Artificial Diets Based on Selective Amino Acid Restriction versus Capecitabine in Mice with Metastatic Colon Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture Conditions
2.2. Chemicals and Drugs
2.3. In Vitro Experiments
2.4. Animals
2.5. In Vivo Colon Cancer Models
2.6. Diet Preparation and Composition
2.7. Statistical Analysis
3. Results
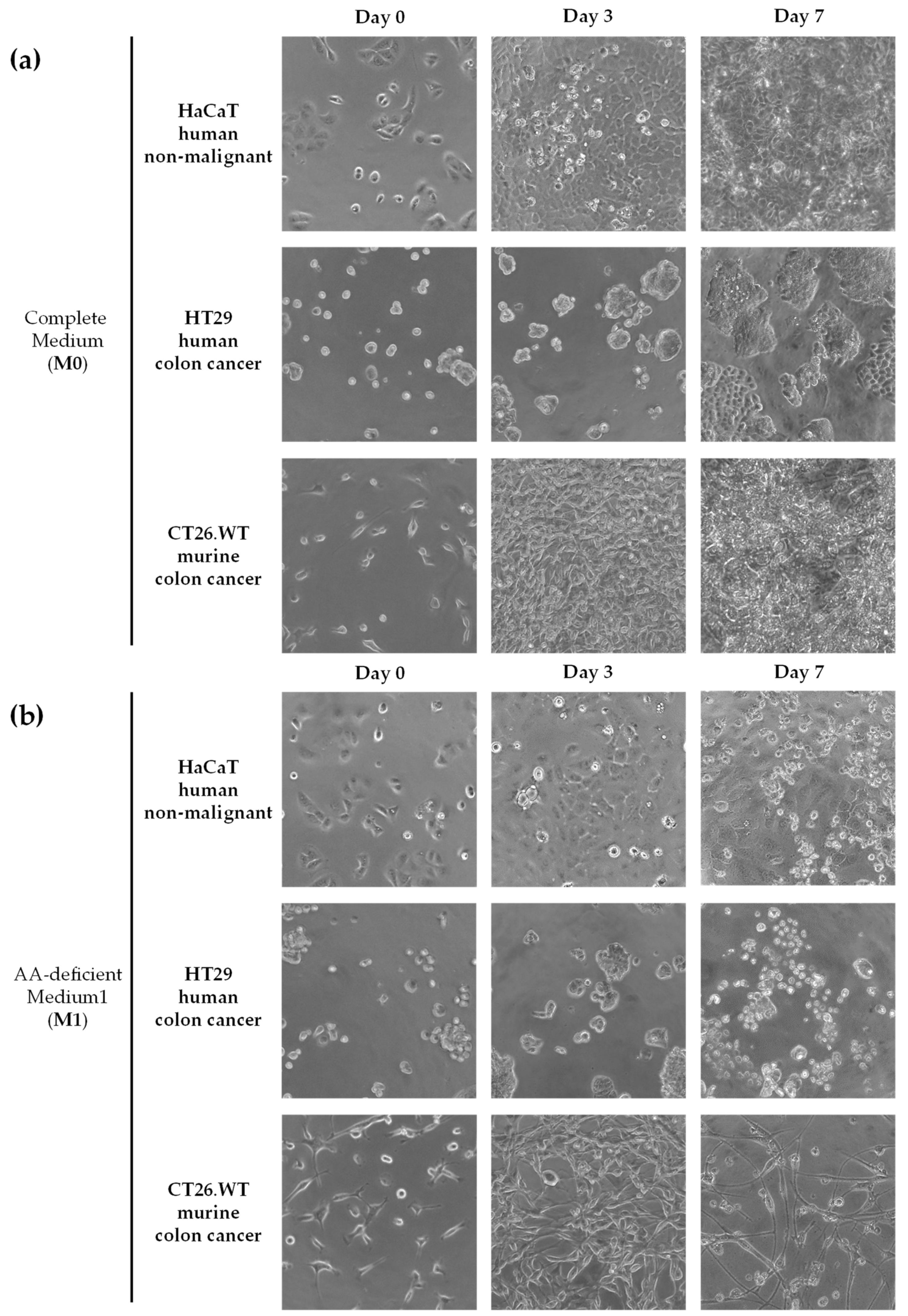
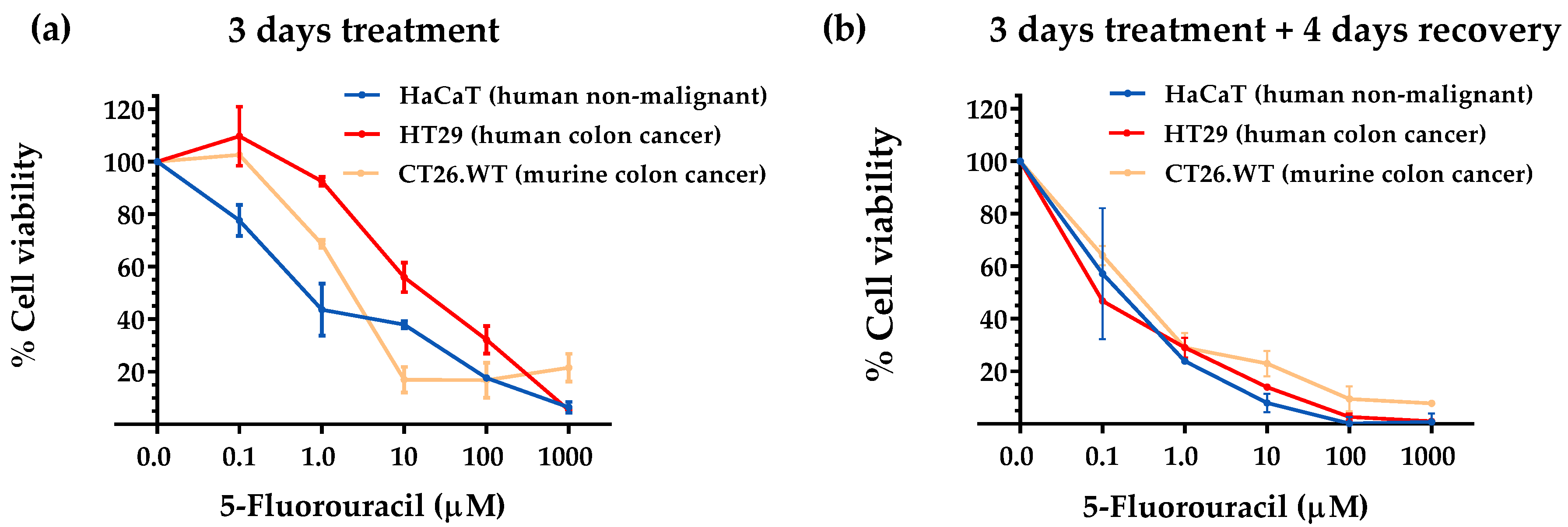
3.1. Selective Amino Acid Restriction Induced Anticancer Activity in Colon Cancer Cells In Vitro
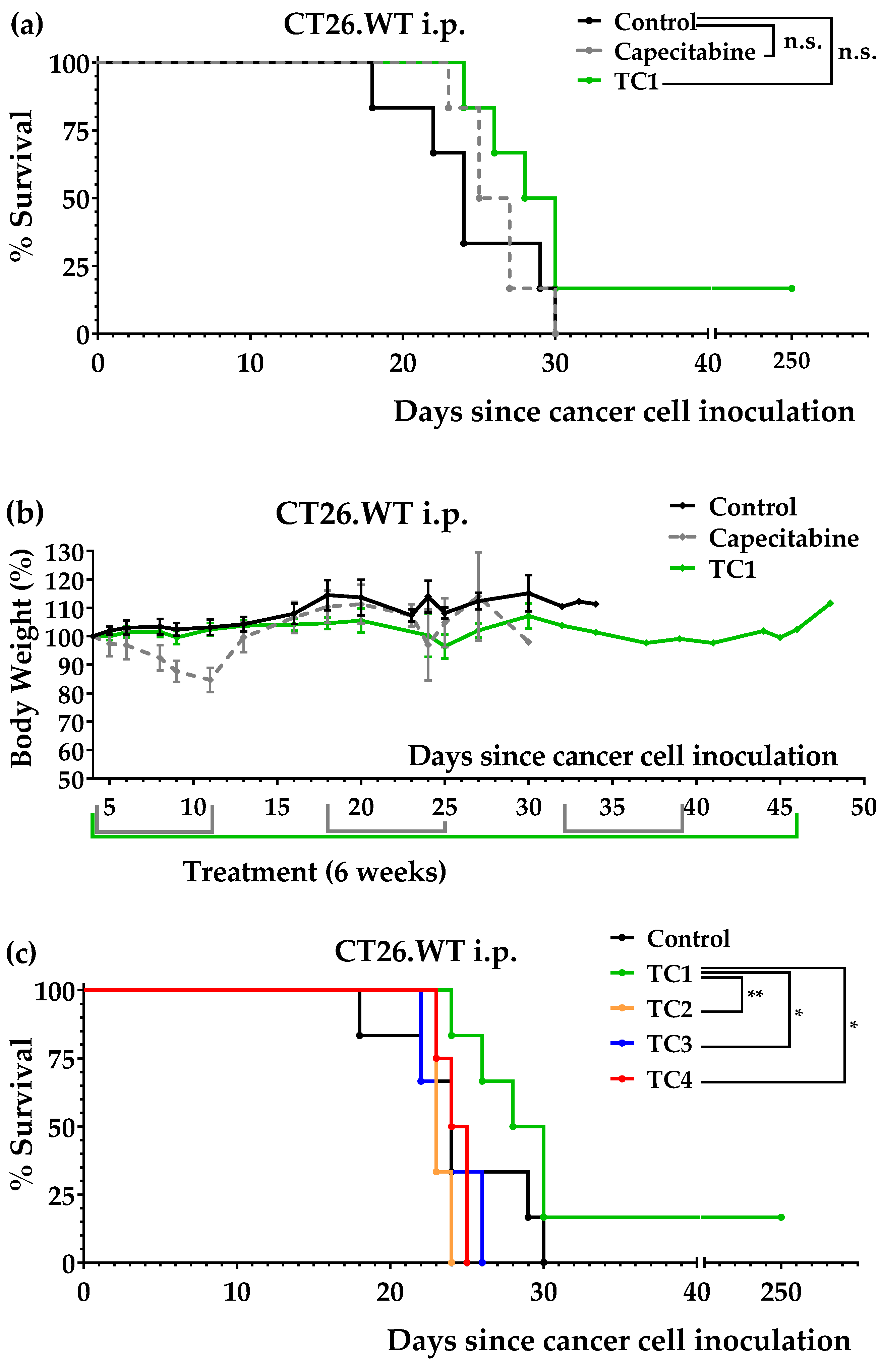
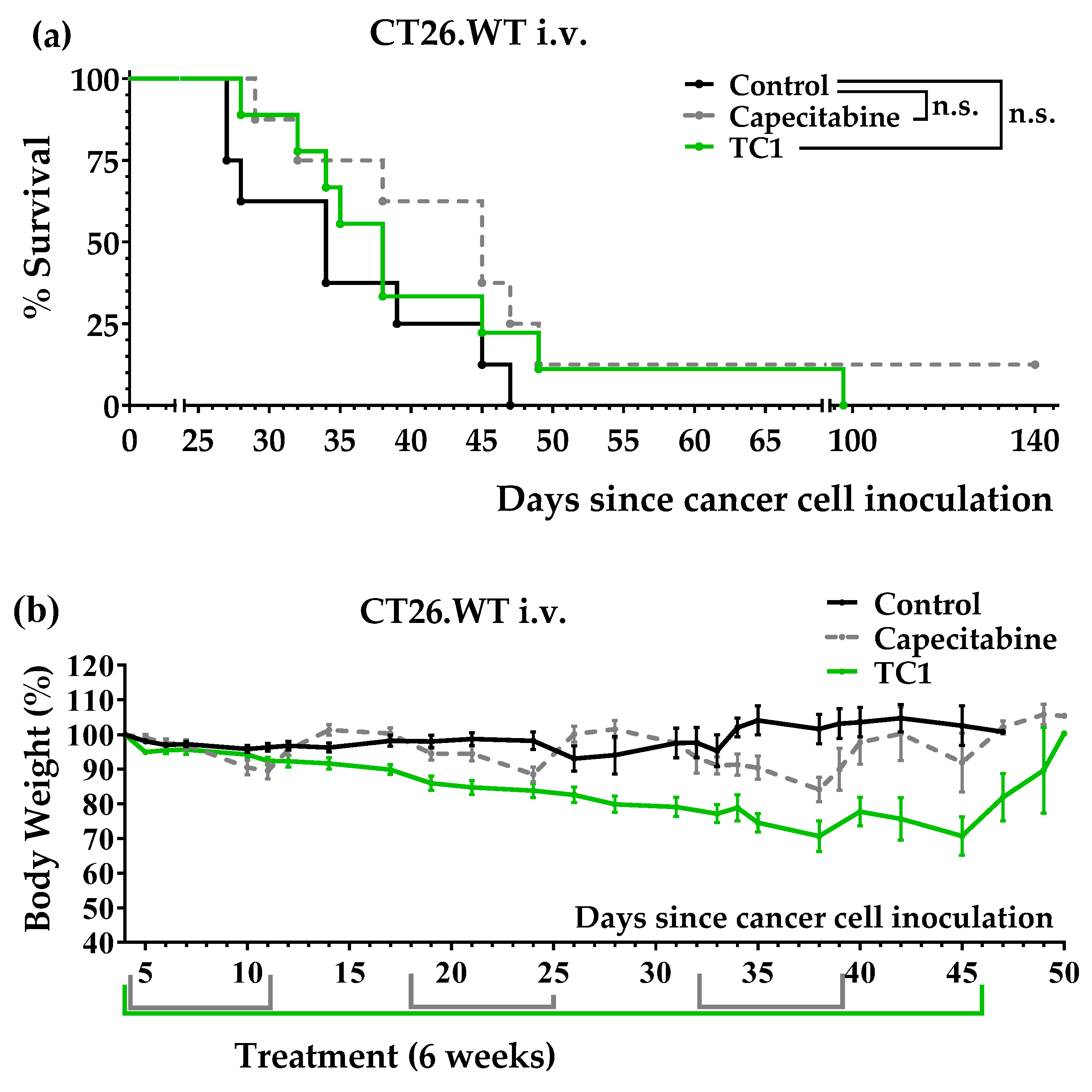
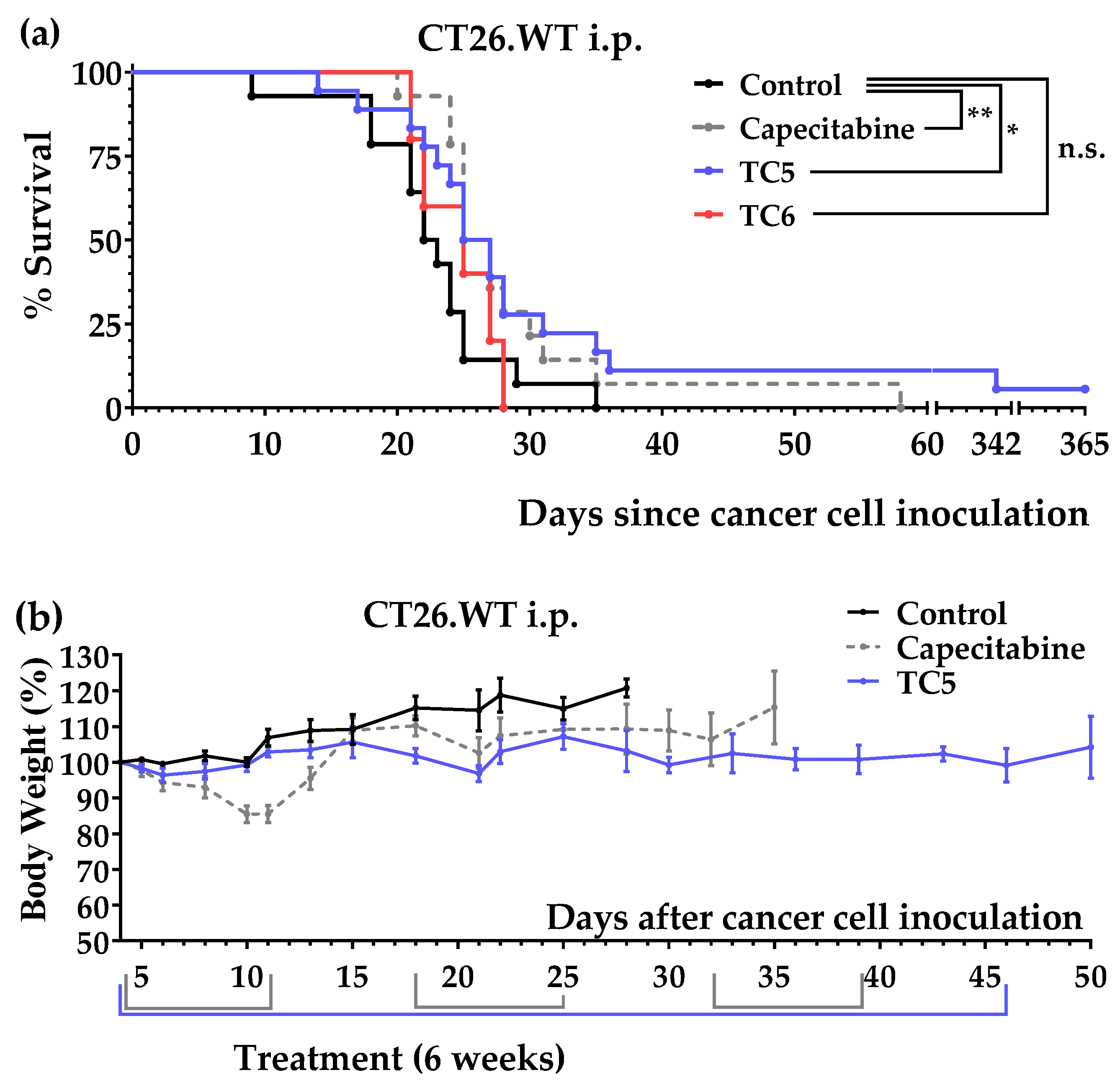
3.2. An Artificial Diet Lacking 10 Amino Acids Induced Anticancer Activity in Mice with Colon Cancer
3.3. An Artificial Diet with 6% Casein, 5% Glutamine, and 2.5% Leucine Induced Marked Anticancer Activity in Mice with Colon Cancer
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cancer Stat Facts: Colorectal Cancer. Available online: https://seer.cancer.gov/statfacts/html/colorect.html (accessed on 15 March 2022).
