Development of Spatial Orientation in Two-to-Three-Year-Old Children in Relation to Lifestyle Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Materials and Procedure
2.2.1. BMI
2.2.2. Questionnaires
FFQ
Vineland Screener
Additional Lifestyle Questionnaire
2.2.3. Spatial Cognition Assessment
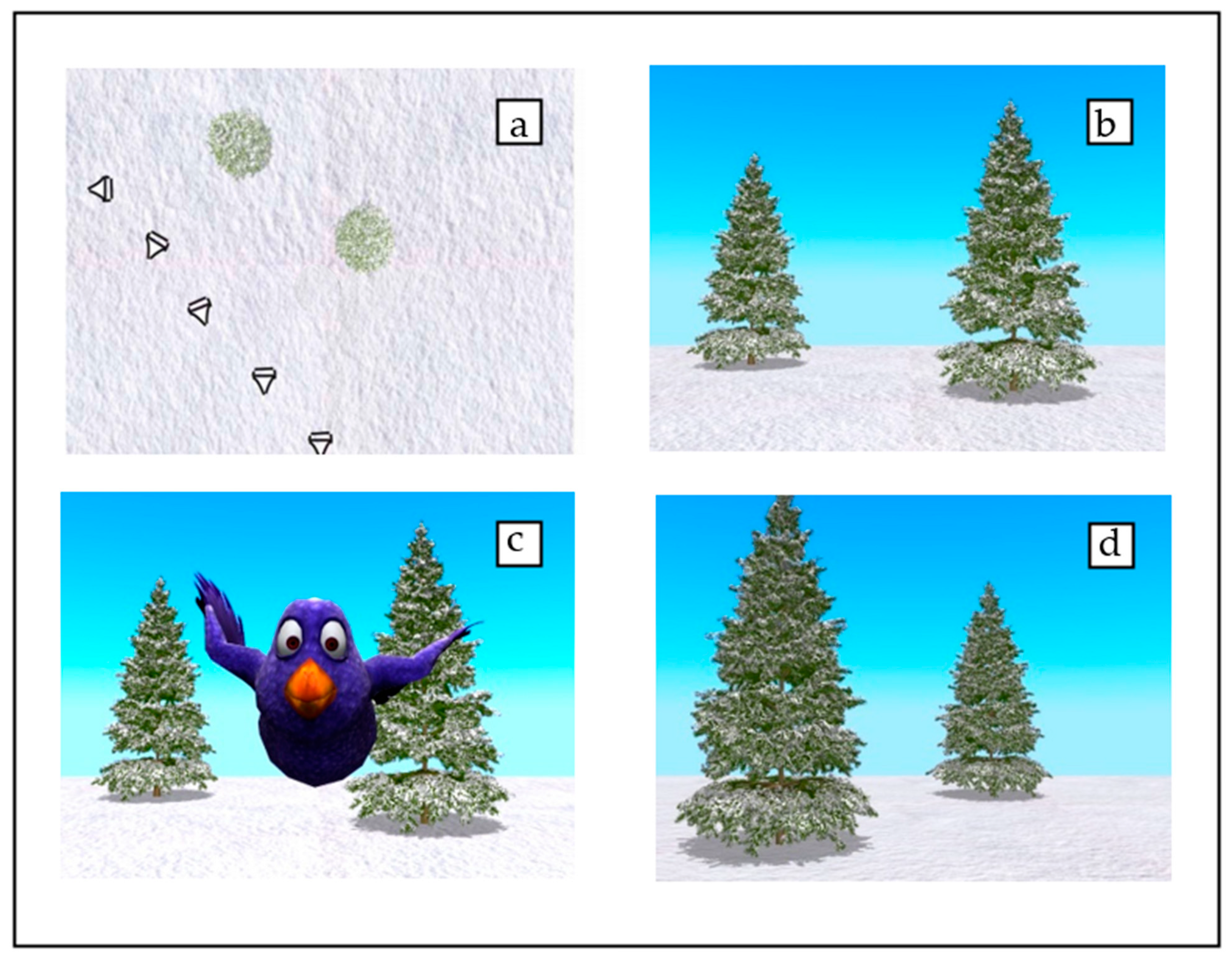
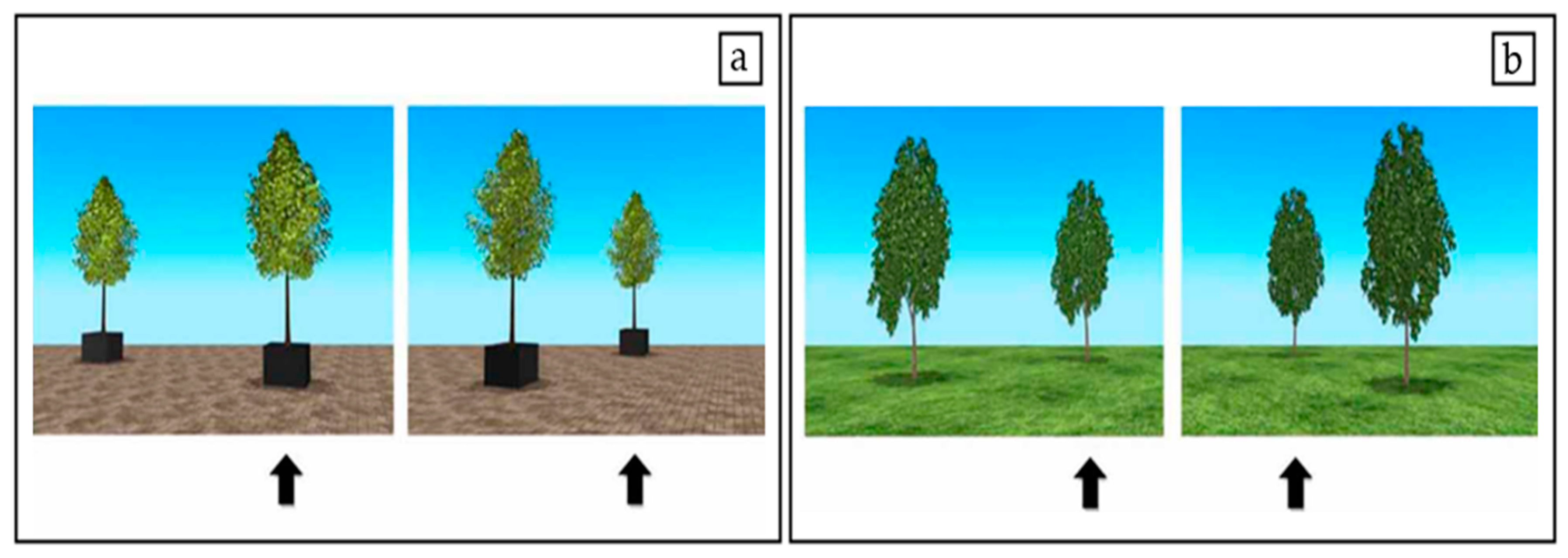
Stimuli
Procedure
2.3. Data Analyses
3. Results
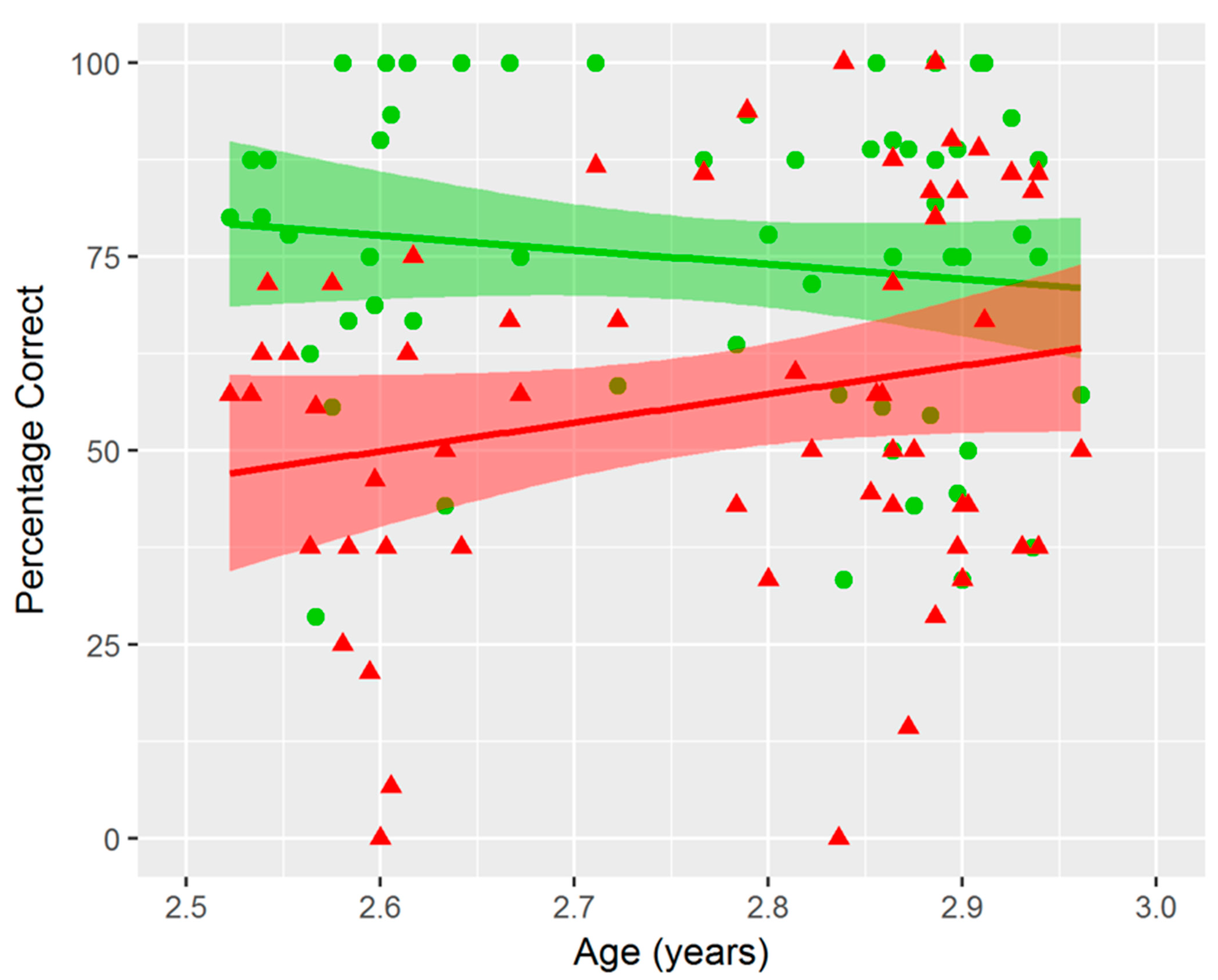
3.1. Spatial Cognition Task Performance and Age
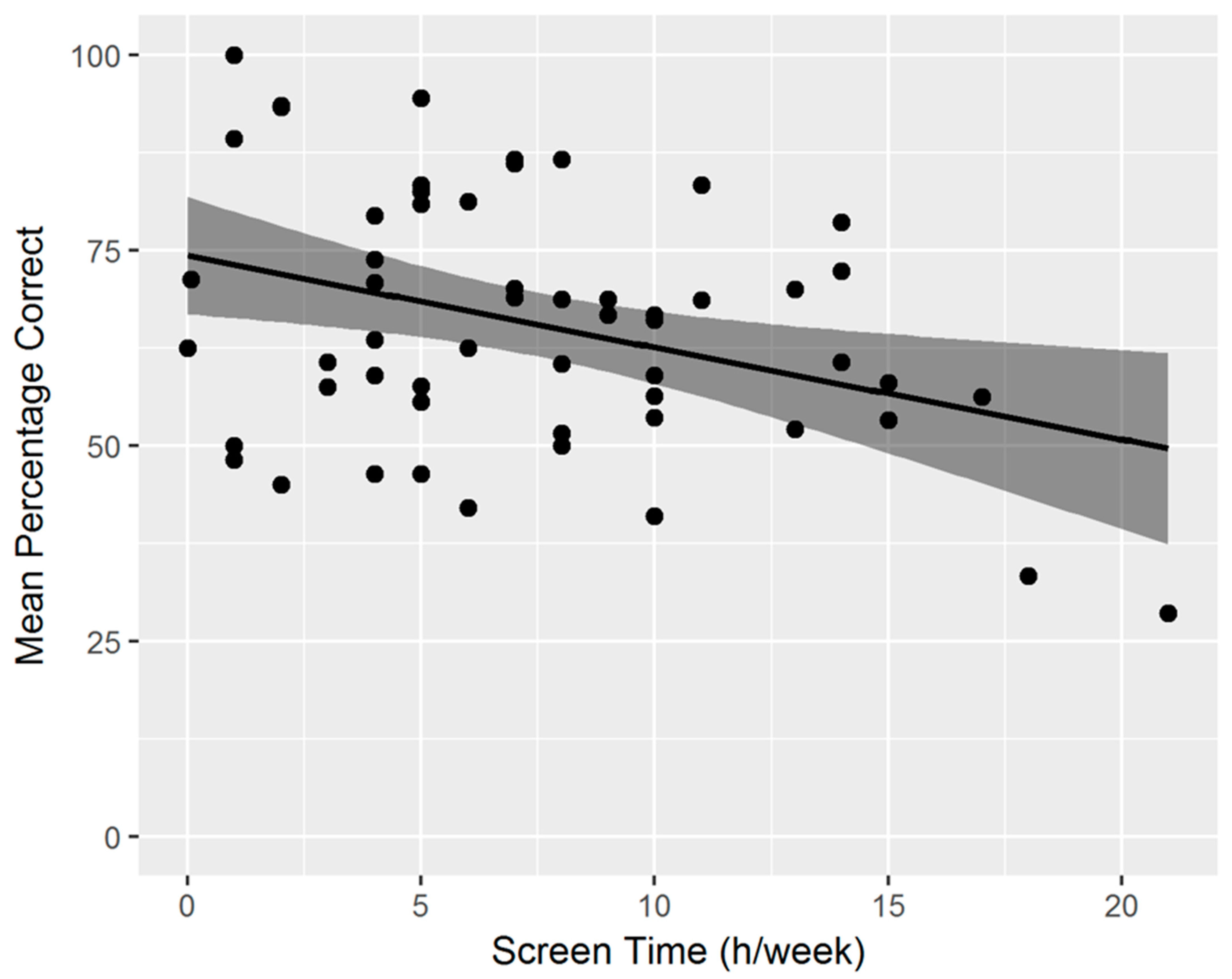
3.2. Relation between Spatial Cognition Task Performance and Lifestyle Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Commission on Ending Childhood Obesity. 2019. Available online: https://www.who.int/publications/i/item/9789241510066 (accessed on 1 October 2021).
