Potential of Egg as Complementary Food to Improve Nutrient Intake and Dietary Diversity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design and Participants
2.3. Intervention Procedures
2.4. Research Procedures and Data Collection
2.5. Data Analysis
2.6. Ethics
3. Results
3.1. Baseline Characteristics of Study Participants
3.2. Dietary Intake
3.2.1. Energy and Nutrient Intake
3.2.2. Food Groups and Dietary Diversity
3.2.3. Frequency of Foods Consumed
3.3. Egg Usage and Perceptions on Egg
3.4. Effect of Lockdown on Feeding and Care of the Baby
3.5. Allergy-Related Symptoms
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNICEF (United Nations Children’s Fund); WHO (World Health Organization); WB (The World Bank Group). Levels and Trends in Child Malnutrition: Key Findings of the 2021 Edition. Available online: https://data.unicef.org/resources/jme-report-2021/ (accessed on 11 June 2022).
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Victora, C.G.; Adair, L.; Fall, C.; Hallal, P.C.; Martorell, R.; Richter, L.; Sachdev, H.S.; Maternal Child Undernutrition Study Group. Maternal and child undernutrition: Consequences for adult health and human capital. Lancet 2008, 371, 340–357. [Google Scholar] [CrossRef]
- UNICEF (United Nations Children Fund). Improving Child Nutrition: The Achievable Imperative for Global Progress. 2013. Available online: https://www.unicef.org/publications/files/Nutrition_Report_final_lo_res_8_April.pdf (accessed on 4 March 2019).
- Dewey, K.G. The challenge of meeting nutrient needs of infants and young children during the period of complementary feeding: An evolutionary perspective. J. Nutr. 2013, 143, 2050–2054. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; West, K.P., Jr.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66 (Suppl. S2), 22–33. [Google Scholar] [CrossRef]
- Parikh, P.; Semba, R.; Manary, M.; Swaminathan, S.; Udomkesmalee, E.; Bos, R.; Poh, B.K.; Rojroongwasinkul, N.; Geurts, J.; Sekartini, R.; et al. Animal source foods, rich in essential amino acids, are important for linear growth and development of young children in low- and middle-income countries. Matern. Child Nutr. 2021, 18, e13264. [Google Scholar] [CrossRef]
- Michaelsen, K.F.; Dewey, K.G.; Perez-Exposito, A.B.; Nurhasan, M.; Lauritzen, L.; Roos, N. Food sources and intake of n-6 and n-3 fatty acids in low-income countries with emphasis on infants, young children (6–24 months), and pregnant and lactating women. Mater. Child Nutr. 2011, 7, 124–140. [Google Scholar] [CrossRef]
- Gatica-Domínguez, G.; Neves, P.A.R.; Barros, A.J.D.; Victora, C.G. Complementary feeding practices in 80 low- and middle-income countries: Prevalence of and socioeconomic inequalities in dietary diversity, meal frequency, and dietary adequacy. J. Nutr. 2021, 151, 1956–1964. [Google Scholar] [CrossRef]
- Pries, A.M.; Filteau, S.; Ferguson, E.L. Snack food and beverage consumption and young child nutrition in low- and middle-income countries: A systematic review. Matern. Child Nutr. 2019, 15 (Suppl. S4), e12729. [Google Scholar] [CrossRef]
- NDoH (National Department of Health); Stats SA (Statistics South Africa); SAMRC (South African Medical Research Council); ICF (Inner City Fund). South Africa Demographic and Health Survey 2016: Key Indicator Report, 2017. Pretoria and Rockville, Maryland. Available online: https://www.statssa.gov.za/publications/Report%2003-00-09/Report%2003-00-092016.pdf (accessed on 10 January 2020).
- Tam, E.; Keats, E.C.; Rind, F.; Das, J.K.; Bhutta, A.Z.A. Micronutrient supplementation and fortification interventions on health and development outcomes among children under-five in low- and middle-income countries: A systematic review and meta-analysis. Nutrients 2020, 12, 289. [Google Scholar] [CrossRef]
- Blasbalg, T.L.; Wispelwey, B.; Deckelbaum, R.J. Econutrition and utilization of food-based approaches for nutritional health. Food Nutr. Bull. 2011, 32 (Suppl. S1), S4–S13. [Google Scholar] [CrossRef]
- Neumann, C.; Harris, D.M.; Rogers, L.M. Contribution of animal source foods in improving diet quality and function in children in the developing world. Nutr. Res. 2002, 22, 193–220. [Google Scholar] [CrossRef]
- Asare, H.; Rosi, A.; Faber, M.; Smuts, C.M.; Ricci, C. Animal-source foods as a suitable complementary food for improved physical growth in 6 to 24-month-old children in low- and middle-income countries: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, L.L.; Lutter, C.K.; Stewart, C.P.; Riofrío, C.A.G.; Malo, C.; Reinhart, G.; Palacios, A.; Karp, C.; Chapnick, M.; Cox, K. Eggs in early complementary feeding and child growth: A randomized controlled trial. Pediatrics 2017, 140, e20163459. [Google Scholar] [CrossRef]
- Fiocchi, A.; Assa’ad, A.; Bahna, S. Food allergy and the introduction of solid foods to infants: A consensus document. Ann. Allergy Asthma Immunol. 2006, 97, 10–21. [Google Scholar] [CrossRef]
- Al-Saud, B.; Sigurdardóttir, S.T. Early introduction of egg and the development of egg allergy in children: A systematic review and meta-analysis. Int. Arch. Allergy Immunol. 2018, 177, 350–359. [Google Scholar] [CrossRef]
- Caffarelli, C.; Giannetti, A.; Rossi, A.; Ricci, G. Egg allergy in children and weaning diet. Nutrients 2022, 14, 1540. [Google Scholar] [CrossRef]
- Levin, M.; Goga, A.; Doherty, T.; Coovadia, H.; Sanders, D.; Green, R.J.; King, S. Allergy and infant feeding guidelines in the context of resource-constrained settings. J. Allergy Clin. Immunol. 2017, 139, 455–458. [Google Scholar] [CrossRef]
- Lutter, C.K.; Iannotti, L.L.; Stewart, C.P. The potential of a simple egg to improve maternal and child Nutrition. Matern. Child Nutr. 2018, 14 (Suppl. S3), e12678. [Google Scholar] [CrossRef]
- White, J.M.; Bégin, F.; Kumapley, R.; Murray, C.; Krasevec, J. Complementary feeding practices: Current global and regional estimates. Matern. Child Nutr. 2017, 13 (Suppl. S2), e12505. [Google Scholar] [CrossRef]
- Nkukwana, T.T. Global poultry production: Current impact and future outlook on the South African poultry industry. S. Afr. J. Anim. Sci. 2018, 48, 869–884. [Google Scholar] [CrossRef]
- SAPA (South African Poultry Association). South African Poultry Association 2019 Industry Profile. 2019. Available online: http://www.sapoultry.co.za/pdf-docs/sapa-industry-profile.pdf (accessed on 25 May 2022).
