Increased vs. Standard Dose of Iron in Ready-to-Use Therapeutic Foods for the Treatment of Severe Acute Malnutrition in a Community Setting: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion/Exclusion Criteria
2.2. Outcomes
2.3. Literature Search
2.4. Data Extraction and Synthesis
2.5. Subgroup Analyses and Sensitivity Analyses
2.6. Patient and Public Involvement
3. Results
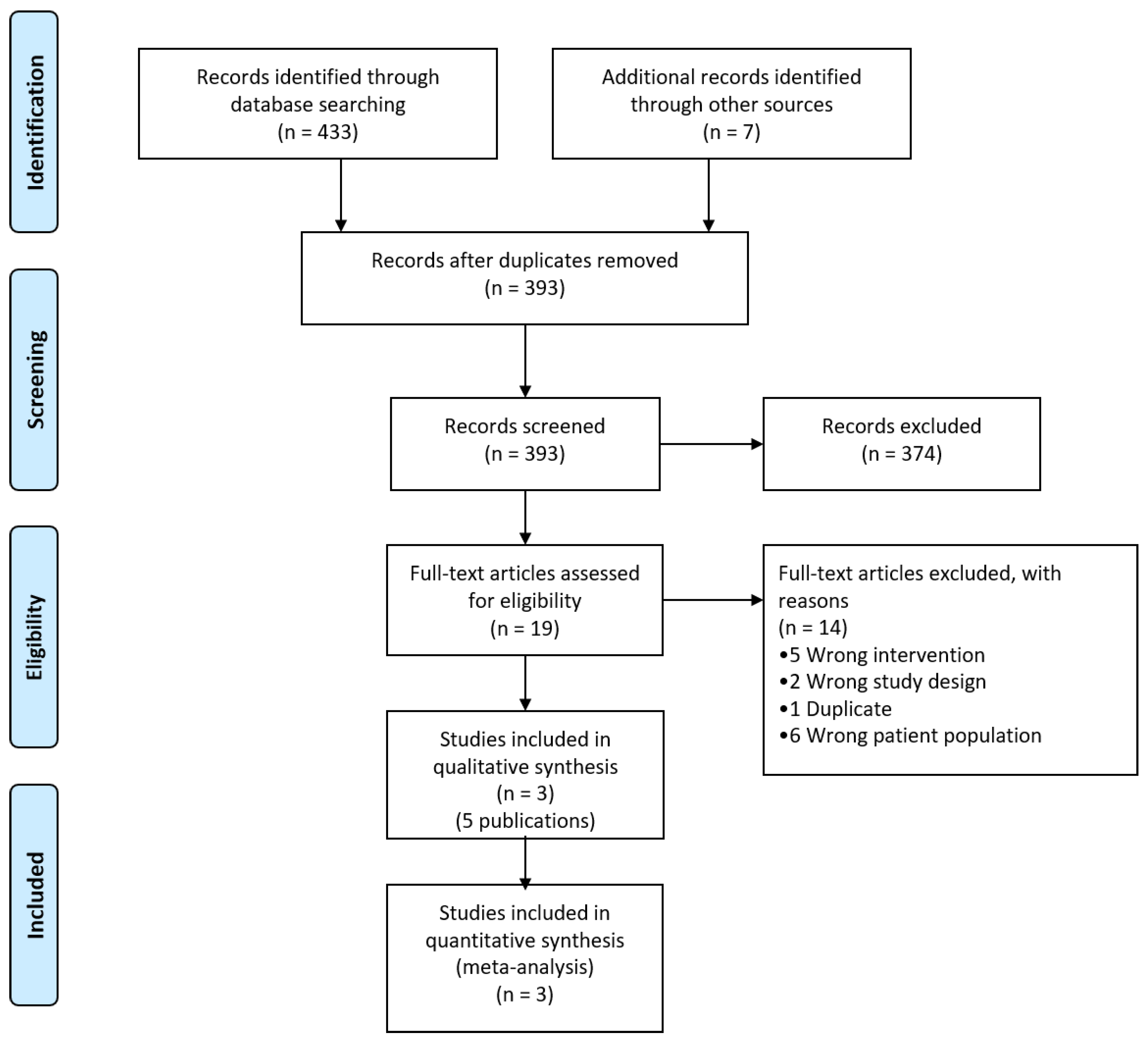
3.1. Literature Search
3.2. Characteristics of Included Studies
3.3. Studies with Multiple Intervention Arms and Missing Data
3.4. Effects of Interventions
3.5. Primary Outcomes
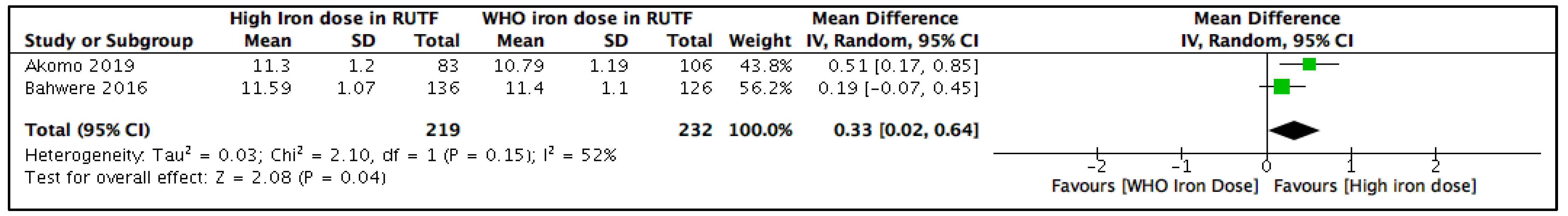
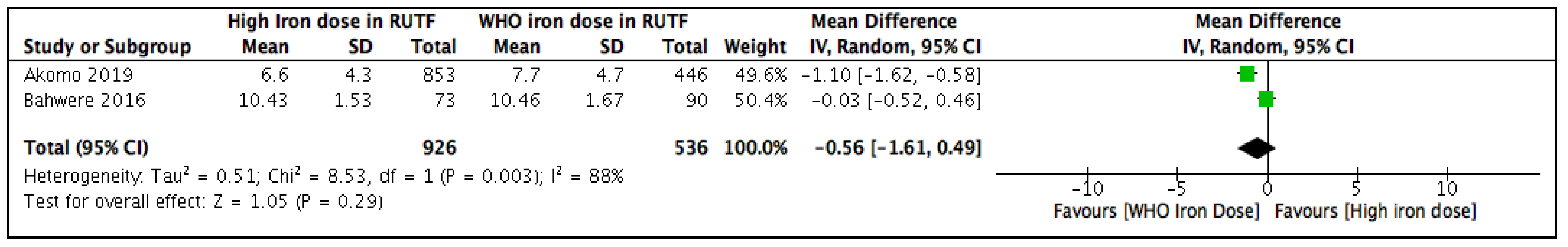
3.5.1. Blood Hemoglobin Concentration (g/dL) at the Longest Follow-Up
3.5.2. Subgroup and Sensitivity Analyses
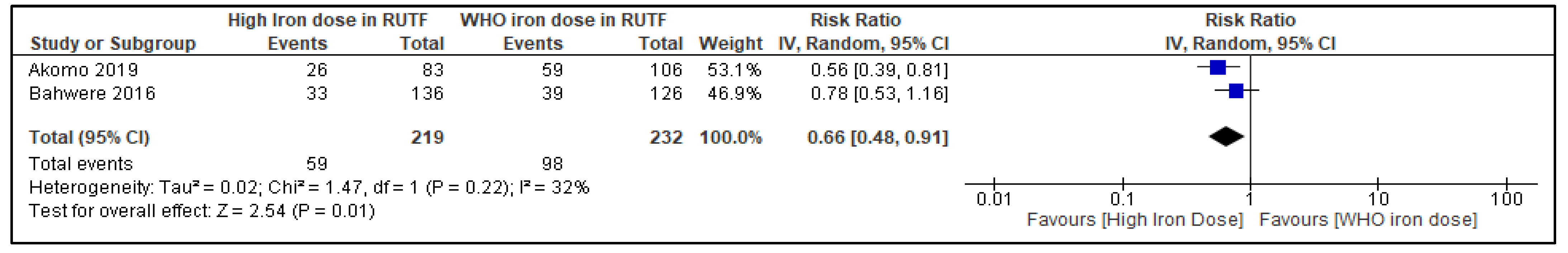
3.5.3. Any Anemia at the Longest Follow-Up
3.6. Subgroup and Sensitivity Analyses
3.6.1. Iron Deficiency Anemia at the Longest Follow-Up
3.6.2. Subgroup and Sensitivity Analyses
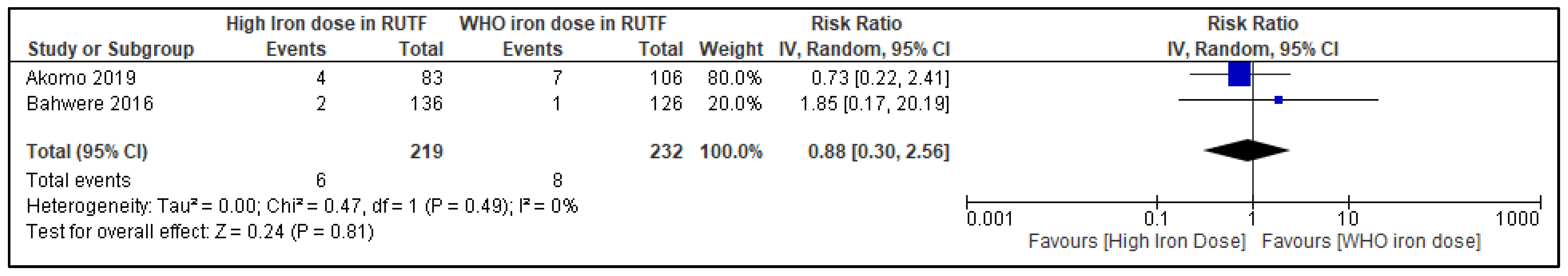
3.6.3. Severe Anemia (Hemoglobin < 9 mg/dL) at the Longest Follow-Up
3.6.4. Subgroup and Sensitivity Analyses
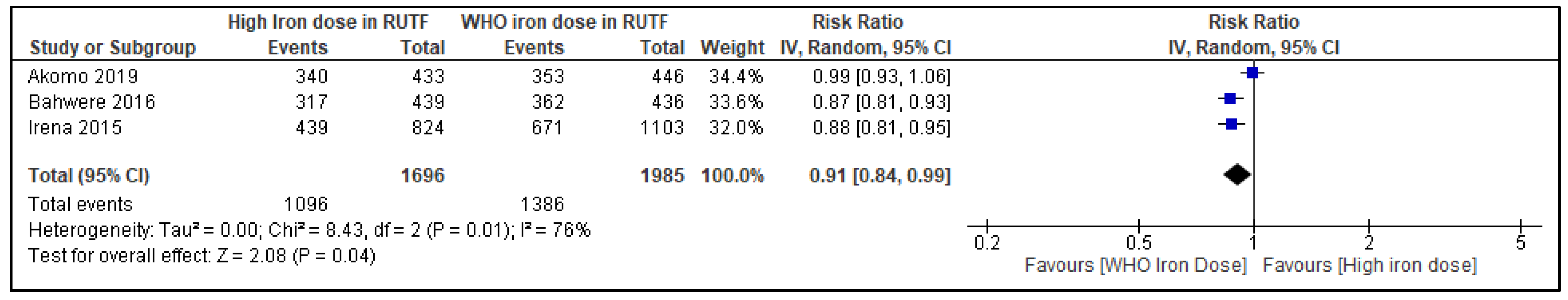
3.6.5. Recovery from SAM at the Longest Follow-Up
3.6.6. Adverse Events
3.7. Secondary Outcomes
3.7.1. All-Cause Mortality at the Longest Follow-Up
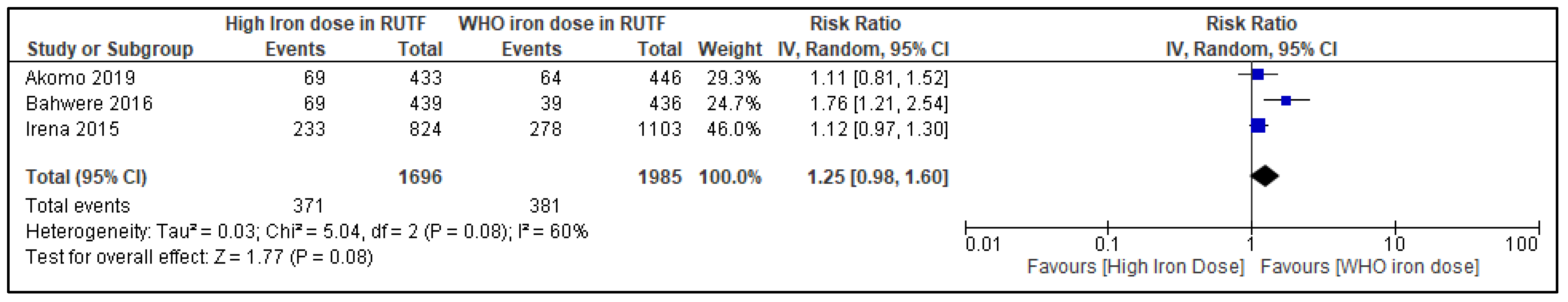
