Home Parenteral Nutrition in Patients with Advanced Cancer: Quality Outcomes from a Centralized Model of Care Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Statistical Analysis
3. Results
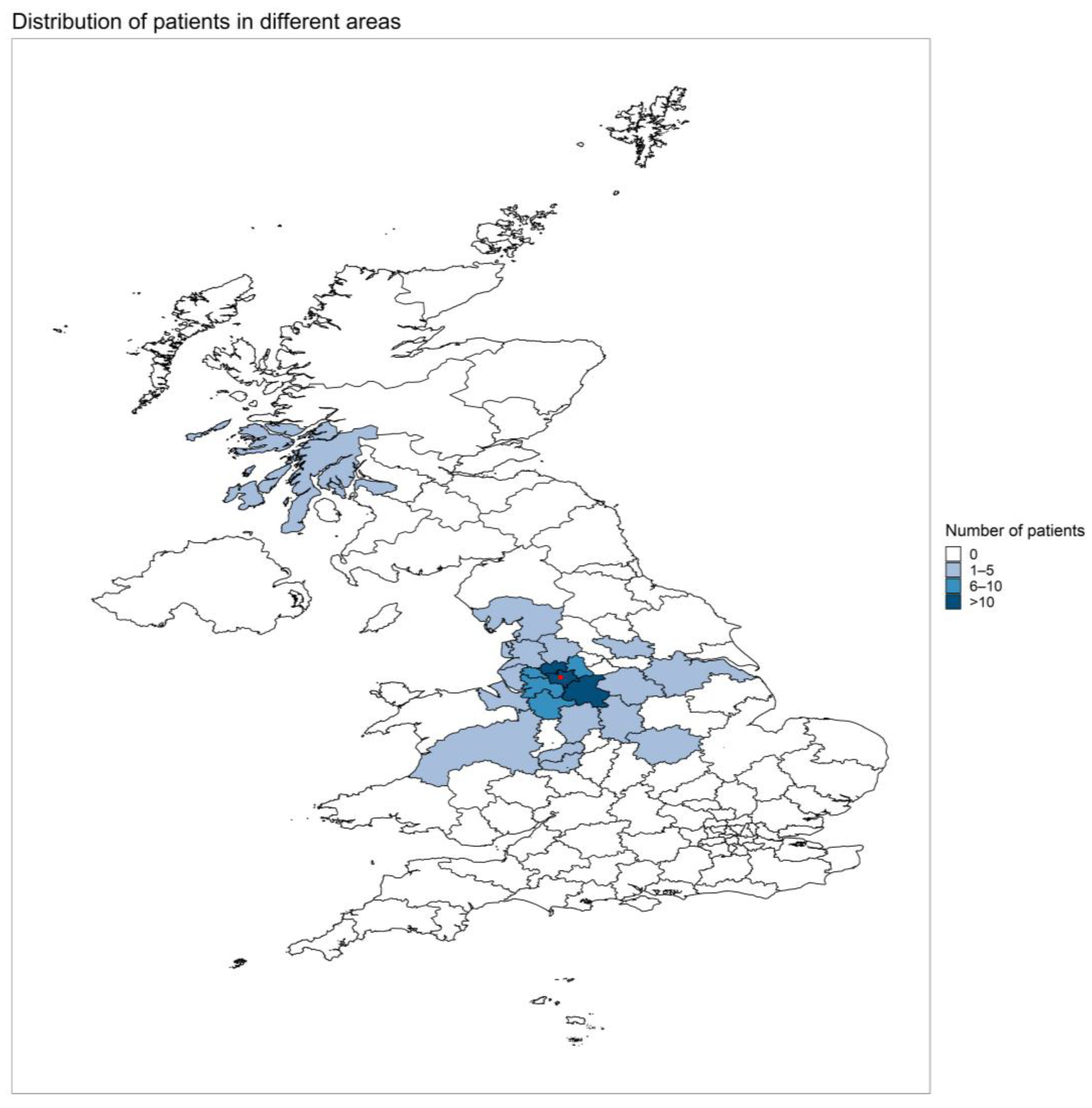
3.1. Patient Demographics and Clinical Characteristics
3.2. Changes over the Study Period
3.3. Catheter Characteristics
3.4. HPN Complications
3.5. Readmission to Hospital
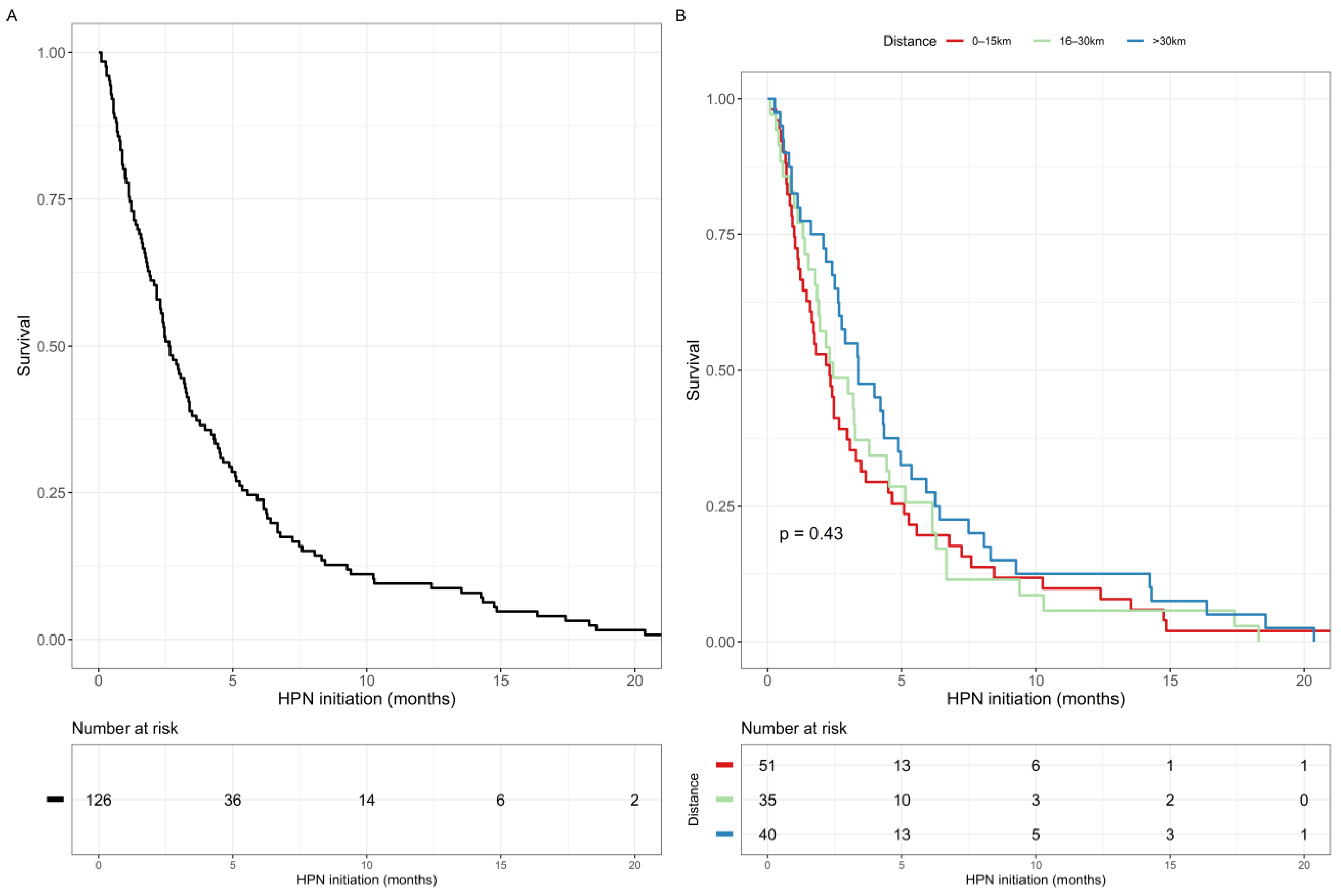
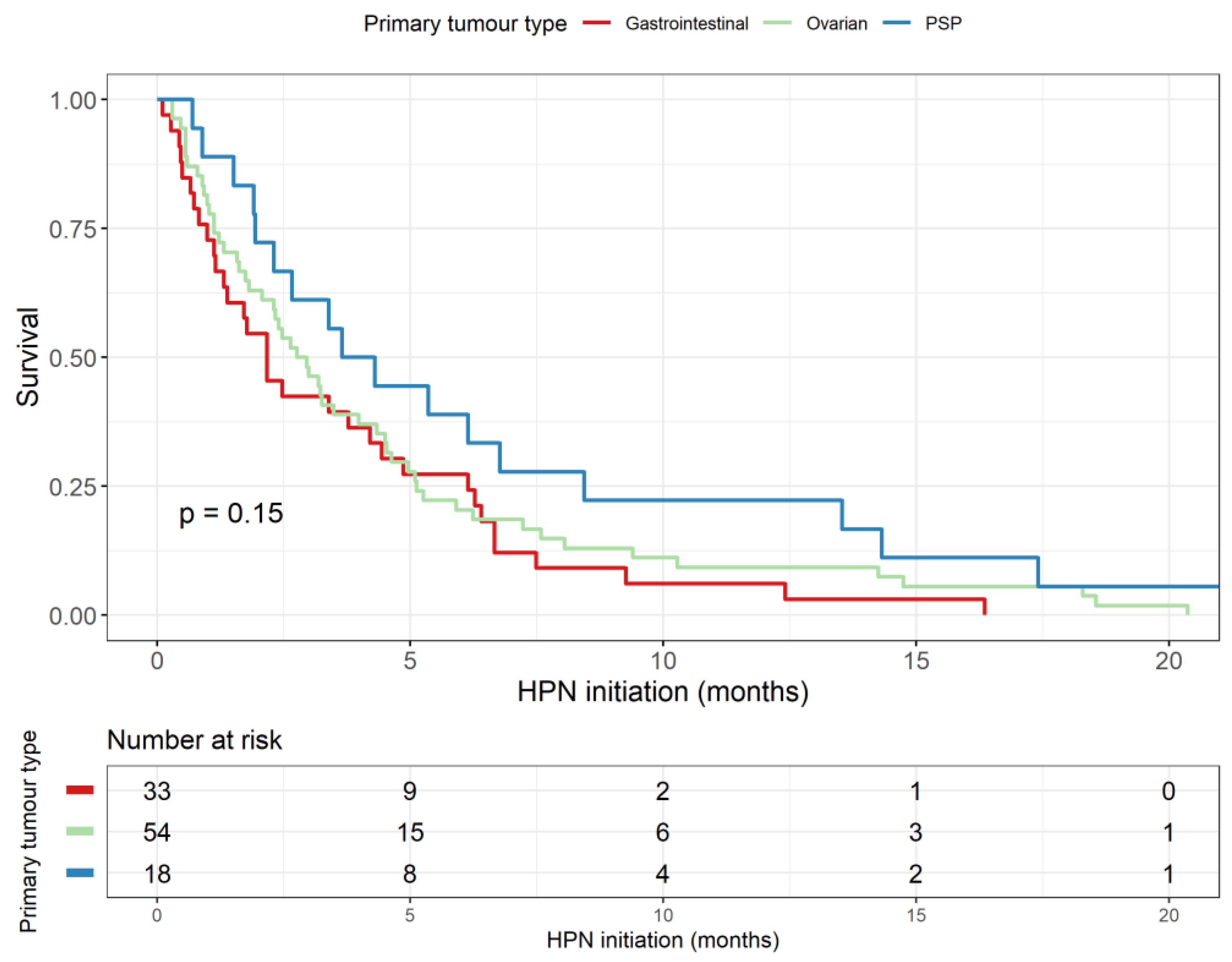
3.6. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Vashi, P.G.; Dahlk, S.; Popiel, B.; Lammersfeld, C.A.; Ireton-Jones, C.; Gupta, D. A longitudinal study investigating quality of life and nutritional outcomes in advanced cancer patients receiving home parenteral nutrition. BMC Cancer 2014, 14, 593. [Google Scholar] [CrossRef] [PubMed]
- Madhok, B.M.; Yeluri, S.; Haigh, K.; Burton, A.; Broadhead, T.; Jayne, D.G. Parenteral nutrition for patients with advanced ovarian malignancy. J. Hum. Nutr. Diet. 2011, 24, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Pelzer, U.; Arnold, D.; Gövercin, M.; Stieler, J.; Doerken, B.; Riess, H.; Oettle, H. Parenteral nutrition support for patients with pancreatic cancer. Results of a phase II study. BMC Cancer 2010, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Keane, N.; Fragkos, K.C.; Patel, P.S.; Bertsch, F.; Mehta, S.J.; Di Caro, S.; Rahman, F. Performance Status, Prognostic Scoring, and Parenteral Nutrition Requirements Predict Survival in Patients with Advanced Cancer Receiving Home Parenteral Nutrition. Nutr. Cancer 2018, 70, 73–82. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlon, F.J.; Fragkos, K.C.; Fini, L.; Patel, P.S.; Mehta, S.J.; Rahman, F.; Di Caro, S. Home Parenteral Nutrition in Patients with Advanced Cancer: A Systematic Review and Meta-Analysis. Nutr. Cancer 2020, 73, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Sowerbutts, A.M.; Lal, S.; Sremanakova, J.; Clamp, A.; Todd, C.; Jayson, G.C.; Teubner, A.; Raftery, A.-M.; Sutton, E.J.; Hardy, L.; et al. Home parenteral nutrition for people with inoperable malignant bowel obstruction. Cochrane Database Syst. Rev. 2018, 8, CD012812. [Google Scholar] [CrossRef] [PubMed]
- BANS Report. Artificial Nutrition Support in the UK 2005–2015. Adult Home Parenteral Nutrition & Home Intravenous Fluids. Available online: https://www.bapen.org.uk/ (accessed on 10 March 2022).
- Bozzetti, F.; Santarpia, L.; Pironi, L.; Thul, P.; Klek, S.; Gavazzi, C.; Tinivella, M.; Joly, F.; Jonkers, C.; Baxter, J.; et al. The prognosis of incurable cachectic cancer patients on home parenteral nutrition: A multi-centre observational study with prospective follow-up of 414 patients. Ann. Oncol. 2014, 25, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Kratzing, C. Parenteral nutrition in oncology: A service review. Clin. Nutr. ESPEN 2015, 10, e206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naghibi, M.; Skinner, C.; Burden, S.T.; Bozzetti, F.; Cuerda, C.; Joly, F.; Jeppesen, P.; Lamprecht, G.; Mundi, M.; Szczepanek, K.; et al. A multi-national survey of experience and attitudes towards commencing home parenteral nutrition for patients with advanced cancer. Clin. Nutr. ESPEN 2022, 47, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Steiger, E.; Joly, F.; Wanten, G.J.A.; Chambrier, C.; Aimasso, U.; Sasdelli, A.S.; Szczepanek, K.; Jukes, A.; Theilla, M.; et al. Intravenous supplementation type and volume are associated with 1-year outcome and major complications in patients with chronic intestinal failure. Gut 2020, 69, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Fuglsang, K.A.; Brandt, C.F.; Scheike, T.; Jeppesen, P.B. Hospitalizations in Patients with Nonmalignant Short-Bowel Syndrome Receiving Home Parenteral Support. Nutr. Clin. Pract. 2020, 35, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.; Teubner, A.; Taylor, M.; Willbraham, L.; Gillespie, L.; Farrer, K.; McMahon, M.; Leahy, G.; Abraham, A.; Soop, M.; et al. A novel discharge pathway for patients with advanced cancer requiring home parenteral nutrition. J. Hum. Nutr. Diet. 2019, 32, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Dibb, M.; Lal, S. Home Parenteral Nutrition: Vascular Access and Related Complications. Nutr. Clin. Pract. 2017, 32, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.; Teubner, A.; Taylor, M.; Cawley, C.; Varden, J.; Abraham, A.; Chadwick, P.R.; Soop, M.; Carlson, G.L.; Lal, S. Catheter-related infections in patients with acute type II intestinal failure admitted to a national centre: Incidence and outcomes. Clin. Nutr. 2019, 38, 1828–1832. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, C.I.; Easson, A.M.; Gerdes, H. Management of malignant bowel obstruction. Eur. J. Cancer 2008, 44, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Arends, J.; Bozzetti, F.; Cuerda, C.; Gillanders, L.; Jeppesen, P.B.; Joly, F.; Kelly, D.; Lal, S.; Staun, M.; et al. ESPEN guidelines on chronic intestinal failure in adults. Clin. Nutr. 2016, 35, 247–307. [Google Scholar] [CrossRef] [PubMed]