- Iannotti, L.L.; Lutter, C.K.; Bunn, D.A.; Stewart, C.P. Eggs: The uncracked potential for improving maternal and young child nutrition among the world’s poor. Nutr. Rev. 2014, 72, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Waters, W.F.; Gallegos, C.A.; Karp, C.; Lutter, C.; Stewart, C.; Iannotti, L. Cracking the egg potential: Traditional knowledge, attitudes, and practices in a food-based nutrition intervention in highland Ecuador. Food Nutr. Bull. 2018, 39, 206–218. [Google Scholar] [CrossRef]
- Stats SA (Statistics South Africa). Demographic Information: City of Matlosana Local Municipality. 2018. Available online: https://municipalities.co.za/demographic/1193/city-of-matlosana-local-municipality (accessed on 11 March 2019).
- Matsungo, T.M.; Kruger, H.S.; Faber, M.; Rothman, M.; Smuts, C.M. The prevalence and factors associated with stunting among infants aged 6 months in a peri-urban South African community. Public Health Nutr. 2017, 20, 3209–3218. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Training Course on Child Growth Assessment; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- WHO (World Health Organization). Indicators for Assessing Infant and Young Child Feeding Practices, Part 2: Measurement; World Health Organization: Geneva, Switzerland, 2010; Available online: http://apps.who.int/iris/bitstream/10665/44368/1/9789241599757_eng.pdf (accessed on 10 January 2017).
- Rothman, M.; Faber, M.; Covic, N.; Matsungo, T.; Cockeran, M.; Kvalsvig, J.; Smuts, C. Infant development at the age of 6 months in relation to feeding practices, iron status, and growth in a peri-urban community of South Africa. Nutrients 2018, 10, 73–85. [Google Scholar] [CrossRef]
- SAFOODS. SAMRC Food Quantities Manual for South Africa, 3rd ed.; South African Medical Research Council: Cape Town, South Africa, 2018. [Google Scholar]
- World Health Organization. Complementary Feeding of Young Children in Developing Countries: A Review of Current Scientific Knowledge. WHO/ NUT/98.1; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- SAFOODS. SAMRC Food Composition Tables for South Africa, 5th ed.; South African Medical Research Council: Cape Town, South Africa, 2017. [Google Scholar]
- Otten, J.J.; Meyers, L.D.; Hellwig, J.P. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Institute of Medicine; The National Academies Press: Washington, WA, USA, 2011; Available online: https://www.nap.edu/search/?term=DRIMAR (accessed on 20 January 2018).
- WHO (World Health Organization); United Nations Children’s Fund (UNICEF). Indicators for Assessing Infant and Young Child Feeding Practices: Definitions and Measurement Methods; World Health Organization: Geneva, Switzerland; The United Nations Children’s Fund (UNICEF): New York, NY, USA, 2021. [Google Scholar]
- Senekal, M.; Nel, J.; Malczyk, S.; Drummond, L.; Steyn, N.P. Provincial Dietary Intake Study (PDIS): Micronutrient intakes of children in a representative/random sample of 1- to <10-year-old children in two economically active and urbanized Provinces in South Africa. Int. J. Environ. Res. Public Health 2020, 17, 5924. [Google Scholar] [CrossRef]
- Lutter, C.K.; Caswell, B.L.; Arnold, C.D.; Iannotti, L.L.; Maleta, K.; Chipatala, R.; Prado, E.L.; Stewart, C.P. Impacts of an egg complementary feeding trial on energy intake and dietary diversity in Malawi. Matern. Child Nutr. 2021, 17, e13055. [Google Scholar] [CrossRef]
- Sayed, N.; Schönfeldt, H.C. A review of complementary feeding practices in South Africa. S. Afr. J. Clin. Nutr. 2020, 33, 36–43. [Google Scholar] [CrossRef]
- Papanikolaou, Y.; Fulgoni, V. Egg consumption in Infants is associated with longer recumbent length and greater intake of several nutrients essential in growth and development. Nutrients 2018, 10, 719–730. [Google Scholar] [CrossRef]
- Schmid, A.; Walther, B. Natural vitamin D content in animal products. Adv. Nutr. 2013, 4, 453–462. [Google Scholar] [CrossRef]
- Caswell, B.L.; Arnold, C.D.; Lutter, C.K.; Iannotti, L.L.; Chipatala, R.; Werner, E.R.; Maleta, K.M.; Stewart, C.P. Impacts of an egg intervention on nutrient adequacy among young Malawian children. Matern. Child Nutr. 2021, 17, e13196. [Google Scholar] [CrossRef]
- Botha, M.; Basera, W.; Facey-Thomas, H.E.; Gaunt, B.; Gray, C.L.; Ramjith, J.; Watkins, A.; Levin, M.E. Rural and urban food allergy prevalence from the South African Food Allergy (SAFFA) study. J. Allergy Clin. Immunol. 2019, 143, 662–668. [Google Scholar] [CrossRef] [PubMed]
- CDC. Salmonella and Eggs. 2022. Available online: https://www.cdc.gov/foodsafety/communication/salmonella-and-eggs.html (accessed on 25 May 2022).