3.7.2. Withdrawal from Trial
3.7.3. Weight Gain
4. Discussion
4.1. Summary of Main Results
4.2. Overall Completeness of Evidence
4.3. Certainty of Evidence
4.4. Potential Bias in the Review Process
4.5. Implications for Practice
4.6. Implications for Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Search Strategies for Different Electronic Databases
Appendix A.1. PubMed
Appendix A.2. CINAHL
Appendix A.3. Embase
Appendix A.4. SCOPUS
Appendix A.5. CENTRAL
Appendix A.6. Web of Science
Appendix A.7. LILACS
Appendix A.8. Global Index Medicus
References
- United Nations Children’s Fund (UNICEF); World Health Organization; International Bank for Reconstruction and Development/The World Bank. Levels and Trends in Child Malnutrition: Key Findings of the 2021 Edition of the Joint Child Malnutrition Estimates; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- WHO; UNICEF. Community-Based Management of Severe Acute Malnutrition; A Joint Statement by the World Health Organization, World Food Programme, United Nations Standing Committee on Nu-trition, United Nations Children’s Fund; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Kangas, S.T.; Salpéteur, C.; Nikièma, V.; Talley, L.; Briend, A.; Ritz, C.; Friis, H.; Kaestel, P. Vitamin A and iron status of children before and after treatment of uncomplicated severe acute malnutrition. Clin. Nutr. 2020, 39, 3512–3519. [Google Scholar] [CrossRef] [PubMed]
