Different Protein Sources Enhance 18FDG-PET/MR Uptake of Brown Adipocytes in Male Subjects
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Procedures
2.2.1. Screening of Subjects
2.2.2. Dietary Assessments
2.2.3. Anthropometric Measurements
2.2.4. Cold Exposure and PET/MR Scanning
2.2.5. Resting Metabolic Rate Measurements
2.2.6. Blood Collection and Biochemical Measurements
2.3. Statistical Analyses
2.4. Ethics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Virtanen, K.A.; Lidell, M.E.; Orava, J.; Heglind, M.; Westergren, R.; Niemi, T.; Taittonen, M.; Laine, J.; Savisto, N.J.; Enerbäck, S.; et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009, 360, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Himms-Hagen, J. Brown adipose tissue thermogenesis: Interdisciplinary studies. FASEB J. 1990, 4, 2890–2898. [Google Scholar] [CrossRef]
- Astrup, A. Thermogenesis in human brown adipose tissue and skeletal muscle induced by sympathomimetic stimulation. Acta Endocrinol. Suppl. 1986, 278, 1–32. [Google Scholar] [CrossRef]
- Maliszewska, K.; Kretowski, A. Brown Adipose Tissue and Its Role in Insulin and Glucose Homeostasis. Int. J. Mol. Sci. 2021, 22, 1530. [Google Scholar] [CrossRef]
- Chen, K.Y.; Brychta, R.J.; Abdul Sater, Z.; Cassimatis, T.M.; Cero, C.; Fletcher, L.A.; Israni, N.S.; Johnson, J.W.; Lea, H.J.; Linderman, J.D.; et al. Opportunities and challenges in the therapeutic activation of human energy expenditure and thermogenesis to manage obesity. J. Biol. Chem. 2020, 295, 1926–1942. [Google Scholar] [CrossRef]
- Thuzar, M.; Ho, K.K. Mechanisms in endocrinology: Brown adipose tissue in humans: Regulation and metabolic significance. Eur. J. Endocrinol. 2016, 175, R11–R25. [Google Scholar] [CrossRef]
- Orava, J.; Nuutila, P.; Noponen, T.; Parkkola, R.; Viljanen, T.; Enerbäck, S.; Rissanen, A.; Pietiläinen, K.H.; Virtanen, K.A. Blunted metabolic responses to cold and insulin stimulation in brown adipose tissue of obese humans. Obesity 2013, 21, 2279–2287. [Google Scholar] [CrossRef]
- Vijgen, G.H.; Bouvy, N.D.; Teule, G.J.; Brans, B.; Hoeks, J.; Schrauwen, P.; van Marken Lichtenbelt, W.D. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J. Clin. Endocrinol. Metab. 2012, 97, E1229–E1233. [Google Scholar] [CrossRef]
- Lee, P.; Greenfield, J.R. Non-pharmacological and pharmacological strategies of brown adipose tissue recruitment in humans. Mol. Cell Endocrinol. 2015, 418 Pt 2, 184–190. [Google Scholar] [CrossRef] [PubMed]
- O’Mara, A.E.; Johnson, J.W.; Linderman, J.D.; Brychta, R.J.; McGehee, S.; Fletcher, L.A.; Fink, Y.A.; Kapuria, D.; Cassimatis, T.M.; Kelsey, N.; et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J. Clin. Investig. 2020, 130, 2209–2219. [Google Scholar] [CrossRef]