- World Health Organization. Infant and Young Child Feeding. Available online: https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding (accessed on 12 June 2022).
- Gray, C.L. Food Allergy in South Africa. Curr. Allergy Asthma Rep. 2017, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Palacios, A.M.; Gallegos, C.A.; Waters, W.F.; Malo, C.; Lutter, C.; Stewart, C.P.; Reinhart, G.A.; Iannotti, L.A. Egg and dairy consumption were not associated with allergic reactions or allergy-like symptoms in 6 to 9 month-old infants from rural Cotopaxi, Ecuador. FASEB J. 2016, 30, 674–713. [Google Scholar]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W. American Academy of Pediatrics Committee on Nutrition; American Academy of Pediatrics Section on Allergy and Immunology. Effects of early nutritional interventions on the development of atopic disease in infants and children: The role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics 2008, 121, 183–191. [Google Scholar]
- FAO. Dietary Assessment: A Resource Guide to Method Selection and Application in Low Resource Settings; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Campbell, R.K.; Hurley, K.M.; Shamim, A.A.; Shaikh, S.; Chowdhury, Z.T.; Mehra, S.; Wu, L.; Christian, P. Complementary food supplements increase dietary nutrient adequacy and do not replace home food consumption in children 6–18 months old in a randomized controlled trial in rural Bangladesh. J. Nutr. 2018, 148, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Onifade, O.M.; Pringle, K.G.; Rollo, M.E.; Collins, C.E.; Schumacher, T.; Rae, K.M.; Gomeroi Gaaynggal Advisory Committee. Dietary intake of Indigenous Australian infants and young children in the Gomeroi gaaynggal cohort. Nutr. Diet. 2021, 78, 386–396. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Egg Group (n = 70) | Control Group (n = 85) |
|---|---|---|
| Infant characteristics | ||
| Sex: Male, n (%) | 34 (48.6) | 44 (51.8) |
| Female, n (%) | 36 (51.4) | 41 (48.2) |
| Age, months | 7.7 ± 0.9 1 | 7.7 ± 0.9 |
| Breastfeeding at enrolment, n (%) | 45 (64.3) | 58 (68.2) |
| Baby already introduced to egg, n (%) | 45 (64.3) | 60 (70.6) |
| Higher risk for allergy 2, n (%) | 18 (26.1) | 15 (18.3) |
| Anthropometric status | ||
| Length-for-age z-score (LAZ) | −1.26 (−1.85, −0.60) 3 | −1.37 (−2.07, −0.48) |
| Stunted (<−2 LAZ), n (%) | 14 (20.0) | 25 (29.4) |
| Weight-for-age z-score (WAZ) | −0.42 (−1.25, 0.17) | −0.61 (−1.51, 0.24) |
| Underweight (<−2 WAZ), n (%) | 7 (10.0) | 12 (14.1) |
| Weight-for-length z-score (WLZ) | 0.54 (−0.40, 1.30) | 0.24 (−0.62, 1.31) |
| Wasted (<−2 WLZ), n (%) | 1 (1.4) | 4 (4.7) |
| Overweight (>+2 WLZ), n (%) | 8 (11.4) | 14 (16.5) |
| Hemoglobin (Hb), g/L | 110 ± 13 | 108 ± 12 |
| Anemic (Hb <110 g/L), n (%) | 32 (45.7) | 42 (49.4) |
| Mother /caregiver characteristics | ||
| Age, y | 30.2 ± 9.5 | 29.3 ± 6.7 |
| Education, ≥Grade 10, n (%) | 54 (78.3) | 71 (83.5) |
| Married, n (%) | 8 (11.4) | 6 (7.1) |
| Household characteristics | ||
