Contribution of Advanced Glycation End Products to PCOS Key Elements: A Narrative Review
Abstract
:1. Introduction
2. Search Strategy and Data Extraction
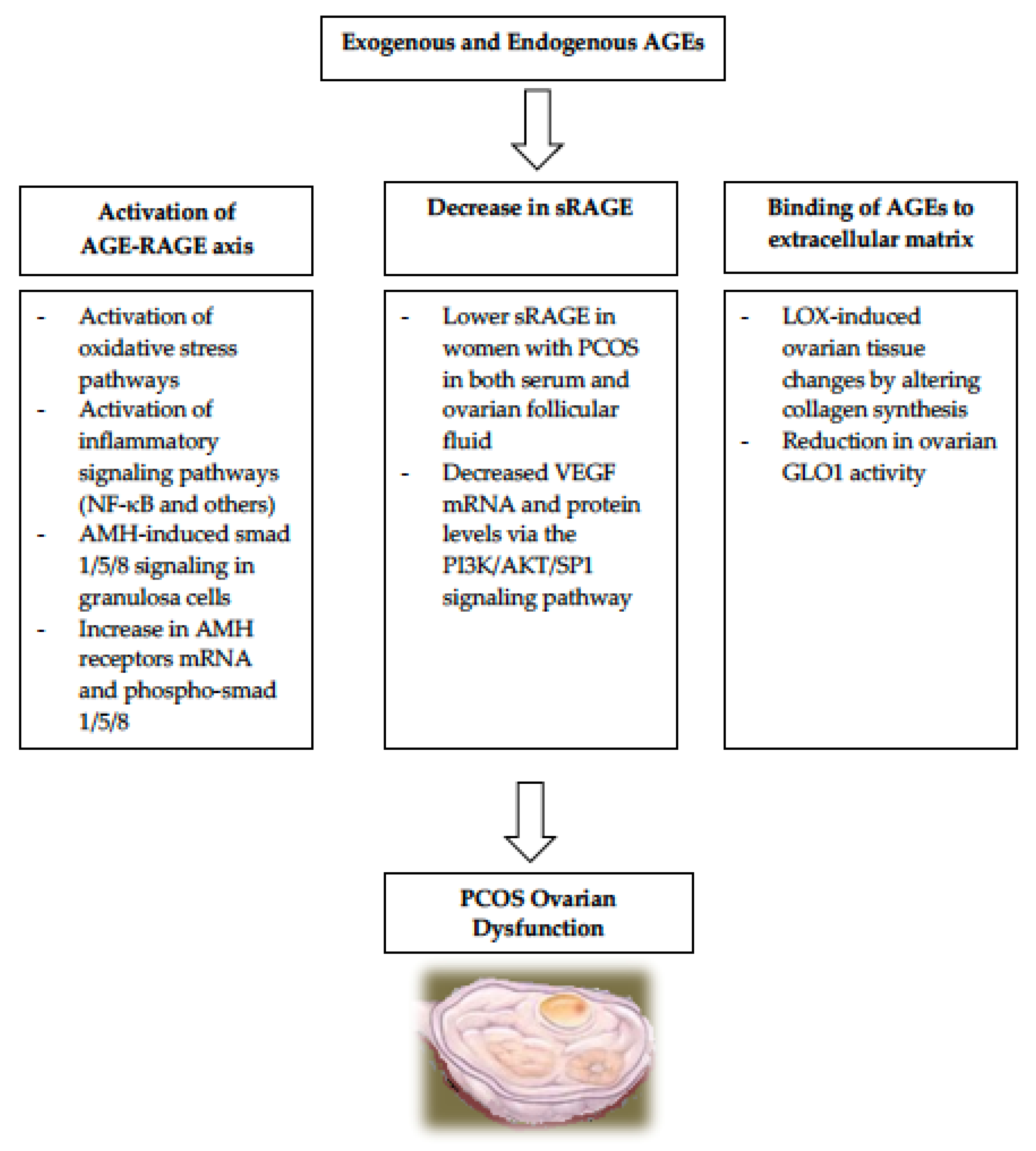
3. AGEs and Ovarian Dysfunction in PCOS
3.1. Activation of the AGE-RAGE Axis in PCOS
3.2. Relationship of sRAGE to PCOS
3.3. AGEs Binding to Extracellular Matrix in PCOS
- (A)
- One enzyme important for normal folliculogenesis is LOX (lysyl oxidase), which is present in granulosa cells and in the para-follicular environment. It regulates the changes to the para-follicular connective tissue, helping the formation and maintenance of the extracellular matrix during follicular development [58,59]. LOX is overexpressed in the ovaries of women with PCOS, causing alteration in enzymes responsible for collagen synthesis and leading to increased volume and density of the ovarian stroma [60]. Since AGEs also induce LOX expression, it is very plausible that the elevated AGEs in women with PCOS are responsible for the LOX-induced ovarian tissue changes observed in PCOS [61].
