Usefulness of Muscle Ultrasonography in the Nutritional Assessment of Adult Patients with Cystic Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Anthropometric and Body Composition Parameters
2.2. Muscle Ultrasonography of the Quadriceps Rectus Femoris (QRF)
2.3. Assessment of Nutritional Status
2.4. Assessment of Respiratory Status
2.5. Statistical Analysis
2.6. Ethics
3. Results
3.1. Body Composition and Other Anthropometric Measurements
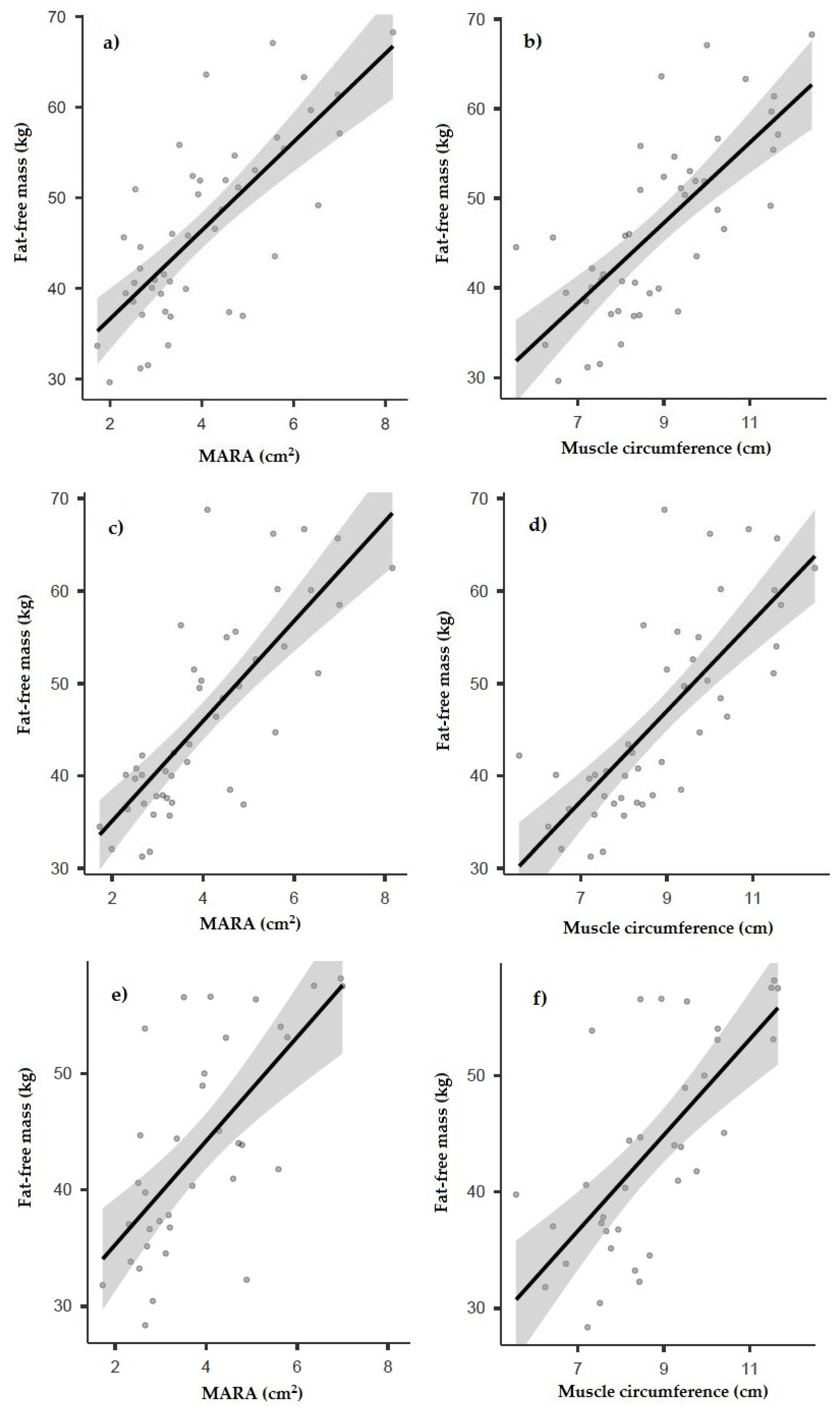
3.2. Correlation between Ultrasound and Other Anthropometric and Body Composition Measurements
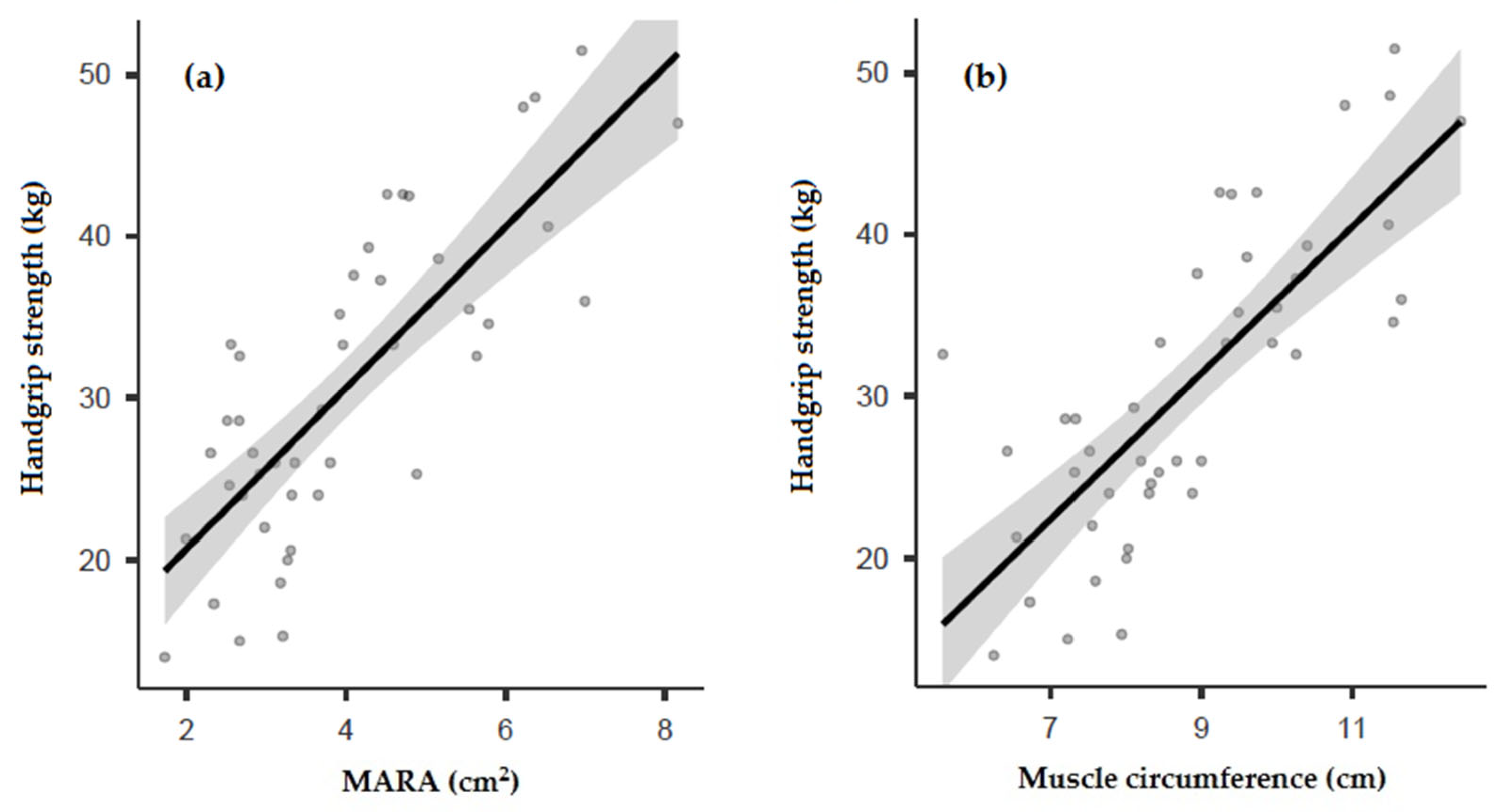
3.3. Correlation between Ultrasound and Muscle Strength
3.4. Respiratory Variables
3.5. Nutritional Status
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Culhane, S.; George, C.; Pearo, B.; Spoede, E. Malnutrition in Cystic Fibrosis: A review. Nutr. Clin. Pract. 2013, 28, 676–683. [Google Scholar] [CrossRef]
- Contreras-Bolívar, V.; Olveira, C.; Porras, N.; García-Olivares, M.; Girón, M.V.; Sánchez-Torralvo, F.J.; Ruiz-García, I.; Alonso-Gallardo, S.P.; Olveira, G.F. Assessment of body composition in cystic fibrosis: Agreement between skinfold measurement and densitometry. Nutr. Hosp. 2022, 39, 376–382. [Google Scholar] [CrossRef]
- Castellani, C.; Duff, A.J.; Bell, S.C.; Heijerman, H.G.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS best practice guidelines: The 2018 revision. J. Cyst. Fibros. 2018, 17, 153–178. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Braegger, C.P.; Colombo, C.; Declercq, D.; Morton, A.; Pancheva, R.; Robberecht, E.; Stern, M.; Strandvik, B.; Wolfe, S.; et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin. Nutr. 2016, 35, 557–577. [Google Scholar] [CrossRef]
- Contreras-Bolívar, V.; Olveira, C.; Blasco Alonso, J.; Olveira, G. Actualización en nutrición en la fibrosis quística. Nutr. Clínica Med. 2019, 13, 19–44. [Google Scholar]
- Gomes, A.; Hutcheon, D.; Ziegler, J. Association Between Fat-Free Mass and Pulmonary Function in Patients with Cystic Fibrosis: A Narrative Review. Nutr. Clin. Pract. 2019, 34, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Calella, P.; Valerio, G.; Thomas, M.; McCabe, H.; Taylor, J.; Brodlie, M.; Siervo, M. Association between body composition and pulmonary function in children and young people with cystic fibrosis. Nutrition 2018, 48, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Kerem, E.; Conway, S.; Elborn, S.; Heijerman, H. Standards of care for patients with cystic fibrosis: A European consensus. J. Cyst. Fibros. 2005, 4, 7–26. [Google Scholar] [CrossRef]