| Electricity at home, n (%) | 59 (84.3) | 75 (88.2) |
| Tap water at home, n (%) 4 | 65 (94.2) | 79 (94.0) |
| Flush toilet at home, n (%) | 65 (92.9) | 74 (87.1) |
| Number of people in household | 5 (4, 7) | 6 (4, 7) |
| Number of child grants per household | 2 (1, 3) | 2 (2, 3) |
| Nutrient | Group 1 | Baseline Median (P25, P75) | Endpoint Median (P25, P75) |
|---|---|---|---|
| Energy (kcal) | Egg | 690 (617, 839) | 931 (767, 1090) |
| Control | 736 (627, 895) | 903 (733, 1098) | |
| p-value 2 | 0.143 | 0.759 | |
| Protein (g) | Egg | 14.6 (10.5, 16.8) | 26.6 (18.1, 35.0) |
| Control | 15.9 (12.10, 21.6) | 23.8 (18.4, 34.8) | |
| p-value | 0.138 | 0.633 | |
| Plant protein (g) | Egg | 3.6 (2.0, 5.7) | 6.9 (51, 10.8) |
| Control | 4.3 (2.2, 6.3) | 8.8 (5.2, 12.4) | |
| p-value | 0.382 | 0.235 | |
| Animal protein (g) | Egg | 9.9 (8.0, 13.8) | 18.1 (10.0, 26.0) |
| Control | 11.2 (8.1, 14.3) | 15.6 (9.4, 22.8) | |
| p-value | 0.349 | 0.255 | |
| Fat (g) | Egg | 32.1 (29.5, 35.5) | 37.5 (24.1, 45.3) |
| Control | 35.3 (29.6, 39.0) | 33.2 (20.0, 40.3) | |
| p-value | 0.070 | 0.151 | |
| Saturated fat (g) | Egg | 14.0 (5.4, 15.2) | 15.0 (6.5, 17.4) |
| Control | 14.2 (6.4, 15.8) | 11.6 (5.4, 16.3) | |
| p-value | 0.426 | 0.157 | |
| MU fat (g) | Egg | 11.7 (4.3, 12.4) | 12.9 (6.6, 15.6) |
| Control | 11.8 (4.8, 13.1) | 10.7 (4.8, 14.2) | |
| p-value | 0.195 | 0.075 | |
| PU fat (g) | Egg | 3.6 (3.3, 4.2) | 5.8 (4.1, 8.0) |
| Control | 3.6 (3.3, 5.6) | 5.5 (3.2, 7.6) | |
| p-value | 0.348 | 0.322 | |
| Cholesterol (mg) | Egg | 94.6 (8.4, 101.5) | 256.0 (90.5, 382.6) |
| Control | 94.5 (23.9, 105.7) | 95.5 (27.8, 151.3) | |
| p-value | 0.702 | <0.001 | |
| Carbohydrates (g) | Egg | 89.0 (75.4, 112.3) | 126.5 (101.1, 145.4) |
| Control | 93.2 (75.6, 119.5) | 130.5 (101.6, 159.0) | |
| p-value | 0.330 | 0.336 | |
| Sugars (g) | Egg | 52.3 (18.6, 61.5) | 49.0 (30.9, 64.8) |
| Control | 53.4 (32.2, 61.9) | 45.8 (23.9, 60.1) | |
| p-value | 0.418 | 0.239 | |
| Fiber (g) | Egg | 1.75 (1.16, 3.09) | 4.96 (3.46, 7.74) |
| Control | 2.14 (0.99, 3.54) | 5.60 (3.72, 9.05) | |
| p-value | 0.664 | 0.217 | |
| Calcium (mg) | Egg | 402 (314, 554) | 380 (244, 532) |
| Control | 460 (325, 556) | 380 (267, 578) | |
| p-value | 0.404 | 0.787 | |
| Iron (mg) | Egg | 6.51 (2.46, 9.35) | 5.13 (3.27, 7.28) |
| Control | 5.95 (2.97, 9.79) | 5.63 (3.40, 8.36) | |
| p-value | 0.905 | 0.407 | |
| Magnesium (mg) | Egg | 49.1 (29.8, 74.7) | 112.6 (85.6, 149.7) |
| Control | 58.2 (38.5, 86.5) | 122.9 (81.9, 159.8) | |
| p-value | 0.046 | 0.444 | |
| Phosphorous (mg) | Egg | 230 (161, 389) | 460 (320, 637) |
| Control | 328 (199, 457) | 447 (364, 673) | |
| p-value | 0.068 | 0.880 | |
| Potassium (mg) | Egg | 649 (458, 933) | 881 (604, 1044) |
| Control | 764 (541, 1063) | 875 (689, 1128) | |
| p-value | 0.011 | 0.582 | |
| Zinc (mg) | Egg | 3.27 (2.39, 5.10) | 4.81 (3.31, 6.10) |
| Control | 3.46 (2.46, 5.34) | 4.82 (3.65, 7.13) | |
| p-value | 0.530 | 0.409 | |
| Copper (mg) | Egg | 0.42 (0.36, 0.50) | 0.57 (0.45, 0.71) |