- Akomo, P.; Bahwere, P.; Murakami, H.; Banda, C.; Maganga, E.; Kathumba, S.; Sadler, K.; Collins, S. Soya, maize and sorghum ready-to-use therapeutic foods are more effective in correcting anaemia and iron deficiency than the standard ready-to-use therapeutic food: Randomized controlled trial. BMC Public Health 2019, 19, 806. [Google Scholar] [CrossRef] [PubMed]
- Savadogo, L.G.B.; Zoetaba, I.; Ilboudo, B.; Kinda, M.; Donnen, P. Impact of anemia on mortality and nutritional recovery among hospitalized severely malnourished children in Burkina Faso. Open J. Pediatrics 2014, 4, 44104. [Google Scholar] [CrossRef][Green Version]
- Thakur, N.; Chandra, J.; Pemde, H.; Singh, V. Anemia in severe acute malnutrition. Nutrition 2014, 30, 440–442. [Google Scholar] [CrossRef]
- Babirekere-Iriso, E.; Mortensen, C.G.; Mupere, E.; Rytter, M.J.H.; Namusoke, H.; Michaelsen, K.F.; Briend, A.; Stark, K.D.; Friis, H.; Lauritzen, L. Changes in whole-blood PUFA and their predictors during recovery from severe acute malnutrition. Br. J. Nutr. 2016, 115, 1730–1739. [Google Scholar] [CrossRef]
- Jones, K.D.; Ali, R.; Khasira, M.A.; Odera, D.; West, A.L.; Koster, G.; Akomo, P.; Talbert, A.W.; Goss, V.M.; Ngari, M.; et al. Ready-to-use therapeutic food with elevated n-3 polyunsaturated fatty acid content, with or without fish oil, to treat severe acute malnutrition: A randomized controlled trial. BMC Med. 2015, 13, 93. [Google Scholar] [CrossRef]