- Ma, S.W.; Foster, D.O. Brown adipose tissue, liver, and diet-induced thermogenesis in cafeteria diet-fed rats. Can. J. Physiol. Pharmacol. 1989, 67, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Leibel, R.L.; Rosenbaum, M.; Hirsch, J. Changes in energy expenditure resulting from altered body weight. N. Engl. J. Med. 1995, 332, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Kinabo, J.L.; Durnin, J.V. Thermic effect of food in man: Effect of meal composition, and energy content. Br. J. Nutr. 1990, 64, 37–44. [Google Scholar] [CrossRef]
- García-Ruiz, E.; Reynés, B.; Díaz-Rúa, R.; Ceresi, E.; Oliver, P.; Palou, A. The intake of high-fat diets induces the acquisition of brown adipocyte gene expression features in white adipose tissue. Int. J. Obes. 2015, 39, 1619–1629. [Google Scholar] [CrossRef]
- Van Baak, M.A. Meal-induced activation of the sympathetic nervous system and its cardiovascular and thermogenic effects in man. Physiol. Behav. 2008, 94, 178–186. [Google Scholar] [CrossRef]
- Smith, R.E. Thermoregulatory and adaptive behavior of brown adipose tissue. Science 1964, 146, 1686–1689. [Google Scholar] [CrossRef]
- Rothwell, N.J.; Stock, M.J. A role for brown adipose tissue in diet-induced thermogenesis. Nature 1979, 281, 31–35. [Google Scholar] [CrossRef]
- Liisberg, U.; Myrmel, L.S.; Fjære, E.; Rønnevik, A.K.; Bjelland, S.; Fauske, K.R.; Holm, J.B.; Basse, A.L.; Hansen, J.B.; Liaset, B.; et al. The protein source determines the potential of high protein diets to attenuate obesity development in C57BL/6J mice. Adipocyte 2016, 5, 196–211. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S.; Lemmens, S.G.; Westerterp, K.R. Dietary protein—Its role in satiety, energetics, weight loss and health. Br. J. Nutr. 2012, 108 (Suppl. S2), S105–S112. [Google Scholar] [CrossRef] [PubMed]
- Westerterp-Plantenga, M.S.; Lemmens, S.G.; Westerterp, K.R. Protein intake trends and conformity with the Dietary Reference Intakes in the United States: Analysis of the National Health and Nutrition Examination Survey, 2001–2014. Am. J. Clin. Nutr. 2018, 108, 405–413. [Google Scholar]
- Pasiakos, S.M.; Agarwal, S.; Lieberman, H.R.; Fulgoni, V.L. Sources and Amounts of Animal, Dairy, and Plant Protein Intake of US Adults in 2007–2010. Nutrients 2015, 7, 7058–7069. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.S.; Delgado, S.; Mittal, J.; Eshraghi, R.S.; Mittal, R.; Eshraghi, A.A. Beneficial Effects of Milk Having A2 β-Casein Protein: Myth or Reality? J. Nutr. 2021, 151, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef] [PubMed]
- Conn, C.S.; Yang, H.; Tom, H.J.; Ikeda, K.; Oses-Prieto, J.A.; Vu, H.; Oguri, Y.; Nair, S.; Gill, R.M.; Kajimura, S.; et al. The major cap-binding protein eIF4E regulates lipid homeostasis and diet-induced obesity. Nat. Metab. 2021, 3, 244–257. [Google Scholar] [CrossRef]