| Control | 0.46 (0.39, 0.55) | 0.55 (0.44, 0.67) | |
| p-value | 0.083 | 0.560 | |
| Vitamin A (µg RE) | Egg | 658 (516, 823) | 552 (412, 762) |
| Control | 676 (523, 891) | 530 (352, 752) | |
| p-value | 0.428 | 0.567 | |
| Thiamin (mg) | Egg | 0.51 (0.32, 1.00) | 0.67 (0.46, 0.95) |
| Control | 0.61 (0.35, 0.87) | 0.74 (0.49, 1.08) | |
| p-value | 0.834 | 0.267 | |
| Riboflavin (mg) | Egg | 0.60 (0.45, 0.93) | 0.95 (0.60, 1.19) |
| Control | 0.71 (0.46, 1.08) | 0.97 (0.59, 1.65) | |
| p-value | 0.448 | 0.486 | |
| Niacin (mg) | Egg | 3.67 (2.59, 5.80) | 6.85 (4.48, 9.49) |
| Control | 4.03 (2.77, 5.82) | 8.01 (5.82, 12.04) | |
| p-value | 0.594 | 0.040 | |
| Vitamin B6 (mg) | Egg | 0.43 (0.26, 0.59) | 0.67 (0.49, 0.90) |
| Control | 0.47 (0.29, 0.72) | 0.84 (0.60, 1.13) | |
| p-value | 0.268 | 0.034 | |
| Folate (µg) | Egg | 98.1 (59.9, 177.6) | 143.7 (102.3, 235.7) |
| Control | 95.2 (63.5, 128.9) | 157.1 (99.0, 233.1) | |
| p-value | 0.437 | 0.769 | |
| Vitamin B12 (µg) | Egg | 0.86 (0.50, 1.32) | 1.63 (0.80, 2.43) |
| Control | 1.05 (0.63, 1.71) | 1.18 (0.71, 1.87) | |
| p-value | 0.402 | 0.199 | |
| Pantothenic acid (mg) | Egg | 2.33 (1.83, 3.16) | 3.20 (1.91, 4.42) |
| Control | 2.95 (2.03, 3.92) | 2.72 (1.95, 4.31) | |
| p-value | 0.167 | 0.545 | |
| Biotin (µg) | Egg | 6.42 (2.29, 15.2) | 14.82 (7.86, 25.22) |
| Control | 7.48 (2.00, 16.4) | 11.17 (6.83, 16.02) | |
| p-value | 0.810 | 0.070 | |
| Vitamin C (mg) | Egg | 71.7 49.3, 92.1) | 35.1 (26.6, 62.4) |
| Control | 67.3 (50.0, 99.8) | 37.7 (29.4, 61.2) | |
| p-value | 0.589 | 0.515 | |
| Vitamin D (µg) | Egg | 3.27 (1.21, 6.57) | 4.60 (1.00, 8.00) |
| Control | 4.25 (1.56, 6.67) | 0.98 (0.64, 4.93) | |
| p-value | 0.564 | 0.002 | |
| Vitamin E (mg) | Egg | 3.61 (1.45, 8.91) | 3.56 (2.12, 6.32) |
| Control | 3.27 (1.63, 7.07) | 3.02 (1.64, 7.00) | |
| p-value | 0.659 | 0.533 |
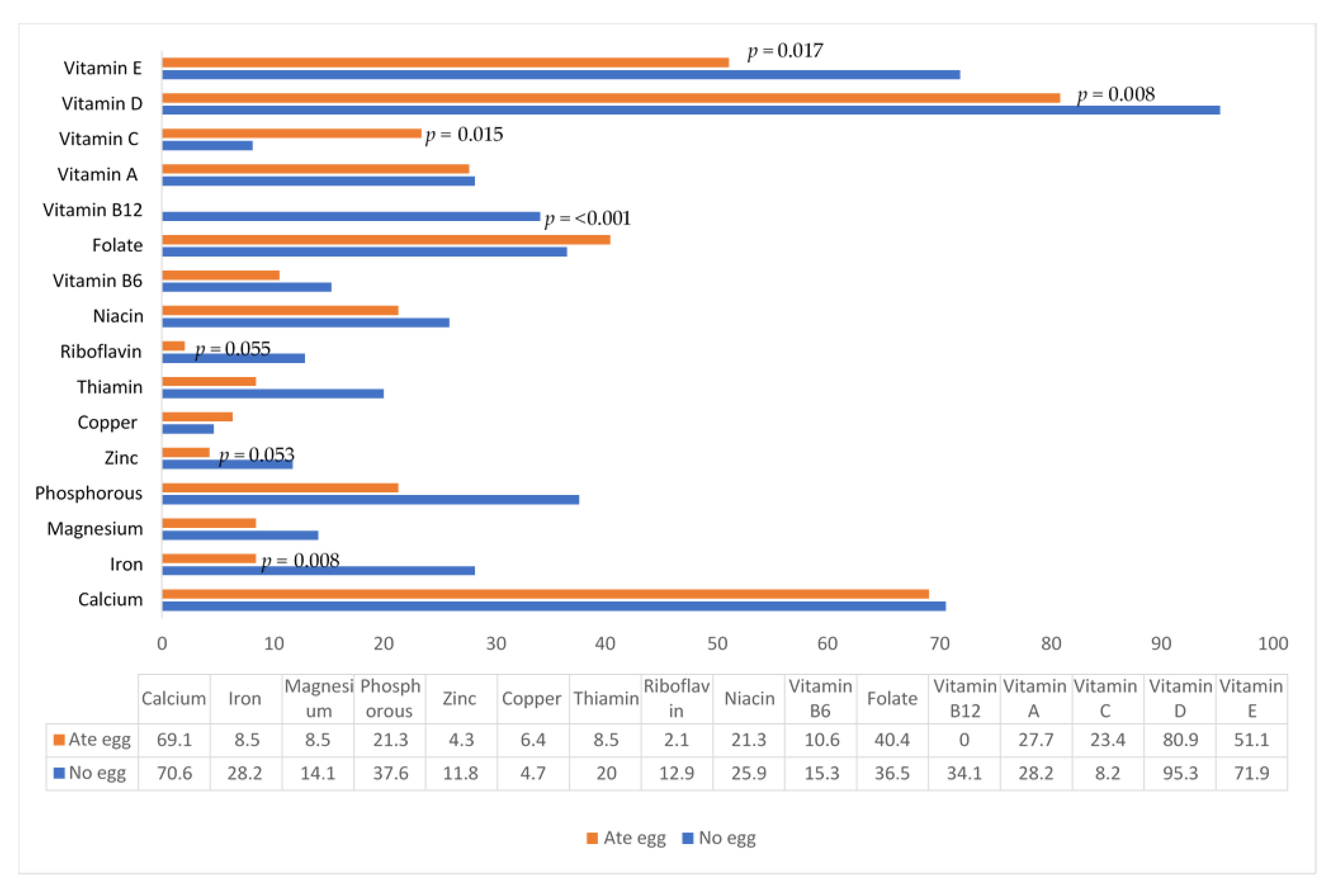
| Nutrient | Per 50 g Egg 1 | Ate Egg (n = 47) | No Egg (n = 85) | p-Value 2 |
|---|---|---|---|---|
| Median (P25, P75) | Median (P25, P75) | |||
| Energy (kcal) | 308 | 985 (754, 1180) | 899 (750, 1080) | 0.282 |
| Protein (g) | 6.3 | 31.7 (23.7, 37.9) | 22.3 (16.7, 29.0) | <0.001 |
| Plant protein (g) | 0 | 7.9 (5.1, 12.4) | 7.8 (5.2, 10.8) | 0.520 |
| Animal protein (g) | 6.3 | 23.7 (15.8, 30.3) | 13.3 (6.9, 19.6) | <0.001 |
| Fat (g) | 5.1 | 38.2 (24.3, 49.9) | 33.1 (19.4, 40.7) | 0.026 |
| Saturated fat (g) | 1.5 | 15.0 (6.5, 18.1) | 12.7 (5.0, 16.3) | 0.069 |
| MU fat (g) | 2.0 | 13.55 (6.9, 15.9) | 10.5 (4.4, 13.8) | 0.006 |
| PU fat (g) | 0.7 | 6.6 (4.5, 9.3) | 5.2 (3.3, 7.3) | 0.005 |
| Cholesterol (mg) | 209.5 | 329.7 (277.6, 452.9) | 86.1 (22.0, 108.6) | <0.001 |
| Carbohydrates (g) | 0.6 | 125.3 (92.4, 145.9) | 131.9 (105.2, 156.0) | 0.197 |
| Sugars (g) | 0.6 | 45.9 (15.9, 65.5) | 47.6 (27.4, 60.3) | 0.702 |
| Fiber (g) | 0.0 | 4.95 (3.48, 7.71) | 5.53 (3.37, 8.93) | 0.434 |
| Calcium (mg) | 19.5 | 393 (242, 562) | 382 (267, 571) | 0.847 |
| Iron (mg) | 0.9 | 5.77 (4.03, 7.64) | 4.89 (2.68, 8.06) | 0.197 |