- Mundi, M.S.; Mercer, D.F.; Iyer, K.; Pfeffer, D.; Zimmermann, L.B.; Berner-Hansen, M.; Bishop, J.; Seidner, D.L. Characteristics of chronic intestinal failure in the USA based on analysis of claims data. JPEN J. Parenter Enteral Nutr. 2022. [Google Scholar] [CrossRef] [PubMed]
- Brandt, C.F.; Hvistendahl, M.; Naimi, R.M.; Tribler, S.; Staun, M.; Brøbech, P.; Jeppesen, P.B. Home Parenteral Nutrition in Adult Patients with Chronic Intestinal Failure: The Evolution Over 4 Decades in a Tertiary Referral Center. JPEN J. Parenter Enteral Nutr. 2017, 41, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | N = 126 1 |
|---|---|
| Age | 58 (49–69) |
| Sex | |
| Female | 94 (75%) |
| Male | 32 (25%) |
| Indication for HPN | |
| Bowel obstruction | 117 (93%) |
| High output stoma | 6 (4.8%) |
| Malnutrition | 1 (0.8%) |
| Dysmotility | 1 (0.8%) |
| Short bowel syndrome | 1 (0.8%) |
| Malignancy | |
| Ovarian | 54 (43%) |
| Pseudomyxoma peritonei | 18 (14%) |
| Colorectal | 14 (11%) |
| Gastric | 10 (7.9%) |
| Small bowel adenocarcinoma | 6 (4.8%) |
| Appendiceal | 4 (3.2%) |
| Lymphoma | 4 (3.2%) |
| Bladder | 3 (2.4%) |
| Oesophageal | 3 (2.4%) |
| Breast | 2 (1.6%) |
| Endometrial | 2 (1.6%) |
| Pancreatic | 2 (1.6%) |
| Unknown primary | 2 (1.6%) |
| Fallopian tube | 1 (0.8%) |
| Lung | 1 (0.8%) |
| Body mass index (kg/m2) | 22.9 (20.3, 26.1) |
| Venting gastrostomy present | 60 (48%) |