- Jo, S.; Lockridge, A.; Mohan, R.; Esch, N.; Schlichting, R.; Panigrahy, N.; Essawy, A.; Gustafson, E.; Alejandro, E.U. Translational Factor eIF4G1 Regulates Glucose Homeostasis and Pancreatic β-Cell Function. Diabetes 2021, 70, 155–170. [Google Scholar] [CrossRef]
- Tsukiyama-Kohara, K.; Poulin, F.; Kohara, M.; DeMaria, C.T.; Cheng, A.; Wu, Z.; Gingras, A.C.; Katsume, A.; Elchebly, M.; Spiegelman, B.M.; et al. Adipose tissue reduction in mice lacking the translational inhibitor 4E-BP1. Nat. Med. 2001, 7, 1128–1132. [Google Scholar] [CrossRef]
- Devlin, M.J. The "Skinny" on brown fat, obesity, and bone. Am. J. Phys. Anthropol. 2015, 156 (Suppl. S59), 98–115. [Google Scholar] [CrossRef]
- Kang, R.; Nagoshi, T.; Kimura, H.; Tanaka, T.D.; Yoshii, A.; Inoue, Y.; Morimoto, S.; Ogawa, K.; Minai, K.; Ogawa, T.; et al. Possible Association Between Body Temperature and B-Type Natriuretic Peptide in Patients with Cardiovascular Diseases. J. Card. Fail. 2021, 27, 75–82. [Google Scholar] [CrossRef]
- Carper, D.; Coué, M.; Nascimento, E.B.M.; Barquissau, V.; Lagarde, D.; Pestourie, C.; Laurens, C.; Petit, J.V.; Soty, M.; Monbrun, L.; et al. Atrial Natriuretic Peptide Orchestrates a Coordinated Physiological Response to Fuel Non-shivering Thermogenesis. Cell Rep. 2020, 32, 108075. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Da Eira, D.; Jani, S.; Sepa-Kishi, D.; Vu, V.; Hunter, H.; Lai, M.; Wheeler, M.B.; Ceddia, R.B.; Sweeney, G. Cardiac Autophagy Deficiency Attenuates ANP Production and Disrupts Myocardial-Adipose Cross Talk, Leading to Increased Fat Accumulation and Metabolic Dysfunction. Diabetes 2021, 70, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ceddia, R.P.; Collins, S. Cardiac natriuretic peptides promote adipose ‘browning’ through mTOR complex-1. Mol. Metab. 2018, 9, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Nagoshi, T.; Oi, Y.; Yoshii, A.; Tanaka, Y.; Takahashi, H.; Kashiwagi, Y.; Tanaka, T.D.; Yoshimura, M. Treatment with atrial natriuretic peptide induces adipose tissue browning and exerts thermogenic actions in vivo. Sci. Rep. 2021, 11, 17466. [Google Scholar] [CrossRef]
- Inuzuka, M.; Tamura, N.; Yamada, N.; Katsuura, G.; Oyamada, N.; Taura, D.; Sonoyama, T.; Fukunaga, Y.; Ohinata, K.; Sone, M.; et al. C-type natriuretic peptide as a new regulator of food intake and energy expenditure. Endocrinology 2010, 151, 3633–3642. [Google Scholar] [CrossRef]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef]
- Szponar, L.; Wolnicka, K.; Rychlik, E. Album of Photographs of Food Products and Dishes; National Food and Nutrition Institute: Warsaw, Poland, 2011. [Google Scholar]
