Liposomal Mineral Absorption: A Randomized Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview
2.2. Participants
2.3. Testing Visits
2.4. Nutrient Analysis
2.5. Statistical Analysis
3. Results
3.1. Mixed Models
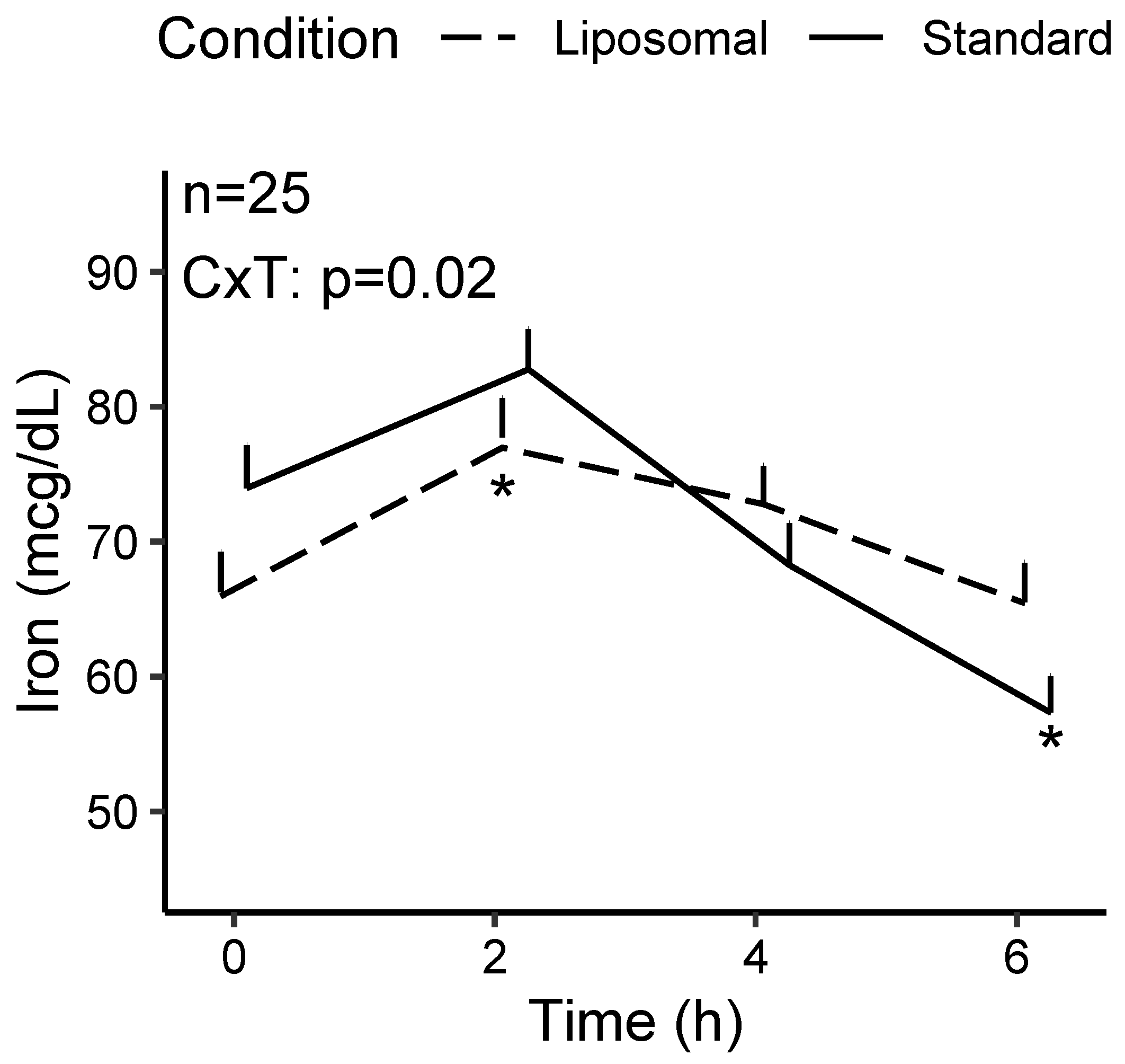
