Coenzyme Q10 Supplementation Increases Removal of the ATXN3 Polyglutamine Repeat, Reducing Cerebellar Degeneration and Improving Motor Dysfunction in Murine Spinocerebellar Ataxia Type 3
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Model
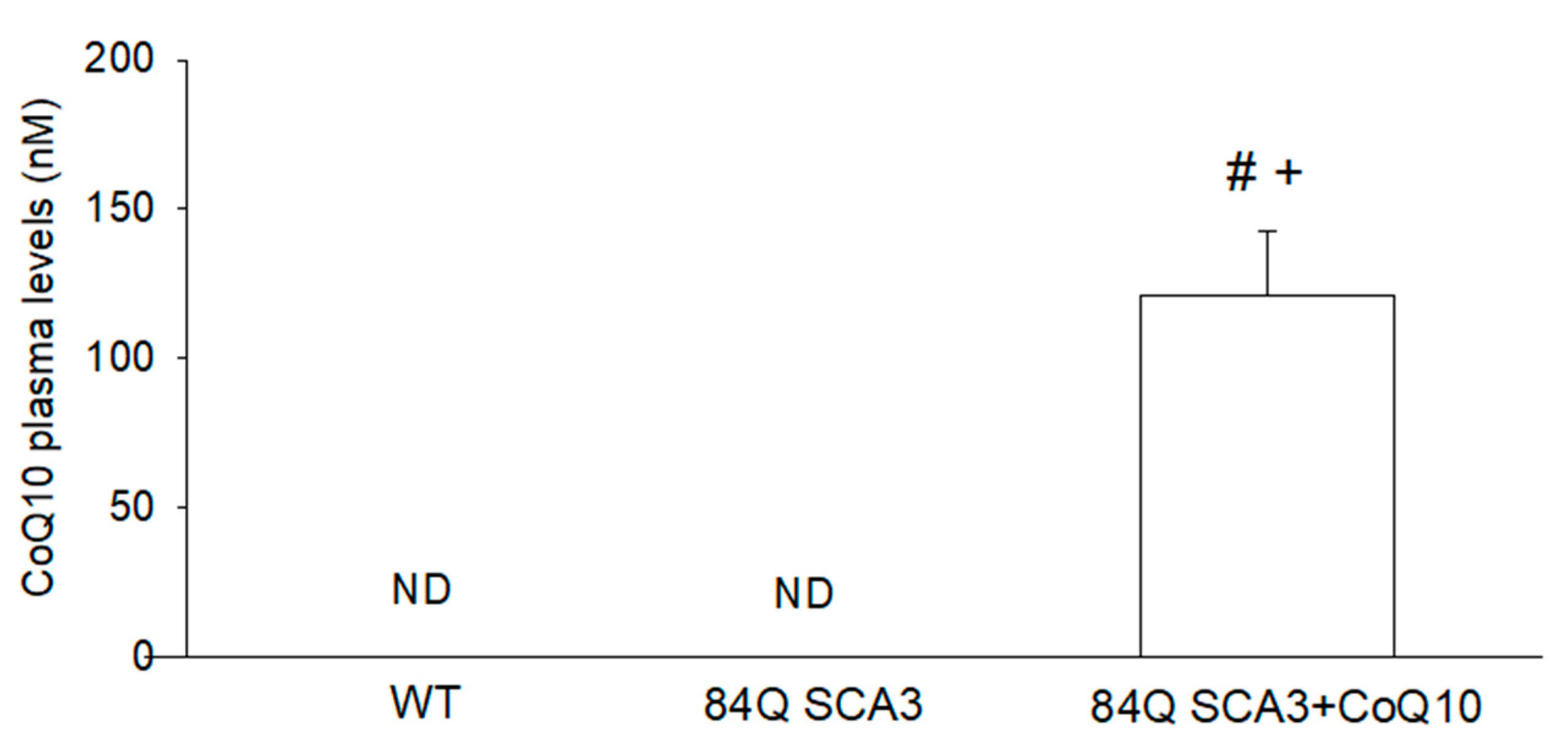
2.3. Plasma CoQ10 Measurement
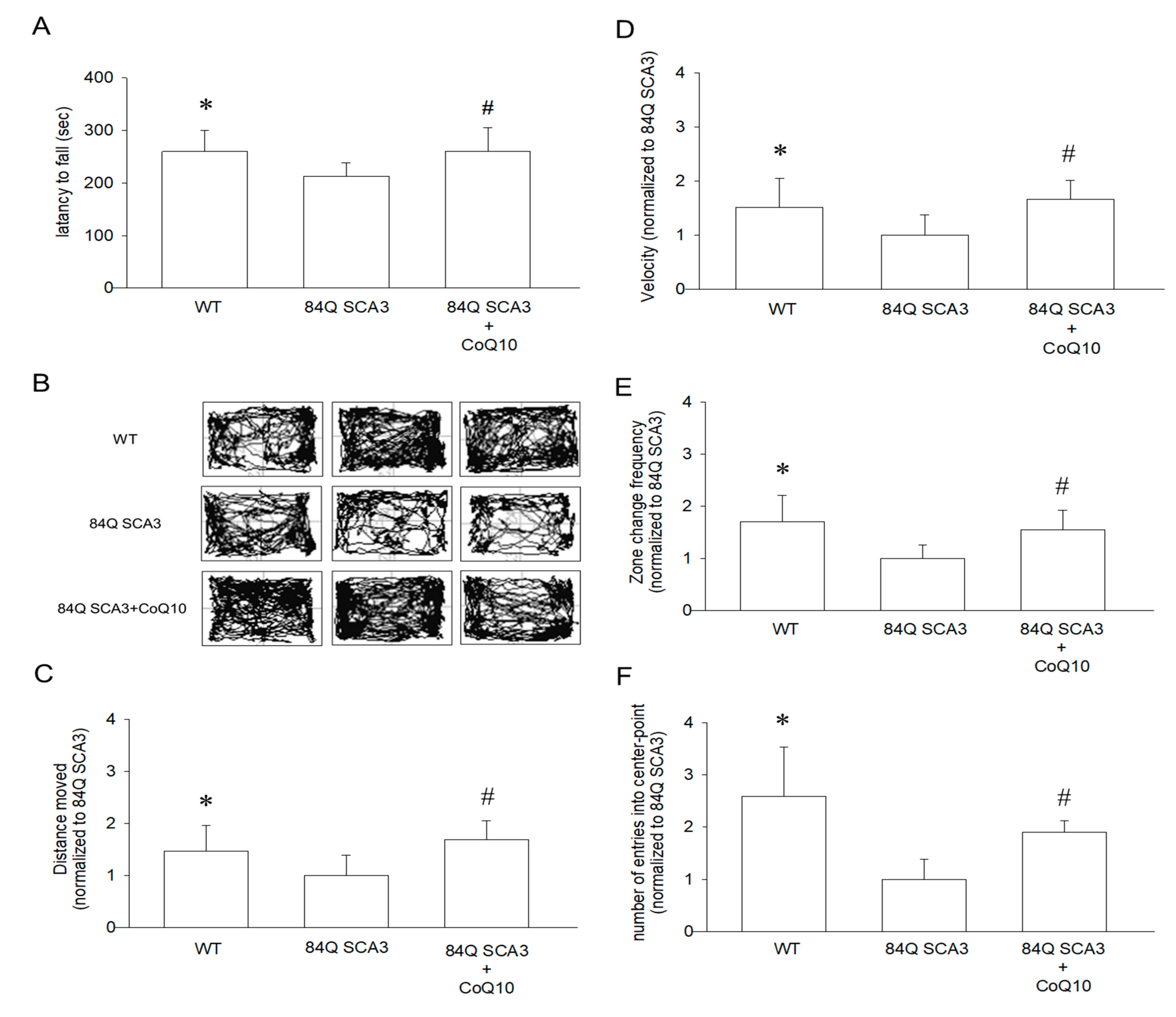
2.4. Rotarod Test
2.5. Open Field Behavior Assay
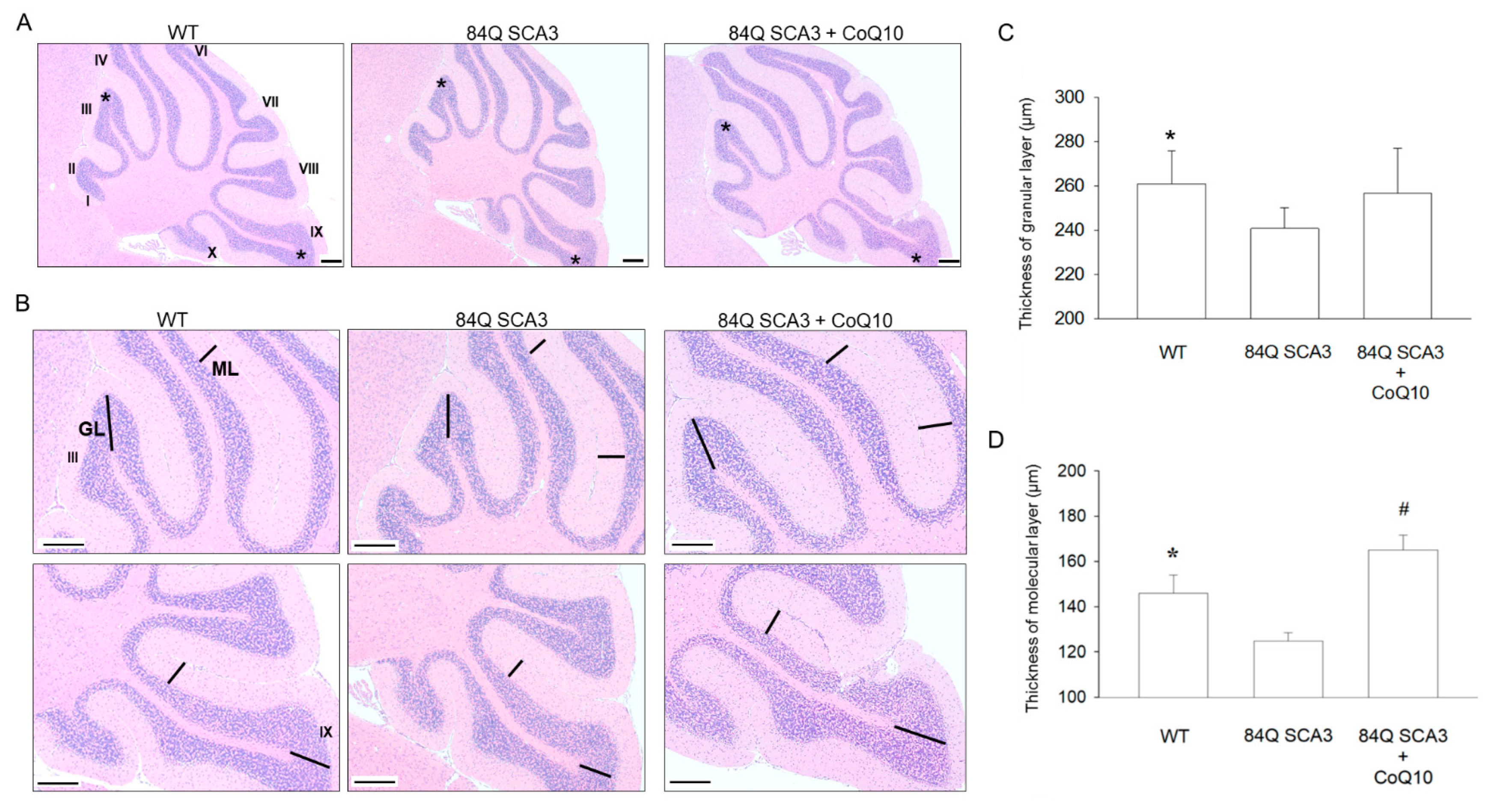
2.6. Preparation of Histological Tissue Sections
2.7. Histological Staining
2.8. Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling (TUNEL) Staining
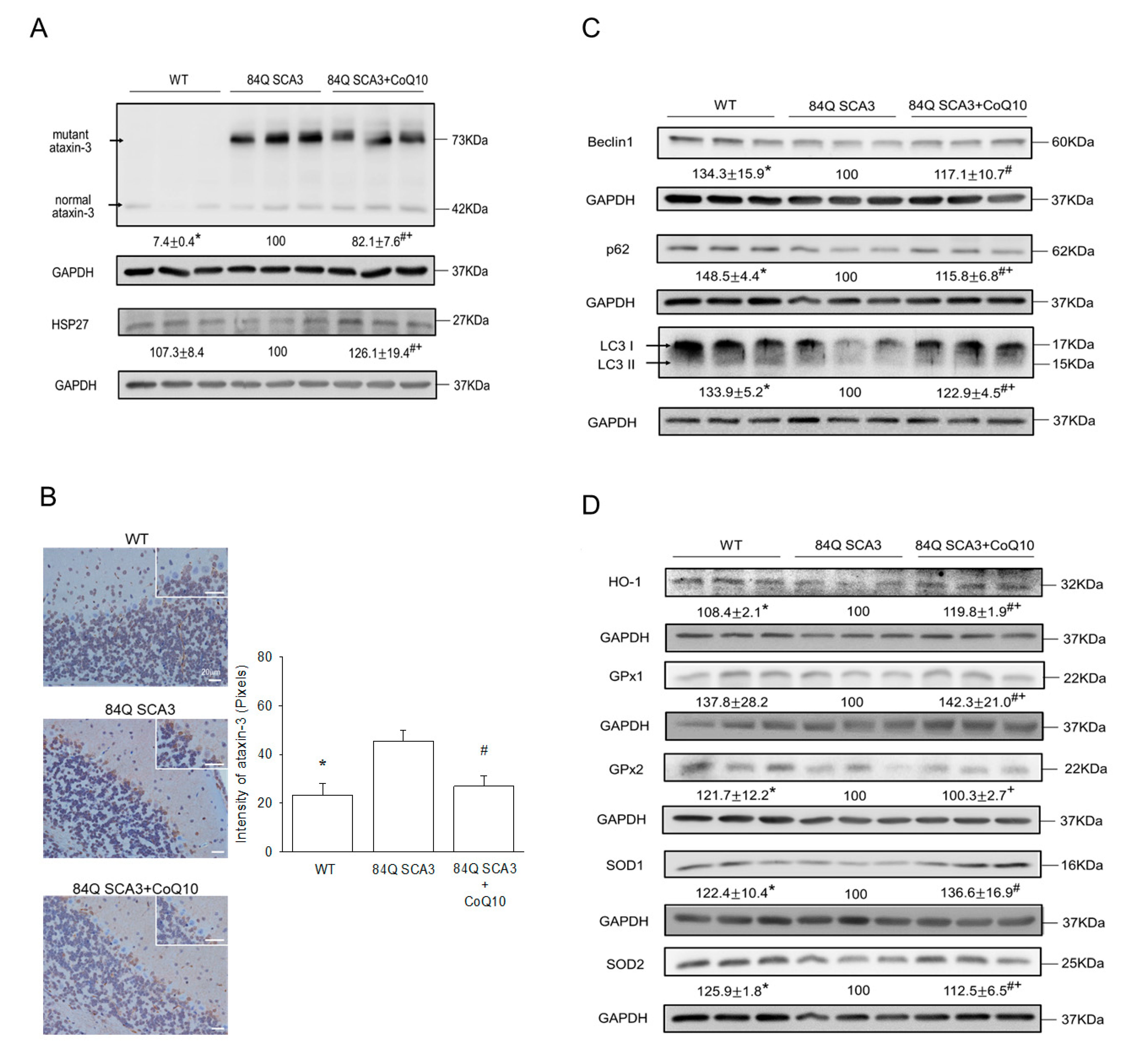
2.9. Protein Extraction and Western Blot
2.10. Statistical Analysis
3. Results
3.1. Effects of CoQ10 on Plasma Concentration in 84Q SCA3 Mice
3.2. Effects of CoQ10 on Rotarod Performance and Open-Field Behavior in 84Q SCA3 Mice
3.3. Effects of CoQ10 on the Granular and Molecular Layers of the Cerebellar Cortex in 84Q SCA3 Mice
3.4. Effects of CoQ10 on Cerebellar Apoptosis in 84Q SCA3 Mice
3.5. Effects of CoQ10 on Mutant Ataxin-3, Hsp27, Autophagy and Antioxidant Protein Expression in 84Q SCA3 Mice
3.6. Effects of CoQ10 on Skeletal Muscle Atrophy of 84Q SCA3 Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matos, C.A.; de Almeida, L.P.; Nóbrega, C. Machado–Joseph disease/spinocerebellar ataxia type 3: Lessons from disease pathogenesis and clues into therapy. J. Neurochem. 2019, 148, 8–28. [Google Scholar] [CrossRef]
- Koeppen, A.H. The pathogenesis of spinocerebellar ataxia. Cerebellum 2005, 4, 62–73. [Google Scholar] [CrossRef]
- Koeppen, A.H. The neuropathology of spinocerebellar ataxia type 3/Machado-Joseph disease. Polyglutamine Disord. 2018, 1049, 233–241. [Google Scholar] [CrossRef]
- Mendonça, N.; França, M.C.; Gonçalves, A.F.; Januário, C. Clinical features of Machado-Joseph disease. Polyglutamine Disord. 2018, 1049, 255–273. [Google Scholar] [CrossRef]
- Hsieh, M.; Tsai, H.F.; Chang, W.H. HSP27 and cell death in spinocerebellar ataxia type 3. Cerebellum 2005, 4, 31–36. [Google Scholar] [CrossRef]