- Schoonees, A.; Lombard, M.J.; Musekiwa, A.; Nel, E.; Volmink, J. Ready-to-use therapeutic food (RUTF) for home-based nutritional rehabilitation of severe acute malnutrition in children from six months to five years of age. Cochrane Database Syst. Rev. 2019, 5, CD009000. [Google Scholar] [CrossRef]
- Weisstaub, G.; Medina, M.; Pizarro, F.; Araya, M. Copper, iron, and zinc status in children with moderate and severe acute malnutrition recovered following WHO protocols. Biol. Trace Elem. Res. 2008, 124, 1. [Google Scholar] [CrossRef]
- Lopriore, C.; Guidoum, Y.; Briend, A.; Branca, F. Spread fortified with vitamins and minerals induces catch-up growth and eradicates severe anemia in stunted refugee children aged 3–6 y. Am. J. Clin. Nutr. 2004, 80, 973–981. [Google Scholar] [CrossRef]
- Bahwere, P.; Balaluka, B.; Wells, J.C.; Mbiribindi, C.N.; Sadler, K.; Akomo, P.; Dramaix-Wilmet, M.; Collins, S. Cereals and pulse-based ready-to-use therapeutic food as an alternative to the standard milk- and peanut paste-based formulation for treating severe acute malnutrition: A noninferiority, individually randomized controlled efficacy clinical trial. Am. J. Clin. Nutr. 2016, 103, 1145–1161. [Google Scholar] [CrossRef]