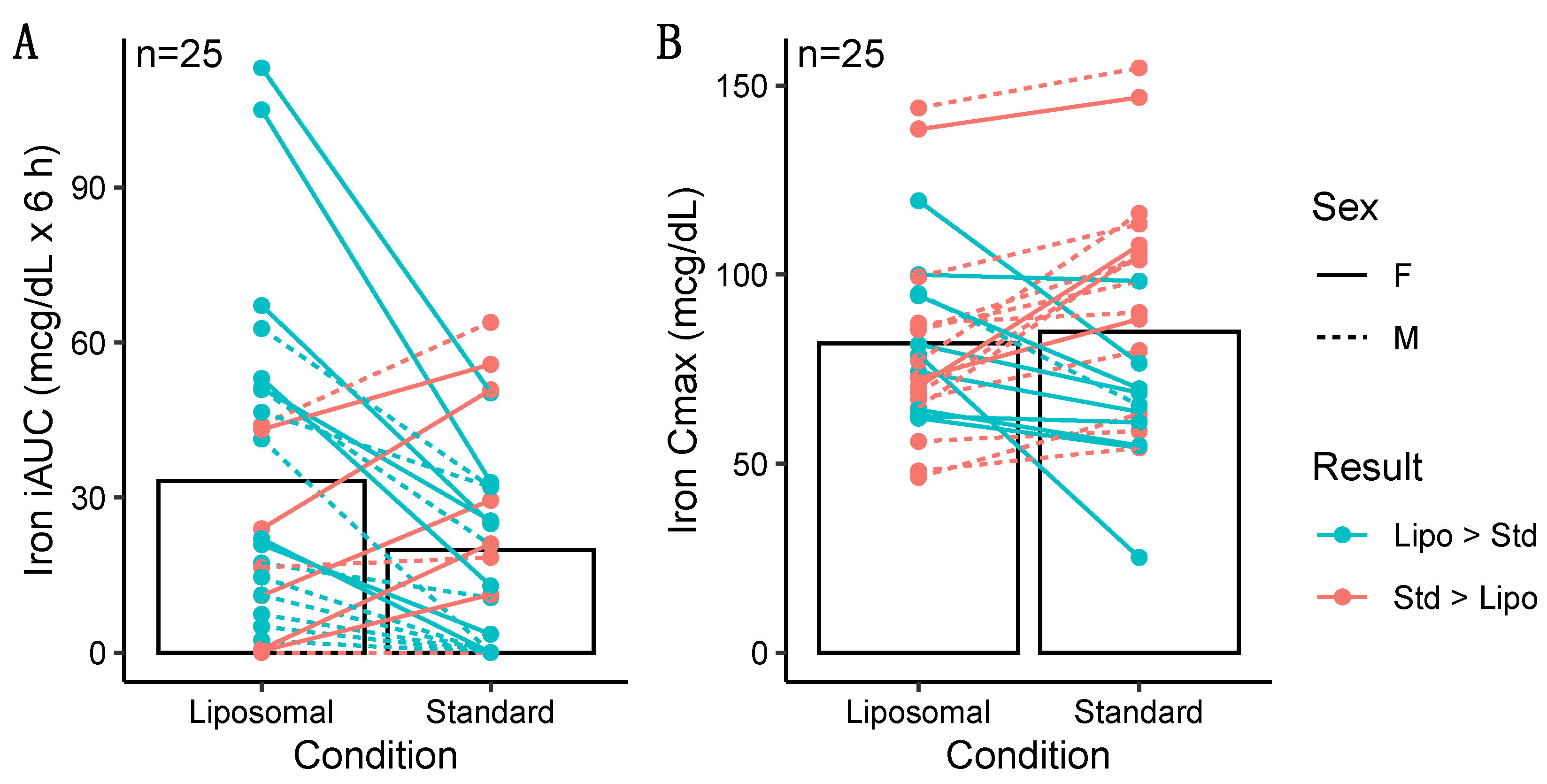
3.1.1. Iron
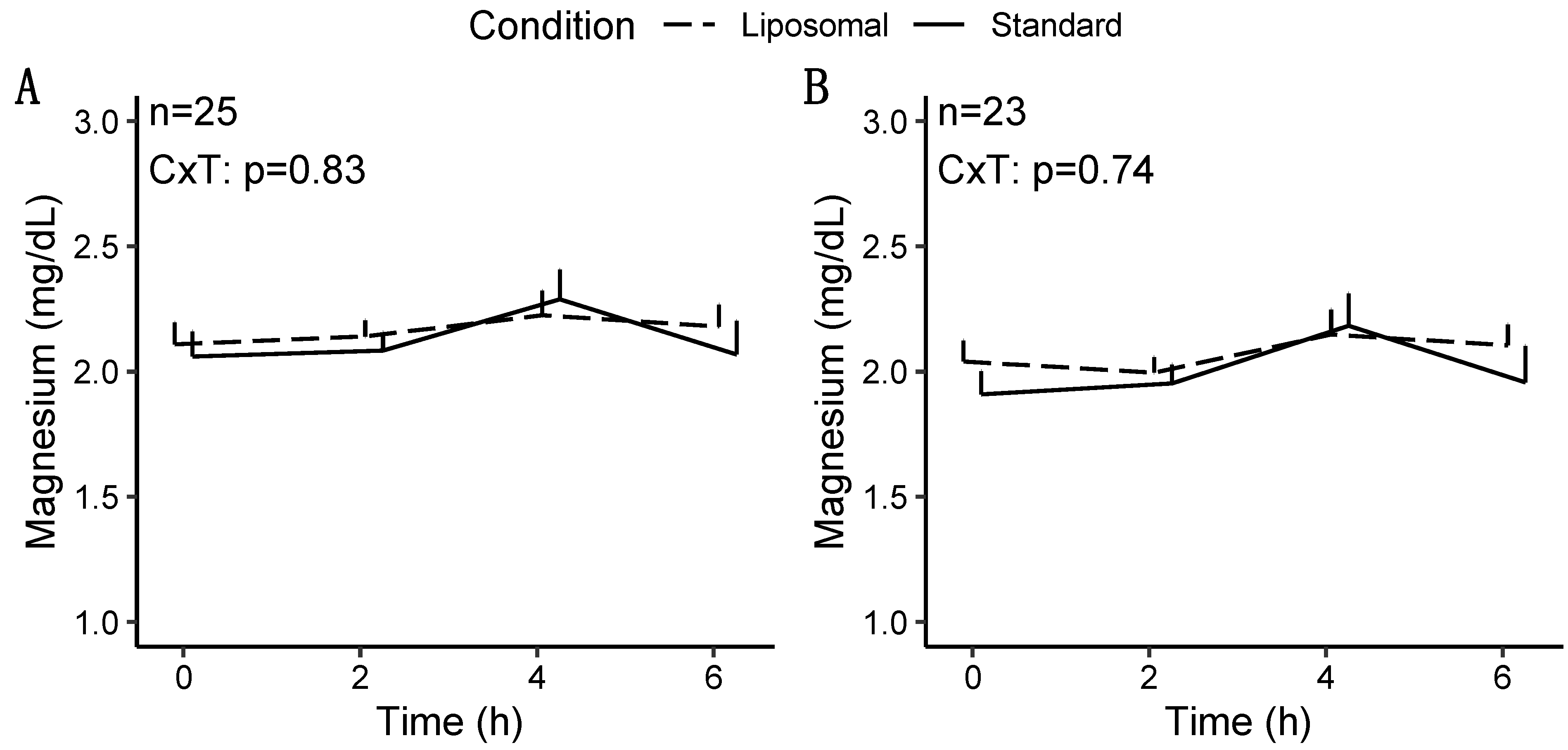
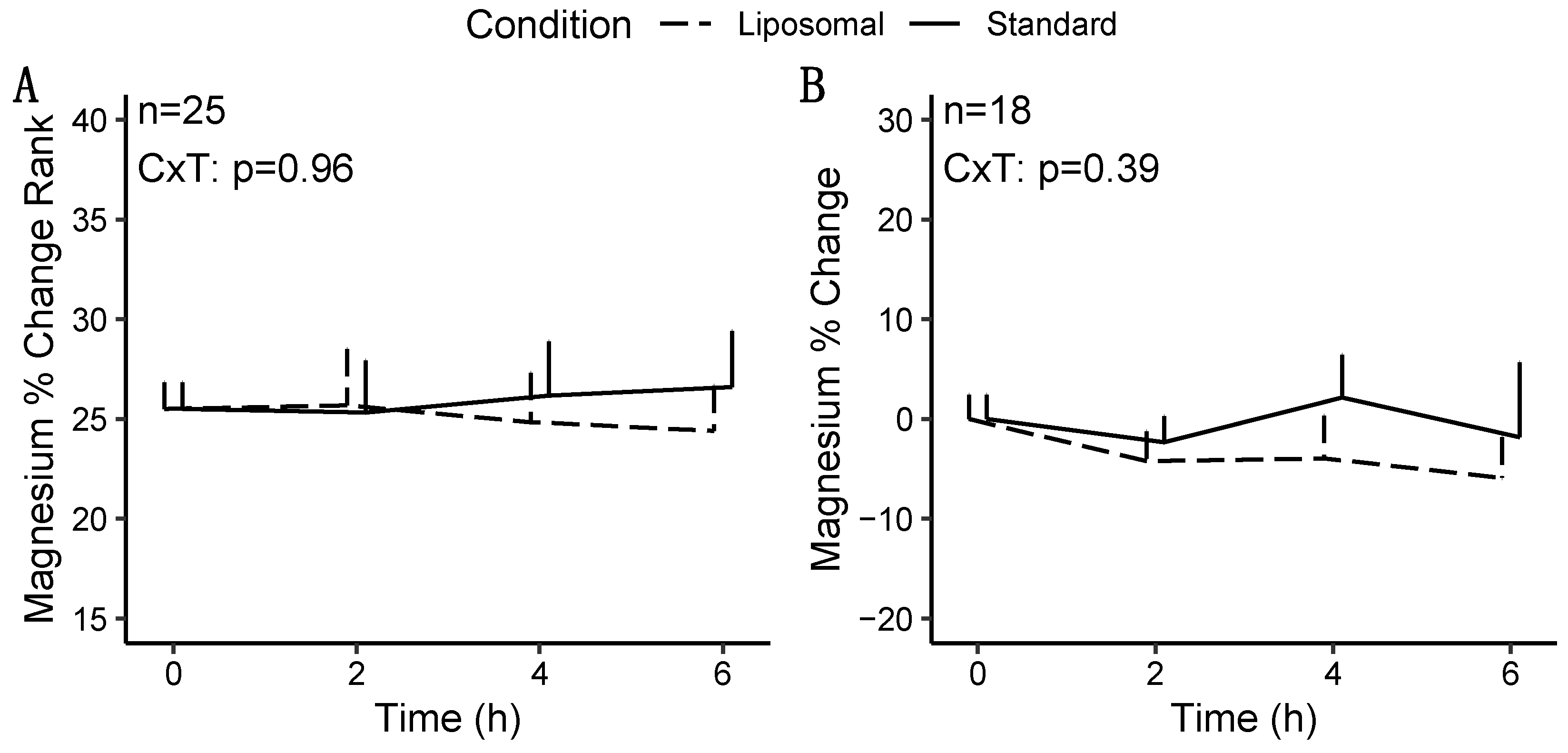
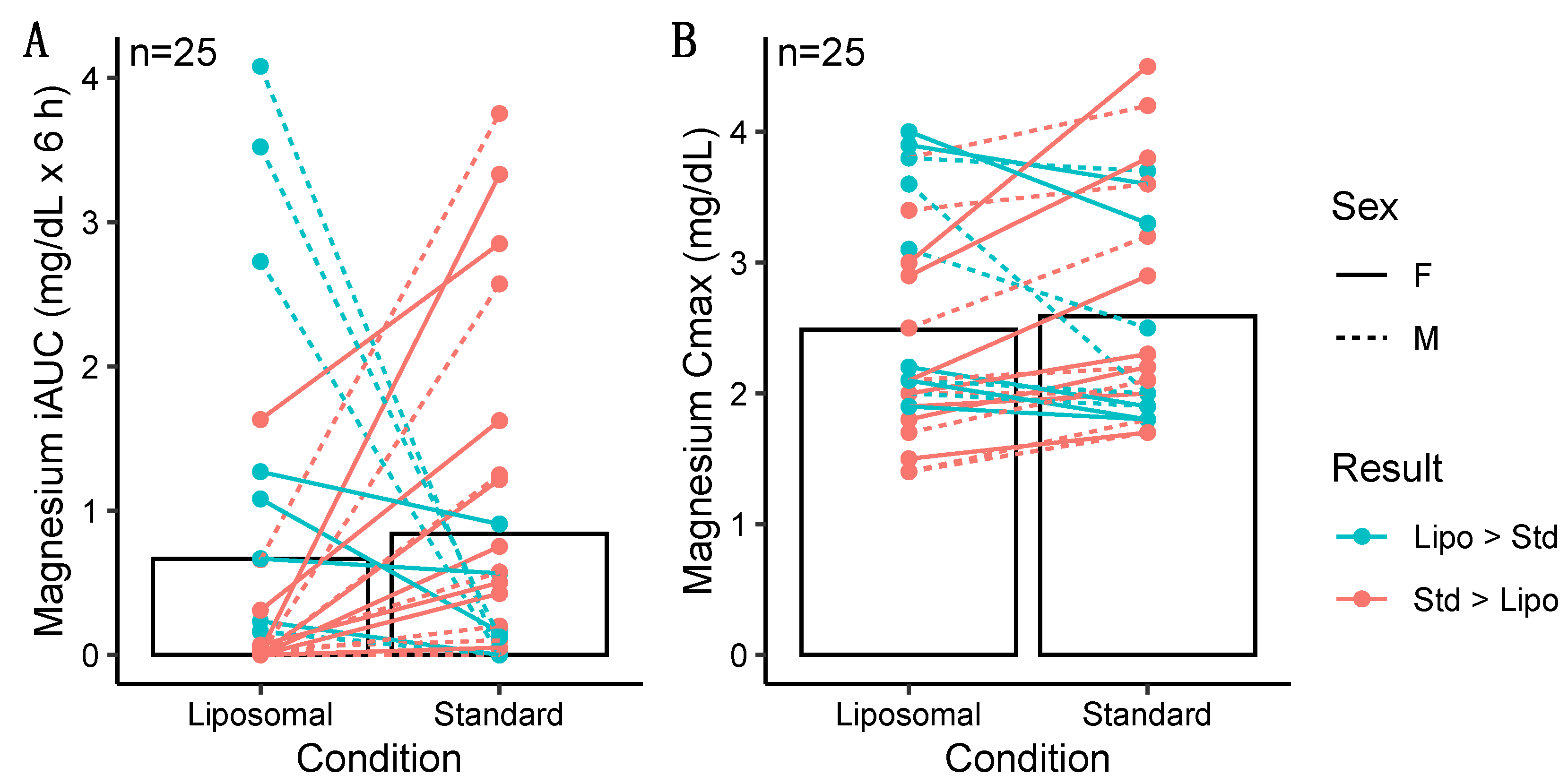
3.1.2. Magnesium
3.2. Pharmacokinetic Analysis
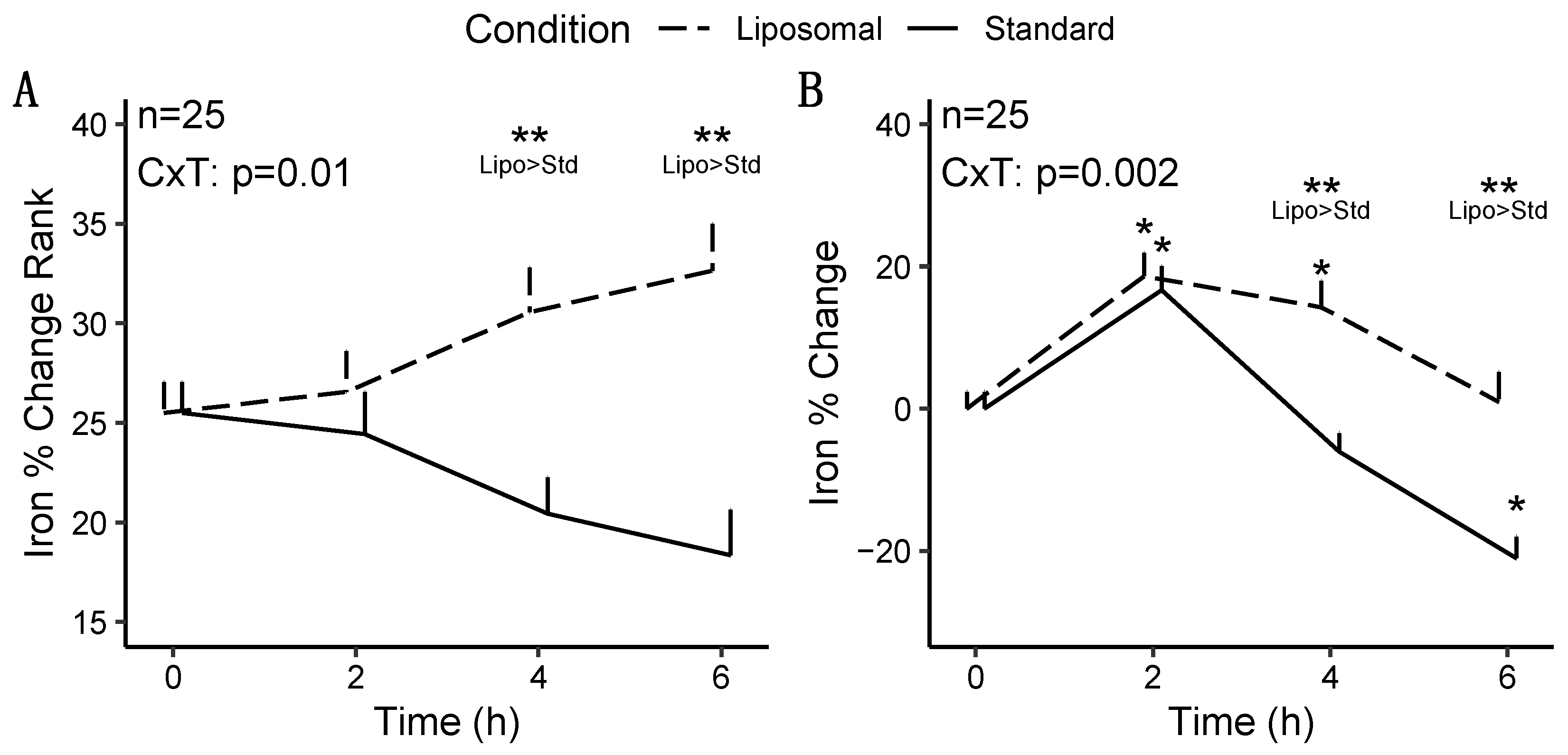