- Wyttenbach, A.; Sauvageot, O.; Carmichael, J.; Diaz-Latoud, C.; Arrigo, A.-P.; Rubinsztein, D.C. Heat shock protein 27 prevents cellular polyglutamine toxicity and suppresses the increase of reactive oxygen species caused by huntingtin. Hum. Mol. Genet. 2002, 11, 1137–1151. [Google Scholar] [CrossRef] [PubMed]
- Intihar, T.A.; Martinez, E.A.; Gomez-Pastor, R. Mitochondrial Dysfunction in Huntington’s Disease; Interplay Between HSF1, p53 and PGC-1α Transcription Factors. Front. Cell. Neurosci. 2019, 13, 103. [Google Scholar] [CrossRef]
- Da Silva, J.D.; Teixeira-Castro, A.; Maciel, P. From Pathogenesis to Novel Therapeutics for Spinocerebellar Ataxia Type 3: Evading Potholes on the Way to Translation. Neurotherapeutics 2019, 16, 1009–1031. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.C.; Kuo, C.L.; Cheng, W.L.; Liu, C.S.; Hsieh, M. Decreased antioxidant enzyme activity and increased mitochondrial DNA damage in cellular models of Machado-Joseph disease. J. Neurosci. Res. 2009, 87, 1884–1891. [Google Scholar] [CrossRef]
- Araujo, J.; Breuer, P.; Dieringer, S.; Krauss, S.; Dorn, S.; Zimmermann, K.; Pfeifer, A.; Klockgether, T.; Wuellner, U.; Evert, B.O. FOXO4-dependent upregulation of superoxide dismutase-2 in response to oxidative stress is impaired in spinocerebellar ataxia type 3. Hum. Mol. Genet. 2011, 20, 2928–2941. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.L.; Chang, J.C.; Lin, W.Y.; Li, C.C.; Hsieh, M.; Chen, H.W.; Wang, T.S.; Wu, W.T.; Liu, C.S.; Liu, K.L. Caffeic acid and resveratrol ameliorate cellular damage in cell and Drosophila models of spinocerebellar ataxia type 3 through upregulation of Nrf2 pathway. Free Radic. Biol. Med. 2018, 115, 309–317. [Google Scholar] [CrossRef] [PubMed]
- da S Hage-Melim, L.I.; Ferreira, J.V.; de Oliveira, N.K.; Correia, L.C.; Almeida, M.R.; Poiani, J.G.; Taft, C.A.; de Paula da Silva, C.H. The impact of natural compounds on the treatment of neurodegenerative diseases. Curr. Org. Chem. 2019, 23, 335–360. [Google Scholar] [CrossRef]
- Wu, Y.L.; Chang, J.C.; Lin, W.Y.; Li, C.C.; Hsieh, M.; Chen, H.W.; Wang, T.S.; Liu, C.S.; Liu, K.L. Treatment with caffeic acid and resveratrol alleviates oxidative stress induced neurotoxicity in cell and drosophila models of spinocerebellar ataxia type3. Sci. Rep. 2017, 7, 11641. [Google Scholar] [CrossRef]