- National Cancer Institute. Colon and Rectum Stage Distribution of SEER Incidence Cases, 2010–2019. Available online: https://seer.cancer.gov/explorer/application.html?site=20&data_type=1&graph_type=4&compareBy=sex&chk_sex_1=1&race=1&age_range=1&advopt_precision=1 (accessed on 15 March 2022).
- Riihimäki, M.; Hemminki, A.; Sundquist, J.; Hemminki, K. Patterns of Metastasis in Colon and Rectal Cancer. Sci. Rep. 2016, 6, 29765. [Google Scholar] [CrossRef] [PubMed]
- American Society Of Clinical Oncology. Colorectal Cancer: Types of Treatment|Cancer.Net. Available online: https://www.cancer.net/cancer-types/colorectal-cancer/types-treatment (accessed on 16 March 2022).
- López-Lázaro, M. Two Preclinical Tests to Evaluate Anticancer Activity and to Help Validate Drug Candidates for Clinical Trials. Oncoscience 2015, 2, 91–98. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Cancer Metabolism: Looking Forward. Nat. Rev. Cancer 2021, 21, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Zhu, J.; Thompson, C.B. The Hallmarks of Cancer Metabolism: Still Emerging. Cell Metab. 2022, 34, 355–377. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M. The Warburg Effect: Why and How Do Cancer Cells Activate Glycolysis in the Presence of Oxygen? Anticancer Agents Med. Chem. 2008, 8, 305–312. [Google Scholar] [CrossRef]
- Tajan, M.; Vousden, K.H. Dietary Approaches to Cancer Therapy. Cancer Cell 2020, 37, 767–785. [Google Scholar] [CrossRef]
- Kanarek, N.; Petrova, B.; Sabatini, D.M. Dietary Modifications for Enhanced Cancer Therapy. Nature 2020, 579, 507–517. [Google Scholar] [CrossRef]
- Snaebjornsson, M.T.; Janaki-Raman, S.; Schulze, A. Greasing the Wheels of the Cancer Machine: The Role of Lipid Metabolism in Cancer. Cell Metab. 2020, 31, 62–76. [Google Scholar] [CrossRef]
- Villa, E.; Ali, E.; Sahu, U.; Ben-Sahra, I. Cancer Cells Tune the Signaling Pathways to Empower de Novo Synthesis of Nucleotides. Cancers 2019, 11, 688. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yeung, S.-C.J.; Liu, S.; Qdaisat, A.; Jiang, D.; Liu, W.; Cheng, Z.; Liu, W.; Wang, H.; Li, L.; et al. Cyst(e)Ine in Nutrition Formulation Promotes Colon Cancer Growth and Chemoresistance by Activating MTORC1 and Scavenging ROS. Signal Transduct. Target. Ther. 2021, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Arensman, M.D.; Yang, X.S.; Leahy, D.M.; Toral-Barza, L.; Mileski, M.; Rosfjord, E.C.; Wang, F.; Deng, S.; Myers, J.S.; Abraham, R.T.; et al. Cystine–Glutamate Antiporter XCT Deficiency Suppresses Tumor Growth While Preserving Antitumor Immunity. Proc. Natl. Acad. Sci. USA 2019, 116, 9533–9542. [Google Scholar] [CrossRef] [PubMed]
- Durando, X.; Farges, M.-C.; Buc, E.; Abrial, C.; Petorin-Lesens, C.; Gillet, B.; Vasson, M.-P.; Pezet, D.; Chollet, P.; Thivat, E. Dietary Methionine Restriction with FOLFOX Regimen as First Line Therapy of Metastatic Colorectal Cancer: A Feasibility Study. Oncology 2010, 78, 205–209. [Google Scholar] [CrossRef]
- Gao, X.; Sanderson, S.M.; Dai, Z.; Reid, M.A.; Cooper, D.E.; Lu, M.; Richie, J.P.; Ciccarella, A.; Calcagnotto, A.; Mikhael, P.G.; et al. Dietary Methionine Influences Therapy in Mouse Cancer Models and Alters Human Metabolism. Nature 2019, 572, 397–401. [Google Scholar] [CrossRef]
- Tan, Y.; Mingxu, X.U.; Guo, H.; Sun, X.; Kubota, T.; Hoffman, R.M. Anticancer Efficacy of Methioninase in Vivo. Anticancer Res. 1996, 16, 3931–3936. [Google Scholar]
- Wallis, K.F.; Morehead, L.C.; Bird, J.T.; Byrum, S.D.; Miousse, I.R. Differences in Cell Death in Methionine versus Cysteine Depletion. Environ. Mol. Mutagen. 2021, 62, 216–226. [Google Scholar] [CrossRef]
- Maddocks, O.D.K.K.; Berkers, C.R.; Mason, S.M.; Zheng, L.; Blyth, K.; Gottlieb, E.; Vousden, K.H. Serine Starvation Induces Stress and P53-Dependent Metabolic Remodelling in Cancer Cells. Nature 2013, 493, 542–546. [Google Scholar] [CrossRef]
- Maddocks, O.D.K.K.; Athineos, D.; Cheung, E.C.; Lee, P.; Zhang, T.; Van Den Broek, N.J.F.F.; Mackay, G.M.; Labuschagne, C.F.; Gay, D.; Kruiswijk, F.; et al. Modulating the Therapeutic Response of Tumours to Dietary Serine and Glycine Starvation. Nature 2017, 544, 372–376. [Google Scholar] [CrossRef]
- Tajan, M.; Hennequart, M.; Cheung, E.C.; Zani, F.; Hock, A.K.; Legrave, N.; Maddocks, O.D.K.; Ridgway, R.A.; Athineos, D.; Suárez-Bonnet, A.; et al. Serine Synthesis Pathway Inhibition Cooperates with Dietary Serine and Glycine Limitation for Cancer Therapy. Nat. Commun. 2021, 12, 366. [Google Scholar] [CrossRef]