- Jirout, J.; LoCasale-Crouch, J.; Turnbull, K.; Gu, Y.; Cubides, M.; Garzione, S.; Evans, T.M.; Weltman, A.L.; Kranz, S. How lifestyle factors affect cognitive and executive function and the ability to learn in children. Nutrients 2019, 11, 1953. [Google Scholar] [CrossRef] [PubMed]
- Bolshakov, A.P.; Tret’yakova, L.V.; Kvichansky, A.A.; Gulyaeva, N.V. Glucocorticoids: Dr. Jekyll and Mr. Hyde of Hippocampal Neuroinflammation. Biochemistry 2021, 86, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, K.A.; Brunson, K.L.; Baram, T.Z. Hippocampal neuroplasticity induced by early-life stress: Functional and molecular aspects. Front. Neuroendocrinol. 2006, 27, 180–192. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.; Dostrovsky, J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971, 34, 171–175. [Google Scholar] [CrossRef]
- Hart, R.A.; Moore, G.T. The development of spatial cognition: A review. In Image and Environment: Cognitive Mapping and Spatial Behavior; Downs, R.M., Stea, D., Eds.; Aldine Transaction: Chicago, IL, USA, 1973; pp. 246–288. [Google Scholar]
- Boitard, C.; Cavaroc, A.; Sauvant, J.; Aubert, A.; Castanon, N.; Layé, S.; Ferreira, G. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain. Behav. Immun. 2014, 40, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Baym, C.L.; Khan, N.A.; Monti, J.M.; Raine, L.B.; Drollette, E.S.; Moore, R.D.; Scudder, M.R.; Kramer, A.F.; Hillman, C.H.; Cohen, N.J. Dietary lipids are differentially associated with hippocampal-dependent relational memory in prepubescent children. Am. J. Clin. Nutr. 2014, 99, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Carbia, C.; Cryan, J.F. Going with the grain: Fiber, cognition, and the microbiota-gut-brain-axis. Exp. Biol. Med. 2021, 246, 796–811. [Google Scholar] [CrossRef]
- Clemente, J.C.; Manasson, J.; Scher, J.U. The role of the gut microbiome in systemic inflammatory disease. BMJ 2018, 360, j5145. [Google Scholar] [CrossRef]
- Nyaradi, A.; Li, J.; Hickling, S.; Foster, J.; Oddy, W.H. The role of nutrition in children’s neurocognitive development, from pregnancy through childhood. Front. Hum. Neurosci. 2013, 7, 97. [Google Scholar] [CrossRef]
- Liang, J.; Matheson, B.E.; Kaye, W.H.; Boutelle, K.N. Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. Int. J. Obes. 2014, 38, 494–506. [Google Scholar] [CrossRef]
- Nimmo, M.A.; Leggate, M.; Viana, J.L.; King, J.A. The effect of physical activity on mediators of inflammation. Diabetes Obes. Metab. 2013, 15, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Carson, V.; Hunter, S.; Kuzik, N.; Wiebe, S.A.; Spence, J.C.; Friedman, A.; Tremblay, M.S.; Slater, L.; Hinkley, T. Systematic review of physical activity and cognitive development in early childhood. J. Sci. Med. Sport 2016, 19, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Duch, H.; Fisher, E.M.; Ensari, I.; Harrington, A. Screen time use in children under 3 years old. Int. J. Behav. Nutr. Phys. Act. 2013, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Haack, M. The sleep-immune crosstalk in health and disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef]
- Bernier, A.; Beauchamp, M.H.; Bouvette-Turcot, A.A.; Carlson, S.M.; Carrier, J. Sleep and cognition in preschool years: Specific links to executive functioning. Child Dev. 2013, 84, 1542–1553. [Google Scholar] [CrossRef]
