Effects of Amino Acids Supplementation on Lipid and Glucose Metabolism in HepG2 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Amino Acids Treatments
2.3. Glucose and Lipid Treatments
2.4. Cell Viability Assays
2.5. Oil Red O Staining
2.6. Quantitative PCR
2.7. Statistical Analysis
3. Results
3.1. Cell Viability
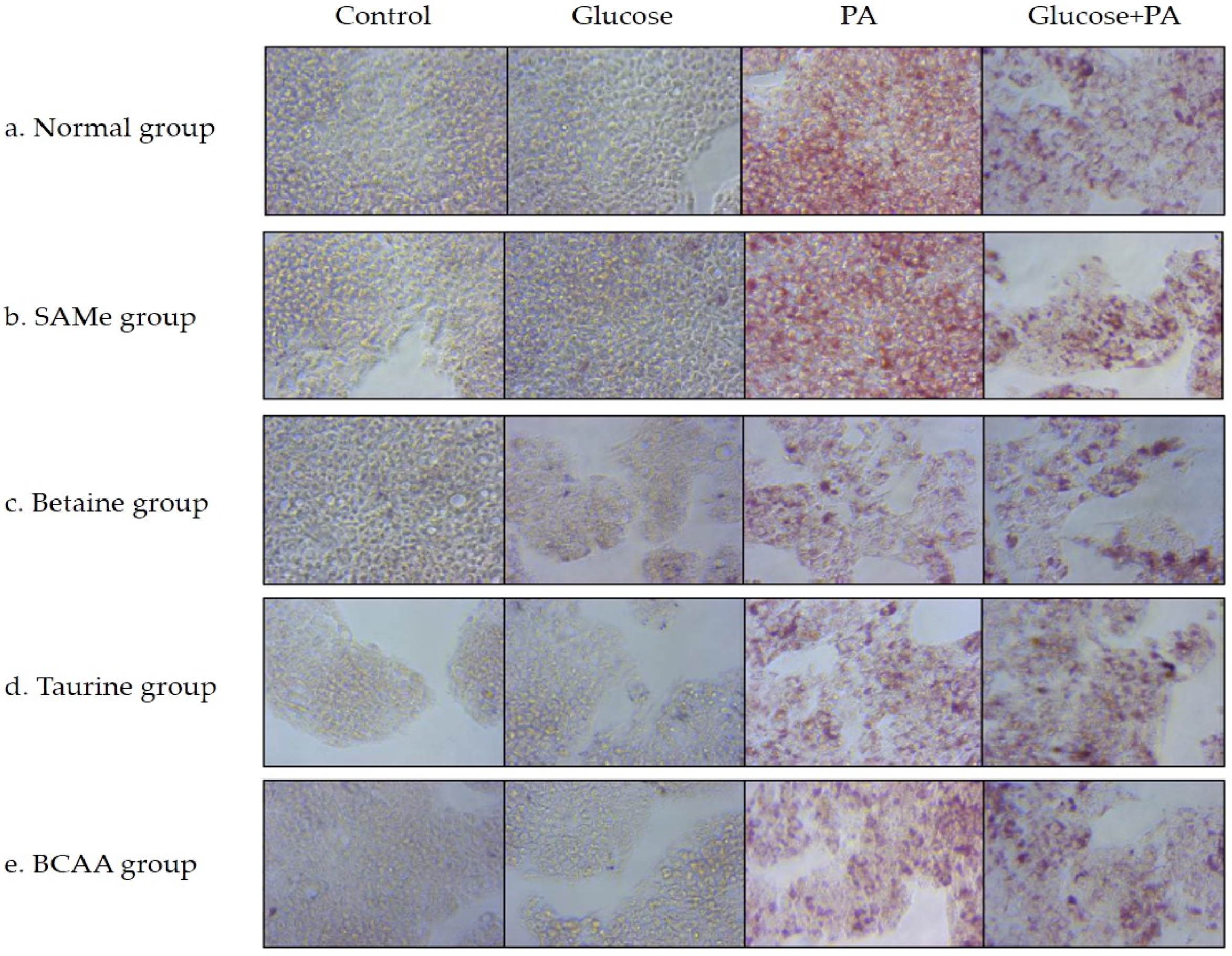
3.2. Effects of Amino Acids on TG Accumulation in Glucose- and/or PA-Treated HepG2 Cells
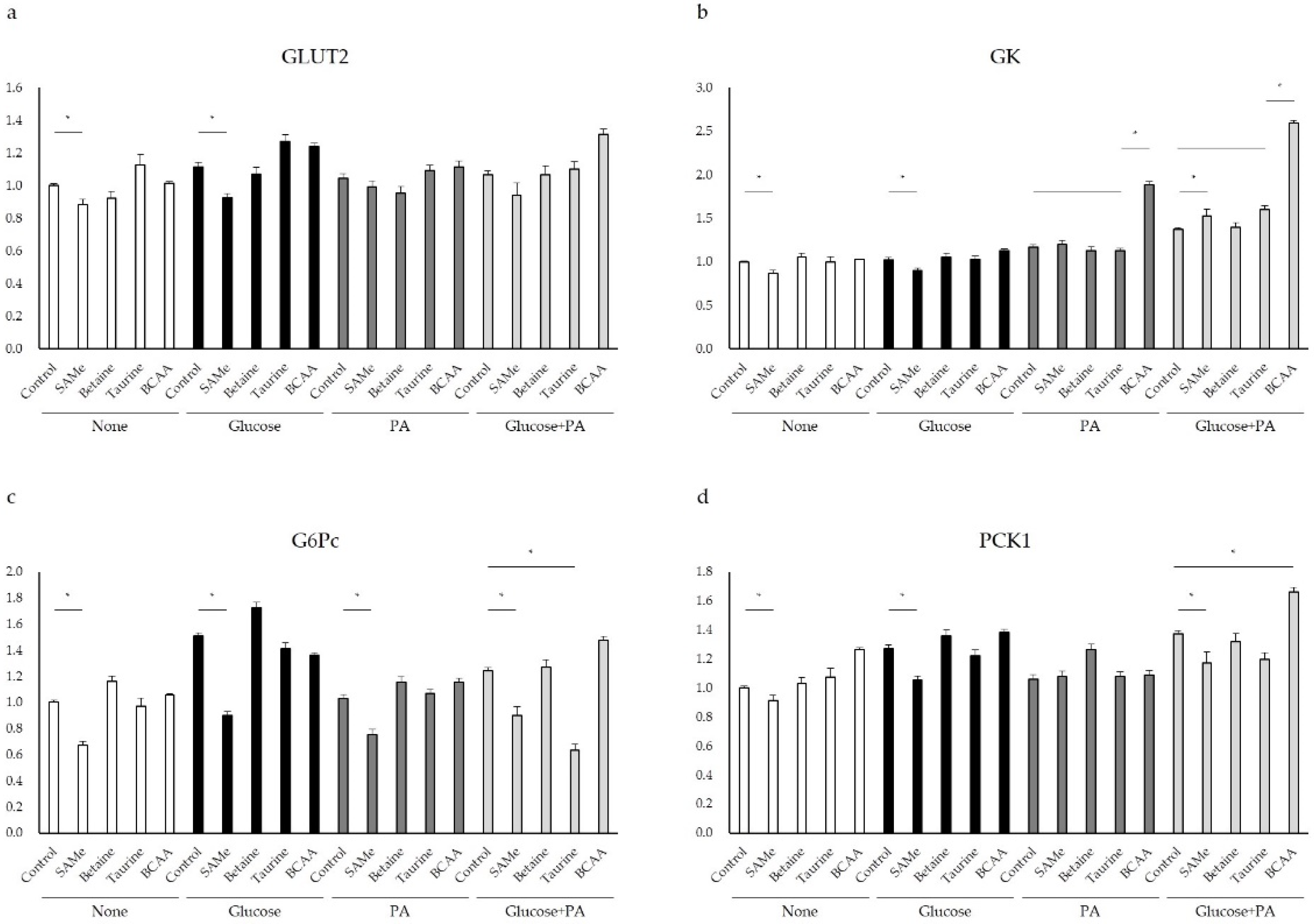
3.3. Glucose Metabolism after Amino Acids Pretreatment with Glucose and/or PA Treatment
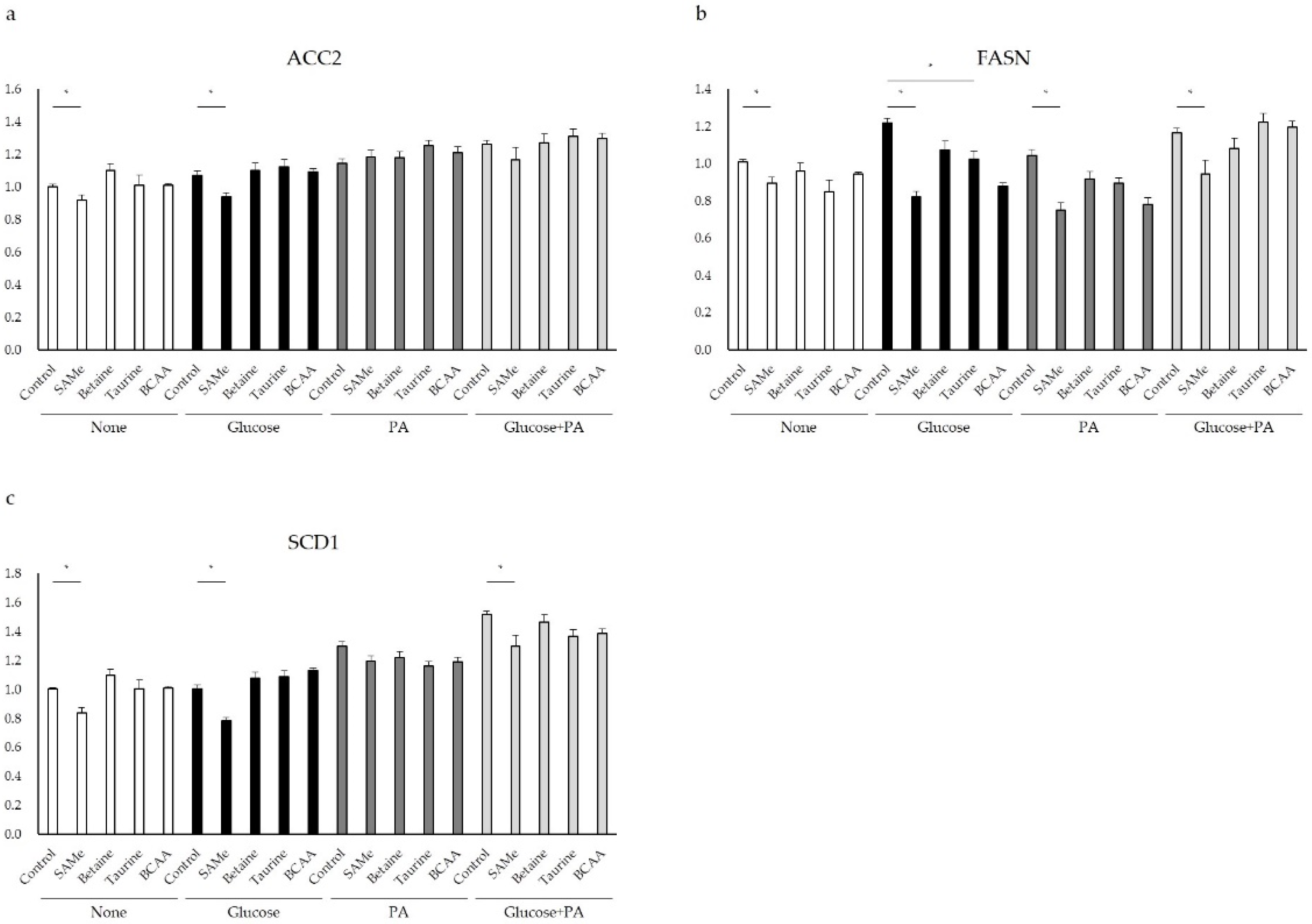
3.4. Genes Involved in Lipid Metabolism after Amino Acids Pretreatment and Glucose and/or PA Treatment
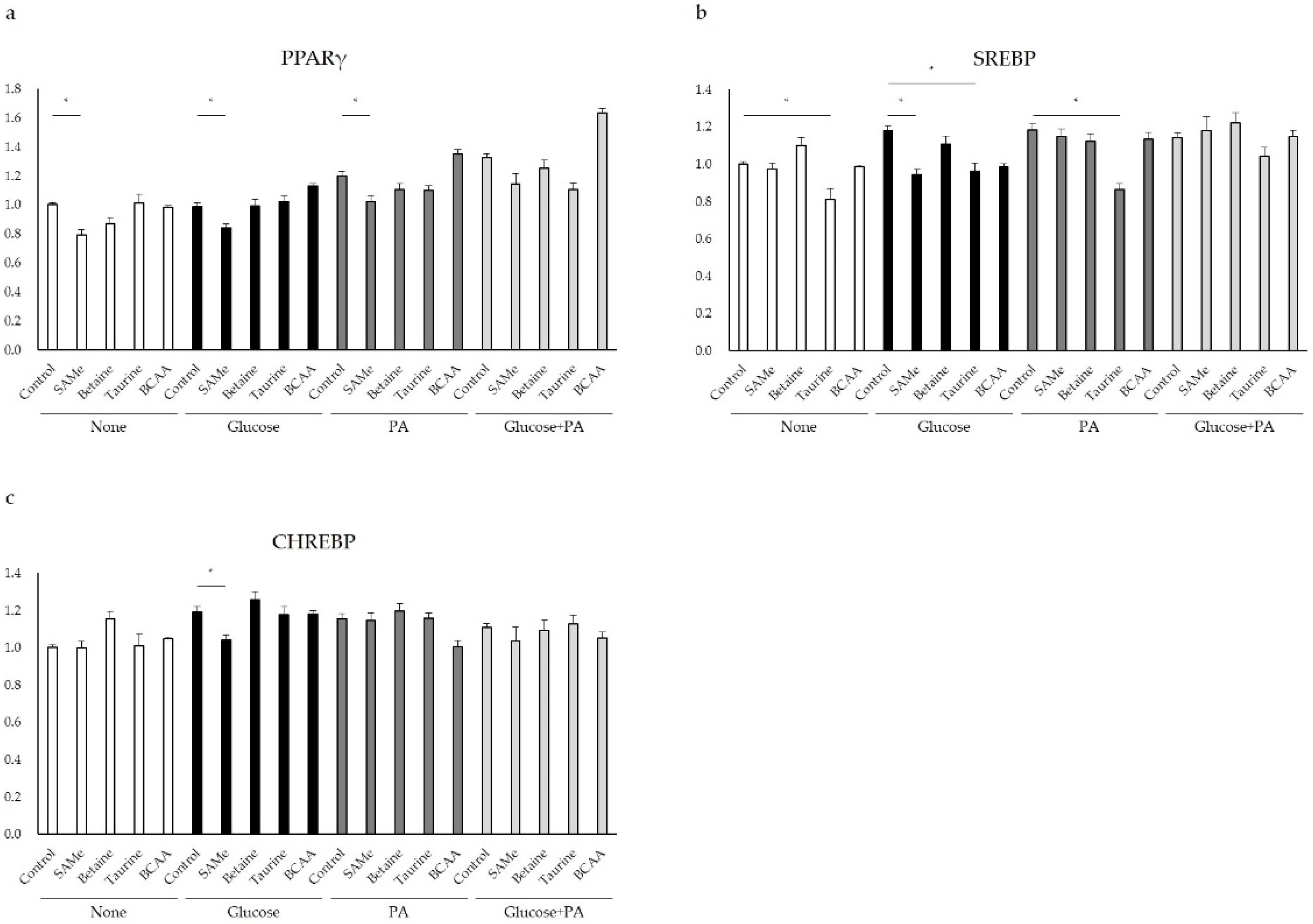
3.5. Regulation of Glucose and Lipid Metabolism after Amino Acids Pretreatment and Glucose and/or PA Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaur, J. A Comprehensive Review on Metabolic Syndrome. Cardiol. Res. Pract. 2014, 2014, 943162. [Google Scholar] [CrossRef]
- Cornier, M.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The Metabolic Syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- De Koning, L.; Malik, V.S.; Kellogg, M.D.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation 2012, 125, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Jump, D.B.; Clarke, S.D. Regulation of gene expression by dietary fat. Ann. Rev. Nutr. 1999, 19, 63–90. [Google Scholar] [CrossRef]
- Jung, Y.S. Metabolism of Sulfur-Containing Amino Acids in the Liver: A Link between Hepatic Injury and Recovery. Biol Pharm Bull. 2015, 38, 971–974. [Google Scholar] [CrossRef]
