The Efficacy of Camelina sativa Defatted Seed Meal against Colitis-Induced Persistent Visceral Hypersensitivity: The Relevance of PPAR α Receptor Activation in Pain Relief
Abstract
1. Introduction
2. Materials and Methods
2.1. Camelina Sativa Defatted Seed Meal Production and Characterization
2.2. Animals
2.3. Induction of Colitis
2.4. Treatments
2.5. Assessment of Visceral Sensitivity by Abdominal Withdrawal Reflex to Colorectal Distension
2.6. Histological Analysis of Colon
2.7. Immunohistochemistry
2.8. Statistics
3. Results
3.1. Characterization of Camelina Sativa Defatted Seed Meal Composition
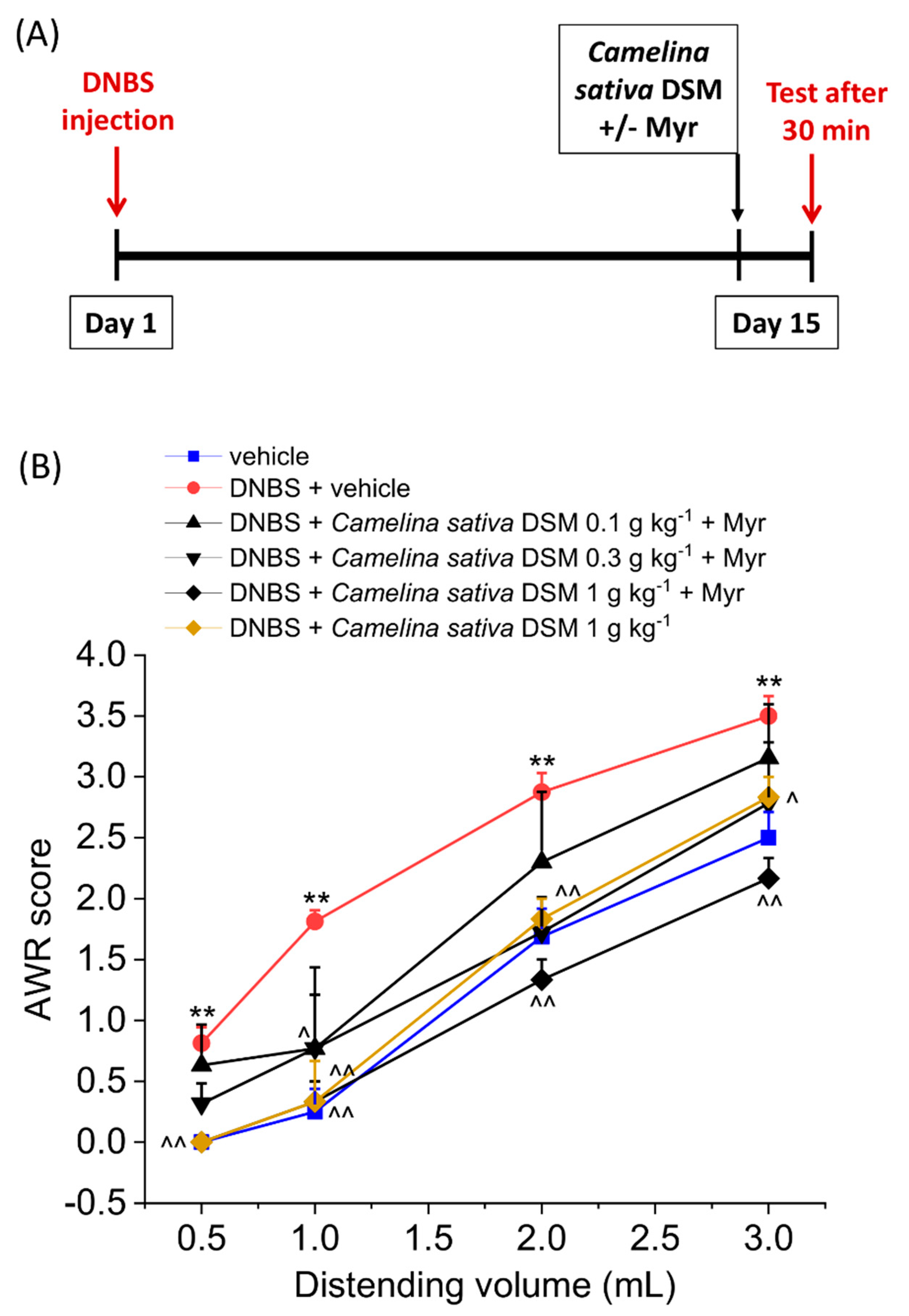
3.2. Acute Anti-Hyperalgesic Efficacy of Camelina Sativa DSM on Pain Associated with Colitis in Rats: Evaluation of the Role of Glucosinolates
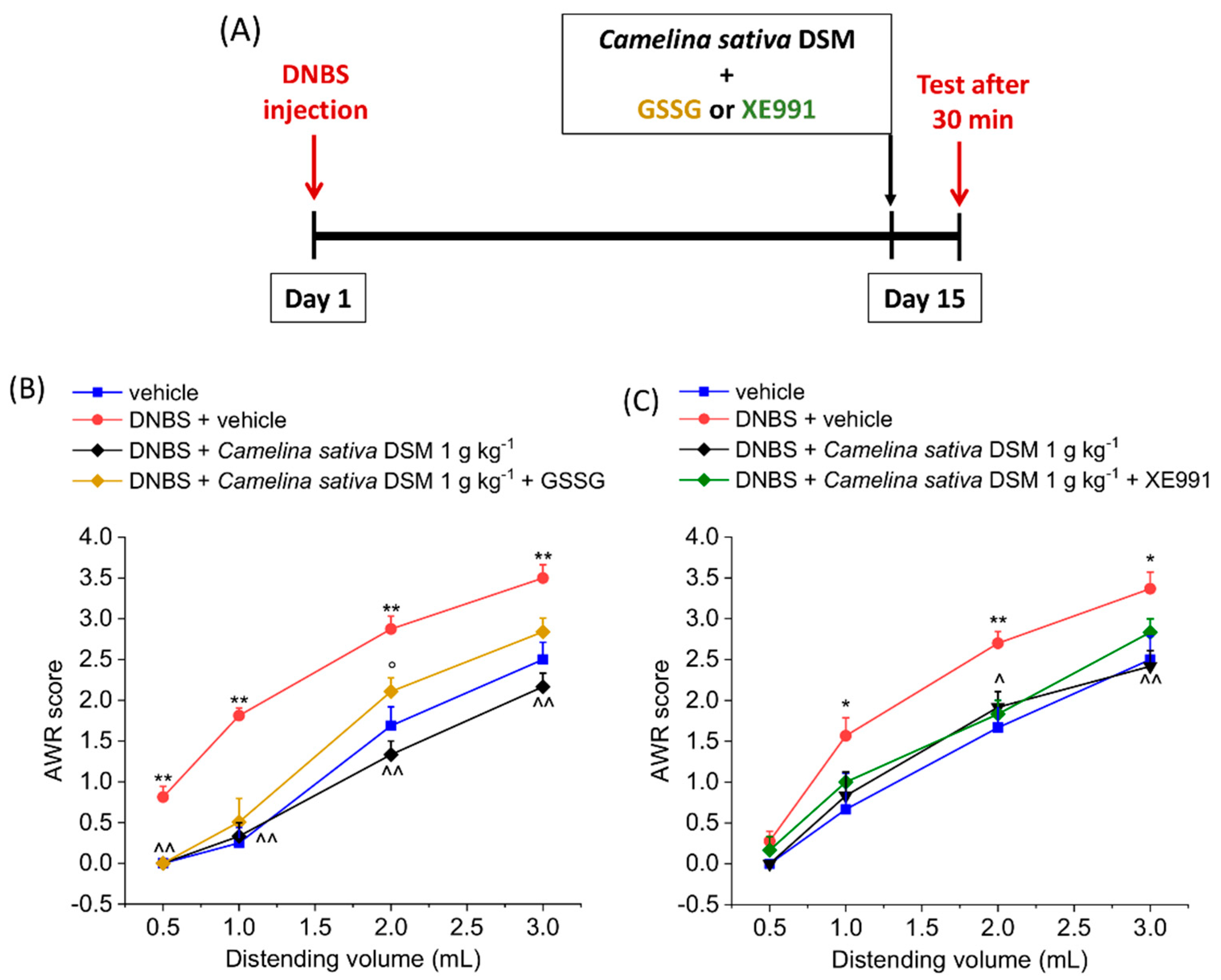
3.3. PPAR-α Activation Mediated the Acute Effect of Camelina Sativa DSM on Visceral Pain
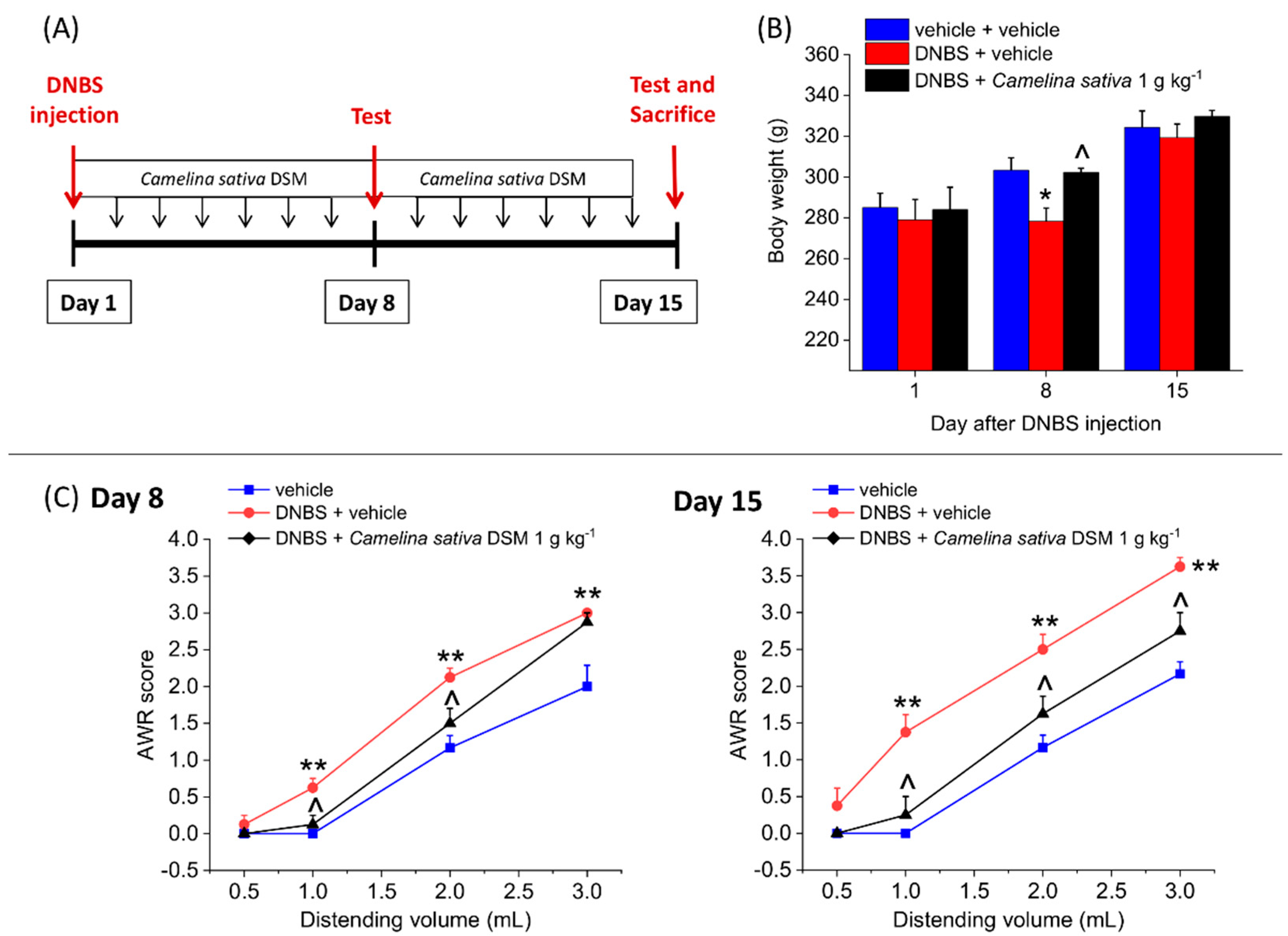
3.4. The Repeated Treatment with Camelina Sativa DSM Prevented the Development and Persistence of Pain Associated with Colitis in Rats
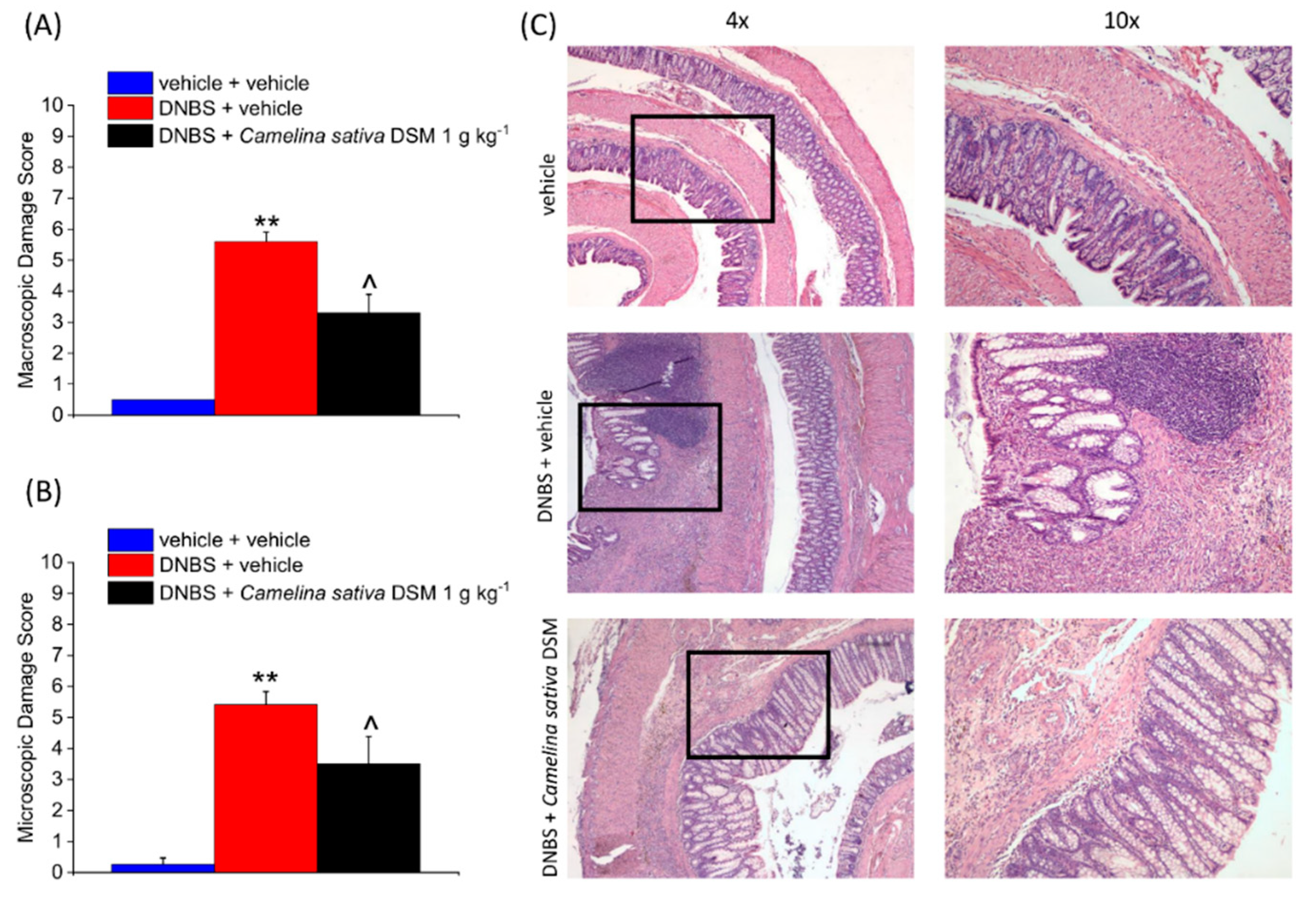
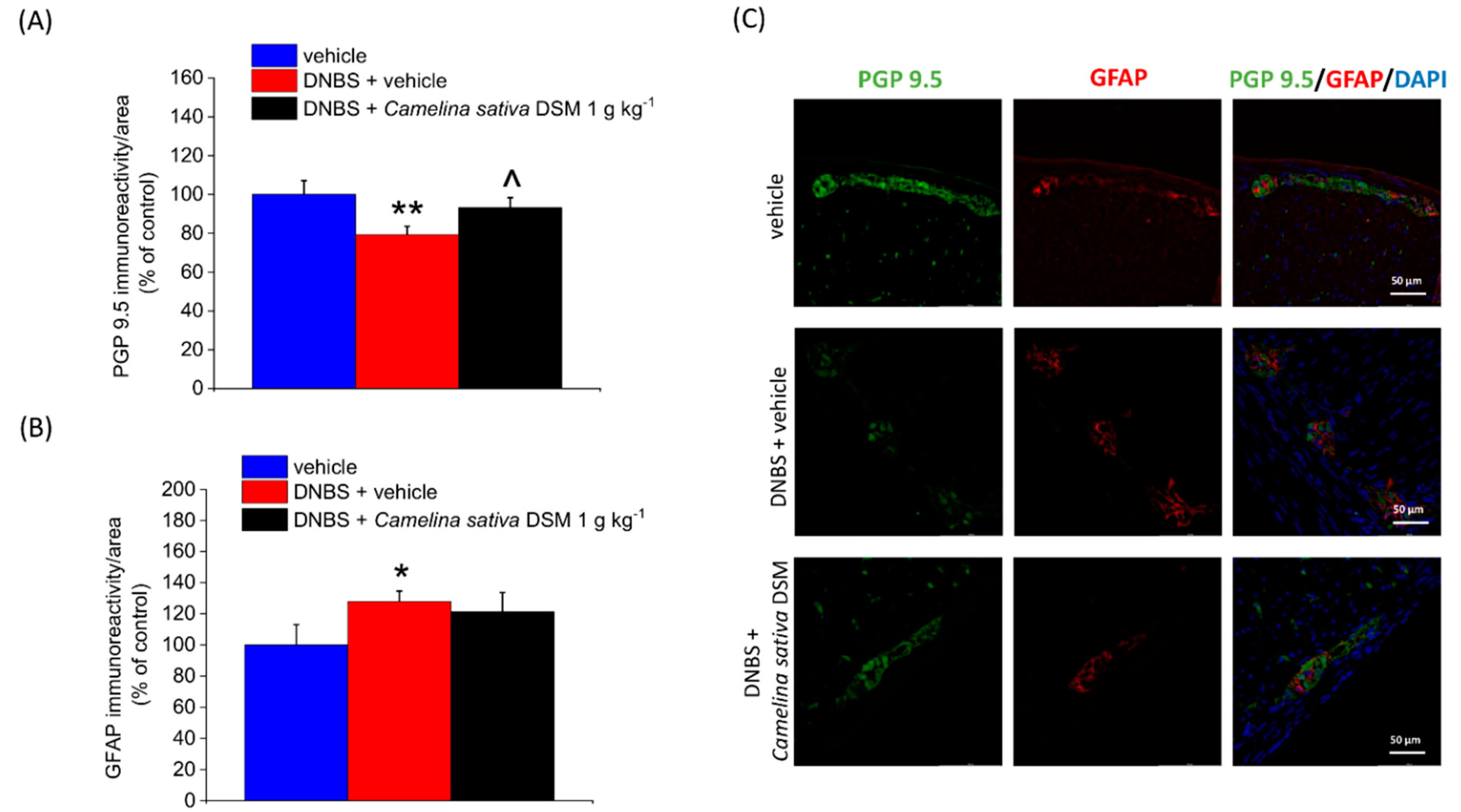
3.5. Camelina Sativa DSM Promoted Tissue Healing and Reduced Mast Cells Infiltration in the Colon
3.6. Neuroprotective Effect of Camelina Sativa DSM on the Colon
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drewes, A.M.; Olesen, A.E.; Farmer, A.D.; Szigethy, E.; Rebours, V.; Olesen, S.S. Gastrointestinal pain. Nat. Rev. Dis. Primers. 2020, 6, 1. [Google Scholar] [CrossRef]
- Spiller, R.; Major, G. IBS and IBD-separate entities or on a spectrum? Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 613–621. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Cui, X.; Wang, M.; Jiao, C.; Li, J.; Yang, Y.; Li, Y.; Zhang, H. A Pilot Study of Clinical Evaluation and Formation Mechanism of Irritable Bowel Syndrome-like Symptoms in Inflammatory Bowel Disease Patients in Remission. J. Neurogastroenterol. Motil. 2021, 27, 612–625. [Google Scholar] [CrossRef]