3.2.1. Iron
3.2.2. Magnesium
3.3. Side Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Iron | Magnesium | ||
|---|---|---|---|
| Raw | Raw | Raw (S) | |
| n | 25 | 25 | 23 |
| Intercept | <0.001 * | <0.001 * | <0.001 * |
| Condition | 0.89 | 0.58 | 0.34 |
| Time | <0.001 | 0.29 | 0.14 |
| Sex | 0.32 | 0.88 | 0.66 |
| Condition × Time | 0.02 * | 0.83 | 0.74 |
| Condition × Sex | <0.001 * | 0.38 | 0.79 |
| Time × Sex | 0.40 | 0.38 | 0.34 |
| Condition × Time × Sex | 0.97 | 0.09 | 0.04 * |
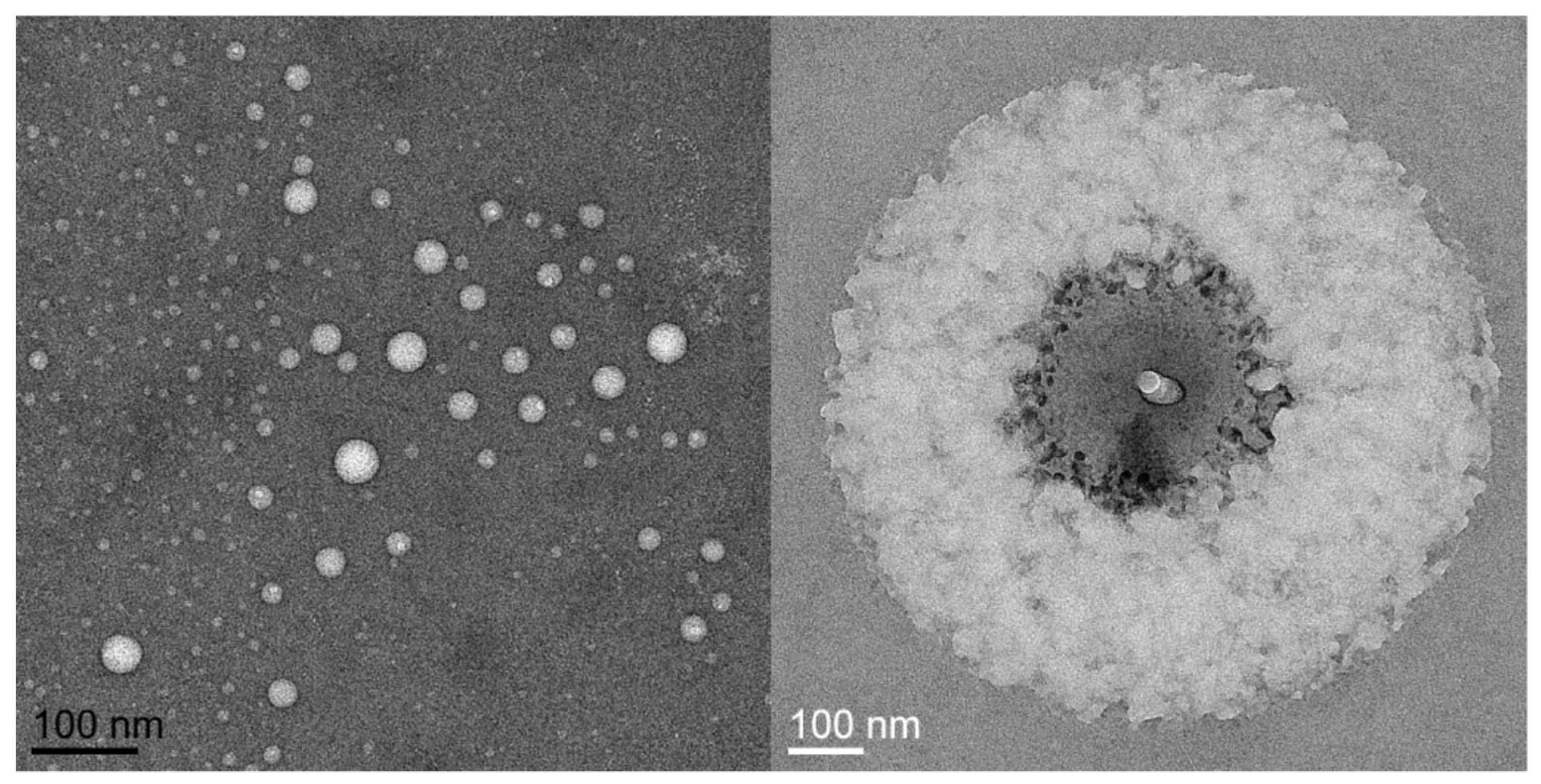
References
- National Institutes of Health Office of Dietary Supplements. Office of Dietary Supplements—Multivitamin/mineral Supplements. Available online: https://ods.od.nih.gov/factsheets/MVMS-Consumer (accessed on 26 January 2022).