- Mato, J.M.; Martínez-Chantar, M.L.; Lu, S.C. S-adenosylmethionine metabolism and liver disease. Ann. Hepatol. 2013, 12, 183–189. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.W.; Wang, L.K.; Li, X.; Zhang, H.; Luo, L.P.; Gong, Z.J. Betaine Protects Against High-Fat-Diet-Induced Liver Injury by Inhibition of High-Mobility Group Box 1 and Toll-Like Receptor 4 Expression in Rats. Dig. Dis. Sci. 2013, 58, 3198–3206. [Google Scholar] [CrossRef]
- Deminice, R.; Da Silva, R.P.; Lamarre, S.G.; Kelly, K.B.; Jacobs, R.L.; Brosnan, M.E.; Brosnan, J.T. Betaine supplementation prevents fatty liver induced by a high-fat diet: Effects on one carbon metabolism. Amino Acids 2015, 47, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Kathirvel, E.; Morgan, K. Betaine improves nonalcoholic fatty liver and associated hepatic insulin resistance: A potential mechanism for hepatoprotection by betaine. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, 1068–1077. [Google Scholar] [CrossRef]
- Ejaz, A.; Martinez-Guino, L.; Goldfine, A.B.; Ribas-Aulinas, F.; De Nigris, V.; Ribó, S.; Lerin, C. Dietary Betaine Supplementation Increases Fgf21 Levels to Improve Glucose Homeostasis and Reduce Hepatic Lipid Accumulation in Mice. Diabetes 2016, 65, 902–912. [Google Scholar] [CrossRef]
- Lee, J.Y.; Da, W.J.; Park, H.A.; Kim, S.J.; Chung, J.H.; Moon, C.K.; Kim, Y.C. Effect of taurine on biliary excretion and metabolism of acetaminophen in male hamsters. Biol. Pharm. Bull. 2004, 27, 1792–1796. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shimada, K.; Jong, C.J.; Takahashi, K.; Schaffer, S.W. Role of ROS production and turnover in the antioxidant activity of taurine. Adv. Exp. Med. Biol. 2015, 803, 581–596. [Google Scholar] [PubMed]