- Bzikowska-Jura, A.; Czerwonogrodzka-Senczyna, A.; Jasińska-Melon, E.; Mojska, H.; Olędzka, G.; Wesołowska, A.; Szostak-Węgierek, D. The Concentration of Omega-3 Fatty Acids in Human Milk Is Related to Their Habitual but Not Current Intake. Nutrients 2019, 11, 1585. [Google Scholar] [CrossRef]
- Virtanen, K.A.; Peltoniemi, P.; Marjamäki, P.; Asola, M.; Strindberg, L.; Parkkola, R.; Huupponen, R.; Knuuti, J.; Lönnroth, P.; Nuutila, P. Human adipose tissue glucose uptake determined using [(18)F]-fluoro-deoxy-glucose ([(18)F]FDG) and PET in combination with microdialysis. Diabetologia 2001, 44, 2171–2179. [Google Scholar] [CrossRef]
- Orava, J.; Nuutila, P.; Lidell, M.E.; Oikonen, V.; Noponen, T.; Viljanen, T.; Scheinin, M.; Taittonen, M.; Niemi, T.; Enerbäck, S.; et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011, 14, 272–279. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Maliszewska, K.; Adamska-Patruno, E.; Miniewska, K.; Bauer, W.; Mojsak, M.; Kretowski, A. PET/MRI-evaluated brown adipose tissue activity may be related to dietary MUFA and omega-6 fatty acids intake. Sci. Rep. 2022, 12, 4112. [Google Scholar] [CrossRef]
- Madsen, L.; Myrmel, L.S.; Fjære, E.; Øyen, J.; Kristiansen, K. Dietary Proteins, Brown Fat, and Adiposity. Front. Physiol. 2018, 9, 1792. [Google Scholar] [CrossRef] [PubMed]
- Weigle, D.S.; Breen, P.A.; Matthys, C.C.; Callahan, H.S.; Meeuws, K.E.; Burden, V.R.; Purnell, J.Q. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am. J. Clin. Nutr. 2005, 82, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Sánchez, M.; Navas-Carrillo, D.; Orenes-Piñero, E. Controversies surrounding high-protein diet intake: Satiating effect and kidney and bone health. Adv. Nutr. 2015, 6, 260–266. [Google Scholar] [CrossRef]
- Sacks, F.M.; Bray, G.A.; Carey, V.J.; Smith, S.R.; Ryan, D.H.; Anton, S.D.; McManus, K.; Champagne, C.M.; Bishop, L.M.; Laranjo, N.; et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N. Engl. J. Med. 2009, 360, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Wycherley, T.P.; Moran, L.J.; Clifton, P.M.; Noakes, M.; Brinkworth, G.D. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 96, 1281–1298. [Google Scholar] [CrossRef] [PubMed]
- Santesso, N.; Akl, E.A.; Bianchi, M.; Mente, A.; Mustafa, R.; Heels-Ansdell, D.; Schünemann, H.J. Effects of higher- versus lower-protein diets on health outcomes: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2012, 66, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Verdich, C.; Flint, A.; Gutzwiller, J.P.; Näslund, E.; Beglinger, C.; Hellström, P.M.; Long, S.J.; Morgan, L.M.; Holst, J.J.; Astrup, A. A meta-analysis of the effect of glucagon-like peptide-1 (7-36) amide on ad libitum energy intake in humans. J. Clin. Endocrinol. Metab. 2001, 86, 4382–4389. [Google Scholar]