| Number of HPN nights per week | |
| 5 | 2 (1.7%) |
| 6 | 1 (0.9%) |
| 7 | 114 (97%) |
| (Missing) | 9 |
| Average daily kcal from HPN (kcal) | 1711 (255) |
| Average daily volume from HPN (mL) | 2172 (504) |
| HPN Catheter Characteristics | N = 126 1 |
|---|---|
| CVC care | |
| Homecare nurse | 114 (90%) |
| Relative | 10 (7.9%) |
| Self | 2 (1.6%) |
| CVC type | |
| PICC | 36 (29%) |
| Tunnelled | 90 (71%) |
| Lumen | |
| Single | 61 (48%) |
| Double | 65 (52%) |
| Concomitant SACT | 35 (28%) |
| SACT via a distinct lumen of the HPN CVC | 13 (10%) |
| Variable | Rate Ratio (95% CI, p Value) | |
|---|---|---|
| Age at HPN initiation | 0.956 (0.888 to 1.024, p = 0.204) | |
| BMI at HPN initiation | 1.127 (0.925 to 1.349, p = 0.203) | |
| CVC lumen | Single | Reference |
| Double | 1.326 (0.176 to 12.796, p = 0.780) | |
| SACT via HPN CVC | No | Reference |
| Yes | 2.775 (0.170 to 20.985, p = 0.359) | |
| Venting gastrostomy present | No | Reference |
| Yes | 0.696 (0.072 to 5.273, p = 0.721) | |
| Distance from patient home to IF centre | 0–15 km | Reference |
| 16–30 km | 0.264 (0.002 to 2.943, p = 0.378) | |
| >30 km | 0.535 (0.041 to 3.873, p = 0.555) | |
| Variable | Rate Ratio (95% CI, p Value) | |
|---|---|---|
| Age at HPN initiation | 1.005 (0.971 to 1.041, p = 0.795) | |
| Sex | Female | Reference |
| Male | 0.611 (0.342 to 1.018, p = 0.074) | |
| CVC type | PICC | Reference |
| Tunnelled | 0.489 (0.207 to 1.198, p = 0.107) | |
| CVC lumen | Single | Reference |
| Double | 1.326 (0.176 to 12.796, p = 0.616) | |
| SACT via HPN CVC | No | Reference |
| Yes | 0.408 (0.028 to 1.86, p = 0.361) | |