- Muthusamy, T.; Cordes, T.; Handzlik, M.K.; You, L.; Lim, E.W.; Gengatharan, J.; Pinto, A.F.M.; Badur, M.G.; Kolar, M.J.; Wallace, M.; et al. Serine Restriction Alters Sphingolipid Diversity to Constrain Tumour Growth. Nature 2020, 586, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Gravel, S.-P.; Hulea, L.; Toban, N.; Birman, E.; Blouin, M.-J.; Zakikhani, M.; Zhao, Y.; Topisirovic, I.; St-Pierre, J.; Pollak, M. Serine Deprivation Enhances Antineoplastic Activity of Biguanides. Cancer Res. 2014, 74, 7521–7533. [Google Scholar] [CrossRef] [PubMed]
- Humpton, T.J.; Hock, A.K.; Maddocks, O.D.K.; Vousden, K.H. P53-Mediated Adaptation to Serine Starvation Is Retained by a Common Tumour-Derived Mutant. Cancer Metab. 2018, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Alexandrou, C.; Al-Aqbi, S.S.; Higgins, J.A.; Boyle, W.; Karmokar, A.; Andreadi, C.; Luo, J.L.; Moore, D.A.; Viskaduraki, M.; Blades, M.; et al. Sensitivity of Colorectal Cancer to Arginine Deprivation Therapy Is Shaped by Differential Expression of Urea Cycle Enzymes. Sci. Rep. 2018, 8, 12096. [Google Scholar] [CrossRef]
- Satoh, Y.; Kotani, H.; Iida, Y.; Taniura, T.; Notsu, Y.; Harada, M. Supplementation of L-Arginine Boosts the Therapeutic Efficacy of Anticancer Chemoimmunotherapy. Cancer Sci. 2020, 111, 2248–2258. [Google Scholar] [CrossRef]
- Yeatman, T.J.; Risley, G.L.; Brunson, M.E. Depletion of Dietary Arginine Inhibits Growth of Metastatic Tumor. Arch. Surg. 1991, 126, 1376–1382. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal Keratinization in a Spontaneously Immortalized Aneuploid Human Keratinocyte Cell Line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M.; López-Lázaro, M. A Simple and Reliable Approach for Assessing Anticancer Activity in vitro. Curr. Med. Chem. 2015, 22, 1324–1334. [Google Scholar] [CrossRef]
- Wang, L.; Hu, X.; Xu, Y.; Liu, Z. Arsenic Trioxide Inhibits Lung Metastasis of Mouse Colon Cancer via Reducing the Infiltration of Regulatory T Cells. Tumor Biol. 2016, 37, 15165–15173. [Google Scholar] [CrossRef]
- Kolinsky, K.; Shen, B.Q.; Zhang, Y.E.; Kohles, J.; Dugan, U.; Zioncheck, T.F.; Heimbrook, D.; Packman, K.; Higgins, B. In vivo Activity of Novel Capecitabine Regimens Alone and with Bevacizumab and Oxaliplatin in Colorectal Cancer Xenograft Models. Mol. Cancer Ther. 2009, 8, 75–82. [Google Scholar] [CrossRef]
- Plaisance, E.P.; Greenway, F.L.; Boudreau, A.; Hill, K.L.; Johnson, W.D.; Krajcik, R.A.; Perrone, C.E.; Orentreich, N.; Cefalu, W.T.; Gettys, T.W. Dietary Methionine Restriction Increases Fat Oxidation in Obese Adults with Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E836–E840. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Stone, K.P.; Forney, L.A.; Sims, L.C.; Gutierrez, G.C.; Ghosh, S.; Gettys, T.W. Implementation of Dietary Methionine Restriction Using Casein after Selective, Oxidative Deletion of Methionine. iScience 2021, 24, 102470. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Berisa, M.; Schwörer, S.; Qin, W.; Cross, J.R.; Thompson, C.B. Transsulfuration Activity Can Support Cell Growth upon Extracellular Cysteine Limitation. Cell Metab. 2019, 30, 865–876.e5. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Batra, S.; Zhang, J. Asparagine: A Metabolite to Be Targeted in Cancers. Metabolites 2021, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Delman, K.A.; Brown, T.D.; Thomas, M.; Ensor, C.M.; Holtsberg, F.W.; Bomalaski, J.S.; Clark, M.A.; Curley, S.A. Phase I/II Trial of Pegylated Arginine Deiminase (ADI-PEG20) in Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2005, 23, 4139. [Google Scholar] [CrossRef]
- Yao, S.; Janku, F.; Subbiah, V.; Stewart, J.; Patel, S.P.; Kaseb, A.; Westin, S.N.; Naing, A.; Tsimberidou, A.M.; Hong, D.; et al. Phase 1 Trial of ADI-PEG20 plus Cisplatin in Patients with Pretreated Metastatic Melanoma or Other Advanced Solid Malignancies. Br. J. Cancer 2021, 124, 1533–1539. [Google Scholar] [CrossRef]
- Cramer, S.L.; Saha, A.; Liu, J.; Tadi, S.; Tiziani, S.; Yan, W.; Triplett, K.; Lamb, C.; Alters, S.E.; Rowlinson, S.; et al. Systemic Depletion of L-Cyst(e)Ine with Cyst(e)Inase Increases Reactive Oxygen Species and Suppresses Tumor Growth. Nat. Med. 2017, 23, 120–127. [Google Scholar] [CrossRef]
- Badgley, M.A.; Kremer, D.M.; Maurer, H.C.; DelGiorno, K.E.; Lee, H.-J.; Purohit, V.; Sagalovskiy, I.R.; Ma, A.; Kapilian, J.; Firl, C.E.M.; et al. Cysteine Depletion Induces Pancreatic Tumor Ferroptosis in Mice. Science 2020, 368, 85–89. [Google Scholar] [CrossRef]
- Saha, A.; Zhao, S.; Chen, Z.; Georgiou, G.; Stone, E.; Kidane, D.; DiGiovanni, J. Combinatorial Approaches to Enhance DNA Damage Following Enzyme-Mediated Depletion of L-Cys for Treatment of Pancreatic Cancer. Mol. Ther. 2021, 29, 775–787. [Google Scholar] [CrossRef]