- (B)
- Another set of enzymes that play a role in protecting the ovarian function is the glyoxalase system. The two enzymes called glyoxalase 1 (GLO1) and glyoxalase 2 (GLO2), both of which are ubiquitously expressed to help in the protection against AGEs-related cellular damage by detoxifying one of the strong glycating agents called methylglyoxal [62]. Elevated levels of dietary AGEs have been linked to a reduction in ovarian GLO1 activity ultimately leading to ovarian dysfunction in PCOS [62]. These findings suggest that it is likely that AGEs play a role in the intraovarian pathophysiology in PCOS, partly via the glyoxalase system.
4. AGEs and Hyperandrogenism in PCOS
5. AGEs and Insulin Resistance (IR) in PCOS
6. AGEs and Obesity in PCOS
7. Pharmacological and Nutritional Therapeutic Options
- 7.1.
- 7.2.
- Women with PCOS and vitamin D deficiency who received vitamin D supplementation showed a significant decrease in the abnormally elevated serum AMH in PCOS and had an increased serum sRAGE levels suggesting that vitamin D supplementation in deficient women with PCOS could reduce the harmful effects of AGEs in PCOS [18,27].
- 7.3.
- Orlistat, a lipase inhibitor, has also been linked with decreased post-meal AGEs levels in women with PCOS as well as fasting insulin and testosterone concentrations [16].
- 7.4.
- Testosterone, which could be elevated in women with PCOS, increases RAGE expression and AGE accumulation in cultured human granulosa lutein cells (GLCs), and this was reduced by treatment with tauroursodeoxycholic acid (TUDCA), which acts as a chemical chaperone that dampens protein misfolding and improves ER stress. [67].
- 7.5.
- Recent studies have shown that L-Carnitine intake in women with PCOS showed improvements in hormonal and metabolic values, increased energy expenditure, and reduced lipids and body weight [80].
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deswal, R.; Narwal, V.; Dang, A.; Pundir, C.S. The Prevalence of Polycystic Ovary Syndrome: A Brief Systematic Review. J. Hum. Reprod. Sci. 2020, 13, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, L.C.; Subasinghe, A.; Jayasinghe, Y.L.; Callegari, E.T.; Garland, S.M.; Gorelik, A.; Wark, J.D. Polycystic ovarian syndrome: Prevalence and impact on the wellbeing of Australian women aged 16-29 years. Aust. N. Z. J. Obstet. Gynaecol. 2018, 58, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Otto-Buczkowska, E.; Grzyb, K.; Jainta, N. Polycystic ovary syndrome (PCOS) and the accompanying disorders of glucose homeostasis among girls at the time of puberty. Pediatr. Endocrinol. Diabetes Metab. 2018, 24, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Group TREAsPcw. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Committee on Practice Bulletins- Gynecology, Polycystic Ovary Syndrome: ACOG Practice Bulletin, Number 194. Obstet. Gynecol. 2018, 131, 1174–1176.