- Thornton, C.S. Body Mass Index and Clinical Outcomes in Persons Living with Cystic Fibrosis—Is Bigger Always Better? JAMA Netw. Open 2022, 5, e220749. [Google Scholar] [CrossRef] [PubMed]
- Olveira, G.; Olveira, C.; Gaspar, I.; Porras, N.; Martín-Núñez, G.; Rubio, E.; Colomo, N.; Rojo-Martínez, G.; Soriguer, F. Fat-Free Mass Depletion and Inflammation in Patients with Bronchiectasis. J. Acad. Nutr. Diet. 2012, 112, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.A.; Nixon, L.S.; Luzio, S.; Lewis-Jenkins, V.; Evans, W.D.; Stone, M.D.; Owens, D.R.; Routledge, P.A.; Shale, D.J. Pulmonary Function, Body Composition, and Protein Catabolism in Adults with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2002, 165, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Chomtho, S.; Fewtrell, M.S.; Jaffe, A.; Williams, J.E.; Wells, J.C.K. Evaluation of Arm Anthropometry for Assessing Pediatric Body Composition: Evidence from Healthy and Sick Children. Pediatr. Res. 2006, 59, 860–865. [Google Scholar] [CrossRef] [PubMed]
- De Meer, K.; Gulmans, V.A.M.; Westerterp, K.R.; Houwen, R.H.J.; Berger, R. Skinfold measurements in children with cystic fibrosis: Monitoring fat-free mass and exercise effects. Eur. J. Pediatr. 1999, 158, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Calella, P.; Valerio, G.; Brodlie, M.; Donini, L.M.; Siervo, M. Cystic fibrosis, body composition, and health outcomes: A systematic review. Nutrition 2018, 55–56, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Schols, A.M.W.J.; Wouters, E.F.; Soeters, P.B.; Westerterp, K.R. Body composition by bioelectrical-impedance analysis compared with deuterium dilution and skinfold anthropometry in patients with chronic obstructive pulmonary disease. Am. J. Clin. Nutr. 1991, 53, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, A.; Scalzo, G.; Masala, S.A.; Tarantino, U.; Guglielmi, G. Body composition assessment by dual-energy X-ray absorptiometry (DXA). Radiol. Med. 2009, 114, 286–300. [Google Scholar] [CrossRef]
- Calella, P.; Valerio, G.; Brodlie, M.; Taylor, J.; Donini, L.M.; Siervo, M. Tools and Methods Used for the Assessment of Body Composition in Patients with Cystic Fibrosis: A Systematic Review. Nutr. Clin. Pract. 2019, 34, 701–714. [Google Scholar] [CrossRef]