- McCarty, M.F. Supplementation with Phycocyanobilin, Citrulline, Taurine, and Supranutritional Doses of Folic Acid and Biotin-potential for Preventing or Slowing the Progression of Diabetic Complications. Healthcare 2017, 5, 15. [Google Scholar] [CrossRef]
- Chen, W.; Guo, J.; Zhang, Y.; Zhang, J. The beneficial effects of taurine in preventing metabolic syndrome. Food Funct. 2016, 7, 1849–1863. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Yoshikawa, N.; Ito, H.; Schaffer, S.W. Impact of taurine depletion on glucose control and insulin secretion in mice. J. Pharmacol. Sci. 2015, 1, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Yamori, Y.; Taguchi, T.; Mori, H.; Mori, M. Low cardiovascular risks in the middle aged males and females excreting greater 24-hour urinary taurine and magnesium in 41 WHO-CARDIAC study populations in the world. J. Biomed. Sci. 2010, 17, 21. [Google Scholar] [CrossRef]
- Arakawa, M.; Masaki, T.; Nishimura, J.; Seike, M.; Yoshimatsu, H. The effects of branched-chain amino acid granules on the accumulation of tissue triglycerides and uncoupling proteins in diet-induced obese mice. Endocr. J. 2011, 58, 161–170. [Google Scholar] [CrossRef]
- Nishitani, S.; Matsumura, T.; Fujitani, S.; Sonaka, I.; Miura, Y.; Yagasaki, K. Leucine promotes glucose uptake in skeletal muscles of rats. Biochem. Biophys. Res. Commun. 2002, 299, 693–696. [Google Scholar] [CrossRef]