- Srinath, A.I.; Walter, C.; Newara, M.C.; Szigethy, E.M. Pain management in patients with inflammatory bowel disease: Insights for the clinician. Therap. Adv. Gastroenterol. 2012, 5, 339–357. [Google Scholar] [CrossRef]
- Camilleri, M.; Boeckxstaens, G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut 2017, 66, 966–974. [Google Scholar] [CrossRef]
- Kaushik, A.S.; Strath, L.J.; Sorge, R.E. Dietary interventions for treatment of chronic pain: Oxidative stress and inflammation. Pain Ther. 2020, 9, 487–498. [Google Scholar] [CrossRef]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef]
- Lucarini, E.; Parisio, C.; Branca, J.J.; Segnani, C.; Ippolito, C.; Pellegrini, C.; Antonioli, L.; Fornai, M.; Micheli, L.; Pacini, A. Deepening the mechanisms of visceral pain persistence: An evaluation of the gut-spinal cord relationship. Cells 2020, 9, 1772. [Google Scholar] [CrossRef]
- Lucarini, E.; Micheli, L.; Di Cesare Mannelli, L.; Ghelardini, C. Naturally occurring glucosinolates and isothiocyanates as a weapon against chronic pain: Potentials and limits. Phytochem. Rev. 2022, 21, 647–665. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef]
- Citi, V.; Martelli, A.; Testai, L.; Marino, A.; Breschi, M.C.; Calderone, V. Hydrogen sulfide releasing capacity of natural isothiocyanates: Is it a reliable explanation for the multiple biological effects of Brassicaceae? Planta Med. 2014, 80, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, J.L.; Liu, X.; Charron, C.S.; Novotny, J.A.; Jeffery, E.H.; Seifried, H.E.; Ross, S.A.; Miller, M.J.; Swanson, K.S.; Holscher, H.D. Broccoli consumption affects the human gastrointestinal microbiota. J. Nutr. Biochem. 