| Magnesium (mg) | 4.5 | 123.0 (910.7, 151.2) | 115.8 (83.0, 156.5) | 0.657 |
| Phosphorous (mg) | 96 | 564 (393, 710) | 428 (302, 580) | 0.003 |
| Potassium (mg) | 49 | 938 606, 1159) | 861 (682,1049) | 0.529 |
| Zinc (mg) | 0.57 | 5.03 (3.72, 6.25) | 4.59 (3.32, 6.68) | 0.440 |
| Copper (mg) | 0.06 | 0.62 (0.43, 0.72) | 0.53 (0.45, 0.662) | 0.130 |
| Vitamin A (µg RE) | 33 | 600 (330, 890) | 530 (374, 682) | 0.216 |
| Thiamin (mg) | 0.05 | 0.72 (0.50, 0.94) | 0.71 (0.46, 1.05) | 0.934 |
| Riboflavin (mg) | 0.19 | 0.99 (0.73, 1.61) | 0.84 (0.54, 1.42) | 0.098 |
| Niacin (mg) | 0.05 | 7.53 (5.11, 0.16) | 7.64 (4.73, 11.6) | 0.926 |
| Vitamin B6 (mg) | 0.02 | 0.71 (0.56, 0.97) | 0.78 (0.57, 1.11) | 0.324 |
| Folate (µg) | 17.5 | 147.7 (103.1, 225.3) | 156.5 (99.9, 243.5) | 0.546 |
| Vitamin B12 (µg) | 0.8 | 1.98 (1.45, 3.37) | 0.95 (0.49, 1.59) | <0.001 |
| Pantothenic acid (mg) | 0.74 | 3.81 (2.84, 4.88) | 2.49 (1.79, 3.64) | 0.001 |
| Biotin (µg) | 9.2 | 18.2 (13.7, 27.9) | 8.4 (5.7, 14.3) | <0.001 |
| Vitamin C (mg) | 0 | 33.9 (16.3, 64.2) | 37.7 (31.8, 59.6) | 0.140 |
| Vitamin D (µg) | 3.97 | 5.37 (4.27, 8.84) | 0.80 (0.62, 2.91) | <0.001 |
| Vitamin E (mg) | 1.73 | 4.78 (3.19, 8.05) | 2.26 (1.42, 5.26) | <0.001 |
| Food Group | Group | Baseline (n) % | Endpoint (n) % |
|---|---|---|---|
| Breast milk | Egg | 42 (66.7) | 34 (38.6) |
| Control | 55 (67.9) | 31 (41.9) | |
| p-value 1 | 0.875 | 0.056 | |
| Cereals, roots, and tubers | Egg | 61 (96.8) | 58 (100) |
| Control | 75 (92.6) | 74 (100) | |
| p-value | 0.271 | - | |
| Legumes | Egg | 3 (4.8) | 2 (3.4) |
| Control | 2 (2.5) | 8 (10.8) | |
| p-value | 0.8751 | - | |
| Dairy | Egg | 42 (66.7) | 45 (77.6) |
| Control | 55 (67.9) | 60 (81.8) | |
| p-value | 0.875 | 0.621 | |
| Flesh foods | Egg | 4 (6.3) | 24 (41.4) |
| Control | 7 (8.6) | 37 (50.0) | |
| p-value | 0.875 | 0.324 | |
| Egg | Egg | 7 (11.1) | 36 (62.1) |
| Control | 8 (9.9) | 11 (14.9) | |
| p-value | 0.810 | <0.001 | |
| Vitamin A-rich fruit and vegetables | Egg | 8 (12.7) | 12 (20.7) |
| Control | 12 (14.8) | 11 (14.9) | |
| p-value | 0.716 | 0.965 | |
| Other fruit and vegetables | Egg | 22 (34.9) | 23 (39.7) |
| Control | 28 (34.6) | 42 (56.8) | |
| p-value | 0.965 | 0.051 | |
| Adequate DDS 2 | Egg | 5 (7.9) | 21 (36.2) |
| Control | 5 (6.2) | 14 (18.9) | |
| p-value | 0.680 | 0.026 | |
| DDS (mean ± SD) | Egg | 3.0 ± 0.9 | 4.0 ± 1.2 |
| Control | 3.0 ± 0.9 | 3.7 ± 1.0 | |
| p-value | 0.939 | 0.094 |
| Food Group | Food | Frequency of Consumption | Group 1 | Baseline, % | Midpoint % | Endpoint % |
|---|---|---|---|---|---|---|
| Formula milk feeds | Formula milk | Every day | Egg | 42.9 | 30.6 | 21.9 |
| Control | 43.5 | 32.8 | 19.8 | |||
| Dairy | Milk | ≥4 days/week | Egg | 10.0 | 12.9 | 18.8 |
| Control | 8.2 | 17.9 | 25.9 | |||
| Yogurt | ≥4 days/week | Egg | 4.3 | 11.3 | 14.1 | |
| Control | 1.2 | 11.9 | 16.1 | |||
| Baby foods | Pureed baby foods | ≥4 days/week | Egg | 20.0 | 29.0 | 7.8 |
| Control | 23.5 | 17.9 | 5.0 | |||
| Infant cereal | ≥4 days/week | Egg | 61.4 | 46.8 | 7.8 | |