- Contreras-Bolívar, V.; Olveira, C.; Porras, N.; Abuín-Fernández, J.; García-Olivares, M.; Sánchez-Torralvo, F.; Girón, M.; Ruiz-García, I.; Olveira, G. Oral Nutritional Supplements in Adults with Cystic Fibrosis: Effects on Intake, Levels of Fat-Soluble Vitamins, and Bone Remodeling Biomarkers. Nutrients 2021, 13, 669. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Barazzoni, R.; Jensen, G.L.; Correia, M.I.T.; Gonzalez, M.C.; Higashiguchi, T.; Shi, H.P.; Bischoff, S.C.; Boirie, Y.; Carrasco, F.; Cruz-Jentoft, A.; et al. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition (GLIM) diagnosis of malnutrition. Clin. Nutr. 2022, 41, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.; Saarinen-Pihkala, U. Evaluation of muscle protein mass in children with solid tumors by muscle thickness measurement with ultrasonography, as compared with anthropometric methods and visceral protein concentrations. Eur. J. Clin. Nutr. 1998, 52, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, U.; Özdemir, M.; Aygencel, G.; Kaya, B.; Türkoğlu, M. The role of maximum compressed thickness of the quadriceps femoris muscle measured by ultrasonography in assessing nutritional risk in critically-ill patients with different volume statuses. Rev. Assoc. Med. Bras. 2019, 65, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, N.; Okawa, N.; Hino, T.; Tsuji, R.; Tamura, K.; Moriyama, H. Higher malnutrition risk is related to increased intramuscular adipose tissue of the quadriceps in older inpatients: A cross-sectional study. Clin. Nutr. 2019, 39, 2586–2592. [Google Scholar] [CrossRef]
- Mueller, N.; Murthy, S.; Tainter, C.; Lee, J.; Riddell, K.; Fintelmann, F.J.; Grabitz, S.D.; Timm, F.P.; Levi, B.; Kurth, T.; et al. Can Sarcopenia Quantified by Ultrasound of the Rectus Femoris Muscle Predict Adverse Outcome of Surgical Intensive Care Unit Patients as well as Frailty? A Prospective, Observational Cohort Study. Ann. Surg. 2016, 264, 1116–1124. [Google Scholar] [CrossRef]
- de Souza, R.P.; Donadio, M.V.F.; Heinzmann-Filho, J.P.; Baptista, R.R.; Pinto, L.A.; Epifanio, M.; Marostica, P.J.C. The use of ultrasonography to evaluate muscle thickness and subcutaneous fat in children and adolescents with cystic fibrosis. Rev. Paul Pediatr. 2018, 36, 457–465. [Google Scholar] [CrossRef]
- Hernández-Socorro, C.R.; Saavedra, P.; López-Fernández, J.C.; Ruiz-Santana, S. Assessment of Muscle Wasting in Long-Stay ICU Patients Using a New Ultrasound Protocol. Nutrients 2018, 10, 1849. [Google Scholar] [CrossRef]
- Siri, W.E. Body composition from fluid spaces and density: Analysis of methods. 1961. Nutrition 1993, 9, 480–491; discussion 480, 492. [Google Scholar]