- Higuchi, N.; Kato, M.; Miyazaki, M.; Tanaka, M.; Kohjima, M.; Ito, T.; Takayanagi, R. Potential Role of Branched-Chain Amino Acids in Glucose Metabolism through the Accelerated Induction of the Glucose-Sensing Apparatus in the Liver. J. Cell. Biochem. 2011, 112, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Buqué, X.; Martínez-Uña, M.; Aurrekoetxea, I.; Menor, A.; García-Rodríguez, J.L.; Aspichueta, P. Methionine adenosyltransferase 1A gene deletion disrupts hepatic very low density lipoprotein assembly in mice. Hepatology 2011, 54, 1975–1986. [Google Scholar] [CrossRef] [PubMed]
- Giesbertz, P.; Daniel, H. Branched-chain amino acids as biomarkers in diabetes. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Sun, L.; Gong, Y.; Zhou, Y.; Yang, P.; Ye, Z.; Zhou, H. Relationship between Branched-Chain Amino Acids, Metabolic Syndrome, and Cardiovascular Risk Profile in a Chinese Population: A Cross-Sectional Study. Int. J. Endocrinol. 2016, 2016, 8173905. [Google Scholar] [CrossRef] [PubMed]
- Ricchi, M.; Odoardi, M.R.; Carulli, L.; Anzivino, C.; Ballestri, S.; Pinetti, A.; Loria, P. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J. Gastroenterol. Hepatol. 2009, 24, 830–840. [Google Scholar] [CrossRef]
- Baumgartner-Parzer, S.M.; Wagner, L.; Pettermann, M.; Grillari, J.; Gessl, A.; Waldhäusl, W. High-Glucose–Triggered Apoptosis in Cultured Endothelial Cells. Diabetes 1995, 44, 1323–1327. [Google Scholar] [CrossRef]
- Di Wu, Q.; Wang, J.H.; Fennessy, F.; Redmond, H.P.; Bouchier-Hayes, D. Taurine prevents high-glucose-induced human vascular endothelial cell apoptosis. Am. J. Phys.-Cell Phys. 1999, 227, c1229. [Google Scholar] [CrossRef] [PubMed]