| Control | 65.7 | 26.9 | 6.2 | |||
| Cereals, roots, tubers | Instant maize porridge | ≥4 days/week | Egg | 20.0 | 27.4 | 25.0 |
| Control | 10.6 | 37.3 | 29.6 | |||
| Maize meal | ≥4 days/week | Egg | 14.3 | 48.4 | 65.6 | |
| Control | 20.0 | 55.2 | 72.8 | |||
| Porridge, other than maize | ≥4 days/week | Egg | 2.9 | 11.3 | 18.7 | |
| Control | 8.2 | 9.0 | 14.8 | |||
| Breakfast cereal | ≥4 days/week | Egg | 2.9 | 21.0 | 17.1 | |
| Control | 5.9 | 16.4 | 19.7 | |||
| Bread (commercial) | ≥4 days/week | Egg | - | 6.4 | 6.2 | |
| Control | 4.7 | 8.9 | 13.6 | |||
| Potato | ≥4 days/week | Egg | 14.3 | 35.5 | 17.2 | |
| Control | 14.1 | 19.4 | 18.5 | |||
| Vegetables and fruit | Vegetables 2 | ≥4 days/week | Egg | 4.3 | 9.7 | 4.7 |
| Control | - | 6.0 | 9.9 | |||
| Fruit 3 | ≥4 days/week | Egg | 4.3 | 16.1 | 7.8 | |
| Control | 4.7 | 13.4 | 12.3 | |||
| Animal source foods | Chicken | ≥1 day/week | Egg | 32.8 | 71.0 | 79.7 |
| Control | 34.1 | 61.2 | 82.7 | |||
| Meat | ≥1 day/week | Egg | 10.0 | 19.4 | 32.8 | |
| Control | 5.9 | 23.9 | 37.0 | |||
| Liver | ≥1 day/week | Egg | 25.7 | 35.5 | 43.8 | |
| Control | 30.6 | 35.8 | 48.1 | |||
| Fish | ≥1 day/week | Egg | 7.1 | 12.9 | 25.0 | |
| Control | 9.4 | 26.9 | 29.6 | |||
| Unhealthy foods | Sweets | ≥4 days/week | Egg | 4.3 | 4.8 | 23.4 |
| Control | 2.4 | 4.5 | 27.2 | |||
| Salty savory snacks | ≥4 days/week | Egg | 7.1 | 19.4 | 25.0 | |
| Control | 4.7 | 14.9 | 29.6 | |||
| Fizzy drinks | ≥4 days/week | Egg | 1.4 | 4.8 | 9.4 | |
| Control | 2.4 | 7.5 | 18.5 | |||
| Cordials (mix with water) | ≥4 days/week | Egg | - | 8.1 | 14.0 | |
| Control | 2.4 | 3.0 | 8.6 |
| Food | Group 1 | Frequency of Consumption | Baseline % | Midpoint % | Endpoint % |
|---|---|---|---|---|---|
| Egg consumption during the previous week | Egg | Every day | 1.4 | 87.1 | 53.1 |
| 4–6 days | 4.3 | 8.1 | 21.9 | ||
| 1–3 days | 41.4 | 4.8 | 15.6 | ||
| Never | 52.9 | 0 | 9.4 | ||
| Control | Every day | 4.7 | 9.0 | 4.9 | |
| 4–6 days | 4.7 | 10.4 | 9.9 | ||
| 1–3 days | 35.3 | 41.8 | 45.7 | ||
| Never | 55.3 | 38.8 | 39.5 |
| Food | Group 1 | Baseline % | Midpoint % | Endpoint % | |
|---|---|---|---|---|---|
| Preparation | Egg | Boiled | 48.5 | 29.0 | 41.4 |
| Scrambled | 18.2 | 24.2 | 19.0 | ||
| Fried | 33.3 | 46.8 | 39.7 | ||
| Control | Boiled | 34.2 | 19.5 | 16.3 | |
| Scrambled | 21.1 | 19.5 | 16.3 | ||
| Fried | 44.7 | 61.0 | 63.3 | ||
| Food items added during preparation | Egg | Oil | 30.3 | 29.0 | 31.0 |
| Margarine | 18.2 | 32.3 | 20.7 | ||
| Control | Oil | 30.3 | 46.3 | 57.1 | |
| Margarine | 18.2 | 31.7 | 18.4 | ||
| Mix egg with other food 2 | Egg | Yes | 9.1 | 75.8 | 51.7 |
| Control | Yes | 23.6 | 40.5 | 32.7 | |
| Portion size usually eaten | Egg | >1 egg | - | - | 13.8 |
| 1 egg | 60.6 | 80.6 | 86.2 | ||
| ½ egg | 24.2 | 16.1 | - | ||
| ¼ egg | 15.2 | 3.2 | - | ||
| Control | >1 egg | - | - | 20.4 | |
| 1 egg | 50.0 | 78.0 | 69.4 | ||
| ½ egg | 44.7 | 17.1 | 10.2 | ||
| ¼ egg | 2.6 | 2.4 | - | ||
| <¼ egg | 2.6 | 2.4 | - | ||