- Wang, Y.; Le, W.D. Autophagy and Ubiquitin-Proteasome System. Adv. Exp. Med. Biol. 2019, 1206, 527–550. [Google Scholar] [CrossRef] [PubMed]
- Onofre, I.; Mendonça, N.; Lopes, S.; Nobre, R.; de Melo, J.B.; Carreira, I.M.; Januário, C.; Gonçalves, A.F.; de Almeida, L.P. Fibroblasts of Machado Joseph Disease patients reveal autophagy impairment. Sci. Rep. 2016, 6, 28220. [Google Scholar] [CrossRef]
- Chang, J.C.; Wu, S.L.; Hoel, F.; Cheng, Y.S.; Liu, K.H.; Hsieh, M.; Hoel, A.; Tronstad, K.J.; Yan, K.C.; Hsieh, C.L. Far-infrared radiation protects viability in a cell model of Spinocerebellar Ataxia by preventing polyQ protein accumulation and improving mitochondrial function. Sci. Rep. 2016, 6, 30436. [Google Scholar] [CrossRef]
- Liu, S.W.; Chang, J.C.; Chuang, S.F.; Liu, K.H.; Cheng, W.L.; Chang, H.J.; Chang, H.S.; Lin, T.T.; Hsieh, C.L.; Lin, W.Y. Far-infrared radiation improves motor dysfunction and neuropathology in spinocerebellar ataxia type 3 mice. Cerebellum 2019, 18, 22–32. [Google Scholar] [CrossRef]
- Nascimento-Ferreira, I.; Santos-Ferreira, T.; Sousa-Ferreira, L.; Auregan, G.; Onofre, I.; Alves, S.; Dufour, N.; Colomer Gould, V.F.; Koeppen, A.; Déglon, N. Overexpression of the autophagic beclin-1 protein clears mutant ataxin-3 and alleviates Machado–Joseph disease. Brain 2011, 134, 1400–1415. [Google Scholar] [CrossRef]
- Menzies, F.M.; Huebener, J.; Renna, M.; Bonin, M.; Riess, O.; Rubinsztein, D.C. Autophagy induction reduces mutant ataxin-3 levels and toxicity in a mouse model of spinocerebellar ataxia type 3. Brain 2010, 133, 93–104. [Google Scholar] [CrossRef]
- Forsmark-Andrée, P.; Lee, C.P.; Dallner, G.; Ernster, L. Lipid peroxidation and changes in the ubiquinone content and the respiratory chain enzymes of submitochondrial particles. Free Radic. Biol. Med. 1997, 22, 391–400. [Google Scholar] [CrossRef]