- De Freitas Araújo, D.; De Almondes, K.M. Sleep and cognitive performance in children and pre-adolescents: A review. Biol. Rhythm Res. 2014, 45, 193–207. [Google Scholar] [CrossRef]
- Gage, S.H.; Lawlor, D.A.; Tilling, K.; Fraser, A. Associations of maternal weight gain in pregnancy with offspring cognition in childhood and adolescence: Findings from the avon longitudinal study of parents and children. Am. J. Epidemiol. 2013, 177, 402–410. [Google Scholar] [CrossRef]
- Pereyra-Elías, R.; Quigley, M.A.; Carson, C. Association between breastfeeding duration and cognitive development up to age 14 among children from the UK millennium cohort study. J. Epidemiol. Community Health 2021, 75, A44–A45. [Google Scholar] [CrossRef]
- Learmonth, A.E.; Newcombe, N.S.; Huttenlocher, J. Toddlers’ use of metric information and landmarks to reorient. J. Exp. Child Psychol. 2001, 80, 225–244. [Google Scholar] [CrossRef]
- Bremner, J.G. Egocentric versus allocentric spatial coding in nine-month-old infants: Factors influencing the choice of code. Dev. Psychol. 1978, 14, 346–355. [Google Scholar] [CrossRef]
- Schmuckler, M.A.; Tsang-Tong, H.Y. The role of visual and body movement information in infant search. Dev. Psychol. 2000, 36, 499. [Google Scholar] [CrossRef] [PubMed]
- Committeri, G.; Galati, G.; Paradis, A.L.; Pizzamiglio, L.; Berthoz, A.; LeBihan, D. Reference frames for spatial cognition: Different brain areas are involved in viewer-, object-, and landmark-centered judgments about object location. J. Cogn. Neurosci. 2004, 16, 1517–1535. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Baizan, C.; Arias, J.L.; Mendez, M. Spatial orientation assessment in preschool children: Egocentric and allocentric frameworks. Appl. Neuropsychol. Child 2021, 10, 171–193. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.G.; Knowles, L.; Andreasen, G. Processes Underlying Young Children′ s Spatial Orientation during Movement. J. Exp. Child Psychol. 1994, 57, 355–376. [Google Scholar] [CrossRef] [PubMed]
- Bullens, J.; Iglói, K.; Berthoz, A.; Postma, A.; Rondi-Reig, L. Developmental time course of the acquisition of sequential egocentric and allocentric navigation strategies. J. Exp. Child Psychol. 2010, 107, 337–350. [Google Scholar] [CrossRef]
- Zaehle, T.; Jordan, K.; Wüstenberg, T.; Baudewig, J.; Dechent, P.; Mast, F.W. The neural basis of the egocentric and allocentric spatial frame of reference. Brain Res. 2007, 1137, 92–103. [Google Scholar] [CrossRef]
- Taube, S.; Muller, U.; Ranck, B. Head-Direction Cells Recorded from the Postsubiculum Moving Rats. II. Effects of Environmental Manipulations in Freely. J. Neurosci. 1990, 10, 436–447. [Google Scholar] [CrossRef]
- Rolls, E.T.; O’ Mara, S.M.O. View-Responsive Neurons in the Primate Hippocampal Complex. Hippocampus 1995, 5, 409–424. [Google Scholar] [CrossRef]
- Lambert, F.R.; Lavenex, P.; Lavenex, P.B. Improvement of allocentric spatial memory resolution in children from 2 to 4 years of age. Int. J. Behav. Dev. 2015, 39, 318–331. [Google Scholar] [CrossRef]
- van den Brink, D.; Janzen, G. Visual spatial cue use for guiding orientation in two-to-three-year-old children. Front. Psychol. 2013, 4, 904. [Google Scholar] [CrossRef][Green Version]
- Dutch Central Bureau for Statistics. Trends in Nederland 2018: Onderwijsniveau Bevolking 15 tot 75 Jaar [Trends in The Netherlands: Education Level Population 15 to 75 Years of Age]. Available online: https://longreads.cbs.nl/trends18/maatschappij/cijfers/onderwijs/ (accessed on 1 October 2021).