- Soofi, S.; Cousens, S.; Iqbal, S.P.; Akhund, T.; Khan, J.; Ahmed, I.; Zaidi, A.K.; Bhutta, Z.A. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: A cluster-randomised trial. Lancet 2013, 382, 29–40. [Google Scholar] [CrossRef]
- Paganini, D.; Zimmermann, M.B. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: A review. Am. J. Clin. Nutr. 2017, 106 (Suppl. 6), 1688S–1693S. [Google Scholar] [CrossRef]
- Sazawal, S.; Black, R.E.; Ramsan, M.; Chwaya, H.M.; Stoltzfus, R.J.; Dutta, A.; Dhingra, U.; Kabole, I.; Deb, S.; Othman, M.K.; et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: Community-based, randomised, placebo-controlled trial. Lancet 2006, 367, 133–143. [Google Scholar] [CrossRef]
- English, M.; Snow, R.W. Iron and folic acid supplementation and malaria risk. Lancet 2006, 367, 90–91. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Imdad, A.; François, M.; Chen, F.F.; Smith, A.; Tsistinas, O.; Tanner-Smith, E.; Das, J.K.; Bhutta, Z.A. Optimal iron content in ready-to-use therapeutic foods for the treatment of severe acute malnutrition in the community settings: A protocol for the systematic review and meta-analysis. BMJ Open 2022, 12, e057389. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Management of Severe Malnutrition: A Manual for Physicians and Other Senior Health Workers. 1999. Available online: http://apps.who.int/iris/bitstream/handle/10665/41999/a57361.pdf;jsessionid=52433E368067A8838754035D5E106335?sequence=1 (accessed on 15 July 2022).