- Cowan, A.E.; Jun, S.; Gahche, J.J.; Tooze, J.A.; Dwyer, J.T.; Eicher-Miller, H.A.; Bhadra, A.; Guenther, P.M.; Potischman, N.; Dodd, K.W.; et al. Dietary Supplement Use Differs by Socioeconomic and Health-Related Characteristics among U.S. Adults, NHANES 2011–2014. Nutrients 2018, 10, 1114. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Stierman, B.; Gahche, J.J.; Potischman, N. Dietary Supplement Use among Adults: United States, 2017–2018; National Center for Health Statistics: Hyattsville, MD, USA, 2021. [Google Scholar] [CrossRef]
- Murphy, S.P.; White, K.K.; Park, S.Y.; Sharma, S. Multivitamin-multimineral supplements’ effect on total nutrient intake. Am. J. Clin. Nutr. 2007, 85, 280s–284s. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Pac, S.G.; Fulgoni, V.L., 3rd; Reidy, K.C.; Catalano, P.M. Estimation of Total Usual Dietary Intakes of Pregnant Women in the United States. JAMA Netw. Open 2019, 2, e195967. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.; Macpherson, H.; Vitetta, L.; Kirk, J.; Sali, A.; Pipingas, A. Effects of a multivitamin, mineral and herbal supplement on cognition and blood biomarkers in older men: A randomised, placebo-controlled trial. Hum. Psychopharmacol. 2012, 27, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Özkaya, M.O.; Nazıroğlu, M.; Barak, C.; Berkkanoglu, M. Effects of multivitamin/mineral supplementation on trace element levels in serum and follicular fluid of women undergoing in vitro fertilization (IVF). Biol. Trace Elem. Res. 2011, 139, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maraini, G.; Williams, S.L.; Sperduto, R.D.; Ferris, F.L.; Milton, R.C.; Clemons, T.E.; Rosmini, F.; Ferrigno, L. Effects of multivitamin/mineral supplementation on plasma levels of nutrients. Report No. 4 of the Italian-American clinical trial of nutritional supplements and age-related cataract. Ann. Ist. Super. Sanita 2009, 45, 119–127. [Google Scholar]
- Yetley, E.A. Multivitamin and multimineral dietary supplements: Definitions, characterization, bioavailability, and drug interactions. Am. J. Clin. Nutr. 2007, 85, 269s–276s. [Google Scholar] [CrossRef]
- Comerford, K.B. Recent developments in multivitamin/mineral research. Adv. Nutr. 2013, 4, 644–656. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shade, C.W. Liposomes as Advanced Delivery Systems for Nutraceuticals. Integr. Med. 2016, 15, 33–36. [Google Scholar]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.L.; Paris, H.L.; Beals, J.W.; Binns, S.E.; Giordano, G.R.; Scalzo, R.L.; Schweder, M.M.; Blair, E.; Bell, C. Liposomal-encapsulated Ascorbic Acid: Influence on Vitamin C Bioavailability and Capacity to Protect Against Ischemia-Reperfusion Injury. Nutr. Metab. Insights 2016, 9, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Łukawski, M.; Dałek, P.; Borowik, T.; Foryś, A.; Langner, M.; Witkiewicz, W.; Przybyło, M. New oral liposomal vitamin C formulation: Properties and bioavailability. J. Liposome Res. 2020, 30, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Gopi, S.; Balakrishnan, P. Evaluation and clinical comparison studies on liposomal and non-liposomal ascorbic acid (vitamin C) and their enhanced bioavailability. J. Liposome Res. 2021, 31, 356–364. [Google Scholar] [CrossRef]
- Nowak, J.K.; Sobkowiak, P.; Drzymała-Czyż, S.; Krzyżanowska-Jankowska, P.; Sapiejka, E.; Skorupa, W.; Pogorzelski, A.; Nowicka, A.; Wojsyk-Banaszak, I.; Kurek, S.; et al. Fat-Soluble Vitamin Supplementation Using Liposomes, Cyclodextrins, or Medium-Chain Triglycerides in Cystic Fibrosis: A Randomized Controlled Trial. Nutrients 2021, 13, 4554. [Google Scholar] [CrossRef]
- National Institutes of Health Office of Dietary Supplements. Office of Dietary Supplements—Iron. Available online: https://ods.od.nih.gov/factsheets/Iron-HealthProfessional (accessed on 10 June 2022).
- National Institutes of Health Office of Dietary Supplements. Office of Dietary Supplements—Magnesium. Available online: https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional (accessed on 10 June 2022).
- World Health Organization. Guideline: Daily Iron Supplementation in Adult Women and Adolescent Girls. Available online: https://apps.who.int/iris/bitstream/handle/10665/204761/9789241510196_eng.pdf?sequence=1&isAllowed=y (accessed on 9 June 2022).
- Cabrera, C.C.; Ekström, M.; Linde, C.; Persson, H.; Hage, C.; Eriksson, M.J.; Wallén, H.; Persson, B.; Tornvall, P.; Lyngå, P. Increased iron absorption in patients with chronic heart failure and iron deficiency. J. Card. Fail. 2020, 26, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Jensen, N.M.; Brandsborg, M.; Boesen, A.M.; Yde, H.; Dahlerup, J.F. Low-dose oral iron absorption test: Establishment of a reference interval. Scand J. Clin. Lab. Investig. 1998, 58, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.; Brunk, D.K.; Goodman, D.A. Scottsdale Magnesium Study: Absorption, Cellular Uptake, and Clinical Effectiveness of a Timed-Release Magnesium Supplement in a Standard Adult Clinical Population. J. Am. Coll. Nutr. 2018, 37, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Karagülle, O.; Kleczka, T.; Vidal, C.; Candir, F.; Gundermann, G.; Külpmann, W.R.; Gehrke, A.; Gutenbrunner, C. Magnesium absorption from mineral waters of different magnesium content in healthy subjects. Complement. Med. Res. 2006, 13, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Uschner, D.; Schindler, D.; Hilgers, R.-D.; Heussen, N. randomizeR: An R Package for the Assessment and Implementation of Randomization in Clinical Trials. J. Stat. Softw. 2018, 85, 1–22. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Cousineau, D. Confidence intervals in within-subject designs: A simpler solution to Loftus and Masson’s method. Tutor. Quant. Methods Psychol. 2005, 1, 42–45. [Google Scholar] [CrossRef]
- Morey, R.D. Confidence intervals from normalized data: A correction to Cousineau (2005). Tutor. Quant. Methods Psychol. 2008, 4, 61–64. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Software: Nlme—Linear and Nonlinear Mixed Effects Models, 2021.