- Durnin, J.V.; Womersley, J. Body fat assessed from total body density and its estimation from skinfold thickness: Measurements on 481 men and women aged from 16 to 72 years. Br. J. Nutr. 1974, 32, 77–97. [Google Scholar] [CrossRef] [PubMed]
- García-Almeida, J.M.; García-García, C.; Vegas-Aguilar, I.M.; Pomar, M.D.B.; Cornejo-Pareja, I.M.; Medina, B.F.; Román, D.A.D.L.; Guerrero, D.B.; Lesmes, I.B.; Madueño, F.J.T. Nutritional ultrasound®: Conceptualisation, technical considerations and standardisation. Endocrinol. Diabetes Nutr. 2022. [CrossRef]
- Douglas, E.; McMillan, D.C. Towards a simple objective framework for the investigation and treatment of cancer cachexia: The Glasgow Prognostic Score. Cancer Treat. Rev. 2014, 40, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Máiz, L.; Baranda, F.; Coll, R.; Prados, C.; Vendrell, M.; Escribano, A.; Gartner, S.; de Gracia, S.; Martínez, M.; Salcedo, A.; et al. Normativa del diagnóstico y el tratamiento de la afección respiratoria en la fibrosis quística. Arch. Bronconeumol. 2001, 37, 316–324. [Google Scholar] [CrossRef]
- Martínez-García, M.A.; Perpiñá-Tordera, M.; Román-Sánchez, P.; Soler-Cataluña, J.J. Quality-of-Life Determinants in Patients with Clinically Stable Bronchiectasis. Chest 2005, 128, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.; Brunzell, C.; Cohen, R.C.; Katz, M.; Marshall, B.C.; Onady, G.; Robinson, K.A.; Sabadosa, K.A.; Stecenko, A.; Slovis, B.; et al. Clinical Care Guidelines for Cystic Fibrosis–Related Diabetes: A position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care 2010, 33, 2697–2708. [Google Scholar] [CrossRef]
- López-Gómez, J.J.; Plaar, K.B.-S.; Izaola-Jauregui, O.; Primo-Martín, D.; Gómez-Hoyos, E.; Torres-Torres, B.; De Luis-Román, D.A. Muscular Ultrasonography in Morphofunctional Assessment of Patients with Oncological Pathology at Risk of Malnutrition. Nutrients 2022, 14, 1573. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Kyle, U.G.; Kondrup, J. Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: Phase Angle and Impedance Ratio. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 330–339. [Google Scholar] [CrossRef]
- Alicandro, G.; Battezzati, A.; Bianchi, M.L.; Loi, S.; Speziali, C.; Bisogno, A.; Colombo, C. Estimating body composition from skinfold thicknesses and bioelectrical impedance analysis in cystic fibrosis patients. J. Cyst. Fibros. 2015, 14, 784–791. [Google Scholar] [CrossRef][Green Version]