2019, 63, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Mota, V.R.; Saavedra, M.J.; Rosa, E.A.; Bennett, R.N. The antimicrobial effects of glucosinolates and their respective enzymatic hydrolysis products on bacteria isolated from the human intestinal tract. J. Appl. Microbiol. 2009, 106, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- Kellingray, L.; Le Gall, G.; Doleman, J.F.; Narbad, A.; Mithen, R.F. Effects of in vitro metabolism of a broccoli leachate, glucosinolates and S-methylcysteine sulphoxide on the human faecal microbiome. Eur. J. Nutr. 2021, 60, 2141–2154. [Google Scholar] [CrossRef]
- Lucarini, E.; Di Pilato, V.; Parisio, C.; Micheli, L.; Toti, A.; Pacini, A.; Bartolucci, G.; Baldi, S.; Niccolai, E.; Amedei, A.; et al. Visceral sensitivity modulation by faecal microbiota transplantation: The active role of gut bacteria in pain persistence. Pain 2021, 163, 861–877. [Google Scholar] [CrossRef]
- Luczynski, P.; Tramullas, M.; Viola, M.; Shanahan, F.; Clarke, G.; O’Mahony, S.; Dinan, T.G.; Cryan, J.F. Microbiota regulates visceral pain in the mouse. Elife 2017, 6, e25887. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, M.M.; Gareau, M.G. Visceral pain: Gut microbiota, a new hope? J. Biomed. Sci. 2018, 25, 73. [Google Scholar] [CrossRef]
- Lucarini, E.; Micheli, L.; Trallori, E.; Citi, V.; Martelli, A.; Testai, L.; De Nicola, G.R.; Iori, R.; Calderone, V.; Ghelardini, C. Effect of glucoraphanin and sulforaphane against chemotherapy-induced neuropathic pain: Kv7 potassium channels modulation by H2S release in vivo. Phytother. Res. 2018, 32, 2226–2234. [Google Scholar] [CrossRef] [PubMed]
- Miceli, F.; Soldovieri, M.V.; Martire, M.; Taglialatela, M. Molecular pharmacology and therapeutic potential of neuronal Kv7-modulating drugs. Curr. Opin. Pharmacol. 2008, 8, 65–74. [Google Scholar] [CrossRef]