- Van Lee, L.; Feskens, E.J.M.; Hooft Van Huysduynen, E.J.C.; de Vries, J.H.M.; van ’t Veer, P.; Geleen, A. The Dutch Healthy Diet index as assessed by 24 h recalls and FFQ: Associations with biomarkers from a cross-sectional study. J. Nutr. Sci. 2013, 2, E40. [Google Scholar] [CrossRef] [PubMed]
- Dutch Health Council. Richtlijnen Goede Voeding 2015 [Dutch Dietary Guidelines 2015]. Dutch Health Council: The Hague, The Netherlands, 2015. [Google Scholar]
- Scholte, E.; Van Duijn, G.; Dijkxhoorn, Y.; Noens, I.; Van Berckelaer-Onnes, I. Handleiding Vineland Screener 0–6 [Manual Vineland Screener 0–6 Year-NL]; PITS: Leiden, The Netherlands, 2008. [Google Scholar]
- Kraha, A.; Turner, H.; Nimon, K.; Zientek, L.R.; Henson, R.K. Tools to support interpreting multiple regression in the face of multicollinearity. Front. Psychol. 2012, 3, 44. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Preacher, K.J. Extreme Groups Designs. In The Encyclopedia of Clinical Psychology; John Wiley & Sons: Hoboken, NJ, USA, 2015; Volume 2, pp. 1–4. [Google Scholar] [CrossRef]
- Ponti, M.; Bélanger, S.; Grimes, R.; Heard, J.; Johnson, M.; Moreau, E.; Norris, M.; Shaw, A.; Stanwick, R.; Van Lankveld, J.; et al. Screen time and young children: Promoting health and development in a digital world. Paediatr. Child Health 2017, 22, 461–477. [Google Scholar] [CrossRef]
- Certain, L.K.; Kahn, R.S. Prevalance, correlates, and trajectory of television viewinf among infants and toddlers. Pediatrics 2002, 109, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, F.J.; Christakis, D.A. Associations between content types of early media exposure and subsequent attentional problems. Pediatrics 2007, 120, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Tavris, D.R.; Read, J.A. Effect of maternal weight gain on fetal, infant, and childhood death and on cognitive development. Obstet. Gynecol. 1982, 60, 689–694. [Google Scholar]
- Keim, S.A.; Pruitt, N.T. Gestational weight gain and child cognitive development. Int. J. Epidemiol. 2012, 41, 414–422. [Google Scholar] [CrossRef]
- Martínez-Hortelano, J.A.; Álvarez-Bueno, C.; Cavero-Redondo, I.; Herráiz-Adillo, Á.; Berlanga-Macías, C.; Martínez-Vizcaíno, V. Gestational weight gain and offspring’s cognitive skills: A systematic review and meta-analysis. BMC Pediatr. 2020, 20, 533. [Google Scholar] [CrossRef] [PubMed]