- Groneberg, D.A.; Kindermann, B.; Althammer, M.; Klapper, M.; Vormann, J.; Littarru, G.P.; Döring, F. Coenzyme Q10 affects expression of genes involved in cell signalling, metabolism and transport in human CaCo-2 cells. Int. J. Biochem. Cell Biol. 2005, 37, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, C.; Lindner, I.; Rimbach, G.; Niklowitz, P.; Menke, T.; Döring, F. Functions of coenzyme Q10 in inflammation and gene expression. Biofactors 2008, 32, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Y.; Xu, H.; Luo, X.; Yu, J.; Liu, J.; Chang, R.C. Neuroprotection of Coenzyme Q10 in Neurodegenerative Diseases. Curr. Top. Med. Chem. 2016, 16, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Jal, M.; Suñé-Negre, J.; García-Montoya, E. Coenzyme Q10 supplementation: Efficacy, safety, and formulation challenges. Compr. Rev. Food Sci. Food Saf. 2020, 19, 574–594. [Google Scholar] [CrossRef]
- Lo, R.Y.; Figueroa, K.P.; Pulst, S.M.; Lin, C.Y.; Perlman, S.; Wilmot, G.; Gomez, C.; Schmahmann, J.; Paulson, H.; Shakkottai, V.G.; et al. Coenzyme Q10 and spinocerebellar ataxias. Mov. Disord. 2015, 30, 214–220. [Google Scholar] [CrossRef]
- Lopes-Ramos, C.M.; Pereira, T.C.; Dogini, D.B.; Gilioli, R.; Lopes-Cendes, I. Lithium carbonate and coenzyme Q10 reduce cell death in a cell model of Machado-Joseph disease. Braz. J. Med. Biol. Res. 2016, 49, e5805. [Google Scholar] [CrossRef]
- Liu, J.; Tang, T.-S.; Tu, H.; Nelson, O.; Herndon, E.; Huynh, D.P.; Pulst, S.M.; Bezprozvanny, I. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 2. J. Neurosci. 2009, 29, 9148–9162. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, K.; Cheng, W.; Su, S.; Lin, Y.; Lin, T.; Cheng, Y.; Chang, J.; Wu, Y.; Liu, C. Growth hormone rescue cerebellar degeneration in SCA3 transgenic mice. Biochem. Biophys. Res. Commun. 2020, 529, 467–473. [Google Scholar] [CrossRef]
- Liu, C.S.; Lii, C.K.; Chang, L.L.; Kuo, C.L.; Cheng, W.L.; Su, S.L.; Tsai, C.W.; Chen, H.W. Atorvastatin increases blood ratios of vitamin E/low-density lipoprotein cholesterol and coenzyme Q10/low-density lipoprotein cholesterol in hypercholesterolemic patients. Nutr. Res. 2010, 30, 118–124. [Google Scholar] [CrossRef]
- Huang, C.H.; Kuo, C.L.; Huang, C.S.; Tseng, W.M.; Lian, I.B.; Chang, C.C.; Liu, C.S. High plasma coenzyme Q10 concentration is correlated with good left ventricular performance after primary angioplasty in patients with acute myocardial infarction. Medicine 2016, 95, e4501. [Google Scholar] [CrossRef]
