse, J.M.; Scheijen, J.L.; Hanssen, N.M.; Dower, J.I.; Afman, L.A.; Stehouwer, C.D.; Hollman, P.C.; Schalkwijk, C.G. Quercetin, but not epicatechin, decreases plasma concentrations of methylglyoxal in adults in a randomized, double-blind, placebo-controlled, crossover trial with pure flavonoids. J. Nutr. 2018, 148, 1911–1916. [Google Scholar] [CrossRef]
- Maasen, K.; Scheijen, J.L.J.M.; Opperhuizen, A.; Stehouwer, C.D.A.; van Greevenbroek, M.M.; Schalkwijk, C.G. Quantification of dicarbonyl compounds in commonly consumed foods and drinks; presentation of a food composition database for dicarbonyls. Food Chem. 2021, 339, 128063. [Google Scholar] [CrossRef]
- Degen, J.; Hellwig, M.; Henle, T. 1,2-Dicarbonyl compounds in commonly consumed foods. J. Agric. Food Chem. 2012, 60, 7071–7079. [Google Scholar] [CrossRef]
- Degen, J.; Vogel, M.; Richter, D.; Hellwig, M.; Henle, T. Metabolic transit of dietary methylglyoxal. J. Agric. Food Chem. 2013, 61, 10253–10260. [Google Scholar] [CrossRef]
- Amoroso, A.; Maga, G.; Daglia, M. Cytotoxicity of α-dicarbonyl compounds submitted to in vitro simulated digestion process. Food Chem. 2013, 140, 654–659. [Google Scholar] [CrossRef]
- Daglia, M.; Ferrari, D.; Collina, S.; Curti, V. Influence of in vitro simulated gastroduodenal digestion on methylglyoxal concentration of manuka (Lectospermum scoparium) honey. J. Agric. Food Chem. 2013, 61, 2140–2145. [Google Scholar] [CrossRef]
- Hamzalıoğlu, A.; Gökmen, V. Investigations on the reactions of α-dicarbonyl compounds with amino acids and proteins during in vitro digestion of biscuits. Food Funct. 2016, 7, 2544–2550. [Google Scholar] [CrossRef]
- Jiang, K.; Huang, C.; Jiao, R.; Bai, W.; Zheng, J.; Ou, S. Adducts formed during protein digestion decreased the toxicity of five carbonyl compounds against Caco-2 cells. J. Hazard. Mater. 2019, 363, 26–33. [Google Scholar] [CrossRef]
- Hamzalıoğlu, A.; Gökmen, V. Potential reactions of thermal process contaminants during digestion. Trends Food Sci. Technol. 2020, 106, 198–208. [Google Scholar] [CrossRef]
- Henle, T.; Zehetner, G.; Klostermeyer, H. Fast and sensitive determination of furosine. Z. Lebensm. Unters. Forsch. 1995, 200, 235–237. [Google Scholar] [CrossRef]
- Van der Lugt, T.; Venema, K.; van Leeuwen, S.; Vrolijk, M.F.; Opperhuizen, A.; Bast, A. Gastrointestinal digestion of dietary advanced glycation endproducts using an in vitro model of the gastrointestinal tract (TIM-1). Food Funct. 2020, 11, 6297–6307. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.; Paolillo, M.; Papetti, A. A new millifluidic-based gastrointestinal platform to evaluate the effect of simulated dietary methylglyoxal intakes. Food Funct. 2019, 10, 4330–4338. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M.; Amoroso, A.; Rossi, D.; Mascherpa, D.; Maga, G. Identification and quantification of α-dicarbonyl compounds in balsamic and traditional balsamic vinegars and their cytotoxicity against human cells. J. Food Compos. Anal. 2013, 31, 67–74. [Google Scholar] [CrossRef]
- Kuntz, S.; Kunz, C.; Rudloff, S. Carbonyl compounds methylglyoxal and glyoxal affect interleukin-8 secretion in intestinal cells by superoxide anion generation and activation of mapk p38. Mol. Nutr. Food Res. 2010, 54, 1458–1467. [Google Scholar] [CrossRef]
- Brighina, S.; Turrado, C.P.; Restuccia, C.; Walton, G.; Fallico, B.; Oruna-Concha, M.J.; Arena, E. Detrimental effect on the gut microbiota of 1,2-dicarbonyl compounds after in vitro gastro-intestinal and fermentative digestion. Food Chem. 2021, 341, 128237. [Google Scholar] [CrossRef]
- Cömert, E.D.; Gökmen, V. Investigation of the methylglyoxal scavenging kinetics of different food matrices under simulated intestinal conditions. Eur. Food Res. Technol. 2020, 246, 2461–2470. [Google Scholar] [CrossRef]
- Hellwig, M.; Geissler, S.; Matthes, R.; Peto, A.; Silow, C.; Brandsch, M.; Henle, T. Transport of free and peptide-bound glycated amino acids: Synthesis, transepithelial flux at Caco-2 cell monolayers, and interaction with apical membrane transport proteins. ChemBioChem 2011, 12, 1270–1279. [Google Scholar] [CrossRef]