- Lenth, R. Software: Emmeans—Estimated Marginal Means, aka Least-Squares Means, 2020.
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef]
- Denney, W.S.; Duvvuri, S.; Buckeridge, C. Simple, Automatic Noncompartmental Analysis: The PKNCA R Package. J. Pharmacokinet. Pharmacodyn. 2015, 42, S65. [Google Scholar] [CrossRef]
- Kassambara, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests, 2020.
- Brilli, E.; Khadge, S.; Fabiano, A.; Zambito, Y.; Williams, T.; Tarantino, G. Magnesium bioavailability after administration of sucrosomial® magnesium: Results of an ex-vivo study and a comparative, double-blinded, cross–over study in healthy subjects. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1843–1851. [Google Scholar] [CrossRef]
- Pineda, O.; Ashmead, H.D. Effectiveness of treatment of iron-deficiency anemia in infants and young children with ferrous bis–glycinate chelate. Nutrition 2001, 17, 381–384. [Google Scholar] [CrossRef]
- Zhuo, Z.; Fang, S.; Yue, M.; Zhang, Y.; Feng, J. Kinetics Absorption Characteristics of Ferrous Glycinate in SD Rats and Its Impact on the Relevant Transport Protein. Biol. Trace Elem. Res. 2014, 158, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Bovell-Benjamin, A.C.; Viteri, F.E.; Allen, L.H. Iron absorption from ferrous bisglycinate and ferric trisglycinate in whole maize is regulated by iron status. Am. J. Clin. Nutr. 2000, 71, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Cogswell, M.E.; Looker, A.C.; Pfeiffer, C.M.; Cusick, S.E.; Lacher, D.A.; Grummer-Strawn, L.M. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Am. J. Clin. Nutr. 2011, 93, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Eicher-Miller, H.A.; Mason, A.C.; Weaver, C.M.; McCabe, G.P.; Boushey, C.J. Food insecurity is associated with iron deficiency anemia in US adolescents. Am. J. Clin. Nutr. 2009, 90, 1358–1371. [Google Scholar] [CrossRef] [PubMed]
- Pisani, A.; Riccio, E.; Sabbatini, M.; Andreucci, M.; Del Rio, A.; Visciano, B. Effect of oral liposomal iron versus intravenous iron for treatment of iron deficiency anaemia in CKD patients: A randomized trial. Nephrol. Dial. Transplant. 2015, 30, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Montagud-Marrahi, E.; Arrizabalaga, P.; Abellana, R.; Poch, E. Liposomal iron in moderate chronic kidney disease. Nefrologia 2020, 40, 446–452. [Google Scholar] [CrossRef] [PubMed]
- de Alvarenga Antunes, C.V.; de Alvarenga Nascimento, C.R.; Campanha da Rocha Ribeiro, T.; de Alvarenga Antunes, P.; de Andrade Chebli, L.; Martins Gonçalves Fava, L.; Malaguti, C.; Maria Fonseca Chebli, J. Treatment of iron deficiency anemia with liposomal iron in inflammatory bowel disease: Efficacy and impact on quality of life. Int. J. Clin. Pharm. 2020, 42, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Parisi, F.; Berti, C.; Mandò, C.; Martinelli, A.; Mazzali, C.; Cetin, I. Effects of different regimens of iron prophylaxis on maternal iron status and pregnancy outcome: A randomized control trial. J. Matern. Fetal Neonatal Med. 2017, 30, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
| All (n = 25) | Males (n = 13) | Females (n = 12) | |
|---|---|---|---|
| Age (y) | 26.0 ± 3.4 | 26.2 ± 3.6 | 25.9 ± 3.5 |
| Height (cm) | 169.8 ± 9.0 | 175.9 ± 6.6 | 163.3 ± 6.1 |
| Weight (kg) | 74.7 ± 15.6 | 83.0 ± 10.9 | 65.8 ± 15.3 |
| BMI (kg/m2) | 25.7 ± 3.9 | 26.8 ± 2.8 | 24.5 ± 4.6 |
| Body fat (%) | 25.4 ± 8.3 | 21.4 ± 7.2 | 29.7 ± 7.5 |
| Raw Material 1 | Nutrient | Specified Amount (Unit) 2 | Std. MVM 2 | Lipo. MVM 2 | |
|---|---|---|---|---|---|
| Vegan Beta Carotene | A | 900 | mcg | 950 | 1098 |
| Methylcobalamin | B12 | 1000 | mcg | 1258 | 1210 |
| Ascorbic Acid | C | 90 | mg | 108.4 | 112.7 |
| Vegan Vitamin D-3 | D3 | 20 | mcg | 22.2 | 23.9 |
| (6S)-5-Methyltetrahydrofolate | Folic Acid 3 | 235.3 | mcg | 320.6 | 326.5 |
| Ferrous Glycinate | Iron * | 9 | mg | 10.1 | 9.37 |