- Hrolfsdottir, L.; Schalkwijk, C.G.; Birgisdottir, B.E.; Gunnarsdottir, I.; Maslova, E.; Granström, C.; Strøm, M.; Olsen, S.F.; Halldorsson, T.I. Maternal diet, gestational weight gain, and inflammatory markers during pregnancy. Obesity 2016, 24, 2133–2139. [Google Scholar] [CrossRef]
- Van Der Burg, J.W.; Sen, S.; Chomitz, V.R.; Seidell, J.C.; Leviton, A.; Dammann, O. The role of systemic inflammation linking maternal BMI to neurodevelopment in children. Pediatr. Res. 2016, 79, 3–12. [Google Scholar] [CrossRef]
- Wai, J.; Lubinski, D.; Benbow, C.P.; Steiger, J.H. Accomplishment in Science, Technology, Engineering, and Mathematics (STEM) and Its Relation to STEM Educational Dose: A 25-Year Longitudinal Study. J. Educ. Psychol. 2010, 102, 860–871. [Google Scholar] [CrossRef]
- Duncan, G.J.; Magnuson, K. Socioeconomic status and cognitive functioning: Moving from correlation to causation. Wiley Interdiscip. Rev. Cogn. Sci. 2012, 3, 377–386. [Google Scholar] [CrossRef] [PubMed]
| Predictor | M | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | 2.77 | 0.12 | |||||||||||||||
| 2. Sex | 0.47 | 0.50 | 0.31 * | ||||||||||||||
| 3. Communication skills | 41.44 | 6.15 | 0.32 * | 0.22 | |||||||||||||
| 4. Social skills | 41.91 | 9.15 | 0.27 * | −0.05 | 0.45 ** | ||||||||||||
| 5. Daily living skills | 36.15 | 8.57 | 0.12 | −0.24 | 0.42 ** | 0.55 ** | |||||||||||
| 6. Motor skills | 41.02 | 6.13 | 0.09 | 0.12 | 0.45 ** | 0.56 ** | 0.48 ** | ||||||||||
| 7. Sleep | 12.21 | 1.05 | −0.05 | −0.04 | −0.30 | −0.29 * | −0.26 | −0.05 | |||||||||
| 8. Exercise | 8.91 | 5.55 | 0.07 | 0.18 | −0.01 | 0.02 | −0.03 | 0.12 | −0.05 | ||||||||
| 9. Screen time (h/week) | 7.51 | 4.82 | 0.24 | 0.006 | 0.03 | 0.32 * | −0.15 | 0.22 | −0.01 | 0.40 ** | |||||||
| 10. Dutch Healthy Diet score | 64.80 | 14.12 | −0.02 | 0.09 | −0.01 | 0.01 | 0.14 | −0.01 | −0.01 | −0.21 | −0.38 ** | ||||||
| 11. Caloric intake | 1111.50 | 287.03 | 0.25 | 0.35 ** | 0.03 | 0.08 | −0.14 | −0.008 | 0.03 | 0.19 | 0.11 | 0.13 | |||||
| 12. Saturated fat ratio | 12.12 | 2.44 | 0.12 | 0.09 | 0.004 | −0.09 | −0.23 | −0.12 | 0.24 | −0.10 | −0.07 | −0.18 | 0.16 | ||||
| 13. Fiber intake | 14.77 | 3.86 | 0.13 | 0.13 | 0.04 | 0.07 | 0.04 | −0.007 | 0.02 | 0.02 | −0.13 | 0.44 ** | 0.63 ** | −0.10 | |||