- Hagar, H.; Al Malki, W. Betaine supplementation protects against renal injury induced by cadmium intoxication in rats: Role of oxidative stress and caspase-3. Environ. Toxicol. Pharmacol. 2014, 37, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Ansorena, E.; Garcıa-Trevijano, E.R.; Martınez-Chantar, M.L.; Huang, Z.Z.; Chen, L.; Mato, J.M.; Avila, M.A. S-adenosylmethionine and methylthioadenosine are antiapoptotic in cultured rat hepatocytes but proapoptotic in human hepatoma cells. Hepatology 2002, 35, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Radziuk, J.; Pye, S. Hepatic glucose uptake, gluconeogenesis and the regulation of glycogen synthesis. Diabetes Metab. Res. 2001, 17, 250–272. [Google Scholar] [CrossRef]
- Weinstein, S.P.; O’Boyle, E.; Fisher, M.; Haber, R.S. Regulation of GLUT2 glucose transporter expression in liver by thyroid hormone: Evidence for hormonal regulation of the hepatic glucose transport system. Endocrinology 1994, 135, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Postic, C.; Burcelin, R.; Rencurel, F.; Pegorier, J.P.; Loizeau, M.; Girard, J.; Leturque, A. Evidence for a transient inhibitory effect of insulin on GLUT2 expression in the liver: Studies In Vivo and In Vitro. Biochem. J. 1993, 293, 119–124. [Google Scholar] [CrossRef]
- Belfiore, F.; Romeo, F.; Iannello, S.; Salamone, C. The glucose-6-phosphatase/glucokinase ratio in the liver of obese-diabetic subjects. Biochem. Med. Metab. Biol. 1989, 41, 77–80. [Google Scholar] [CrossRef]