- Tan, Y.; Zavala, J.; Mingxu, X.U.; Zavala, J.; Hoffman, R.M. Serum Methionine Depletion without Side Effects by Methioninase in Metastatic Breast Cancer Patients. Anticancer Res. 1996, 16, 3937–3942. [Google Scholar]
- Tan, Y.; Zavala, J.; Han, Q.; Xu, M.; Sun, X.; Tan, X.; Tan, X.; Magana, R.; Geller, J.; Hoffman, R.M. Recombinant Methioninase Infusion Reduces the Biochemical Endpoint of Serum Methionine with Minimal Toxicity in High-Stage Cancer Patients. Anticancer Res. 1997, 17, 3857–3860. [Google Scholar] [PubMed]
- Stone, E.; Paley, O.; Hu, J.; Ekerdt, B.; Cheung, N.K.; Georgiou, G. De Novo Engineering of a Human Cystathionine-γ-Lyase for Systemic l-Methionine Depletion Cancer Therapy. ACS Chem. Biol. 2012, 7, 1822–1829. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.C.; Saha, A.; Yan, W.; Garrison, K.; Lamb, C.; Pandey, R.; Irani, S.; Lodi, A.; Lu, X.; Tiziani, S.; et al. Enzyme-Mediated Depletion of Serum L-Met Abrogates Prostate Cancer Growth via Multiple Mechanisms without Evidence of Systemic Toxicity. Proc. Natl. Acad. Sci. USA 2020, 117, 13000–13011. [Google Scholar] [CrossRef]
- Jiang, J.; Srivastava, S.; Zhang, J. Starve Cancer Cells of Glutamine: Break the Spell or Make a Hungry Monster? Cancers 2019, 11, 804. [Google Scholar] [CrossRef]
- Ngo, B.; Kim, E.; Osorio-Vasquez, V.; Doll, S.; Bustraan, S.; Liang, R.J.; Luengo, A.; Davidson, S.M.; Ali, A.; Ferraro, G.B.; et al. Limited Environmental Serine and Glycine Confer Brain Metastasis Sensitivity to PHGDH Inhibition. Cancer Discov. 2020, 10, 1352–1373. [Google Scholar] [CrossRef]
- Ducker, G.S.; Ghergurovich, J.M.; Mainolfi, N.; Suri, V.; Jeong, S.K.; Li, S.H.J.; Friedman, A.; Manfredi, M.G.; Gitai, Z.; Kim, H.; et al. Human SHMT Inhibitors Reveal Defective Glycine Import as a Targetable Metabolic Vulnerability of Diffuse Large B-Cell Lymphoma. Proc. Natl. Acad. Sci. USA 2017, 114, 11404–11409. [Google Scholar] [CrossRef]
- García-Cañaveras, J.C.; Lancho, O.; Ducker, G.S.; Ghergurovich, J.M.; Xu, X.; da Silva-Diz, V.; Minuzzo, S.; Indraccolo, S.; Kim, H.; Herranz, D.; et al. SHMT Inhibition Is Effective and Synergizes with Methotrexate in T-Cell Acute Lymphoblastic Leukemia. Leukemia 2021, 35, 377–388. [Google Scholar] [CrossRef]
- Timmerman, L.A.; Holton, T.; Yuneva, M.; Louie, R.J.; Padró, M.; Daemen, A.; Hu, M.; Chan, D.A.; Ethier, S.P.; van’tVeer, L.J.; et al. Glutamine Sensitivity Analysis Identifies the XCT Antiporter as a Common Triple-Negative Breast Tumor Therapeutic Target. Cancer Cell 2013, 24, 450–465. [Google Scholar] [CrossRef]
- Li, Y.; Yan, H.; Xu, X.; Liu, H.; Wu, C.; Zhao, L. Erastin/Sorafenib Induces Cisplatin-Resistant Non-Small Cell Lung Cancer Cell Ferroptosis through Inhibition of the Nrf2/XCT Pathway. Oncol. Lett. 2020, 19, 323–333. [Google Scholar] [CrossRef]
- Zhang, T.; Bauer, C.; Newman, A.C.; Uribe, A.H.; Athineos, D.; Blyth, K.; Maddocks, O.D.K. Polyamine Pathway Activity Promotes Cysteine Essentiality in Cancer Cells. Nat. Metab. 2020, 2, 1062–1076. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, W.; Wang, K.; Wang, X.; Yin, F.; Li, C.; Wang, C.; Zhao, B.; Zhong, C.; Zhang, J.; et al. Methionine and Cystine Double Deprivation Stress Suppresses Glioma Proliferation via Inducing ROS/Autophagy. Toxicol. Lett. 2015, 232, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-T.T.; Qi, Y.; Wang, Y.-C.C.; Chi, K.K.; Chung, Y.; Ouyang, C.; Chen, Y.-R.Y.-H.Y.H.R.; Oh, M.E.; Sheng, X.; Tang, Y.; et al. Arginine Starvation Kills Tumor Cells through Aspartate Exhaustion and Mitochondrial Dysfunction. Commun. Biol. 2018, 1, 178. [Google Scholar] [CrossRef] [PubMed]
- LeBoeuf, S.E.; Wu, W.L.; Karakousi, T.R.; Karadal, B.; Jackson, S.R.E.; Davidson, S.M.; Wong, K.K.; Koralov, S.B.; Sayin, V.I.; Papagiannakopoulos, T. Activation of Oxidative Stress Response in Cancer Generates a Druggable Dependency on Exogenous Non-Essential Amino Acids. Cell Metab. 2020, 31, 339–350.e4. [Google Scholar] [CrossRef]
- Krall, A.S.; Mullen, P.J.; Surjono, F.; Momcilovic, M.; Schmid, E.W.; Halbrook, C.J.; Thambundit, A.; Mittelman, S.D.; Lyssiotis, C.A.; Shackelford, D.B.; et al. Asparagine Couples Mitochondrial Respiration to ATF4 Activity and Tumor Growth. Cell Metab. 2021, 33, 1013–1026.e6. [Google Scholar] [CrossRef] [PubMed]
- Knott, S.R.V.; Wagenblast, E.; Khan, S.; Kim, S.Y.; Soto, M.; Wagner, M.; Turgeon, M.O.; Fish, L.; Erard, N.; Gable, A.L.; et al. Asparagine Bioavailability Governs Metastasis in a Model of Breast Cancer. Nature 2018, 554, 378–381. [Google Scholar] [CrossRef]