- Lucarini, E.; Pagnotta, E.; Micheli, L.; Parisio, C.; Testai, L.; Martelli, A.; Calderone, V.; Matteo, R.; Lazzeri, L.; Di Cesare Mannelli, L.; et al. Eruca sativa Meal against Diabetic Neuropathic Pain: An H(2)S-Mediated Effect of Glucoerucin. Molecules 2019, 24, 3006. [Google Scholar] [CrossRef]
- Mannelli, L.D.C.; Lucarini, E.; Micheli, L.; Mosca, I.; Ambrosino, P.; Soldovieri, M.V.; Martelli, A.; Testai, L.; Taglialatela, M.; Calderone, V. Effects of natural and synthetic isothiocyanate-based H2S-releasers against chemotherapy-induced neuropathic pain: Role of Kv7 potassium channels. Neuropharmacology 2017, 121, 49–59. [Google Scholar] [CrossRef]
- Brown, D.A.; Passmore, G.M. Neural KCNQ (kv7) channels. Br. J. Pharmacol. 2009, 156, 1185–1195. [Google Scholar] [CrossRef]
- Nickerson, A.J.; Rottgen, T.S.; Rajendran, V.M. Activation of KCNQ (KV7) K+ channels in enteric neurons inhibits epithelial Cl− secretion in mouse distal colon. Am. J. Physiol.-Cell Physiol. 2021, 320, C1074–C1087. [Google Scholar] [CrossRef]
- Hirano, K.; Kuratani, K.; Fujiyoshi, M.; Tashiro, N.; Hayashi, E.; Kinoshita, M. Kv7.2-7.5 voltage-gated potassium channel (KCNQ2-5) opener, retigabine, reduces capsaicin-induced visceral pain in mice. Neurosci. Lett. 2007, 413, 159–162. [Google Scholar] [CrossRef]
- Lucarini, E.; Micheli, L.; Pagnotta, E.; Matteo, R.; Parisio, C.; Toti, A.; Ferrara, V.; Ciampi, C.; Martelli, A.; Testai, L. Beneficial Effects of Eruca sativa Defatted Seed Meal on Visceral Pain and Intestinal Damage Resulting from Colitis in Rats. Foods 2022, 11, 580. [Google Scholar]
- Zubr, J. Oil-seed crop: Camelina sativa. Ind. Crops Prod. 1997, 6, 113–119. [Google Scholar]
- Pilgeram, A.L. Camelina Sativa, a Montana Omega-3 and Fuel Crop. 2007. Available online: https://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.571.9006 (accessed on 27 June 2022).