- Huupponen, R.; Karvonen, I.; Sotaniemi, E. Activity of hepatic glucose phosphorylating and NADPH generating enzymes in Zucker rat. Diabetes Res. 1989, 10, 143–146. [Google Scholar] [PubMed]
- Hakimi, P.; Johnson, M.T.; Yang, J.; Lepage, D.F.; Conlon, R.A.; Kalhan, S.C.; Hanson, R.W. Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism. Nutr. Metab. 2005, 2, 33. [Google Scholar] [CrossRef] [PubMed]
- Gautier-Stein, A. Glucotoxicity Induces Glucose-6-Phosphatase Catalytic Unit Expression by Acting on the Interaction of HIF-1a with CREB-Binding Protein. Diabetes 2012, 61, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Clore, J.N. Glucose-6-phosphatase flux In Vitro is increased in type 2 diabetes. Diabetes 2000, 49, 969–974. [Google Scholar] [CrossRef]
- Dentin, R.; Girard, J.; Postic, C. Carbohydrate responsive element binding protein (ChREBP) and sterol regulatory element binding protein-1c (SREBP-1c): Two key regulators of glucose metabolism and lipid synthesis in liver. Biochimie 2005, 87, 81–86. [Google Scholar] [CrossRef]
- Linden, A.G.; Li, S.; Choi, H.Y.; Fang, F.; Fukasawa, M.; Uyeda, K.; Liang, G. Interplay between ChREBP and SREBP-1c coordinates postprandial glycolysis and lipogenesis in livers of mice. J. Lipid Res. 2018, 59, 475–487. [Google Scholar] [CrossRef]
- Shimomura, I.; Matsuda, M.; Hammer, R.E.; Bashmakov, Y.; Brown, M.S.; Goldstein, J.L. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol. Cell 2000, 6, 77–86. [Google Scholar] [CrossRef]
- Higuchi, N.; Kato, M.; Shundo, Y.; Tajiri, H.; Tanaka, M.; Yamashita, N.; Enjoji, M. Liver X receptor in cooperation with SREBP-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol. Res. 2008, 38, 1122–1129. [Google Scholar] [CrossRef]