- King, S.; Wilson, J.; Kotsimbos, T.; Bailey, M.; Nyulasi, I. Body composition assessment in adults with cystic fibrosis: Comparison of dual-energy X-ray absorptiometry with skinfolds and bioelectrical impedance analysis. Nutrition 2005, 21, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Doña, E.; Olveira, C.; Palenque, F.J.; Porras, N.; Dorado, A.; Martín-Valero, R.; Godoy, A.M.; Espíldora, F.; Contreras, V.; Olveira, G. Body Composition Measurement in Bronchiectasis: Comparison between Bioelectrical Impedance Analysis, Skinfold Thickness Measurement, and Dual-Energy X-ray Absorptiometry before and after Pulmonary Rehabilitation. J. Acad. Nutr. Diet. 2018, 118, 1464–1473. [Google Scholar] [CrossRef]
- Papalexopoulou, N.; Dassios, T.G.; Lunt, A.; Bartlett, F.; Perrin, F.; Bossley, C.J.; Wyatt, H.A.; Greenough, A. Nutritional status and pulmonary outcome in children and young people with cystic fibrosis. Respir. Med. 2018, 142, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Bolívar, V.; Sánchez-Torralvo, F.J.; Ruiz-Vico, M.; González-Almendros, I.; Barrios, M.; Padín, S.; Alba, E.; Olveira, G. GLIM Criteria Using Hand Grip Strength Adequately Predict Six-Month Mortality in Cancer Inpatients. Nutrients 2019, 11, 2043. [Google Scholar] [CrossRef]
- Sánchez Torralvo, F.J.; Porras, N.; Abuín Fernández, J.; García Torres, F.; Tapia, M.J.; Lima, F.; Soriguer, F.; Gonzalo, M.; Martínez, G.R.; Olveira, G. Normative reference values for hand grip dynamometry in Spain. Association with lean mass. Nutr. Hosp. 2018, 35, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Bolívar, V.; Olveira, C.; Ruiz-García, I.; Porras, N.; García-Olivares, M.; Sánchez-Torralvo, F.J.; Girón, M.V.; Alonso-Gallardo, S.P.; Olveira, G. Handgrip Strength: Associations with Clinical Variables, Body Composition, and Bone Mineral Density in Adults with Cystic Fibrosis. Nutrients 2021, 13, 4107. [Google Scholar] [CrossRef] [PubMed]
- Bouma, S.F.; Iwanicki, C.; McCaffery, H.; Nasr, S.Z. The Association of Grip Strength, Body Mass Index, and Lung Function in Youth with Cystic Fibrosis. Nutr. Clin. Pract. 2020, 35, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Rovedder, P.M.E.; Borba, G.C.; Anderle, M.; Flores, J.; Ziegler, B.; Barreto, S.S.M.; Dalcin, P.D.T.R. Peripheral muscle strength is associated with lung function and functional capacity in patients with cystic fibrosis. Physiother. Res. Int. 2019, 24, e1771. [Google Scholar] [CrossRef]