- Waraich, E.A.; Ahmed, Z.; Ahmad, R.; Ashraf, M.Y.; Naeem, M.S.; Rengel, Z. ‘Camelina sativa’, a climate proof crop, has high nutritive value and multiple-uses: A review. Aust. J. Crop Sci. 2013, 7, 1551–1559. [Google Scholar]
- Das, N.; Berhow, M.A.; Angelino, D.; Jeffery, E.H. Camelina sativa defatted seed meal contains both alkyl sulfinyl glucosinolates and quercetin that synergize bioactivity. J. Agric. Food Chem. 2014, 62, 8385–8391. [Google Scholar]
- Russo, R.; Reggiani, R. Glucosinolates and Sinapine in camelina meal. Food Nutr. Sci. 2017, 8, 1063–1073. [Google Scholar] [CrossRef]
- Berhow, M.A.; Polat, U.; Glinski, J.A.; Glensk, M.; Vaughn, S.F.; Isbell, T.; Ayala-Diaz, I.; Marek, L.; Gardner, C. Optimized analysis and quantification of glucosinolates from Camelina sativa seeds by reverse-phase liquid chromatography. Ind. Crops Prod. 2013, 43, 119–125. [Google Scholar] [CrossRef]
- Cojocariu, R.O.; Balmus, I.M.; Lefter, R.; Hritcu, L.; Ababei, D.C.; Ciobica, A.; Copaci, S.; Mot, S.E.L.; Copolovici, L.; Copolovici, D.M.; et al. Camelina sativa Methanolic and Ethanolic Extract Potential in Alleviating Oxidative Stress, Memory Deficits, and Affective Impairments in Stress Exposure-Based Irritable Bowel Syndrome Mouse Models. Oxid. Med. Cell. Longev. 2020, 2020, 9510305. [Google Scholar] [CrossRef]
- Morampudi, V.; Bhinder, G.; Wu, X.; Dai, C.; Sham, H.P.; Vallance, B.A.; Jacobson, K. DNBS/TNBS colitis models: Providing insights into inflammatory bowel disease and effects of dietary fat. J. Vis. Exp. 2014, e51297. [Google Scholar] [CrossRef]
- Lazzeri, L.; Malaguti, L.; Bagatta, M.; D’Avino, L.; Ugolini, L.; De Nicola, G.R.; Casadei, N.; Cinti, S.; Matteo, R.; Iori, R. Characterization of the main Glucosinolate Content and Fatty Acid Composition in Non-food Brassicaceae Seeds. Acta Hortic. 2013, 1005, 331–338. [Google Scholar] [CrossRef]
- Russo, R.; Galasso, I.; Reggiani, R. Variability in glucosinolate content among Camelina species. Am. J. Plant Sci. 2014, 2014, 42529. [Google Scholar]
- Testai, L.; Pagnotta, E.; Piragine, E.; Flori, L.; Citi, V.; Martelli, A.; Mannelli, L.D.C.; Ghelardini, C.; Matteo, R.; Suriano, S. Cardiovascular benefits of Eruca sativa mill. Defatted seed meal extract: Potential role of hydrogen sulfide. Phytother. Res. 2022, 36, 2616–2627. [Google Scholar] [CrossRef]
- McGrath, J.C.; Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef] [PubMed]
- Gado, F.; Di Cesare Mannelli, L.; Lucarini, E.; Bertini, S.; Cappelli, E.; Digiacomo, M.; Stevenson, L.A.; Macchia, M.; Tuccinardi, T.; Ghelardini, C. Identification of the first synthetic allosteric modulator of the CB2 receptors and evidence of its efficacy for neuropathic pain relief. J. Med. Chem. 2018, 62, 276–287. [Google Scholar] [CrossRef]
- Matteo, R.; Ramirez-Cando, L.J.; Pagnotta, E.; Angelini, L.G.; Spugnoli, P.; Tavarini, S.; Ugolini, L.; Foschi, L.; Lazzeri, L. Camelina (Camelina sativa L. Crantz) under low-input management systems in northern Italy: Yields, chemical characterization and environmental sustainability. Ital. J. Agron. 2020, 15, 132–143. [Google Scholar] [CrossRef]
- Berti, M.; Gesch, R.; Eynck, C.; Anderson, J.; Cermak, S. Camelina uses, genetics, genomics, production, and management. Ind. Crops Prod. 2016, 94, 690–710. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, B.; Shan, S.; Zhao, X. Neuroprotective effects of vitexin against isoflurane-induced neurotoxicity by targeting the TRPV1 and NR2B signaling pathways. Mol. Med. Rep. 2016, 14, 5607–5613. [Google Scholar] [CrossRef]
- Borghi, S.M.; Carvalho, T.T.; Staurengo-Ferrari, L.; Hohmann, M.S.; Pinge-Filho, P.; Casagrande, R.; Verri, W.A., Jr. Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines. J. Nat. Prod. 2013, 76, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.; Lee, B.-B.; Lee, S.; Park, Y.; Yeom, H.D.; Kim, T.-H.; Nam, S.-H.; Lee, J.H. Antioxidative and Analgesic Effects of Naringin through Selective Inhibition of Transient Receptor Potential Vanilloid Member 1. Antioxidants 2021, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Cannabinoid receptors and pain. Prog. Neurobiol. 2001, 63, 569–611. [Google Scholar] [CrossRef]
- Maeda, T.; Kishioka, S. PPAR and Pain. Int. Rev. Neurobiol. 2009, 85, 165–177. [Google Scholar] [PubMed]
- Boeckxstaens, G.E. The Emerging Role of Mast Cells in Irritable Bowel Syndrome. Gastroenterol. Hepatol. 2018, 14, 250–252. [Google Scholar]