- Shiotsuki, H.; Yoshimi, K.; Shimo, Y.; Funayama, M.; Takamatsu, Y.; Ikeda, K.; Takahashi, R.; Kitazawa, S.; Hattori, N. A rotarod test for evaluation of motor skill learning. J. Neurosci. Methods. 2010, 189, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Beedholm, K.; Dam, V.S.; Bae, S.-H.; Noble, D.J.; Garraway, S.M.; Aalkjaer, C.; Boedtkjer, E. Sodium bicarbonate cotransporter NBCn1/Slc4a7 affects locomotor activity and hearing in mice. Behav. Brain Res. 2021, 401, 113065. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Liu, C.-S.; Lin, L.-Y.; Lin, Y.-C.; Sun, H.-L.; Li, C.-C.; Chen, H.-W.; Wang, T.-S.; Wang, J.; Liu, K.-L. Borage oil supplementation decreases lipopolysaccharide-induced inflammation and skeletal muscle wasting in mice. RSC Adv. 2016, 6, 100174–100185. [Google Scholar] [CrossRef]
- Chuang, C.S.; Chang, J.C.; Soong, B.W.; Chuang, S.F.; Lin, T.T.; Cheng, W.L.; Orr, H.T.; Liu, C.S. Treadmill training increases the motor activity and neuron survival of the cerebellum in a mouse model of spinocerebellar ataxia type 1. Kaohsiung J. Med. Sci. 2019, 35, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Pu, H.; Wang, X.; Zhang, J.; Ma, C.; Su, Y.; Li, X.; Liu, X.; Su, L. Cerebellar neuronal apoptosis in heroin-addicted rats and its molecular mechanism. Int. J. Clin. Exp. 2015, 8, 8260. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J. Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Trzesniewski, J.; Altmann, S.; Jäger, L.; Kapfhammer, J.P. Reduced Purkinje cell size is compatible with near normal morphology and function of the cerebellar cortex in a mouse model of spinocerebellar ataxia. Exp. Neurol. 2019, 311, 205–212. [Google Scholar] [CrossRef]
- Pedroso, J.L.; França, M.C., Jr.; Braga-Neto, P.; D’Abreu, A.; Saraiva-Pereira, M.L.; Saute, J.A.; Teive, H.A.; Caramelli, P.; Jardim, L.B.; Lopes-Cendes, I.; et al. Nonmotor and extracerebellar features in Machado-Joseph disease: A review. Mov. Disord. 2013, 28, 1200–1208. [Google Scholar] [CrossRef]