- Niklison-Chirou, M.V.; Erngren, I.; Engskog, M.; Haglöf, J.; Picard, D.; Remke, M.; McPolin, P.H.R.; Selby, M.; Williamson, D.; Clifford, S.C.; et al. TAp73 Is a Marker of Glutamine Addiction in Medulloblastoma. Genes Dev. 2017, 31, 1738–1753. [Google Scholar] [CrossRef]
- Sahu, N.; Dela Cruz, D.; Gao, M.; Sandoval, W.; Haverty, P.M.; Liu, J.; Stephan, J.-P.; Haley, B.; Classon, M.; Hatzivassiliou, G.; et al. Proline Starvation Induces Unresolved ER Stress and Hinders MTORC1-Dependent Tumorigenesis. Cell Metab. 2016, 24, 753–761. [Google Scholar] [CrossRef]
- Xu, Q.; Li, Y.; Gao, X.; Kang, K.; Williams, J.G.; Tong, L.; Liu, J.; Ji, M.; Deterding, L.J.; Tong, X.; et al. HNF4α Regulates Sulfur Amino Acid Metabolism and Confers Sensitivity to Methionine Restriction in Liver Cancer. Nat. Commun. 2020, 11, 3978. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, J.H.; Lee, E.; Jang, Y.J.; Son, J.E.; Kwon, J.Y.; Lim, T.G.; Kim, S.; Park, J.H.; Kim, J.E.; et al. Methionine Deprivation Suppresses Triple-Negative Breast Cancer Metastasis in vitro and in vivo. Oncotarget 2016, 7, 67223. [Google Scholar] [CrossRef]
- Hens, J.R.; Sinha, I.; Perodin, F.; Cooper, T.; Sinha, R.; Plummer, J.; Perrone, C.E.; Orentreich, D. Methionine-Restricted Diet Inhibits Growth of MCF10AT1-Derived Mammary Tumors by Increasing Cell Cycle Inhibitors in Athymic Nude Mice. BMC Cancer 2016, 16, 349. [Google Scholar] [CrossRef]
- Thivat, E.; Farges, M.-C.; Bacin, F.; D’Incan, M.; Mouret-Reynier, M.-A.; Cellarier, E.; Madelmont, J.-C.; Vasson, M.-P.; Chollet, P.; Durando, X. Phase II Trial of the Association of a Methionine-Free Diet with Cystemustine Therapy in Melanoma and Glioma. Anticancer Res. 2009, 29, 5235–5240. [Google Scholar] [PubMed]
- Xiao, F.; Wang, C.; Yin, H.; Yu, J.; Chen, S.; Fang, J.; Guo, F. Leucine Deprivation Inhibits Proliferation and Induces Apoptosis of Human Breast Cancer Cells via Fatty Acid Synthase. Oncotarget 2016, 7, 63679–63689. [Google Scholar] [CrossRef] [PubMed]
- Sheen, J.-H.; Zoncu, R.; Kim, D.; Sabatini, D.M. Defective Regulation of Autophagy upon Leucine Deprivation Reveals a Targetable Liability of Human Melanoma Cells in vitro and in vivo. Cancer Cell 2011, 19, 613–628. [Google Scholar] [CrossRef]
- López-Lázaro, M. Selective Amino Acid Restriction Therapy (SAART): A Non- Pharmacological Strategy against All Types of Cancer Cells. Oncoscience 2015, 2, 857. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Brown, J. Mutations, Evolution and the Central Role of a Self-Defined Fitness Function in the Initiation and Progression of Cancer. Biochim. Biophys. Acta-Rev. Cancer 2017, 1867, 162–166. [Google Scholar] [CrossRef]
- Sullivan, M.R.; Danai, L.V.; Lewis, C.A.; Chan, S.H.; Gui, D.Y.; Kunchok, T.; Dennstedt, E.A.; Vander Heiden, M.G.; Muir, A. Quantification of Microenvironmental Metabolites in Murine Cancers Reveals Determinants of Tumor Nutrient Availability. Elife 2019, 8, e44235. [Google Scholar] [CrossRef]
- Leney-Greene, M.A.; Boddapati, A.K.; Su, H.C.; Cantor, J.R.; Lenardo, M.J. Human Plasma-like Medium Improves T Lymphocyte Activation. iScience 2020, 23, 100759. [Google Scholar] [CrossRef]
- Ruiz-Rodado, V.; Dowdy, T.; Lita, A.; Kramp, T.; Zhang, M.; Jung, J.; Dios-Esponera, A.; Zhang, L.; Herold-Mende, C.C.; Camphausen, K.; et al. Cysteine Is a Limiting Factor for Glioma Proliferation and Survival. Mol. Oncol. 2022, 16, 1777–1794. [Google Scholar] [CrossRef]
- Daher, B.; Vučetić, M.; Pouysségur, J. Cysteine Depletion, a Key Action to Challenge Cancer Cells to Ferroptotic Cell Death. Front. Oncol. 2020, 10, 723. [Google Scholar] [CrossRef]
- López-Lázaro, M. Dual Role of Hydrogen Peroxide in Cancer: Possible Relevance to Cancer Chemoprevention and Therapy. Cancer Lett. 2007, 252, 1–8. [Google Scholar] [CrossRef]
- Atherton, P.J.; Smith, K.; Etheridge, T.; Rankin, D.; Rennie, M.J. Distinct Anabolic Signalling Responses to Amino Acids in C2C12 Skeletal Muscle Cells. Amino Acids 2010, 38, 1533–1539. [Google Scholar] [CrossRef]
- Han, J.M.; Jeong, S.J.; Park, M.C.; Kim, G.; Kwon, N.H.; Kim, H.K.; Ha, S.H.; Ryu, S.H.; Kim, S. Leucyl-TRNA Synthetase Is an Intracellular Leucine Sensor for the MTORC1-Signaling Pathway. Cell 2012, 149, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Osburn, S.C.; Vann, C.G.; Church, D.D.; Ferrando, A.A.; Roberts, M.D. Proteasome- and Calpain-Mediated Proteolysis, but Not Autophagy, Is Required for Leucine-Induced Protein Synthesis in C2C12 Myotubes. Physiologia 2021, 1, 22–33. [Google Scholar] [CrossRef]