- Journel, M.; Chaumontet, C.; Darcel, N.; Fromentin, G.; Tomé, D. Brain responses to high-protein diets. Adv. Nutr. 2012, 3, 322–329. [Google Scholar] [CrossRef]
- Pesta, D.H.; Samuel, V.T. A high-protein diet for reducing body fat: Mechanisms and possible caveats. Nutr. Metab. 2014, 11, 53. [Google Scholar] [CrossRef]
- Whitehead, J.M.; McNeill, G.; Smith, J.S. The effect of protein intake on 24-h energy expenditure during energy restriction. Int. J. Obes. Relat. Metab. Disord. 1996, 20, 727–732. [Google Scholar]
- Bray, G.A.; Redman, L.M.; de Jonge, L.; Covington, J.; Rood, J.; Brock, C.; Mancuso, S.; Martin, C.K.; Smith, S.R. Effect of protein overfeeding on energy expenditure measured in a metabolic chamber. Am. J. Clin. Nutr. 2015, 101, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Raiko, J.; Holstila, M.; Virtanen, K.A.; Orava, J.; Saunavaara, V.; Niemi, T.; Laine, J.; Taittonen, M.; Borra, R.J.; Nuutila, P.; et al. Brown adipose tissue triglyceride content is associated with decreased insulin sensitivity, independently of age and obesity. Diabetes Obes. Metab. 2015, 17, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Petzke, K.J.; Friedrich, M.; Metges, C.C.; Klaus, S. Long-term dietary high protein intake up-regulates tissue specific gene expression of uncoupling proteins 1 and 2 in rats. Eur. J. Nutr. 2005, 44, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Petzke, K.J.; Riese, C.; Klaus, S. Short-term, increasing dietary protein and fat moderately affect energy expenditure, substrate oxidation and uncoupling protein gene expression in rats. J. Nutr. Biochem. 2007, 18, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Lillefosse, H.H.; Fjaere, E.; Myrmel, L.S.; Midtbø, L.K.; Jarlsby, R.H.; Ma, T.; Jia, B.; Petersen, R.K.; Sonne, S.B.; et al. High-glycemic index carbohydrates abrogate the antiobesity effect of fish oil in mice. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1097–E1112. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L.; Pedersen, L.M.; Liaset, B.; Ma, T.; Petersen, R.K.; van den Berg, S.; Pan, J.; Müller-Decker, K.; Dülsner, E.D.; Kleemann, R.; et al. cAMP-dependent signaling regulates the adipogenic effect of n-6 polyunsaturated fatty acids. J. Biol. Chem. 2008, 283, 7196–7205. [Google Scholar] [CrossRef]
- Bendtsen, L.Q.; Lorenzen, J.K.; Bendsen, N.T.; Rasmussen, C.; Astrup, A. Effect of dairy proteins on appetite, energy expenditure, body weight, and composition: A review of the evidence from controlled clinical trials. Adv. Nutr. 2013, 4, 418–438. [Google Scholar] [CrossRef]