- Tarabees, R.; Hill, D.; Rauch, C.; Barrow, P.; Loughna, P. Endotoxin transiently inhibits protein synthesis through Akt and MAPK mediating pathways in C2C12 myotubes. Am. J. Physiol. Cell Physiol. 2011, 301, C895–C902. [Google Scholar] [CrossRef]
- Eley, H.L.; Tisdale, M.J. Skeletal muscle atrophy, a link between depression of protein synthesis and increase in degradation. J. Biol. Chem. 2007, 282, 7087–7097. [Google Scholar] [CrossRef]
- Ohta, Y.; Hayashi, T.; Nagai, M.; Okamoto, M.; Nagotani, S.; Nagano, I.; Ohmori, N.; Takehisa, Y.; Murakami, T.; Shoji, M. Two cases of spinocerebellar ataxia accompanied by involvement of the skeletal motor neuron system and bulbar palsy. Intern. Med. 2007, 46, 751–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, M.M.S.; Leite, C.; Schieferdecker, M.E.M.; Teive, H.A.G.; Vieira, B.D.; Moro, A. Estimation of skeletal muscle mass in patients with spinocerebellar ataxia type 3 and 10. Int. J. Neurosci. 2019, 129, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Yamaura, H.; Hirai, H.; Yanagihara, D. Postural dysfunction in a transgenic mouse model of spinocerebellar ataxia type 3. Neuroscience 2013, 243, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Szybińska, A.; Leśniak, W. P53 Dysfunction in Neurodegenerative Diseases—The Cause or Effect of Pathological Changes? Aging Dis. 2017, 8, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.H.; Lin, A.C.; Hong, K.Y.; Hu, S.H.; Chen, Y.L.; Chen, J.Y.; Wang, H.L. p53 activation mediates polyglutamine-expanded ataxin-3 upregulation of Bax expression in cerebellar and pontine nuclei neurons. Neurochem. Int. 2011, 58, 145–152. [Google Scholar] [CrossRef]
- Duarte-Neves, J.; Cavadas, C.; Pereira de Almeida, L. Neuropeptide Y (NPY) intranasal delivery alleviates Machado–Joseph disease. Sci. Rep. 2021, 11, 3345. [Google Scholar] [CrossRef]
- Sarkar, S.; Ravikumar, B.; Rubinsztein, D.C. Autophagic clearance of aggregate-prone proteins associated with neurodegeneration. Methods Enzymol. 2009, 453, 83–110. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lin, S.-Y.; Liu, J.-W.; Lin, S.-Z.; Harn, H.-J.; Chiou, T.-W. N-Butylidenephthalide modulates autophagy to ameliorate neuropathological progress of spinocerebellar ataxia type 3 through mTOR pathway. Int. J. Mol. Sci. 2021, 22, 6339. [Google Scholar] [CrossRef]