- Kota, B.P.; Huang, T.H.W.; Roufogalis, B.D. Roufogalis BD: An overview of biological mechanisms of PPARs. Pharmacol. Res. 2005, 51, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Matsusue, K.; Kashireddy, P.; Cao, W.Q.; Yeldandi, V.; Yeldandi, A.V.; Reddy, J.K. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J. Biol. Chem. 2003, 278, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.A.; Lee, D.P. Enzymes of triacylglycerol synthesis and their regulation. Prog. Lipid Res. 2004, 43, 134–176. [Google Scholar] [CrossRef]
- Okekunle, A.P.; Zhang, M.; Wang, Z.; Onwuka, J.U.; Wu, X.; Feng, R.; Li, C. Dietary branched-chain amino acids intake exhibited a different relationship with type 2 diabetes and obesity risk: A meta-analysis. Acta Diabetol. 2019, 56, 187–195. [Google Scholar] [CrossRef]
- Matsusue, K.; Haluzik, M.; Lambert, G.; Yim, S.H.; Gavrilova, O.; Ward, J.M.; Gonzalez, F.J. Liver specific disruption of PPARγ in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J. Clin. Investig. 2003, 111, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, H.I.; Kim, T.H.; Im, S.S.; Park, S.K.; Lee, I.K.; Ahn, Y.H. SREBP-1c mediates the insulin dependent hepatic glucokinase expression. J. Biol. Chem. 2004, 279, 30823–30829. [Google Scholar] [CrossRef]

| Gene | mRNA No. | Primer Sequence | |
|---|---|---|---|
| ACTB | NM_001101.5 | Forward | GGACTTCGAGCAAGAGATGG |
| Reverse | AGGAAGGAAGGCTGGAAGAG | ||
| GLUT2 | NM_000340.2 | Forward | GCGACGTTCTCTCTTTCTAAT |
| Reverse | GGCTATCATGCTCACATAACT | ||
| GK | NM_000167.5 | Forward | GAAACTACACTGTCCCATCTC |