- Covidence Systematic Review Software VHI, Melbourne, Australia. Available online: www.covidence.org (accessed on 15 July 2022).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; TJ, C.J.; Cumpston, M. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2. 2021. Available online: https://training.cochrane.org/handbook/PDF/v6.2 (accessed on 15 July 2022).
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2020 (Developed by Evidence Prime, Inc.). Available online: https://www.gradepro.org/ (accessed on 15 March 2022).
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Irena, A.H.; Bahwere, P.; Owino, V.O.; Diop, E.I.; Bachmann, M.O.; Mbwili-Muleya, C.; Dibari, F.; Sadler, K.; Collins, S. Comparison of the effectiveness of a milk-free soy-maize-sorghum-based ready-to-use therapeutic food to standard ready-to-use therapeutic food with 25% milk in nutrition management of severely acutely malnourished Zambian children: An equivalence non-blinded cluster randomised controlled trial. Matern. Child Nutr. 2015, 11 (Suppl. 4), 105–119. [Google Scholar]
- Bahwere, P.; Akomo, P.; Mwale, M.; Murakami, H.; Banda, C.; Kathumba, S.; Banda, C.; Jere, S.; Sadler, K.; Collins, S. Soya, maize, and sorghum-based ready-to-use therapeutic food with amino acid is as efficacious as the standard milk and peanut paste-based formulation for the treatment of severe acute malnutrition in children: A noninferiority individually randomized controlled efficacy clinical trial in Malawi. Am. J. Clin. Nutr. 2017, 106, 1100–1112. [Google Scholar]
- Owino, V.O.; Irena, A.H.; Dibari, F.; Collins, S. Development and acceptability of a novel milk-free soybean-maize-sorghum ready-to-use therapeutic food (SMS-RUTF) based on industrial extrusion cooking process. Matern. Child Nutr. 2014, 10, 126–134. [Google Scholar] [CrossRef]
- Potani, I.; Spiegel-Feld, C.; Brixi, G.; Bendabenda, J.; Siegfried, N.; Bandsma, R.H.J.; Briend, A.; Daniel, A.I. Ready-to-Use Therapeutic Food (RUTF) Containing Low or No Dairy Compared to Standard RUTF for Children with Severe Acute Malnutrition: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 1930–1943. [Google Scholar] [CrossRef]
- Akomo, P.; Collins, S. Comment on RUTF and correction of anaemia and iron deficiency in severe acute malnutrition. Clin. Nutr. 2020, 39, 2935. [Google Scholar] [CrossRef]
- Borg, B.; Sok, D.; Mihrshahi, S.; Griffin, M.; Chamnan, C.; Berger, J.; Laillou, A.; Roos, N.; Wieringa, F.T. Effectiveness of a locally produced ready-to-use supplementary food in preventing growth faltering for children under 2 years in Cambodia: A cluster randomised controlled trial. Matern. Child Nutr. 2020, 16, e12896. [Google Scholar] [CrossRef] [PubMed]
- Delimont, N.M.; Vahl, C.I.; Kayanda, R.; Msuya, W.; Mulford, M.; Alberghine, P.; Praygod, G.; Mngara, J.; Alavi, S.; Lindshield, B.L. Complementary Feeding of Sorghum-Based and Corn-Based Fortified Blended Foods Results in Similar Iron, Vitamin A, and Anthropometric Outcomes in the MFFAPP Tanzania Efficacy Study. Curr. Dev. Nutr. 2019, 3, nzz027. [Google Scholar] [CrossRef] [PubMed]
- Hieu, N.T.; Sandalinas, F.; de Sesmaisons, A.; Laillou, A.; Tam, N.P.; Khan, N.C.; Bruyeron, O.; Wieringa, F.T.; Berger, J. Multi-micronutrient-fortified biscuits decreased the prevalence of anaemia and improved iron status, whereas weekly iron supplementation only improved iron status in Vietnamese school children. Br. J. Nutr. 2012, 108, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Jayatissa, R.; Bekele, A.; Kethiswaran, A.; De Silva, A.H. Community-based management of severe and moderate acute malnutrition during emergencies in Sri Lanka: Challenges of implementation. Food Nutr. Bull. 2012, 33, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Maleta, K.; Kuittinen, J.; Duggan, M.B.; Briend, A.; Manary, M.; Wales, J.; Kulmala, T.; Ashorn, P. Supplementary feeding of underweight, stunted Malawian children with a ready-to-use food. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 152–158. [Google Scholar] [CrossRef]
- Community-Based Follow-Up of Severely Malnourished Children. Available online: https://ClinicalTrials.gov/show/NCT01157741 (accessed on 15 July 2022).