| Magnesium Glycinate | Magnesium * | 20 | mg | 23.3 | 22.0 |
| Manganese Citrate | Manganese | 2.3 | mg | 2.6 | 2.6 |
| Zinc Citrate | Zinc | 11 | mg | 12.8 | 15.0 |
| Benfotiamine | B1 | 1.2 | mg | 1.0 | 0.9 |
| Riboflavin-5-Phosphate | B2 | 1.3 | mg | 0.9 | 1.1 |
| Niacinamide | B3 | 16 | mg | 18.1 | 17.1 |
| Calcium d-Pantothenate | B5 | 5 | mg | 6.4 | 5.1 |
| Pyridoxine-5-Phospate | B6 | 1.7 | mg | 1.0 | 0.6 |
| Biotin | Biotin | 30 | mcg | 56.8 | 33.0 |
| Choline Bitartrate | Choline | 25 | mg | 57.6 | 44.8 |
| Chromium Glycinate | Chromium | 35 | mcg | 54.7 | 58.0 |
| Co-Q10 | Co-Q10 | 5 | mg | 4.3 | 3.8 |
| Sunflower Vitamin E (a-Tocopherol) | E | 15 | mg | 16.4 | 16.3 |
| Inositol | Inositol | 25 | mg | 19.2 | 22.3 |
| Potassium Iodide | Iodine | 150 | mcg | 171.9 | 244.0 |
| K1 | K1 | 120 | mcg | 129 | 74.5 |
| Vegan Lutein Beadlet | Lutein | 1 | mg | 1.27 | 1.25 |
| Molybdenum Glycinate | Molybdenum | 45 | mcg | 54.1 | 53.6 |
| P-Aminobenzoic Acid | PABA | 5 | mg | 3.4 | 4.1 |
| Selenium | Selenium | 55 | mg | 54.7 | 51.8 |
| Acerola CherryJuice Powder | - | 2 | mg | NT | NT |
| Trace Minerals | - | 5 | mg | NT | NT |
| Rosehips | - | 2 | mg | NT | NT |
| Iron | Magnesium | |||||
|---|---|---|---|---|---|---|
| Δ Rank | Δ | Δ (S) | Δ Rank | Δ | Δ (S) | |
| n | 25 | 25 | 24 | 25 | 25 | 18 |
| Intercept | <0.001 * | 0.72 | 0.98 | <0.001 * | 0.13 | 0.14 |
| Condition | <0.001 * | 0.005 * | 0.02 * | 0.66 | 0.93 | 1.00 |
| Time | 1.00 | <0.001 * | <0.001 * | 1.00 | 0.14 | 0.12 |
| Sex | 0.07 | 0.045 * | 0.08 | 0.25 | 0.80 | 0.77 |
| Condition × Time | 0.01 * | 0.002 * | 0.004 * | 0.96 | 0.79 | 0.74 |
| Condition × Sex | 0.80 | 0.72 | 0.64 | 0.34 | 0.41 | 0.44 |
| Time × Sex | 0.006 * | 0.006 * | 0.001 * | 0.58 | 0.36 | 0.41 |
| Condition × Time × Sex | 0.37 | 0.27 | 0.29 | 0.16 | 0.06 | 0.04 * |
| Liposomal MVM | Standard MVM | All | Females | Males | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | sd | Med | IQR | Mean | sd | Med | IQR | p | ES | p | ES | p | ES | |
| Iron | 33.22 | 30.90 | 22.00 | 39.84 | 19.84 | 19.75 | 18.40 | 32.01 | 0.02 * | 0.52 | 0.13 | 0.47 | 0.03 * | 0.68 |
| Mag. | 0.67 | 1.15 | 0.07 | 0.67 | 0.84 | 1.13 | 0.43 | 1.20 | 0.30 | 0.22 | 0.09 | 0.54 | 1.00 | 0.02 |
| Liposomal MVM | Standard MVM | All | Females | Males | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | sd | Med | IQR | Mean | sd | Med | IQR | p | ES | p | ES | p | ES | |
| Iron | 81.72 | 24.72 | 77.07 | 29.60 | 84.94 | 30.39 | 79.87 | 41.89 | 0.51 | 0.13 | 0.25 | 0.35 | 0.01 * | 0.69 |
| Mag. | 2.49 | 0.84 | 2.10 | 1.20 | 2.59 | 0.88 | 2.20 | 1.40 | 0.41 | 0.17 | 0.27 | 0.34 | 0.46 | 0.21 |
| Liposomal MVM | Standard MVM | All | Females | Males | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | sd | Med | IQR | Mean | sd | Med | IQR | p | ES | p | ES | p | ES | |
| Iron | 3.0 | 1.5 | 2.2 | 2.0 | 1.5 | 1.0 | 2.2 | 2.2 | 0.002 * | 0.68 | 0.10 | 0.57 | 0.01 * | 0.77 |
| Mag. | 3.2 | 2.7 | 4.2 | 6.2 | 3.6 | 2.5 | 4.2 | 4.0 | 0.62 | 0.08 | 0.48 | 0.13 | 1.00 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinsley, G.M.; Harty, P.S.; Stratton, M.T.; Siedler, M.R.; Rodriguez, C. Liposomal Mineral Absorption: A Randomized Crossover Trial. Nutrients 2022, 14, 3321. https://doi.org/10.3390/nu14163321
Tinsley GM, Harty PS, Stratton MT, Siedler MR, Rodriguez C. Liposomal Mineral Absorption: A Randomized Crossover Trial. Nutrients. 2022; 14(16):3321. https://doi.org/10.3390/nu14163321
Chicago/Turabian StyleTinsley, Grant M., Patrick S. Harty, Matthew T. Stratton, Madelin R. Siedler, and Christian Rodriguez. 2022. "Liposomal Mineral Absorption: A Randomized Crossover Trial" Nutrients 14, no. 16: 3321. https://doi.org/10.3390/nu14163321
APA StyleTinsley, G. M., Harty, P. S., Stratton, M. T., Siedler, M. R., & Rodriguez, C. (2022). Liposomal Mineral Absorption: A Randomized Crossover Trial. Nutrients, 14(16), 3321. https://doi.org/10.3390/nu14163321