- Krieger, J.P.; Santos da Conceição, E.P.; Sanchez-Watts, G.; Arnold, M.; Pettersen, K.G.; Mohammed, M.; Modica, S.; Lossel, P.; Morrison, S.F.; Madden, C.J.; et al. Glucagon-like peptide-1 regulates brown adipose tissue thermogenesis via the gut-brain axis in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R708–R720. [Google Scholar] [CrossRef]
- Lockie, S.H.; Heppner, K.M.; Chaudhary, N.; Chabenne, J.R.; Morgan, D.A.; Veyrat-Durebex, C.; Ananthakrishnan, G.; Rohner-Jeanrenaud, F.; Drucker, D.J.; DiMarchi, R.; et al. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling. Diabetes 2012, 61, 2753–2762. [Google Scholar] [CrossRef]
- Heppner, K.M.; Marks, S.; Holland, J.; Ottaway, N.; Smiley, D.; Dimarchi, R.; Perez-Tilve, D. Contribution of brown adipose tissue activity to the control of energy balance by GLP-1 receptor signalling in mice. Diabetologia 2015, 58, 2124–2132. [Google Scholar] [CrossRef][Green Version]
- Kooijman, S.; Wang, Y.; Parlevliet, E.T.; Boon, M.R.; Edelschaap, D.; Snaterse, G.; Pijl, H.; Romijn, J.A.; Rensen, P.C. Central GLP-1 receptor signalling accelerates plasma clearance of triacylglycerol and glucose by activating brown adipose tissue in mice. Diabetologia 2015, 58, 2637–2646. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, B.; Li, M.; Speakman, J.R. Switching on the furnace: Regulation of heat production in brown adipose tissue. Mol. Aspects. Med. 2019, 68, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Bordicchia, M.; Liu, D.; Amri, E.Z.; Ailhaud, G.; Dessì-Fulgheri, P.; Zhang, C.; Takahashi, N.; Sarzani, R.; Collins, S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Investig. 2012, 122, 1022–1036. [Google Scholar] [CrossRef] [PubMed]
- Arndt, A.K.; Schafer, S.; Drenckhahn, J.D.; Sabeh, M.K.; Plovie, E.R.; Caliebe, A.; Klopocki, E.; Musso, G.; Werdich, A.A.; Kalwa, H.; et al. Fine mapping of the 1p36 deletion syndrome identifies mutation of PRDM16 as a cause of cardiomyopathy. Am. J. Hum. Genet. 2013, 93, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xia, M.; Duan, Y.; Zhang, L.; Jiang, H.; Hu, X.; Yan, H.; Zhang, Y.; Gu, Y.; Shi, H.; et al. Berberine promotes the recruitment and activation of brown adipose tissue in mice and humans. Cell Death Dis. 2019, 10, 468. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Falvo, M.J. Protein - Which is Best? J. Sports Sci. Med. 2004, 3, 118–130. [Google Scholar]
- Campbell, W.W.; Barton, M.L.; Cyr-Campbell, D.; Davey, S.L.; Beard, J.L.; Parise, G.; Evans, W.J. Effects of an omnivorous diet compared with a lactoovovegetarian diet on resistance-training-induced changes in body composition and skeletal muscle in older men. Am. J. Clin. Nutr. 1999, 70, 1032–1039. [Google Scholar] [CrossRef]
- Pannemans, D.L.; Wagenmakers, A.J.; Westerterp, K.R.; Schaafsma, G.; Halliday, D. Effect of protein source and quantity on protein metabolism in elderly women. Am. J. Clin. Nutr. 1998, 68, 1228–1235. [Google Scholar] [CrossRef]