- Liang, S.; Ping, Z.; Ge, J. Coenzyme Q10 regulates antioxidative stress and autophagy in acute myocardial ischemia-reperfusion injury. Oxid. Med. Cell. Longev. 2017, 2017, 9863181. [Google Scholar] [CrossRef]
- Parcellier, A.; Gurbuxani, S.; Schmitt, E.; Solary, E.; Garrido, C. Heat shock proteins, cellular chaperones that modulate mitochondrial cell death pathways. Biochem. Biophys. Res. Commun. 2003, 304, 505–512. [Google Scholar] [CrossRef]
- Matthews, R.T.; Yang, L.; Browne, S.; Baik, M.; Beal, M.F. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc. Natl. Acad. Sci. USA 1998, 95, 8892–8897. [Google Scholar] [CrossRef] [Green Version]
- Villalba, J.M.; Parrado, C.; Santos-Gonzalez, M.; Alcain, F.J. Therapeutic use of coenzyme Q10 and coenzyme Q10-related compounds and formulations. Expert Opin. Investig. Drugs 2010, 19, 535–554. [Google Scholar] [CrossRef]
- Shults, C.W.; Beal, M.F.; Song, D.; Fontaine, D. Pilot trial of high dosages of coenzyme Q10 in patients with Parkinson’s disease. Exp. Neurol. 2004, 188, 491–494. [Google Scholar] [CrossRef]
- Ferrante, K.; Shefner, J.; Zhang, H.; Betensky, R.; O’brien, M.; Yu, H.; Fantasia, M.; Taft, J.; Beal, M.; Traynor, B. Tolerance of high-dose (3,000 mg/day) coenzyme Q10 in ALS. Neurology 2005, 65, 1834–1836. [Google Scholar] [CrossRef]
- Hart, P.E.; Lodi, R.; Rajagopalan, B.; Bradley, J.L.; Crilley, J.G.; Turner, C.; Blamire, A.M.; Manners, D.; Styles, P.; Schapira, A.H.; et al. Antioxidant treatment of patients with friedreich ataxia: Four-year follow-up. Arch. Neurol. 2005, 62, 621–626. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Li, G.; Wang, J.; Yang, E.S. Coenzyme Q10 attenuates beta-amyloid pathology in the aged transgenic mice with Alzheimer presenilin 1 mutation. J. Mol. Neurosci. 2008, 34, 165–171. [Google Scholar] [CrossRef]
- Smith, K.M.; Matson, S.; Matson, W.R.; Cormier, K.; Del Signore, S.J.; Hagerty, S.W.; Stack, E.C.; Ryu, H.; Ferrante, R.J. Dose ranging and efficacy study of high-dose coenzyme Q10 formulations in Huntington’s disease mice. Biochim. Biophys. Acta—Mol. Basis Dis. 2006, 1762, 616–626. [Google Scholar] [CrossRef] [Green Version]