- Treibmann, S.; Händler, S.; Hofmann, T.; Henle, T. MG-HCr, the methylglyoxal-derived hydroimidazolone of creatine, a biomarker for the dietary intake of animal source food. J. Agric. Food Chem. 2020, 68, 4966–4972. [Google Scholar] [CrossRef]
- Rückriemen, J.; Klemm, O.; Henle, T. Manuka honey (Leptospermum scoparium) inhibits jack bean urease activity due to methylglyoxal and dihydroxyacetone. Food Chem. 2017, 230, 540–546. [Google Scholar] [CrossRef]
- Klöpfer, A.; Spanneberg, R.; Glomb, M.A. Formation of arginine modifications in a model system of Nα-tert-butoxycarbonyl (Boc)-arginine with methylglyoxal. J. Agric. Food Chem. 2011, 59, 394–401. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Frye, E.B.; Degenhardt, T.P.; Thorpe, S.R.; Baynes, J.W. Nε-(Carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem. J. 1997, 324, 565–570. [Google Scholar] [CrossRef]
- Baskaran, S.; Balasubramanian, K.A. Purification and active site modification studies on glyoxalase I from monkey intestinal mucosa. Biochim. Biophys. Acta 1987, 913, 377–385. [Google Scholar] [CrossRef]
- MacNeil, L.; Hill, L.; MacDonald, D.; Keefe, L.; Cormier, J.F.; Burke, D.G.; Smith-Palmer, T. Analysis of creatine, creatinine, creatine-d3 and creatinine-d3 in urine, plasma, and red blood cells by HPLC and GC-MS to follow the fate of ingested creatine-d3. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 827, 210–215. [Google Scholar] [CrossRef]
- Clarke, R.E.; Dordevic, A.L.; Tan, S.M.; Ryan, L.; Coughlan, M.T. Dietary advanced glycation end products and risk factors for chronic disease: A systematic review of randomised controlled trials. Nutrients 2016, 8, 125. [Google Scholar] [CrossRef]
- Nowotny, K.; Schröter, D.; Schreiner, M.; Grune, T. Dietary advanced glycation end products and their relevance for human health. Ageing Res. Rev. 2018, 447, 55–66. [Google Scholar] [CrossRef]
- Scheijen, J.L.; Hanssen, N.M.; van Greevenbroek, M.M.; Van der Kallen, C.J.; Feskens, E.J.; Stehouwer, C.D.; Schalkwijk, C.G. Dietary intake of advanced glycation endproducts is associated with higher levels of advanced glycation endproducts in plasma and urine: The CODAM study. Clin. Nutr. 2018, 37, 919–925. [Google Scholar] [CrossRef]
| Transition | Fragmentor Voltage [V] | Collision Energy [eV] | Dwell Time [ms] | Q/q 1 | |
|---|---|---|---|---|---|
| MG-HCr | 186 → 87 | 110 | 20 | 180 | Q |
| 186 → 44 | 110 | 25 | 180 | q | |
| D3-MG-HCr | 189 → 90 | 110 | 20 | 130 | Q |
| 189 → 44 | 110 | 25 | 130 | q | |
| CEL | 219 → 130 | 100 | 10 | 70 | q |
| 219 → 84 | 100 | 20 | 70 | Q | |
| 13C3-CEL | 222 → 130 | 90 | 10 | 70 | q |
| 222 → 84 | 90 | 20 | 70 | Q | |
| Creatine | 132 → 90 | 75 | 10 | 60 | Q |
| 132 → 44 | 75 | 20 | 60 | q | |
| D3-Creatine | 135 → 93 | 90 | 10 | 60 | Q |
| 135 → 47 | 90 | 20 | 60 | q | |
| Creatinine | 114 → 86 | 105 | 10 | 50 | Q |
| 114 → 44 | 105 | 14 | 50 | q | |
| D3-Creatinine | 117 → 89 | 105 | 10 | 50 | Q |
| 117 → 47 | 105 | 14 | 50 | q | |
| MG-H1 | 229 → 166 | 75 | 10 | 120 | q |
| 229 → 114 | 75 | 10 | 120 | Q | |
| 13C6-MG-H1 | 235 → 171 | 120 | 10 | 120 | q |
| 235 → 115 | 120 | 10 | 120 | Q |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Treibmann, S.; Groß, J.; Pätzold, S.; Henle, T. Studies on the Reaction of Dietary Methylglyoxal and Creatine during Simulated Gastrointestinal Digestion and in Human Volunteers. Nutrients 2022, 14, 3598. https://doi.org/10.3390/nu14173598
Treibmann S, Groß J, Pätzold S, Henle T. Studies on the Reaction of Dietary Methylglyoxal and Creatine during Simulated Gastrointestinal Digestion and in Human Volunteers. Nutrients. 2022; 14(17):3598. https://doi.org/10.3390/nu14173598
Chicago/Turabian StyleTreibmann, Stephanie, Julia Groß, Susann Pätzold, and Thomas Henle. 2022. "Studies on the Reaction of Dietary Methylglyoxal and Creatine during Simulated Gastrointestinal Digestion and in Human Volunteers" Nutrients 14, no. 17: 3598. https://doi.org/10.3390/nu14173598
APA StyleTreibmann, S., Groß, J., Pätzold, S., & Henle, T. (2022). Studies on the Reaction of Dietary Methylglyoxal and Creatine during Simulated Gastrointestinal Digestion and in Human Volunteers. Nutrients, 14(17), 3598. https://doi.org/10.3390/nu14173598