- Roden, M. Future of muscle research in diabetes: A look into the crystal ball. Diabetologia 2015, 58, 1693–1698. [Google Scholar] [CrossRef][Green Version]
- Maliszewska, K.; Adamska-Patruno, E.; Goscik, J.; Lipinska, D.; Citko, A.; Krahel, A.; Miniewska, K.; Fiedorczuk, J.; Moroz, M.; Gorska, M.; et al. The Role of Muscle Decline in Type 2 Diabetes Development: A 5-Year Prospective Observational Cohort Study. Nutrients 2019, 11, 834. [Google Scholar] [CrossRef]
- Sanchez-Delgado, G.; Acosta, F.M.; Martinez-Tellez, B.; Finlayson, G.; Gibbons, C.; Labayen, I.; Llamas-Elvira, J.M.; Gil, A.; Blundell, J.E.; Ruiz, J.R. Brown adipose tissue volume and 18F-fluorodeoxyglucose uptake are not associated with energy intake in young human adults. Am. J. Clin. Nutr. 2020, 111, 329–339. [Google Scholar] [CrossRef]
- Saito, M.; Matsushita, M.; Yoneshiro, T.; Okamatsu-Ogura, Y. Brown Adipose Tissue, Diet-Induced Thermogenesis, and Thermogenic Food Ingredients: From Mice to Men. Front. Endocrinol. 2020, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Minokoshi, Y.; Shimazu, T. Metabolic and sympathetic nerve activities of brown adipose tissue in tube-fed rats. Am. J. Physiol. 1989, 257 Pt 1, E374–E378. [Google Scholar] [CrossRef] [PubMed]
- Von Essen, G.; Lindsund, E.; Cannon, B.; Nedergaard, J. Adaptive facultative diet-induced thermogenesis in wild-type but not in UCP1-ablated mice. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E515–E527. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Schopman, J.E.; Admiraal, W.M.; Soeters, M.R.; Nieuwdorp, M.; Verberne, H.J.; Holleman, F. Fasting and postprandial activity of brown adipose tissue in healthy men. J. Nucl. Med. 2012, 53, 1407–1410. [Google Scholar] [CrossRef]
- Vosselman, M.J.; Brans, B.; van der Lans, A.A.; Wierts, R.; van Baak, M.A.; Mottaghy, F.M.; Schrauwen, P.; van Marken Lichtenbelt, W.D. Brown adipose tissue activity after a high-calorie meal in humans. Am. J. Clin. Nutr. 2013, 98, 57–64. [Google Scholar] [CrossRef]
- Heenan, K.A.; Carrillo, A.E.; Fulton, J.L.; Ryan, E.J.; Edsall, J.R.; Rigopoulos, D.; Markofski, M.M.; Flouris, A.D.; Dinas, P.C. Effects of Nutrition/Diet on Brown Adipose Tissue in Humans: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2752. [Google Scholar] [CrossRef]
- Muzik, O.; Mangner, T.J.; Leonard, W.R.; Kumar, A.; Janisse, J.; Granneman, J.G. 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat. J. Nucl. Med. 2013, 54, 523–531. [Google Scholar] [CrossRef]
- U Din, M.; Saari, T.; Raiko, J.; Kudomi, N.; Maurer, S.F.; Lahesmaa, M.; Fromme, T.; Amri, E.Z.; Klingenspor, M.; Solin, O.; et al. Postprandial Oxidative Metabolism of Human Brown Fat Indicates Thermogenesis. Cell Metab. 2018, 28, 207–216.e3. [Google Scholar] [CrossRef]