- Newly Formulated, Extruded Fortified-Blended Foods for Food Aid: The MFFAPP Tanzania Efficacy Study. Available online: https://ClinicalTrials.gov/show/NCT02847962 (accessed on 15 July 2022).
- Olney, D.K.; Bliznashka, L.; Becquey, E.; Birba, O.; Ruel, M.T. Adding a Water, Sanitation and Hygiene Intervention and a Lipid-Based Nutrient Supplement to an Integrated Agriculture and Nutrition Program Improved the Nutritional Status of Young Burkinabé Children. FASEB J. 2017, 31, 455.1. [Google Scholar]
- Semba, R.D.; Moench-Pfanner, R.; Sun, K.; De Pee, S.; Akhter, N.; Rah, J.H.; Campbell, A.A.; Badham, J.; Bloem, M.W.; Kraemer, K. Iron-fortified milk and noodle consumption is associated with lower risk of anemia among children aged 6-59 mo in Indonesia. Am. J. Clin. Nutr. 2010, 92, 170–176. [Google Scholar] [CrossRef]
- Siega-Riz, A.M.; Estrada Del Campo, Y.; Kinlaw, A.; Reinhart, G.A.; Allen, L.H.; Shahab-Ferdows, S.; Heck, J.; Suchindran, C.M.; Bentley, M.E. Effect of supplementation with a lipid-based nutrient supplement on the micronutrient status of children aged 6-18 months living in the rural region of Intibuca, Honduras. Paediatr. Perinat. Epidemiol. 2014, 28, 245–254. [Google Scholar] [CrossRef]
- Sood, M.; Sharada, D. Iron food supplement. Indian J. Pediatr. 2002, 69, 943–946. [Google Scholar] [CrossRef]
- Van Stuijvenberg, M.E.; Kvalsvig, J.D.; Faber, M.; Kruger, M.; Kenoyer, D.G.; Benade, A.J. Effect of iron-, iodine-, and beta-carotene-fortified biscuits on the micronutrient status of primary school children: A randomized controlled trial. Am. J. Clin. Nutr. 1999, 69, 497–503. [Google Scholar] [CrossRef]


| Author | Type of Study | Country | Years of Data Collection | Total Number Randomized in All Study Groups | % Female | Inclusion Criteria/Exclusion Criteria a | Notes |
|---|---|---|---|---|---|---|---|
| Irena 2015 [25] | RCT | Zambia | 2009–2010 | 1927 | 47.9 | Inclusion: SAM (MUAC < 11.0 cm or pitting edema) without complications. Exclusion: Previously included in the study | About 14.5% of the study population were HIV positive, 65% had edema |
| Bahwere 2016 [12] | RCT | Democratic Republic of Congo | 2013–2014 | 886 | 47.1 | Inclusion: SAM (MUAC < 115 mm or bilateral pitting edema assessed), Presence of appetite and no medical complications. Exclusion: Congenital or acquired disorder, food allergies, visiting families | About 20% had edema at baseline. Study site was highland plains and hills at elevations ranging between 900 and 1900 m. |