| Number of times egg eaten per day | Egg | Once | 87.9 | 87.1 | 84.5 |
| 2 times | 9.1 | 9.7 | 12.1 | ||
| 3 times | 3.0 | 3.2 | 3.4 | ||
| Control | Once | 84.2 | 66.7 | 83.7 | |
| 2 times | 10.5 | 26.2 | 14.3 | ||
| 3 times | 5.3 | 4.8 | 2.0 |
| Total Group % | ||
|---|---|---|
| Benefits of giving egg to baby | Improves growth | 22.3 |
| Improves weight | 12.9 | |
| Improves health | 10.8 | |
| Provides protein | 28.8 | |
| Provides vitamins/nutrients | 8.7 | |
| Provide energy | 2.9 | |
| Other 1 | 6.5 | |
| Don’t know | 7.2 | |
| Risks of giving egg to baby | None | 95.2 |
| May cause allergy | 3.4 | |
| Other | 1.4 | |
| Best time to start giving egg to baby | Before 6 months | 30.1 |
| 6 months | 54.5 | |
| After 6 months | 15.4 |
| Allergy-Related Symptoms | Incidence, n (%) Duration, Median (25th, 75th) | Association with Frequency of Egg Consumption | |||
|---|---|---|---|---|---|
| Unadjusted | Adjusted 2 | ||||
| Total (n = 155) | Egg Group (n = 70) | Control Group (n = 85) | Exp B 95% CI)/ β p-Value 1 (n = 155) | Exp B 95% CI)/ β p-Value 1 (n = 155) | |
| Possible allergy reported by caregiver | 29 (4) | 10 (3.0) | 19 (4.4) | 1.049 (0.896, 1.227); 0.553 | 0.790 (0.804, 1.180); 0.790 |
| Eczema | 15 (2) | 7 (2.1) | 8 (1.9) | 0.991 (0.796, 1.234); 0.939 | 0.875 (0.667, 1.147); 0.334 |
| Eczema duration (d) | 5 (3, 8) | 6 (3, 12) | 7 (4, 50) | 0.234; 0.489 | 0.836, 0.102 |
| Other rash | 9 (1) | 4 (1.2) | 5 (1.2) | 1.119 (0.849, 1.474); 0.426 | 1.105 (0.752, 1.626); 0.611 |
| Other rash duration (d) | 7 (3, 11) | 8 (4, 13) | 3 (2, 11) | −0.294; 0.443 | −0.269; 0.797 |
| Angioedema | 2 (0.3) | 1 (0.3) | 1 (0.2) | 0.961 (0.527, 1.754); 0.898 | 0.896 (0.472, 1703); 0.737 |
| Wheeze | 31 (4) | 14 (4.2) | 17 (3.9) | 0.969 (0.830, 1.132); 0.690 | 0.978 (0.806, 1.185); 0.817 |
| Wheeze duration (d) | 11 (4, 28) | 4.5 (3, 16) | 15 (7, 28) | 0.413; 0.021 | −0.155; 0.580 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faber, M.; Malan, L.; Kruger, H.S.; Asare, H.; Visser, M.; Mukwevho, T.; Ricci, C.; Smuts, C.M. Potential of Egg as Complementary Food to Improve Nutrient Intake and Dietary Diversity. Nutrients 2022, 14, 3396. https://doi.org/10.3390/nu14163396
Faber M, Malan L, Kruger HS, Asare H, Visser M, Mukwevho T, Ricci C, Smuts CM. Potential of Egg as Complementary Food to Improve Nutrient Intake and Dietary Diversity. Nutrients. 2022; 14(16):3396. https://doi.org/10.3390/nu14163396
Chicago/Turabian StyleFaber, Mieke, Linda Malan, Herculina S. Kruger, Hannah Asare, Marina Visser, Tshiphiri Mukwevho, Cristian Ricci, and Cornelius M. Smuts. 2022. "Potential of Egg as Complementary Food to Improve Nutrient Intake and Dietary Diversity" Nutrients 14, no. 16: 3396. https://doi.org/10.3390/nu14163396
APA StyleFaber, M., Malan, L., Kruger, H. S., Asare, H., Visser, M., Mukwevho, T., Ricci, C., & Smuts, C. M. (2022). Potential of Egg as Complementary Food to Improve Nutrient Intake and Dietary Diversity. Nutrients, 14(16), 3396. https://doi.org/10.3390/nu14163396