| Venting gastrostomy present | No | Reference |
| Yes | 1.193 (0.496 to 2.94, p = 0.694) | |
| Distance from patient home to IF centre | 0–15 km | Reference |
| 16–30 km | 1.658 (0.682 to 4.105, p = 0.263) | |
| >30 km | 0.256 (0.039 to 0.984, p = 0.081) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopczynska, M.; Teubner, A.; Abraham, A.; Taylor, M.; Bond, A.; Clamp, A.; Wight, R.; Salih, Z.; Hasan, J.; Mitchell, C.; et al. Home Parenteral Nutrition in Patients with Advanced Cancer: Quality Outcomes from a Centralized Model of Care Delivery. Nutrients 2022, 14, 3379. https://doi.org/10.3390/nu14163379
Kopczynska M, Teubner A, Abraham A, Taylor M, Bond A, Clamp A, Wight R, Salih Z, Hasan J, Mitchell C, et al. Home Parenteral Nutrition in Patients with Advanced Cancer: Quality Outcomes from a Centralized Model of Care Delivery. Nutrients. 2022; 14(16):3379. https://doi.org/10.3390/nu14163379
Chicago/Turabian StyleKopczynska, Maja, Antje Teubner, Arun Abraham, Michael Taylor, Ashley Bond, Andrew Clamp, Rebecca Wight, Zena Salih, Jurjees Hasan, Claire Mitchell, and et al. 2022. "Home Parenteral Nutrition in Patients with Advanced Cancer: Quality Outcomes from a Centralized Model of Care Delivery" Nutrients 14, no. 16: 3379. https://doi.org/10.3390/nu14163379
APA StyleKopczynska, M., Teubner, A., Abraham, A., Taylor, M., Bond, A., Clamp, A., Wight, R., Salih, Z., Hasan, J., Mitchell, C., Jayson, G. C., & Lal, S. (2022). Home Parenteral Nutrition in Patients with Advanced Cancer: Quality Outcomes from a Centralized Model of Care Delivery. Nutrients, 14(16), 3379. https://doi.org/10.3390/nu14163379