- Hibi, M.; Oishi, S.; Matsushita, M.; Yoneshiro, T.; Yamaguchi, T.; Usui, C.; Yasunaga, K.; Katsuragi, Y.; Kubota, K.; Tanaka, S.; et al. Brown adipose tissue is involved in diet-induced thermogenesis and whole-body fat utilization in healthy humans. Int. J. Obes. 2016, 40, 1655–1661. [Google Scholar] [CrossRef]
- McNeill, B.T.; Morton, N.M.; Stimson, R.H. Substrate Utilization by Brown Adipose Tissue: What’s Hot and What’s Not? Front. Endocrinol. 2020, 11, 571659. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.S. Responses of the hands and feet to cold exposure. Temperature 2015, 2, 105–120. [Google Scholar] [CrossRef] [PubMed]
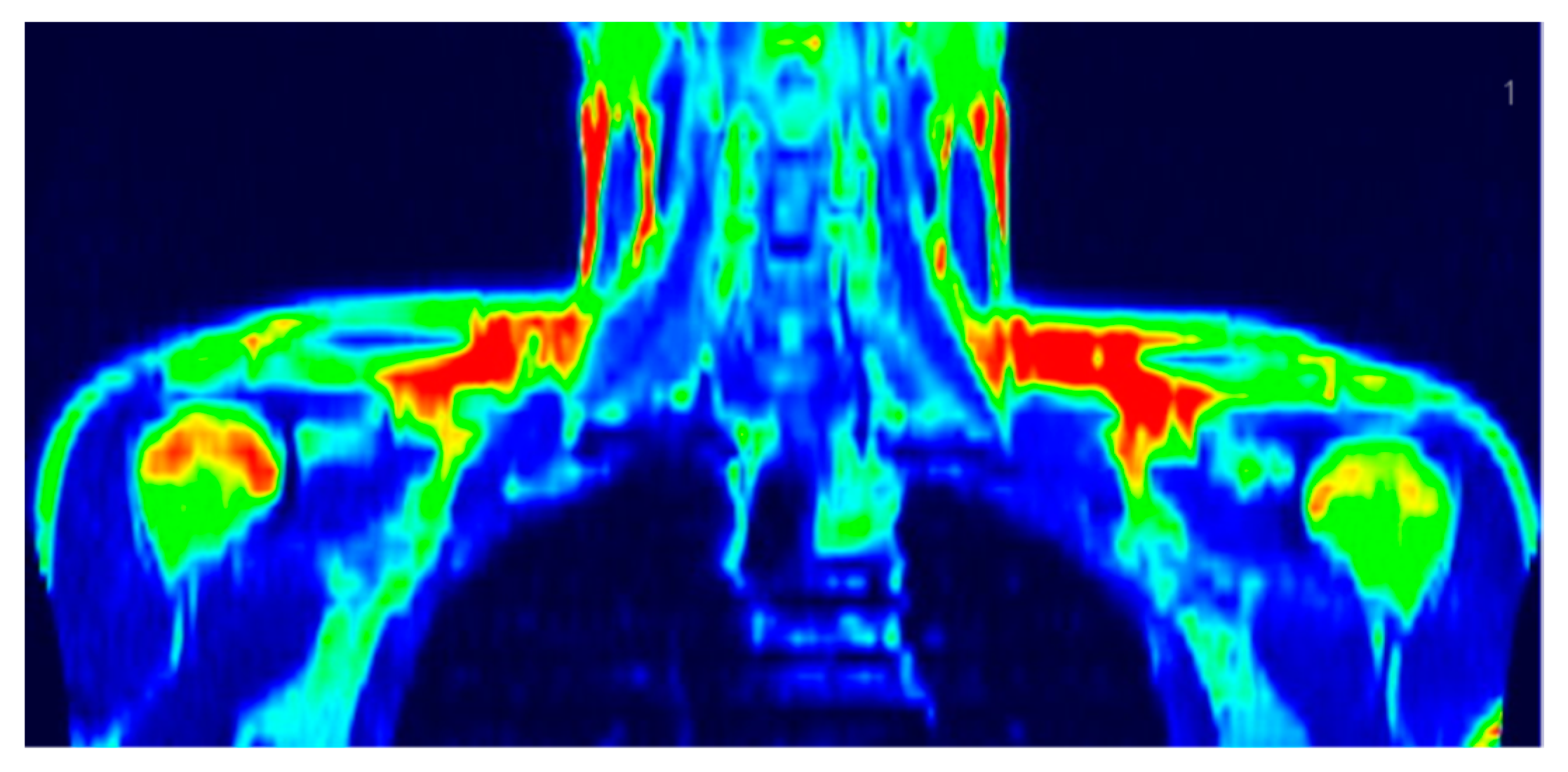
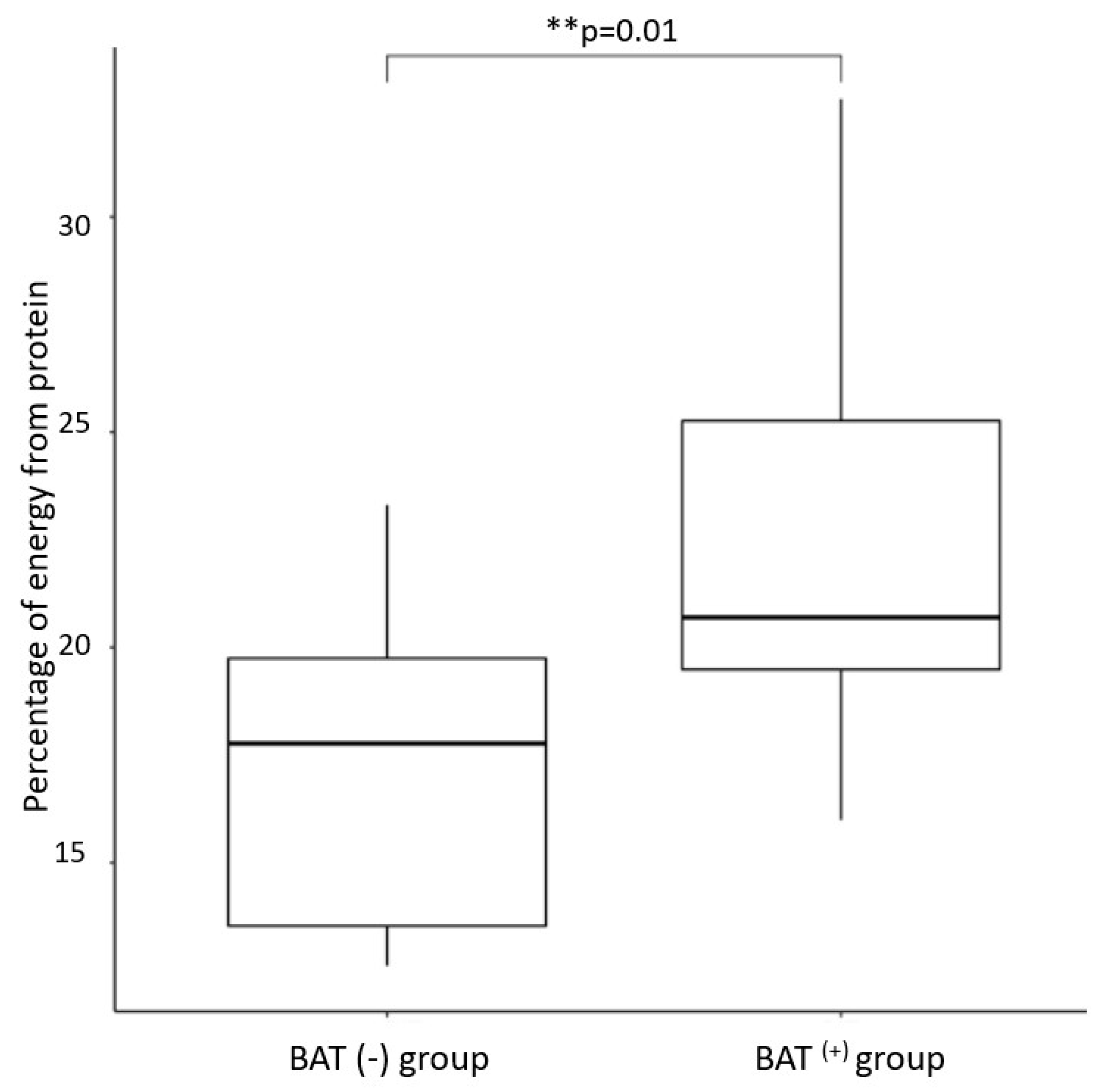
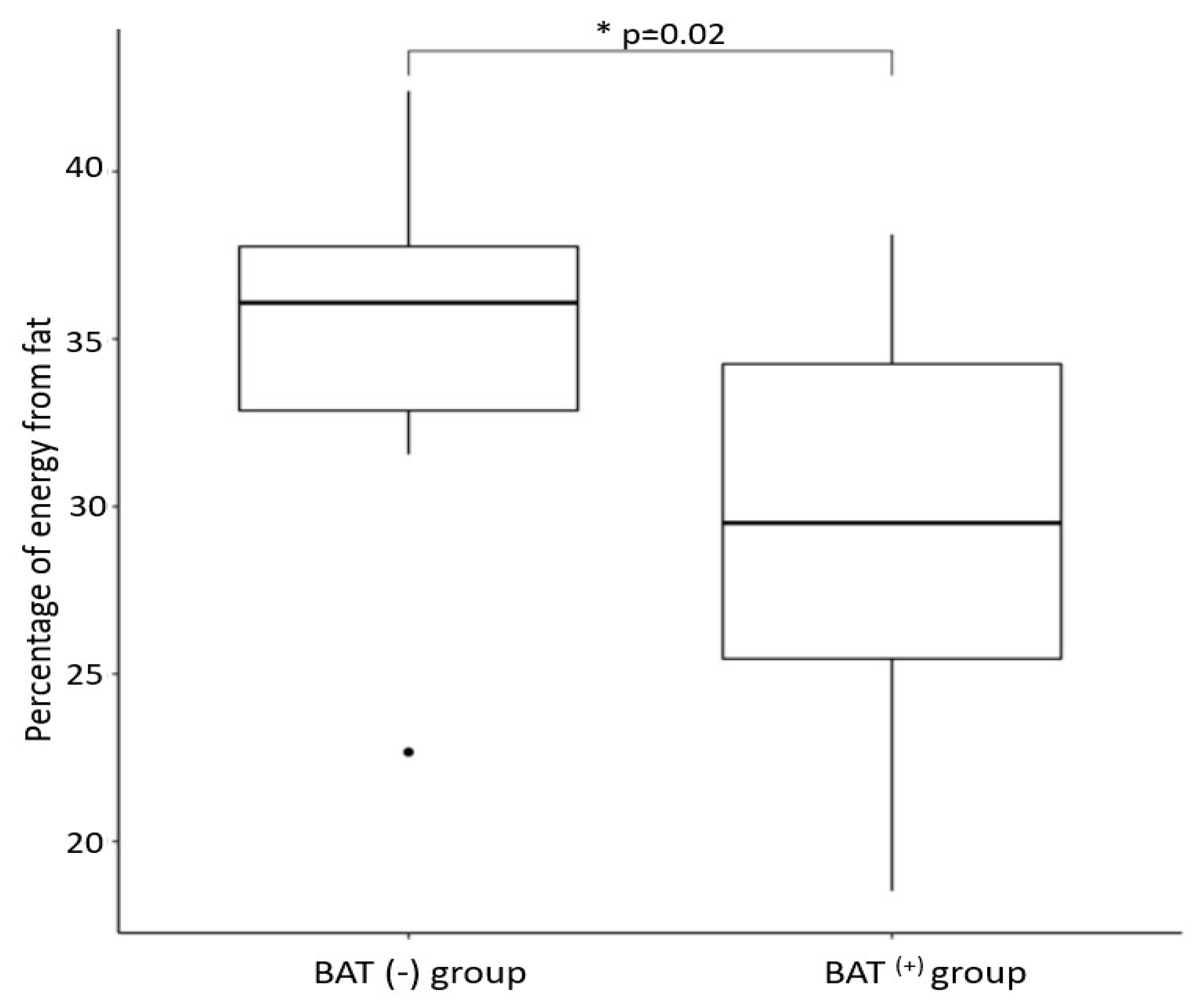
| Variables ¹ | BAT(+) 2 | BAT(−) 3 | p-Value 4 |
|---|---|---|---|
| Age (years) | 24.7 ± 2.4 | 30.3 ± 6.7 | 0.006 |
| BMI < 25 kg/m2 (N) | 12 | 4 | |
| BMI > 25 kg/m2(N) | 6 | 6 | |
| IB weight | 86.7 ± 16.5 | 94.01 ± 15.0 | 0.1 |
| IB AT percentage (%) | 18.4 ± 6.5 | 22.5 ± 5.9 | 0.06 |
| IB SM mass | 39.9 ± 5.5 | 41.1 ± 5.2 | 0.1 |
| IB SM percentage | 46.4 ± 3.7 | 44.0 ± 3.3 | 0.04 |
| IB VAT (cm2) | 71.6 ± 41.3 | 99.3 ± 38.6 | 0.05 |
| Percentage of daily energy obtained from protein | 22.6 ± 4.9 | 17.2 ± 3.9 | 0.01 |
| Percentage of daily energy obtained from fat | 29.3 ± 5.2 | 34.9 ± 6.1 | 0.02 |
| Percentage of daily energy obtained from carbohydrates | 46.0 ± 6.7 | 45.4 ± 7.6 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maliszewska, K.; Adamska-Patruno, E.; Miniewska, K.; Bauer, W.; Buczyńska, A.; Mojsak, M.; Kretowski, A. Different Protein Sources Enhance 18FDG-PET/MR Uptake of Brown Adipocytes in Male Subjects. Nutrients 2022, 14, 3411. https://doi.org/10.3390/nu14163411
Maliszewska K, Adamska-Patruno E, Miniewska K, Bauer W, Buczyńska A, Mojsak M, Kretowski A. Different Protein Sources Enhance 18FDG-PET/MR Uptake of Brown Adipocytes in Male Subjects. Nutrients. 2022; 14(16):3411. https://doi.org/10.3390/nu14163411
Chicago/Turabian StyleMaliszewska, Katarzyna, Edyta Adamska-Patruno, Katarzyna Miniewska, Witold Bauer, Angelika Buczyńska, Małgorzata Mojsak, and Adam Kretowski. 2022. "Different Protein Sources Enhance 18FDG-PET/MR Uptake of Brown Adipocytes in Male Subjects" Nutrients 14, no. 16: 3411. https://doi.org/10.3390/nu14163411
APA StyleMaliszewska, K., Adamska-Patruno, E., Miniewska, K., Bauer, W., Buczyńska, A., Mojsak, M., & Kretowski, A. (2022). Different Protein Sources Enhance 18FDG-PET/MR Uptake of Brown Adipocytes in Male Subjects. Nutrients, 14(16), 3411. https://doi.org/10.3390/nu14163411