- Huttunen, H.J.; Fages, C.; Rauvala, H. Receptor for advanced glycation end products (RAGE)-mediated neurite outgrowth and activation of NF-kappaB require the cytoplasmic domain of the receptor but different downstream signaling pathways. J. Biol. Chem. 1999, 274, 19919–19924. [Google Scholar] [CrossRef]
- Merhi, Z. Advanced glycation end products and their relevance in female reproduction. Hum. Reprod. 2014, 29, 135–145. [Google Scholar] [CrossRef]
- John, W.G.; Lamb, E.J. The Maillard or browning reaction in diabetes. Eye 1993, 7 Pt 2, 230–237. [Google Scholar] [CrossRef]
- Sibbersen, C.; Johannsen, M. Dicarbonyl derived post-translational modifications: Chemistry bridging biology and aging-related disease. Essays Biochem. 2020, 64, 97–110. [Google Scholar] [CrossRef]
- Tessier, F.J. The Maillard reaction in the human body. The main discoveries and factors that affect glycation. Pathol. Biol. 2010, 58, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Botros, N.; Sluik, D.; Van Waateringe, R.P.; De Vries, J.H.M.; Geelen, A.; Feskens, E.J.M. Advanced glycation end-products (AGEs) and associations with cardio-metabolic, lifestyle, and dietary factors in a general population: The NQplus study. Diabetes Metab. Res. Rev. 2017, 33, e2892. [Google Scholar] [CrossRef] [PubMed]
- Soro-Paavonen, A.; Watson, A.M.D.; Li, J.; Paavonen, K.; Koitka, A.; Calkin, A.C.; Barit, D.; Coughlan, M.T.; Drew, B.G.; Lancaster, G.I.; et al. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes 2008, 57, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Katsikis, I.; Piperi, C.; Kandaraki, E.; Piouka, A.; Papavassiliou, A.G.; Panidis, D. Increased serum advanced glycation end-products is a distinct finding in lean women with polycystic ovary syndrome (PCOS). Clin. Endocrinol. 2008, 69, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Piperi, C.; Kalofoutis, A.; Creatsas, G. Increased levels of serum advanced glycation end-products in women with polycystic ovary syndrome. Clin. Endocrinol. 2005, 62, 37–43. [Google Scholar] [CrossRef]
- Garg, D.; Merhi, Z. Advanced Glycation End Products: Link between Diet and Ovulatory Dysfunction in PCOS? Nutrients 2015, 7, 10129–10144. [Google Scholar] [CrossRef]
- Goldberg, T.; Cai, W.; Peppa, M.; Dardaine, V.; Baliga, B.S.; Uribarri, J.; Vlassara, H. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc. 2004, 104, 1287–1291. [Google Scholar] [CrossRef]
- Gill, V.; Kumar, V.; Singh, K.; Kumar, A.; Kim, J.J. Advanced Glycation End Products (AGEs) May Be a Striking Link between Modern Diet and Health. Biomolecules 2019, 9, 888. [Google Scholar] [CrossRef]
- Cerami, C.; Founds, H.; Nicholl, I.; Mitsuhashi, T.; Giordano, D.; Vanpatten, S.; Lee, A.; Al-Abed, Y.; Vlassara, H.; Bucala, R.; et al. Tobacco smoke is a source of toxic reactive glycation products. Proc. Natl. Acad. Sci. USA 1997, 94, 13915–13920. [Google Scholar] [CrossRef] [PubMed]
- Guilbaud, A.; Niquet-Leridon, C.; Boulanger, E.; Tessier, F.J. How Can Diet Affect the Accumulation of Advanced Glycation End-Products in the Human Body? Foods 2016, 5, 84. [Google Scholar] [CrossRef]
- Bettiga, A.; Fiorio, F.; Di Marco, F.; Trevisani, F.; Romani, A.; Porrini, E.; Salonia, A.; Montorsi, F.; Vago, R. The Modern Western Diet Rich in Advanced Glycation End-Products (AGEs): An Overview of Its Impact on Obesity and Early Progression of Renal Pathology. Nutrients 2019, 11, 1748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamanti-Kandarakis, E.; Piperi, C.; Korkolopoulou, P.; Kandaraki, E.; Levidou, G.; Papalois, A.; Patsouris, E.; Papavassiliou, A.G. Accumulation of dietary glycotoxins in the reproductive system of normal female rats. J. Mol. Med. 2007, 85, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.; Wu, L. Methylglyoxal and advanced glycation endproducts: New therapeutic horizons? Recent Pat. Cardiovasc. Drug Discov. 2007, 2, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Piperi, C.; Patsouris, E.; Korkolopoulou, P.; Panidis, D.; Pawelczyk, L.; Papavassiliou, A.G.; Duleba, A.J. Immunohistochemical localization of advanced glycation end-products (AGEs) and their receptor (RAGE) in polycystic and normal ovaries. Histochem. Cell Biol. 2007, 127, 581–589. [Google Scholar] [CrossRef]