| Reverse | GGATTGAGGTGTTGCCTATC | ||
| PCK1 | NM_002591.4 | Forward | GCCTGGATGAAGTTTGACGC |
| Reverse | ATGGCATTGGGGTTGGTCTT | ||
| G6PC | NM_000151.4 | Forward | GCAATGGGCACTGGTATT |
| Reverse | GGAGTCACACATGGGAATAAG | ||
| ACC2 | NM_001093.4 | Forward | GGAACATCCCTACGCTAAAC |
| Reverse | GACAAGGTGGAGTGAATGAG | ||
| SCD1 | NM_005063.5 | Forward | CAACTACCACCACTCCTTTC |
| Reverse | GAGACTTTCTTCCGGTCATAG | ||
| FASN | NM_004104.5 | Forward | GGTTTGATGCCTCCTTCTT |
| Reverse | GGAGTGAATCTGGGTTGATG | ||
| SREBP-1 | NM_001018067.2 | Forward | TACCGCTCCTCCATCAAT |
| Reverse | GTGTTGCAGAAAGCGAATG | ||
| ChREBP | NM_032951.3 | Forward | GACAGCTGAGTACATCCTTATG |
| Reverse | TGCTGGCACAGGTTAATG | ||
| PPARγ | NM_001330615.1 | Forward | GTCGTGTCTGTGGAGATAAAG |
| Reverse | GGATCCGACAGTTAAGATCAC | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Jung, S.; Ko, K.S. Effects of Amino Acids Supplementation on Lipid and Glucose Metabolism in HepG2 Cells. Nutrients 2022, 14, 3050. https://doi.org/10.3390/nu14153050
Wang S, Jung S, Ko KS. Effects of Amino Acids Supplementation on Lipid and Glucose Metabolism in HepG2 Cells. Nutrients. 2022; 14(15):3050. https://doi.org/10.3390/nu14153050
Chicago/Turabian StyleWang, Shuang, Soohan Jung, and Kwang Suk Ko. 2022. "Effects of Amino Acids Supplementation on Lipid and Glucose Metabolism in HepG2 Cells" Nutrients 14, no. 15: 3050. https://doi.org/10.3390/nu14153050
APA StyleWang, S., Jung, S., & Ko, K. S. (2022). Effects of Amino Acids Supplementation on Lipid and Glucose Metabolism in HepG2 Cells. Nutrients, 14(15), 3050. https://doi.org/10.3390/nu14153050