- Combaret, L.; Dardevet, D.; Rieu, I.; Pouch, M.-N.; Béchet, D.; Taillandier, D.; Grizard, J.; Attaix, D. A Leucine-Supplemented Diet Restores the Defective Postprandial Inhibition of Proteasome-Dependent Proteolysis in Aged Rat Skeletal Muscle. J. Physiol. 2005, 569, 489–499. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of Leucine and Its Metabolite β-Hydroxy-β-Methylbutyrate on Human Skeletal Muscle Protein Metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef]
- Hodson, N.; Brown, T.; Joanisse, S.; Aguirre, N.; West, D.; Moore, D.; Baar, K.; Breen, L.; Philp, A. Characterisation of L-Type Amino Acid Transporter 1 (LAT1) Expression in Human Skeletal Muscle by Immunofluorescent Microscopy. Nutrients 2017, 10, 23. [Google Scholar] [CrossRef]
- Nichenametla, S.N.; Mattocks, D.A.L.; Malloy, V.L.; Pinto, J.T. Sulfur Amino Acid Restriction-Induced Changes in Redox-Sensitive Proteins Are Associated with Slow Protein Synthesis Rates. Ann. N. Y. Acad. Sci. 2018, 1418, 80–94. [Google Scholar] [CrossRef]
- Kang, J.-S. Dietary Restriction of Amino Acids for Cancer Therapy. Nutr. Metab. 2020, 17, 20. [Google Scholar] [CrossRef]
- Wanders, D.; Hobson, K.; Ji, X. Methionine Restriction and Cancer Biology. Nutrients 2020, 12, 684. [Google Scholar] [CrossRef]
- Newman, A.C.; Maddocks, O.D.K. One-Carbon Metabolism in Cancer. Br. J. Cancer 2017, 116, 1499–1504. [Google Scholar] [CrossRef]

| Amino Acid | M0 | M1 |
|---|---|---|
| L-phenylalanine | 192 | 192 |
| L-histidine | 80 | 80 |
| L-lysine | 240 | 240 |
| L-threonine | 160 | 160 |
| L-isoleucine | 96 | 96 |
| L-valine | 235 | 235 |
| L-leucine | 528 | 528 |
| L-tryptophan | 16 | 16 |
| L-methionine | 48 | 48 |
| L-glutamine | 1000 | 1000 |
| L-arginine | 100 | |
| Glycine | 200 | |
| L-alanine | 20 | |
| L-aspartic acid | 20 | |
| L-serine | 40 | |
| L-tyrosine | 100 | |
| L-cysteine dihydrochloride | 60 | |
| L-asparagine-1-hydrate | 50 | |
| L-glutamic acid | 20 | |
| L-proline | 20 |
| DIET | TC1 | TC2 | TC3 | TC4 | TC5 | TC6 | TC7 | TC8 |
|---|---|---|---|---|---|---|---|---|
| Casein | 6 | 6 | 6 | 6 | ||||
| Glutamine (Gln) | 2 | 2 | 2 | 2 | 5 | 5 | 5 | |
| Leucine (Leu) | 3 | 3 | 0.6 | 3 | 2.5 | 5 | 2.5 | |
| Methionine (Met) | 0.18 | 0.18 | 0.18 | 0.15 | ||||
| Phenylalanine (Phe) | 0.6 | 0.6 | 0.6 | 0.6 | ||||
| Histidine (His) | 0.2 | 0.2 | 0.2 | 0.2 | ||||
| Lysine (Lys) | 0.5 | 0.5 | 0.5 | 0.5 | ||||
| Threonine (Thr) | 0.25 | 0.25 | 0.25 | 0.25 | ||||
| Isoleucine (Iso) | 0.3 | 0.3 | 0.3 | 0.3 | ||||
| Valine (Val) | 0.35 | 0.35 | 0.35 | 0.35 | ||||
| Tryptophan (Trp) | 0.07 | 0.07 | 0.07 | 0.07 | ||||
| Cystine (CySS) | 0.2 | 0.2 | 0.2 | 0.2 | ||||
| Arginine (Arg) | ||||||||
| Glycine (Gly) | ||||||||
| Serine (Ser) | ||||||||
| Tyrosine (Tyr) | ||||||||
| Alanine (Ala) | ||||||||
| Aspartate (Asp) | ||||||||
| Proline (Pro) | ||||||||
| Asparagine (Asn) | ||||||||
| Glutamate (Glu) | ||||||||
| Salmon oil | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Choline | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Tert-butylhydroquinone | 0.0008 | 0.0008 | 0.0008 | 0.0008 | 0.0008 | 0.0008 | 0.0008 | 0.0008 |
| Vitamin Mix | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Mineral Mix | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| Sucrose | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| Cellulose | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Corn starch | 66.80 | 66.60 | 69.00 | 66.63 | 60.75 | 68.25 | 58.25 | 60.75 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Alonso, J.J.; Guillén-Mancina, E.; Calderón-Montaño, J.M.; Jiménez-González, V.; Díaz-Ortega, P.; Burgos-Morón, E.; López-Lázaro, M. Artificial Diets Based on Selective Amino Acid Restriction versus Capecitabine in Mice with Metastatic Colon Cancer. Nutrients 2022, 14, 3378. https://doi.org/10.3390/nu14163378
Jiménez-Alonso JJ, Guillén-Mancina E, Calderón-Montaño JM, Jiménez-González V, Díaz-Ortega P, Burgos-Morón E, López-Lázaro M. Artificial Diets Based on Selective Amino Acid Restriction versus Capecitabine in Mice with Metastatic Colon Cancer. Nutrients. 2022; 14(16):3378. https://doi.org/10.3390/nu14163378
Chicago/Turabian StyleJiménez-Alonso, Julio José, Emilio Guillén-Mancina, José Manuel Calderón-Montaño, Víctor Jiménez-González, Patricia Díaz-Ortega, Estefanía Burgos-Morón, and Miguel López-Lázaro. 2022. "Artificial Diets Based on Selective Amino Acid Restriction versus Capecitabine in Mice with Metastatic Colon Cancer" Nutrients 14, no. 16: 3378. https://doi.org/10.3390/nu14163378
APA StyleJiménez-Alonso, J. J., Guillén-Mancina, E., Calderón-Montaño, J. M., Jiménez-González, V., Díaz-Ortega, P., Burgos-Morón, E., & López-Lázaro, M. (2022). Artificial Diets Based on Selective Amino Acid Restriction versus Capecitabine in Mice with Metastatic Colon Cancer. Nutrients, 14(16), 3378. https://doi.org/10.3390/nu14163378