- Rutkowska, A.Z.; Diamanti-Kandarakis, E. Do Advanced Glycation End Products (AGEs) Contribute to the Comorbidities of Polycystic Ovary Syndrome (PCOS)? Curr. Pharm. Des. 2016, 22, 5558–5571. [Google Scholar] [CrossRef]
- Pertynska-Marczewska, M.; Diamanti-Kandarakis, E.; Zhang, J.; Merhi, Z. Advanced glycation end products: A link between metabolic and endothelial dysfunction in polycystic ovary syndrome? Metabolism 2015, 64, 1564–1573. [Google Scholar] [CrossRef]
- Merhi, Z. Crosstalk between advanced glycation end products and vitamin D: A compelling paradigm for the treatment of ovarian dysfunction in PCOS. Mol. Cell. Endocrinol. 2019, 479, 20–26. [Google Scholar] [CrossRef]
- Kalea, A.Z.; Schmidt, A.M.; Hudson, B.I. RAGE: A novel biological and genetic marker for vascular disease. Clin. Sci. 2009, 116, 621–637. [Google Scholar] [CrossRef]
- Tatone, C.; Amicarelli, F. The aging ovary--the poor granulosa cells. Fertil. Steril. 2013, 99, 12–17. [Google Scholar] [CrossRef]
- Basta, G. Receptor for advanced glycation endproducts and atherosclerosis: From basic mechanisms to clinical implications. Atherosclerosis 2008, 196, 9–21. [Google Scholar] [CrossRef]
- Fujii, E.Y.; Nakayama, M. The measurements of RAGE, VEGF, and AGEs in the plasma and follicular fluid of reproductive women: The influence of aging. Fertil. Steril. 2010, 94, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, K.; Fukagawa, T.; Matsumoto, K.-I.; Mita, K.; Takahashi, E.-I.; Ando, A.; Inoko, H.; Ikemura, T. Three genes in the human MHC class III region near the junction with the class II: Gene for receptor of advanced glycosylation end products, PBX2 homeobox gene and a notch homolog, human counterpart of mouse mammary tumor gene int-3. Genomics 1994, 23, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Meghnani, V.; Wagh, A.; Indurthi, V.S.; Koladia, M.; Vetter, S.W.; Law, B.; Leclerc, E. The receptor for advanced glycation end products influences the expression of its S100 protein ligands in melanoma tumors. Int. J. Biochem. Cell Biol. 2014, 57, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Neeper, M.; Schmidt, A.M.; Brett, J.; Yan, S.D.; Wang, F.E.N.G.; Pan, Y.C.; Elliston, K.; Stern, D.; Shaw, A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J. Biol. Chem. 1992, 267, 14998–15004. [Google Scholar] [CrossRef]
- Bohlender, J.M.; Franke, S.; Stein, G.; Wolf, G. Advanced glycation end products and the kidney. Am. J. Physiol. Ren. Physiol. 2005, 289, F645–F659. [Google Scholar] [CrossRef]
- Kumar Pasupulati, A.; Chitra, P.S.; Reddy, G.B. Advanced glycation end products mediated cellular and molecular events in the pathology of diabetic nephropathy. Biomol. Concepts 2016, 7, 293–309. [Google Scholar] [CrossRef]
- Stinghen, A.E.; Massy, Z.A.; Vlassara, H.; Striker, G.E.; Boullier, A. Uremic Toxicity of Advanced Glycation End Products in CKD. J. Am. Soc. Nephrol. 2016, 27, 354–370. [Google Scholar] [CrossRef]
- Kandaraki, E.A.; Chatzigeorgiou, A.; Papageorgiou, E.; Piperi, C.; Adamopoulos, C.; Papavassiliou, A.G.; Koutsilieris, M.; Diamanti-Kandarakis, E. Advanced glycation end products interfere in luteinizing hormone and follicle stimulating hormone signaling in human granulosa KGN cells. Exp. Biol. Med. 2018, 243, 29–33. [Google Scholar] [CrossRef]