- Aguilera-Lizarraga, J.; Florens, M.; Hussein, H.; Boeckxstaens, G. Local immune response as novel disease mechanism underlying abdominal pain in patients with irritable bowel syndrome. Acta Clin. Belg. 2021, 1–8. [Google Scholar] [CrossRef]
- Wouters, M.M.; Vicario, M.; Santos, J. The role of mast cells in functional GI disorders. Gut 2016, 65, 155–168. [Google Scholar] [CrossRef]
- Ippolito, C.; Segnani, C.; Errede, M.; Virgintino, D.; Colucci, R.; Fornai, M.; Antonioli, L.; Blandizzi, C.; Dolfi, A.; Bernardini, N. An integrated assessment of histopathological changes of the enteric neuromuscular compartment in experimental colitis. J. Cell Mol. Med. 2015, 19, 485–500. [Google Scholar] [CrossRef]
- Grubišić, V.; McClain, J.L.; Fried, D.E.; Grants, I.; Rajasekhar, P.; Csizmadia, E.; Ajijola, O.A.; Watson, R.E.; Poole, D.P.; Robson, S.C.; et al. Enteric Glia Modulate Macrophage Phenotype and Visceral Sensitivity following Inflammation. Cell Rep. 2020, 32, 108100. [Google Scholar] [CrossRef]
- Morales-Soto, W.; Gulbransen, B.D. Enteric Glia: A New Player in Abdominal Pain. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Seguella, L.; Gulbransen, B.D. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 571–587. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, E.; Seguella, L.; Vincenzi, M.; Parisio, C.; Micheli, L.; Toti, A.; Corpetti, C.; Del Re, A.; Squillace, S.; Maftei, D.; et al. Role of Enteric Glia as Bridging Element between Gut Inflammation and Visceral Pain Consolidation during Acute Colitis in Rats. Biomedicines 2021, 9, 1671. [Google Scholar] [CrossRef] [PubMed]
- Grubišić, V.; Gulbransen, B.D. Enteric glia: The most alimentary of all glia. J. Physiol. 2017, 595, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Musazadeh, V.; Dehghan, P.; Saleh-Ghadimi, S.; Abbasalizad Farhangi, M. Omega 3-rich Camelina sativa oil in the context of a weight loss program improves glucose homeostasis, inflammation and oxidative stress in patients with NAFLD: A randomised placebo-controlled clinical trial. Int. J. Clin. Pract. 2021, 75, e14744. [Google Scholar] [CrossRef] [PubMed]
- Bonvicini, F.; Pagnotta, E.; Punzo, A.; Calabria, D.; Simoni, P.; Mirasoli, M.; Passerini, N.; Bertoni, S.; Ugolini, L.; Lazzeri, L.; et al. Effect of Lactobacillus acidophilus Fermented Broths Enriched with Eruca sativa Seed Extracts on Intestinal Barrier and Inflammation in a Co-Culture System of an Enterohemorrhagic Escherichia coli and Human Intestinal Cells. Nutrients 2020, 12, 3064. [Google Scholar] [CrossRef]
- de Mello, V.D.; Dahlman, I.; Lankinen, M.; Kurl, S.; Pitkänen, L.; Laaksonen, D.E.; Schwab, U.S.; Erkkilä, A.T. The effect of different sources of fish and Camelina sativa oil on immune cell and adipose tissue mRNA expression in subjects with abnormal fasting glucose metabolism: A randomized controlled trial. Nutr. Diabetes 2019, 9, 1. [Google Scholar] [CrossRef]
- Piomelli, D.; Hohmann, A.G.; Seybold, V.; Hammock, B.D. A lipid gate for the peripheral control of pain. J. Neurosci. 2014, 34, 15184–15191. [Google Scholar] [CrossRef]
- Lucarini, E.; Micheli, L.; Martelli, A.; Testai, L.; Calderone, V.; Ghelardini, C.; Mannelli, L.D.C. Efficacy of isothiocyanate-based compounds on different forms of persistent pain. J. Pain Res. 2018, 11, 2905. [Google Scholar] [CrossRef]
- Martelli, A.; Testai, L.; Breschi, M.C.; Lawson, K.; McKay, N.G.; Miceli, F.; Taglialatela, M.; Calderone, V. Vasorelaxation by hydrogen sulphide involves activation of Kv7 potassium channels. Pharmacol. Res. 2013, 70, 27–34. [Google Scholar] [CrossRef]
- Nodera, H.; Spieker, A.; Sung, M.; Rutkove, S. Neuroprotective effects of Kv7 channel agonist, retigabine, for cisplatin-induced peripheral neuropathy. Neurosci. Lett. 2011, 505, 223–227. [Google Scholar] [CrossRef]
- Peiris, M.; Hockley, J.R.; Reed, D.E.; Smith, E.S.J.; Bulmer, D.C.; Blackshaw, L.A. Peripheral K(V)7 channels regulate visceral sensory function in mouse and human colon. Mol. Pain 2017, 13, 1744806917709371. [Google Scholar] [CrossRef] [PubMed]
- Schwab, U.S.; Lankinen, M.A.; de Mello, V.D.; Manninen, S.M.; Kurl, S.; Pulkki, K.J.; Laaksonen, D.E.; Erkkilä, A.T. Camelina sativa oil, but not fatty fish or lean fish, improves serum lipid profile in subjects with impaired glucose metabolism—A randomized controlled trial. Mol. Nutr. Food Res. 2018, 62, 1700503. [Google Scholar] [CrossRef]