| 14. Child BMI | 16.34 | 1.39 | −0.04 | 0.005 | −0.001 | −0.20 | −0.20 | −0.13 | 0.16 | −0.02 | −0.23 | −0.02 | −0.18 | 0.23 | 0.02 | ||
| 15. Breastfeeding time | 8.65 | 8.71 | −0.21 | −0.38 | −0.02 | −0.05 | 0.13 | −0.10 | −0.02 | −0.19 | −0.25 | 0.19 | −0.32 * | −0.22 | −0.04 | 0.11 | |
| 16. Gestational weight gain | 12.95 | 5.88 | −0.01 | 0.12 | −0.24 | 0.02 | −0.03 | 0.08 | −0.06 | 0.17 | 0.07 | 0.09 | 0.26 | −0.11 | 0.18 | −0.21 | −0.15 |
| Predictor | β | p | r | p |
|---|---|---|---|---|
| Age | 0.05 | 0.75 | 0.05 | 0.72 |
| Sex | −0.26 | 0.13 | 0.003 | 0.98 |
| Communication skills | 0.22 | 0.21 | 0.42 | 0.001 ** |
| Social skills | 0.18 | 0.35 | 0.15 | 0.28 |
| Daily living skills | −0.21 | 0.31 | 0.28 | 0.04 * |
| Motor skills | 0.12 | 0.49 | 0.11 | 0.41 |
| Sleep | 0.01 | 0.93 | −0.07 | 0.59 |
| Exercise | 0.22 | 0.14 | −0.08 | 0.58 |
| Screen time | −0.69 | <0.001 *** | −0.58 | <0.001 *** |
| Dutch Healthy Diet total score | −0.006 | 0.97 | 0.21 | 0.12 |
| Caloric intake | 0.30 | 0.14 | 0.33 | 0.02* |
| Saturated fat ratio | 0.009 | 0.95 | 0.16 | 0.25 |
| Fiber intake | −0.02 | 0.92 | 0.29 | 0.03 * |
| Child BMI | −0.17 | 0.24 | −0.04 | 0.77 |
| Breastfeeding time | −0.10 | 0.48 | −0.01 | 0.94 |
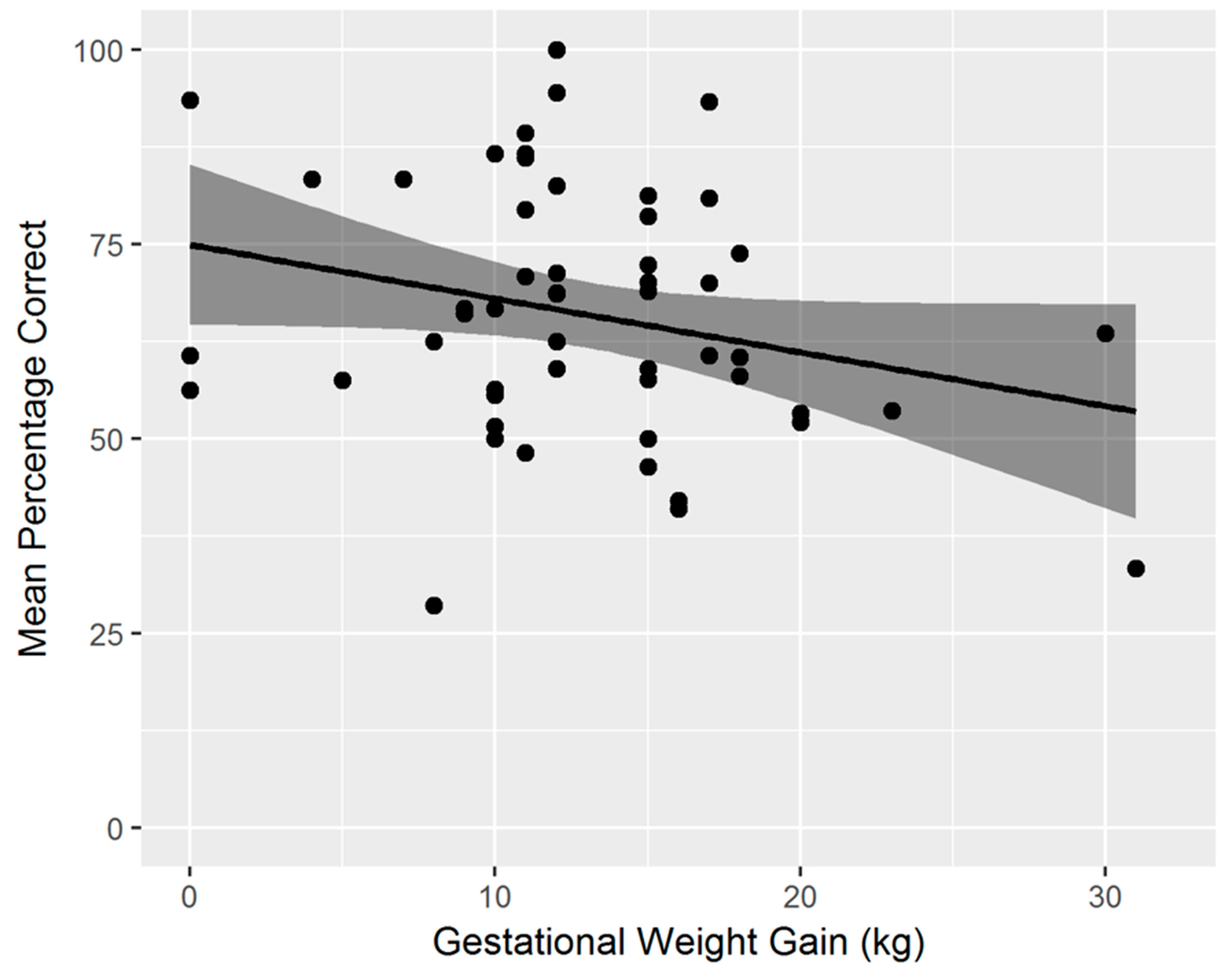
| Gestational weight gain | −0.31 | 0.03 * | −0.38 | 0.005 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Dun, C.; Lisi, I.; van Diepen, J.; Gross, G.; Janzen, G.; Aarts, E. Development of Spatial Orientation in Two-to-Three-Year-Old Children in Relation to Lifestyle Factors. Nutrients 2022, 14, 3322. https://doi.org/10.3390/nu14163322
van Dun C, Lisi I, van Diepen J, Gross G, Janzen G, Aarts E. Development of Spatial Orientation in Two-to-Three-Year-Old Children in Relation to Lifestyle Factors. Nutrients. 2022; 14(16):3322. https://doi.org/10.3390/nu14163322
Chicago/Turabian Stylevan Dun, Claudia, Ilaria Lisi, Janna van Diepen, Gabriele Gross, Gabriele Janzen, and Esther Aarts. 2022. "Development of Spatial Orientation in Two-to-Three-Year-Old Children in Relation to Lifestyle Factors" Nutrients 14, no. 16: 3322. https://doi.org/10.3390/nu14163322
APA Stylevan Dun, C., Lisi, I., van Diepen, J., Gross, G., Janzen, G., & Aarts, E. (2022). Development of Spatial Orientation in Two-to-Three-Year-Old Children in Relation to Lifestyle Factors. Nutrients, 14(16), 3322. https://doi.org/10.3390/nu14163322