- Verma, N.; Manna, S.K. Advanced Glycation End Products (AGE) Potently Induce Autophagy through Activation of RAF Protein Kinase and Nuclear Factor κB (NF-κB). J. Biol. Chem. 2016, 291, 1481–1491. [Google Scholar] [CrossRef]
- Guimarães, E.L.; Empsen, C.; Geerts, A.; Van Grunsven, L.A. Advanced glycation end products induce production of reactive oxygen species via the activation of NADPH oxidase in murine hepatic stellate cells. J. Hepatol. 2010, 52, 389–397. [Google Scholar] [CrossRef]
- Durlinger, A.L.; Gruijters, M.J.; Kramer, P.; Karels, B.; Ingraham, H.A.; Nachtigal, M.W.; Uilenbroek, J.T.; Grootegoed, J.A.; Themmen, A.P. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology 2002, 143, 1076–1084. [Google Scholar] [CrossRef]
- Josso, N.; Clemente, N. Transduction pathway of anti-Mullerian hormone, a sex-specific member of the TGF-beta family. Trends Endocrinol. Metab. 2003, 14, 91–97. [Google Scholar] [CrossRef]
- Josso, N.; Di Clemente, N.; Gouedard, L. Anti-Mullerian hormone and its receptors. Mol. Cell. Endocrinol. 2001, 179, 25–32. [Google Scholar] [CrossRef]
- Pellatt, L.; Rice, S.; Mason, H.D. Anti-Mullerian hormone and polycystic ovary syndrome: A mountain too high? Reproduction 2010, 139, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Merhi, Z.; Fadiel, A.; Buyuk, E.; Naftolin, F.; Cipolla, M. Vitamin D attenuates the adverse effect of advanced glycation end products on human granulosa cells: Implications for women with PCOS. Fertil. Steril. 2015, 104, e106. [Google Scholar] [CrossRef]
- Reynolds, P.R.; Kasteler, S.D.; Cosio, M.G.; Sturrock, A.; Huecksteadt, T.; Hoidal, J.R. RAGE: Developmental expression and positive feedback regulation by Egr-1 during cigarette smoke exposure in pulmonary epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 294, L1094–L1101. [Google Scholar] [CrossRef]
- Konishi, H.; Nakatsuka, M.; Chekir, C.; Noguchi, S.; Kamada, Y.; Sasaki, A.; Hiramatsu, Y. Advanced glycation end products induce secretion of chemokines and apoptosis in human first trimester trophoblasts. Hum. Reprod. 2004, 19, 2156–2162. [Google Scholar] [CrossRef]
- Erusalimsky, J.D. The use of the soluble receptor for advanced glycation-end products (sRAGE) as a potential biomarker of disease risk and adverse outcomes. Redox Biol. 2021, 42, 101958. [Google Scholar] [CrossRef]
- Wang, B.; Li, J.; Yang, Q.; Zhang, F.; Hao, M.; Guo, Y. Decreased levels of sRAGE in follicular fluid from patients with PCOS. Reproduction 2017, 153, 285–292. [Google Scholar] [CrossRef]
- Asadipooya, K.; Uy, E.M. Advanced Glycation End Products (AGEs), Receptor for AGEs, Diabetes, and Bone: Review of the Literature. J. Endocr. Soc. 2019, 3, 1799–1818. [Google Scholar] [CrossRef]
- Schmidt, A.M. Soluble RAGEs-Prospects for treating & tracking metabolic and inflammatory disease. Vasc. Pharmacol. 2015, 72, 1–8. [Google Scholar]
- Selvin, E.; Halushka, M.K.; Rawlings, A.M.; Hoogeveen, R.C.; Ballantyne, C.M.; Coresh, J.; Astor, B.C. sRAGE and risk of diabetes, cardiovascular disease, and death. Diabetes 2013, 62, 2116–2121. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wang, J.; Liu, Y.; Wang, L.; Du, M.; Zhang, Z.; Guan, Y. sRAGE downregulates the VEGF expression in OHSS ovarian granulosa cells. Gynecol. Endocrinol. 2021, 37, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Merhi, Z.; Buyuk, E.; Cipolla, M.J. Advanced glycation end products alter steroidogenic gene expression by granulosa cells: An effect partially reversible by vitamin D. Mol. Hum. Reprod. 2018, 24, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Basta, G.; Sironi, A.M.; Lazzerini, G.; Del Turco, S.; Buzzigoli, E.; Casolaro, A.; Natali, A.; Ferrannini, E.; Gastaldelli, A. Circulating soluble receptor for advanced glycation end products is inversely associated with glycemic control and S100A12 protein. J. Clin. Endocrinol. Metab. 2006, 91, 4628–4634. [Google Scholar] [CrossRef]