- Campbell, C.G.; Picotte, M.S.; Syndergaard, S.; Filipowicz, R.; Thorland, W.G. Anti-inflammatory effects of Camelina sativa oil in postmenopausal women. FASEB J. 2009, 23, 910–914. [Google Scholar] [CrossRef]
- Perna, E.; Aguilera-Lizarraga, J.; Florens, M.V.; Jain, P.; Theofanous, S.A.; Hanning, N.; De Man, J.G.; Berg, M.; De Winter, B.; Alpizar, Y.A. Effect of resolvins on sensitisation of TRPV1 and visceral hypersensitivity in IBS. Gut 2021, 70, 1275–1286. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Zhang, L.; Liu, T.; Park, J.Y.; Berta, T.; Yang, R.; Serhan, C.N.; Ji, R.R. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 2010, 16, 592–597. [Google Scholar] [CrossRef]
- Shehata, A.H.; Ahmed, A.-S.F.; Abdelrehim, A.B.; Heeba, G.H. The impact of single and combined PPAR-α and PPAR-γ activation on the neurological outcomes following cerebral ischemia reperfusion. Life Sci. 2020, 252, 117679. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, H.; Huang, J.; Ding, S.; Huang, B.; Zhou, P.; Jiang, Q. EETs/PPARs activation together mediates the preventive effect of naringenin in high glucose-induced cardiomyocyte hypertrophy. Biomed. Pharmacother. 2019, 109, 1498–1505. [Google Scholar] [CrossRef]
- Sasso, O.; Moreno-Sanz, G.; Martucci, C.; Realini, N.; Dionisi, M.; Mengatto, L.; Duranti, A.; Tarozzo, G.; Tarzia, G.; Mor, M. Antinociceptive effects of the N-acylethanolamine acid amidase inhibitor ARN077 in rodent pain models. Pain 2013, 154, 350–360. [Google Scholar] [CrossRef][Green Version]
- Wójtowicz, S.; Strosznajder, A.K.; Jeżyna, M.; Strosznajder, J.B. The Novel Role of PPAR Alpha in the Brain: Promising Target in Therapy of Alzheimer’s Disease and Other Neurodegenerative Disorders. Neurochem. Res. 2020, 45, 972–988. [Google Scholar] [CrossRef]
- Zhou, G.; Fu, X.; Wang, L.; Cao, Y.; Zhuang, J.; Hu, J.; Li, Y.; Xu, C.; Gao, S.; Shao, A.; et al. Palmitoylethanolamide ameliorates neuroinflammation via modulating PPAR-α to promote the functional outcome after intracerebral hemorrhage. Neurosci. Lett. 2022, 781, 136648. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Iskandar, S.; Kooshki, M.; Sharpe, J.G.; Payne, V.; Robbins, M.E. Knocking out peroxisome proliferator-activated receptor (PPAR) alpha inhibits radiation-induced apoptosis in the mouse kidney through activation of NF-kappaB and increased expression of IAPs. Radiat. Res. 2007, 167, 581–591. [Google Scholar] [CrossRef] [PubMed]
- McClain, J.L.; Mazzotta, E.A.; Maradiaga, N.; Duque-Wilckens, N.; Grants, I.; Robison, A.J.; Christofi, F.L.; Moeser, A.J.; Gulbransen, B.D. Histamine-dependent interactions between mast cells, glia, and neurons are altered following early-life adversity in mice and humans. Am. J. Physiol. Gastrointest Liver Physiol. 2020, 319, G655–G668. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Choi, Y.E.; Kim, H.S. Eruca sativa and its flavonoid components, quercetin and isorhamnetin, improve skin barrier function by activation of peroxisome proliferator-activated receptor (PPAR)-α and suppression of inflammatory cytokines. Phytother. Res. 2014, 28, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Özkay, Ü.D.; Can, Ö.D. Anti-nociceptive effect of vitexin mediated by the opioid system in mice. Pharmacol. Biochem. Behav. 2013, 109, 23–30. [Google Scholar] [CrossRef]
- Bordet, R.; Gelé, P.; Duriez, P.; Fruchart, J. PPARs: A New Target for Neuroprotection; BMJ Publishing Group Ltd.: London, UK, 2006; Volume 77, pp. 285–287. [Google Scholar]
- Deplanque, D.; Gelé, P.; Pétrault, O.; Six, I.; Furman, C.; Bouly, M.; Nion, S.; Dupuis, B.; Leys, D.; Fruchart, J.-C. Peroxisome proliferator-activated receptor-α activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J. Neurosci. 2003, 23, 6264–6271. [Google Scholar] [CrossRef]
- Assaf, N.; El-Shamarka, M.E.; Salem, N.A.; Khadrawy, Y.A.; El Sayed, N.S. Neuroprotective effect of PPAR alpha and gamma agonists in a mouse model of amyloidogenesis through modulation of the Wnt/beta catenin pathway via targeting alpha-and beta-secretases. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 97, 109793. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; D’Agostino, G.; Pacini, A.; Russo, R.; Zanardelli, M.; Ghelardini, C.; Calignano, A. Palmitoylethanolamide is a disease-modifying agent in peripheral neuropathy: Pain relief and neuroprotection share a PPAR-alpha-mediated mechanism. Mediat. Inflamm. 2013, 2013, 328797. [Google Scholar] [CrossRef]