- Hauschild, D.B.; Barbosa, E.; Moreira, E.A.M.; Neto, N.L.; Platt, V.B.; Filho, E.P.; Wazlawik, E.; Moreno, Y.M.F. Nutrition Status Parameters and Hydration Status by Bioelectrical Impedance Vector Analysis Were Associated with Lung Function Impairment in Children and Adolescents with Cystic Fibrosis. Nutr. Clin. Pract. 2016, 31, 378–386. [Google Scholar] [CrossRef]

| Overall | Normo-Nourished | Malnourished | p-Value | ||
|---|---|---|---|---|---|
| n = 48 | n = 23 | n = 25 | |||
| Age (years) | m ± SD | 34.1 ± 8.8 | 35 ± 8.8 | 32.9 ± 8.9 | 0.43 |
| Gender | n (%) | ||||
| Men | 24 (50) | 15 (62.2) | 9 (36) | ||
| Women | 24 (50) | 8 (34.8) | 16 (64) | 0.02 | |
| Mutation | n (%) | ||||
| Homozygous for ΔF508 | 11 (22.9) | 6 (26.1) | 5 (20) | ||
| Heterozygous for ΔF508 | 24 (50) | 9 (39.1) | 15 (60) | ||
| Negative for ΔF508 | 13 (27.1) | 8 (34.8) | 5 (20) | 0.26 | |
| Cystic fibrosis-related diabetes [34] | n (%) | 25 (52.1) | 11 (47.8) | 14 (56) | 0.68 |
| Pancreatic insufficiency | n (%) | 37 (77.1) | 17 (73.9) | 20 (80) | 0.76 |
| Bronchorrhea (mL) | m ± SD | 22 ± 22.5 | 20.5 ± 24.1 | 22.3 ± 20.3 | 0.81 |
| Total exacerbations | m ± SD | 0.63 ± 0.93 | 0.73 ± 1.03 | 0.54 ± 0.83 | 0.51 |
| Severe exacerbations | m ± SD | 0.13 ± 0.4 | 0.18 ± 0.5 | 0.08 ± 0.28 | 0.41 |
| FEV 1 (%) | m ± SD | 59.4 ± 24.1 | 65.7 ± 22.6 | 52.5 ± 24.5 | 0.09 |
| FVC (%) | m ± SD | 67.6 ± 19.8 | 70.3 ± 18.4 | 64.5 ± 21.3 | 0.38 |
| FEV1/FVC (%) | m ± SD | 0.69 ± 0.11 | 0.73 ± 0.09 | 0.64 ± 0.11 | 0.01 |
| Colonizations | n (%) | 41 (87.2) | 21 (91.3) | 20 (80) | 0.13 |
| Pseudomonas aeruginosa | 37 (77.1) | 19 (82.6) | 18 (72) | 0.14 | |
| Staphylococcus aureus | 38 (79.2) | 20 (86.9) | 18 (72) | 0.10 | |
| Haemophilus influenzae | 23 (47.9) | 12 (52.2) | 11 (44) | 0.47 |
| Men (n = 24) | Women (n = 24) | p-Value | ||
|---|---|---|---|---|
| BMI (kg/m2) | Mean ± SD | 23.2 ± 4.2 | 21.8 ± 3.4 | 0.21 |
| Triceps skinfold (mm) | Mean ± SD | 9.26 ± 4.4 | 17.3 ± 5.6 | p < 0.001 |
| Arm muscle circumference (cm) | Mean ± SD | 24.8 ± 2.4 | 20.5 ± 2.4 | p < 0.001 |
| Fat-free mass (anthropometry) (kg) | Mean ± SD | 54.1 ± 7.1 | 39.1 ± 5.8 | p < 0.001 |
| FFMI (anthropometry) (kg/m2) | Mean ± SD | 18.9 ± 1.9 | 15.6 ± 1.4 | p < 0.001 |
| Phase angle (°) | Mean ± SD | 6.07 ± 0.6 | 5.02 ± 0.54 | p < 0.001 |
| Fat-free mass (BIA) (kg) | Mean ± SD | 54.4 ± 8.1 | 38.6 ± 5.7 | p < 0.001 |
| FFMI (BIA) (kg/m2) | Mean ± SD | 18.9 ± 2.2 | 15.4 ± 1.5 | p < 0.001 |
| Fat-free mass (DXA) (kg) | Mean ± SD | 51.8 ± 6 | 37.3 ± 6 | p < 0.001 |
| FFMI (DXA) (kg/m2) | Mean ± SD | 18.2 ± 1.4 | 14.6 ± 1.6 | p < 0.001 |
| Handgrip strength (kg) | Mean ± SD | 38.3 ± 7.1 | 23.8 ± 5.5 | p < 0.001 |