- Wang, B.; Hao, M.; Yang, Q.; Li, J.; Guo, Y. Follicular fluid soluble receptor for advanced glycation endproducts (sRAGE): A potential protective role in polycystic ovary syndrome. J. Assist. Reprod. Genet. 2016, 33, 959–965. [Google Scholar] [CrossRef]
- Garg, D.; Grazi, R.; Lambert-Messerlian, G.M.; Merhi, Z. Correlation between follicular fluid levels of sRAGE and vitamin D in women with PCOS. J. Assist. Reprod. Genet. 2017, 34, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Smith-Mungo, L.I.; Kagan, H.M. Lysyl oxidase: Properties, regulation and multiple functions in biology. Matrix Biol. J. Int. Soc. Matrix Biol. 1998, 16, 387–398. [Google Scholar] [CrossRef]
- Slee, R.B.; Hillier, S.G.; Largue, P.; Harlow, C.R.; Miele, G.; Clinton, M. Differentiation-dependent expression of connective tissue growth factor and lysyl oxidase messenger ribonucleic acids in rat granulosa cells. Endocrinology 2001, 142, 1082–1089. [Google Scholar] [CrossRef]
- Endo, H.H.T.; Nagasawa, K.; Hayashi, T.; Chida, M.; Akutagawa, N.; Iwasaki, M.; Kitajima, Y.; Kiya, T.; Nishikawa, A.; Manase, K.; et al. Lysyl oxidase and MMP-2 expression in dehydroepiandrosterone-induced polycystic ovary in rats. Biol. Reprod. 2001, 64, 157–162. [Google Scholar] [CrossRef]
- Papachroni, K.K.; Piperi, C.; Levidou, G.; Korkolopoulou, P.; Pawelczyk, L.; Diamanti-Kandarakis, E.; Papavassiliou, A.G. Lysyl oxidase interacts with AGE signalling to modulate collagen synthesis in polycystic ovarian tissue. J. Cell. Mol. Med. 2010, 14, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- Kandaraki, E.; Chatzigeorgiou, A.; Piperi, C.; Palioura, E.; Palimeri, S.; Korkolopoulou, P.; Koutsilieris, M.; Papavassiliou, A.G. Reduced ovarian glyoxalase-I activity by dietary glycotoxins and androgen excess: A causative link to polycystic ovarian syndrome. Mol. Med. 2012, 18, 1183–1189. [Google Scholar] [CrossRef]
- Tantalaki, E.; Piperi, C.; Livadas, S.; Kollias, A.; Adamopoulos, C.; Koulouri, A.; Christakou, C.; Diamanti-Kandarakis, E. Impact of dietary modification of advanced glycation end products (AGEs) on the hormonal and metabolic profile of women with polycystic ovary syndrome (PCOS). Hormones 2014, 13, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-H.; Chang, C.-C.; Wu, K.-H.; Shih, C.-K.; Chiang, W.; Chen, H.-Y.; Wang, K.-L.; Hong, Y.-H.; Shieh, T.-M.; Hsia, S.-M. Dietary Glycotoxins, Advanced Glycation End Products, Inhibit Cell Proliferation and Progesterone Secretion in Ovarian Granulosa Cells and Mimic PCOS-like Symptoms. Biomolecules 2019, 9, 327. [Google Scholar] [CrossRef] [PubMed]
- Merhi, Z.; Wang, S.; Cipolla, M.J. Vitamin d reverses the adverse effects of advanced glycation end products on granulosa cells. Fertil. Steril. 2016, 106, e76. [Google Scholar] [CrossRef]
- Chatzigeorgiou, A.; Kandaraki, E.; Piperi, C.; Livadas, S.; Papavassiliou, A.G.; Koutsilieris, M.; Papalois, A.; Diamanti-Kandarakis, E. Dietary glycotoxins affect scavenger receptor expression and the hormonal profile of female rats. J. Endocrinol. 2013, 218, 331–337. [Google Scholar] [CrossRef]
- Azhary, J.M.; Harada, M.; Kunitomi, C.; Kusamoto, A.; Takahashi, N.; Nose, E.; Oi, N.; Wada-Hiraike, O.; Urata, Y.; Hirata, T.; et al. Androgens Increase Accumulation of Advanced Glycation End Products in Granulosa Cells by Activating ER Stress in PCOS. Endocrinology 2020, 161, bqaa015. [Google Scholar] [CrossRef]