- Ruiz-Medina, J.; Flores, J.A.; Tasset, I.; Tunez, I.; Valverde, O.; Fernandez-Espejo, E. Alteration of neuropathic and visceral pain in female C57BL/6J mice lacking the PPAR-α gene. Psychopharmacology 2012, 222, 477–488. [Google Scholar] [CrossRef]
- Murugesan, N.; Woodard, K.; Ramaraju, R.; Greenway, F.L.; Coulter, A.A.; Rebello, C.J. Naringenin Increases Insulin Sensitivity and Metabolic Rate: A Case Study. J. Med. Food 2020, 23, 343–348. [Google Scholar] [CrossRef]
- Goldwasser, J.; Cohen, P.Y.; Yang, E.; Balaguer, P.; Yarmush, M.L.; Nahmias, Y. Transcriptional regulation of human and rat hepatic lipid metabolism by the grapefruit flavonoid naringenin: Role of PPARalpha, PPARgamma and LXRalpha. PLoS ONE 2010, 5, e12399. [Google Scholar] [CrossRef]
- Zhu, Q.; Mao, L.-N.; Liu, C.-P.; Sun, Y.-H.; Jiang, B.; Zhang, W.; Li, J.-X. Antinociceptive effects of vitexin in a mouse model of postoperative pain. Sci. Rep. 2016, 6, 19266. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.; Zhang, J.; Sun, A.; Zhang, E.; Ding, L.; Mukherjee, S.; Wei, X.; Chou, G.; Wang, Z.-T.; Mani, S. Protective effect of naringenin against experimental colitis via suppression of Toll-like receptor 4/NF-κB signalling. Br. J. Nutr. 2013, 110, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, F.; Alberghini, B.; Marjanović Jeromela, A.; Grahovac, N.; Rajković, D.; Kiprovski, B.; Monti, A. Camelina, an ancient oilseed crop actively contributing to the rural renaissance in Europe. A review. Agron. Sustain. Dev. 2021, 41, 2. [Google Scholar] [CrossRef]



| Glucosinolate | Side Chain (R) Structure | Content (μmol g−1) |
|---|---|---|
| Glucoarabin | 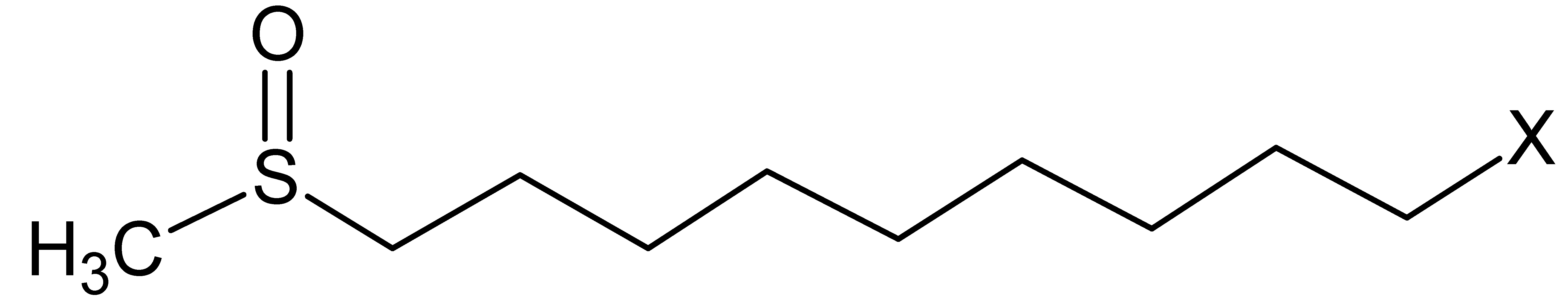 | 10.9 ± 0.9 |
| Glucocamelinin | 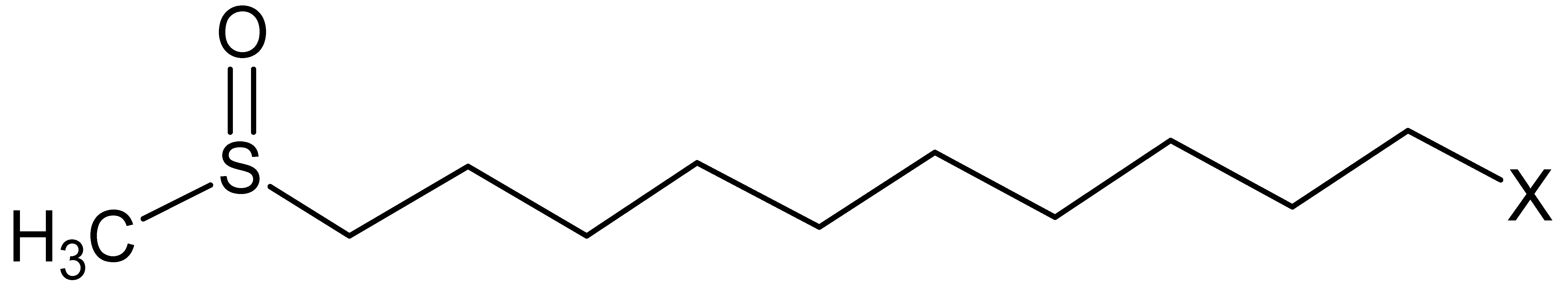 | 29 ± 2 |
| 11-methylsulfinylundecyl Glucosinolate |  | 5.0 ± 0.7 |
| Soluble Conjugated Phenolic Acids and Aldehyde | Insoluble Conjugated Phenolic Acids and Aldehyde | |
|---|---|---|
| Gallic acid | 273 ± 27 | 17.4 ± 4 |
| Protocatechuic acid | 4.4 ± 0.5 | n.d. |
| p-hydroxybenzoic acid | 443.1 ± 0.8 | n.d. |
| Vanillic acid | 29.7 ± 0.2 | n.d. |
| Syringic acid | 13.2 ± 0.2 | 1.8 ± 0.3 |
| Vanillin | 44 ± 3 | 3.1 ± 0.3 |
| Caffeic acid | 9.8 ± 0.1 | 8.5 ± 1.7 |
| p-coumaric acid | 177 ± 16 | 34 ± 6 |
| Sinapic acid | 461 ± 25 | 758 ± 78 |
| trans-cinnamic acid | 853.2 ± 0.4 | 50.1 ± 1.4 |
| Soluble Conjugated Flavonoids | Insoluble Conjugated Flavonoids | |
|---|---|---|
| Luteolin | 287 ± 40 | n.d. |
| Vitexin | 7486 ± 46 | 1.8 ± 0.3 |
| Apigenin | 473 ± 13 | 10 ± 3 |
| Naringenin | 2038 ± 46 | n.d. |
| Rutin | 302 ± 22 | 6.0 ± 0.1 |
| Quercetin | 209 ± 1 | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucarini, E.; Micheli, L.; Pagnotta, E.; Toti, A.; Ferrara, V.; Ciampi, C.; Margiotta, F.; Martelli, A.; Testai, L.; Calderone, V.; et al. The Efficacy of Camelina sativa Defatted Seed Meal against Colitis-Induced Persistent Visceral Hypersensitivity: The Relevance of PPAR α Receptor Activation in Pain Relief. Nutrients 2022, 14, 3137. https://doi.org/10.3390/nu14153137
Lucarini E, Micheli L, Pagnotta E, Toti A, Ferrara V, Ciampi C, Margiotta F, Martelli A, Testai L, Calderone V, et al. The Efficacy of Camelina sativa Defatted Seed Meal against Colitis-Induced Persistent Visceral Hypersensitivity: The Relevance of PPAR α Receptor Activation in Pain Relief. Nutrients. 2022; 14(15):3137. https://doi.org/10.3390/nu14153137
Chicago/Turabian StyleLucarini, Elena, Laura Micheli, Eleonora Pagnotta, Alessandra Toti, Valentina Ferrara, Clara Ciampi, Francesco Margiotta, Alma Martelli, Lara Testai, Vincenzo Calderone, and et al. 2022. "The Efficacy of Camelina sativa Defatted Seed Meal against Colitis-Induced Persistent Visceral Hypersensitivity: The Relevance of PPAR α Receptor Activation in Pain Relief" Nutrients 14, no. 15: 3137. https://doi.org/10.3390/nu14153137
APA StyleLucarini, E., Micheli, L., Pagnotta, E., Toti, A., Ferrara, V., Ciampi, C., Margiotta, F., Martelli, A., Testai, L., Calderone, V., Matteo, R., Suriano, S., Troccoli, A., Pecchioni, N., Manera, C., Mannelli, L. D. C., & Ghelardini, C. (2022). The Efficacy of Camelina sativa Defatted Seed Meal against Colitis-Induced Persistent Visceral Hypersensitivity: The Relevance of PPAR α Receptor Activation in Pain Relief. Nutrients, 14(15), 3137. https://doi.org/10.3390/nu14153137