| Muscular area rectus anterior (MARA) (cm2) | Mean ± SD | 4.97 ± 1.4 | 3.11 ± 0.89 | p < 0.001 |
| Muscular area index (MARAI) (cm2/m2) | Mean ± SD | 1.73 ± 0.48 | 1.24± 0.34 | p < 0.001 |
| X-axis (cm) | Mean ± SD | 3.96 ± 0.52 | 3.1 ± 0.41 | p < 0.001 |
| Y-axis (cm) | Mean ± SD | 1.47 ± 0.33 | 1.16 ± 0.31 | p < 0.001 |
| Muscular circumference rectus (cm) | Mean ± SD | 9.93 ± 1.26 | 7.73 ± 1.03 | p < 0.001 |
| Subcutaneous adipose tissue (SCAT) (cm) | Mean ± SD | 0.55 ± 0.28 | 1.11 ± 0.38 | p < 0.001 |
| MARA (cm2) | MARAI (cm2/m2) | X-Axis (cm) | Y-Axis (cm) | Muscular Circumference (cm) | SCAT (cm) | |
|---|---|---|---|---|---|---|
| BMI (kg/m2) | r = 0.385 p = 0.008 | r = 0.339 p = 0.02 | r = 0.183 p = 0.217 | r = 0.380 p = 0.008 | r = 0.280 p = 0.057 | r = 0.330 p = 0.023 |
| Fat-free mass (anthropometry) (kg) | r = 0.747 p < 0.001 | r = 0.574 p < 0.001 | r = 0.688 p < 0.001 | r = 0.554 p < 0.001 | r = 0.736 p < 0.001 | r = −0.381 p = 0.009 |
| FFMI (anthropometry) (kg/m2) | r = 0.712 p < 0.001 | r = 0.642 p < 0.001 | r = 0.605 p < 0.001 | r = 0.568 p < 0.001 | r = 0.659 p < 0.001 | r = −0.286 p = 0.009 |
| Fat-free mass (BIA) (kg) | r = 0.780 p < 0.001 | r = 0.612 p < 0.001 | r = 0.703 p < 0.001 | r = 0.607 p < 0.001 | r = 0.763 p < 0.001 | r = −0.405 p = 0.006 |
| FFMI (BIA) (kg/m2) | r = 0.774 p < 0.001 | r = 0.710 p < 0.001 | r = 0.635 p < 0.001 | r = 0.660 p < 0.001 | r = 0.714 p < 0.001 | r = −0.325 p = 0.029 |
| Phase angle (°) | r = 0.695 p < 0.001 | r = 0.675 p < 0.001 | r = 0.578 p < 0.001 | r = 0.623 p < 0.001 | r = 0.632 p < 0.001 | r = −0.589 p < 0.001 |
| Fat-free mass (DXA) (kg) | r = 0.670 p < 0.001 | r = 0.505 p = 0.002 | r = 0.480 p = 0.004 | r = 0.616 p < 0.001 | r = 0.677 p < 0.001 | r = −0.570 p = 0.005 |
| FFMI (DXA) (kg/m2) | r = 0.678 p < 0.001 | r = 0.567 p < 0.001 | r = 0.491 p = 0.003 | r = 0.576 p < 0.001 | r = 0.680 p < 0.001 | r = −0.610 p = 0.002 |
| Handgrip strength (kg) | r = 0.790 p < 0.001 | r = 0.687 p < 0.001 | r = 0.718 p < 0.001 | r = 0.625 p < 0.001 | r = 0.779 p < 0.001 | r = −0.589 p < 0.001 |
| Total Exacerbations | Severe Exacerbations | FEV1 (%) | FVC (%) | FEV1/FVC (%) | |
|---|---|---|---|---|---|
| Muscular area rectus anterior (MARA) (cm2) | r = 0.019 p = 0.89 | r = 0.161 p = 0.286 | r = 0.445 p = 0.005 | r = 0.376 p = 0.02 | r = 0.344 p = 0.037 |
| Muscular area index (MARAI) (cm2/m2) | r = 0.052 p = 0.730 | r = 0.148 p = 0.326 | r = 0.328 p = 0.044 | r = 0.299 p = 0.068 | r = 0.234 p = 0.164 |