- Deligeoroglou, E.; Vrachnis, N.; Athanasopoulos, N.; Iliodromiti, Z.; Sifakis, S.; Iliodromiti, S.; Siristatidis, C.; Creatsas, G. Mediators of chronic inflammation in polycystic ovarian syndrome. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2012, 28, 974–978. [Google Scholar] [CrossRef]
- Moghetti, P.; Tosi, F. Insulin resistance and PCOS: Chicken or egg? J. Endocrinol. Investig. 2021, 44, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Arnone, A.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Salzano, C.; Pugliese, G.; Colao, A.; Savastano, S. Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women with Polycystic Ovary Syndrome (PCOS). Nutrients 2019, 11, 2278. [Google Scholar] [CrossRef] [PubMed]
- De Courten, B.; De Courten, M.P.; Soldatos, G.; Dougherty, S.L.; Straznicky, N.; Schlaich, M.; Sourris, K.C.; Chand, V.; Scheijen, J.L.; Kingwell, B.A.; et al. Diet low in advanced glycation end products increases insulin sensitivity in healthy overweight individuals: A double-blind, randomized, crossover trial. Am. J. Clin. Nutr. 2016, 103, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Cai, W.; Goodman, S.; Pyzik, R.; Yong, A.; Chen, X.; Zhu, L.; Neade, T.; Beeri, M.; Silverman, J.M.; et al. Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: Role of the antiinflammatory AGE receptor-1. J. Clin. Endocrinol. Metab. 2009, 94, 4483–4491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlassara, H.; Striker, G.E. AGE restriction in diabetes mellitus: A paradigm shift. Nat. Rev. Endocrinol. 2011, 7, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Häring, H.U.; Hu, F.B.; Schulze, M.B. Metabolically healthy obesity: Epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013, 1, 152–162. [Google Scholar] [CrossRef]
- Cai, W.; Ramdas, M.; Zhu, L.; Chen, X.; Striker, G.E.; Vlassara, H. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc. Natl. Acad. Sci. USA 2012, 109, 15888–15893. [Google Scholar] [CrossRef]
- Mark, A.B.; Poulsen, M.W.; Andersen, S.; Andersen, J.M.; Bak, M.J.; Ritz, C.; Holst, J.J.; Nielsen, J.; De Courten, B.; Dragsted, L.O.; et al. Consumption of a diet low in advanced glycation end products for 4 weeks improves insulin sensitivity in overweight women. Diabetes Care 2014, 37, 88–95. [Google Scholar] [CrossRef]
- Baye, E.; Kiriakova, V.; Uribarri, J.; Moran, L.J.; De Courten, B. Consumption of diets with low advanced glycation end products improves cardiometabolic parameters: Meta-analysis of randomised controlled trials. Sci. Rep. 2017, 7, 2266. [Google Scholar] [CrossRef]
- Karimi, F.; Omrani, G.R. Effects of selenium and vitamin C on the serum level of antithyroid peroxidase antibody in patients with autoimmune thyroiditis. J. Endocrinol. Investig. 2019, 42, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Jud, P.; Sourij, H. Therapeutic options to reduce advanced glycation end products in patients with diabetes mellitus: A review. Diabetes Res. Clin. Pract. 2019, 148, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Di Emidio, G.; Santini, S.J.; D’Alessandro, A.M.; Vetuschi, A.; Sferra, R.; Artini, P.G.; Carta, G.; Falone, S.; Amicarelli, F.; Tatone, C. SIRT1 participates in the response to methylglyoxal-dependent glycative stress in mouse oocytes and ovary. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1389–1401. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.M.; Leiva Balich, L.; Concha, M.J.; Mizón, C.; Bunout Barnett, D.; Barrera Acevedo, G.; Hirsch Birn, S.; Jiménez Jaime, T.; Henríquez, S.; Uribarri, J.; et al. Reduction of serum advanced glycation end-products with a low calorie Mediterranean diet. Nutr. Hosp. 2015, 31, 2511–2517. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moreno, J.; Quintana-Navarro, G.M.; Delgado-Lista, J.; Garcia-Rios, A.; Alcala-Diaz, J.F.; Gomez-Delgado, F.; Camargo, A.; Perez-Martinez, P.; Tinahones, F.J.; Striker, G.E.; et al. Mediterranean Diet Supplemented with Coenzyme Q10 Modulates the Postprandial Metabolism of Advanced Glycation End Products in Elderly Men and Women. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 340–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebeková, K.; Krajcoviová-Kudlácková, M.; Schinzel, R.; Faist, V.; Klvanová, J.; Heidland, A. Plasma levels of advanced glycation end products in healthy, long-term vegetarians and subjects on a western mixed diet. Eur. J. Nutr. 2001, 40, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Peyroux, J.; Sternberg, M. Advanced glycation endproducts (AGEs): Pharmacological inhibition in diabetes. Pathol. Biol. 2006, 54, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P.; Beyaz, A. Inhibitors of the Maillard reaction and AGE breakers as therapeutics for multiple diseases. Drug Discov. Today 2006, 11, 646–654. [Google Scholar] [CrossRef]
- Rasheed, Z.; Anbazhagan, A.N.; Akhtar, N.; Ramamurthy, S.; Voss, F.R.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate inhibits advanced glycation end product-induced expression of tumor necrosis factor-alpha and matrix metalloproteinase-13 in human chondrocytes. Arthritis Res. Ther. 2009, 11, R71. [Google Scholar] [CrossRef] [Green Version]
| Authors and Study Type | Mode of Delivery of AGEs | Outcome | Comorbid Conditions |
|---|---|---|---|
| Po-Han Lin, et al.: Preclinical and Clinical [64] |
|
| Mimic DHEA-induced PCOS phenotypes. |
| Diamanti-Kandarakis E, et al.: Preclinical [22] | Female rats given a high-AGE diet for six months. | Rats had significantly elevated deposition of AGEs in their theca interna cells, increased RAGE expression in their granulosa cells, and higher blood T levels compared to rats on low-AGE diet. | A positive correlation between serum AGEs and ovarian tissue weight, and between serum AGEs and serum T levels. |
| Azhary JMK, et al.: Clinical [67] | T increased RAGE expression and AGE accumulation in cultured human luteinized granulosa cells. | Androgens induced the action of AGEs by upregulating RAGE expression. | Reduced by pretreatment with an agent that inhibits ER stress. |
| De Courten B, et al.: Clinical [71] | Women were given a low- or a high-AGE diet for two weeks. | Decrease in insulin sensitivity. | No changes in body weight or insulin secretion. |
| Cai W, et al.: Preclinical [75] | Mice were given an isocaloric diet, with or without AGEs. | Mice on a diet that contains AGEs manifested increased adiposity and IR in their white adipose tissue, skeletal muscle, and liver. | Significant changes in insulin receptor. |
| Mark AB, et al.: Clinical [76] | Overweight women on a low-AGE diet for four weeks. | Had significantly lower fasting serum insulin levels and lower HOMA-IR compared to overweight women on high-AGE diet. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouanness, M.; Nava, H.; Dagher, C.; Merhi, Z. Contribution of Advanced Glycation End Products to PCOS Key Elements: A Narrative Review. Nutrients 2022, 14, 3578. https://doi.org/10.3390/nu14173578
Mouanness M, Nava H, Dagher C, Merhi Z. Contribution of Advanced Glycation End Products to PCOS Key Elements: A Narrative Review. Nutrients. 2022; 14(17):3578. https://doi.org/10.3390/nu14173578
Chicago/Turabian StyleMouanness, Marco, Henry Nava, Christelle Dagher, and Zaher Merhi. 2022. "Contribution of Advanced Glycation End Products to PCOS Key Elements: A Narrative Review" Nutrients 14, no. 17: 3578. https://doi.org/10.3390/nu14173578
APA StyleMouanness, M., Nava, H., Dagher, C., & Merhi, Z. (2022). Contribution of Advanced Glycation End Products to PCOS Key Elements: A Narrative Review. Nutrients, 14(17), 3578. https://doi.org/10.3390/nu14173578