| Treatment | WT | 84Q SCA3 | 84Q SCA3 + CoQ10 |
|---|---|---|---|
| Quadriceps muscle (mg/gw) | 15.5 ± 1.3 * | 12.5 ± 0.9 | 14.7 ± 0.5 # |
| Tibialis muscle (mg/gw) | 2.3 ± 0.6 * | 1.4 ± 0.1 | 1.7 ± 0.3 #, + |
| Gastrocnemius muscle (mg/gw) | 11.0 ± 1.5 * | 8.2 ± 1.5 | 9.9 ± 0.2 |
| Soleus muscle (mg/gw) | 1.4 ± 0.3 * | 0.9 ± 0.1 | 1.1 ± 0.2 # |
| Extensor digitorum longus muscle (mg/gw) | 1.1 ± 0.1 * | 0.8 ± 0.2 | 1.0 ± 0.2 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-L.; Chang, J.-C.; Sun, H.-L.; Cheng, W.-L.; Yen, Y.-P.; Lin, Y.-S.; Chao, Y.-C.; Liu, K.-H.; Huang, C.-S.; Liu, K.-L.; et al. Coenzyme Q10 Supplementation Increases Removal of the ATXN3 Polyglutamine Repeat, Reducing Cerebellar Degeneration and Improving Motor Dysfunction in Murine Spinocerebellar Ataxia Type 3. Nutrients 2022, 14, 3593. https://doi.org/10.3390/nu14173593
Wu Y-L, Chang J-C, Sun H-L, Cheng W-L, Yen Y-P, Lin Y-S, Chao Y-C, Liu K-H, Huang C-S, Liu K-L, et al. Coenzyme Q10 Supplementation Increases Removal of the ATXN3 Polyglutamine Repeat, Reducing Cerebellar Degeneration and Improving Motor Dysfunction in Murine Spinocerebellar Ataxia Type 3. Nutrients. 2022; 14(17):3593. https://doi.org/10.3390/nu14173593
Chicago/Turabian StyleWu, Yu-Ling, Jui-Chih Chang, Hai-Lun Sun, Wen-Ling Cheng, Yu-Pei Yen, Yong-Shiou Lin, Yi-Chun Chao, Ko-Hung Liu, Ching-Shan Huang, Kai-Li Liu, and et al. 2022. "Coenzyme Q10 Supplementation Increases Removal of the ATXN3 Polyglutamine Repeat, Reducing Cerebellar Degeneration and Improving Motor Dysfunction in Murine Spinocerebellar Ataxia Type 3" Nutrients 14, no. 17: 3593. https://doi.org/10.3390/nu14173593
APA StyleWu, Y.-L., Chang, J.-C., Sun, H.-L., Cheng, W.-L., Yen, Y.-P., Lin, Y.-S., Chao, Y.-C., Liu, K.-H., Huang, C.-S., Liu, K.-L., & Liu, C.-S. (2022). Coenzyme Q10 Supplementation Increases Removal of the ATXN3 Polyglutamine Repeat, Reducing Cerebellar Degeneration and Improving Motor Dysfunction in Murine Spinocerebellar Ataxia Type 3. Nutrients, 14(17), 3593. https://doi.org/10.3390/nu14173593