| X-axis (cm) | r = 0.019 p = 0.899 | r = 0.092 p = 0.545 | r = 0.279 p = 0.090 | r = 0.270 p = 0.101 | r = 0.107 p = 0.530 |
| Y-axis (cm) | r = −0.057 p = 0.708 | r = 0.038 p = 0.801 | r = 0.444 p = 0.005 | r = 0.398 p = 0.013 | r = 0.350 p = 0.034 |
| Muscular circumference rectus (cm) | r = −0.016 p = 0.917 | r = 0.069 p = 0.646 | r = 0.348 p = 0.032 | r = 0.304 p = 0.064 | r = 0.214 p = 0.203 |
| Subcutaneous adipose tissue (SCAT) (cm) | r = 0.122 p = 0.420 | r = 0.072 p = 0.634 | r = −0.065 p = 0.698 | r = −0.015 p = 0.931 | r = −0.224 p = 0.183 |
| Normo-Nourished (n = 21) | Malnourished (n = 27) | p-Value | ||
|---|---|---|---|---|
| Muscular area rectus anterior (MARA) (cm2) | m ± SD | 4.75 ± 1.65 | 3.37 ± 1.04 | p = 0.014 |
| Muscular area index (MARAI) (cm2/m2) | m ± SD | 1.71 ± 0.51 | 1.28 ± 0.36 | p = 0.016 |
| X-axis (cm) | m ± SD | 3.74 ± 0.65 | 3.37 ± 0.61 | p = 0.279 |
| Y-axis (cm) | m ± SD | 1.45 ± 0.36 | 1.17 ± 0.28 | p = 0.010 |
| Muscular circumference rectus (cm) | m ± SD | 9.48 ± 1.69 | 8.26 ± 1.34 | p = 0.097 |
| Subcutaneous adipose tissue (SCAT) (cm) | m ± SD | 0.73 ± 0.37 | 0.95 ± 0.46 | p = 0.872 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Torralvo, F.J.; Porras, N.; Ruiz-García, I.; Maldonado-Araque, C.; García-Olivares, M.; Girón, M.V.; Gonzalo-Marín, M.; Olveira, C.; Olveira, G. Usefulness of Muscle Ultrasonography in the Nutritional Assessment of Adult Patients with Cystic Fibrosis. Nutrients 2022, 14, 3377. https://doi.org/10.3390/nu14163377
Sánchez-Torralvo FJ, Porras N, Ruiz-García I, Maldonado-Araque C, García-Olivares M, Girón MV, Gonzalo-Marín M, Olveira C, Olveira G. Usefulness of Muscle Ultrasonography in the Nutritional Assessment of Adult Patients with Cystic Fibrosis. Nutrients. 2022; 14(16):3377. https://doi.org/10.3390/nu14163377
Chicago/Turabian StyleSánchez-Torralvo, Francisco José, Nuria Porras, Ignacio Ruiz-García, Cristina Maldonado-Araque, María García-Olivares, María Victoria Girón, Montserrat Gonzalo-Marín, Casilda Olveira, and Gabriel Olveira. 2022. "Usefulness of Muscle Ultrasonography in the Nutritional Assessment of Adult Patients with Cystic Fibrosis" Nutrients 14, no. 16: 3377. https://doi.org/10.3390/nu14163377
APA StyleSánchez-Torralvo, F. J., Porras, N., Ruiz-García, I., Maldonado-Araque, C., García-Olivares, M., Girón, M. V., Gonzalo-Marín, M., Olveira, C., & Olveira, G. (2022). Usefulness of Muscle Ultrasonography in the Nutritional Assessment of Adult Patients with Cystic Fibrosis. Nutrients, 14(16), 3377. https://doi.org/10.3390/nu14163377