| Akomo 2019 [4] | RCT | Malawi | 2015–2016 | 392 | 48.5 | Inclusion: SAM (MUAC < 115 mm or bilateral pitting edema), good appetite and no medical complications. Exclusion: Parent refusal, congenital or acquired disorder, food allergies, visting families | About 54% had edema, 48% had anemia, and 33% had iron deficiency anemia at baseline. The altitude of the study area ranges from 578 to 1300 m above sea level. |
| Author | Iron Dose a in the Experimental Group | Zinc Dose a in the Experimental Group | Frequency of RUTF | Duration of Intervention RUTF | Iron Content in Comparison Standard RUTF | Reported Outcomes |
|---|---|---|---|---|---|---|
| Irena 2015 [25] | SMS-RUTF 52.5 | 18.5 | “1-week ration of RUTF and health and nutrition advice. Total calories 200 kcal/kg/day.” | 2 weeks | 12 mg/100 g RUTF | Recovery rates, mortality, default, non-recovered, mean rate of weight gain (g/kg/day), anemia |
| Bahwere 2016 [12] | SMS-RUTF 43.8 | 18.5 | Ad libitum | – | 11.1 mg/100 g RUTF | Recovery rates, mortality, mean daily weight gain, hemoglobin changes, plasma concentrations of eight key amino acids |
| Akomo 2019 [4] | FSMS-RUTF: 35.1 MSMS-RUTF: 31.6 | FSMS-RUTF: 19.5 MSMS-RUTF: 19.9 | Ad libitum | – | 10.5 mg/100 g RUTF | Recovery rates, mortality, anemia |
| Ingredients | Unit ^ | WHO Standard Peanut-Based RUTF | Irena 2015 | Bahwere 2016 | Akomo 2019 | |
|---|---|---|---|---|---|---|
| SMS-RUTF | SMS-RUTF | FSMS-RUTF | PM-RUTF | |||
| Soybean | g | 0.0 | 29.7 | 38.6 | – | – |
| Maize | g | 0.0 | 18.2 | 4.0 | – | – |
| Sorghum | g | 0.0 | 6.5 | 10.0 | – | – |
| Dried skim milk | g | 25.0 | 0.0 | 0.0 | – | – |
| Water | g | – | – | – | 2.2 | 1.1 |
| Ash | g | – | – | – | 3.9 | 3.9 |
| Sugar | g | 27.4 | 14.6 | 16.7 | 22.5 | 25.0 |
| Peanut paste | g | 26.0 | 0.0 | 0.0 | – | – |
| Palm oil | g | 0.0 | 22.4 | 21.6 | – | – |
| Soybean oil | g | 20.0 | 0.0 | – | – | – |
| Linseed oil | g | – | – | 2.1 | – | – |
| Palm stearin | g | 0.0 | 5.6 | 4.0 | – | – |
| Vitamin and minerals premix | g | 1.6 | 3.0 | 3.0 | 2.5 | 1.6 |
| Nutrients | ||||||
| Energy | Kcal | 530 | 521 | 553 | 532 | 545 |
| Protein | g | 15.9 | 11.1 | 16.5 | 18.4 | 15.6 |
| Fat | g | 33.0 | 33.0 | 36.3 | 34.2 | 33.8 |
| Carbohydrate | g | – | 55.0 | – | 41.3 | 45.0 |
| Fibre | g | – | – | – | 7.1 | 1.9 |
| Protein/energy ratio | % | 12 | 8.5 | 11.9 | 13.8 | 11.4 |
| Fat/energy ratio | % | 56.0 | 57.0 | 59.1 | 57.9 | 55.8 |
| Omega-6/energy ratio | % | – | 10.4 | 12.3 | 5.15 | 5.01 |
| Omega-3/energy ratio | % | – | 1.1 | 3.1 | 0.43 | 0.50 |
| Omega-6/omega-3 ratio | % | – | 9.6 | 4.0 | 12.0 | 10.0 |
| SFAs | g | – | – | – | 13.5 | 11.0 |
| MUFAs | g | – | – | – | 11.1 | 18.2 |
| PUFAs | g | – | – | – | 5.58 | 3.16 |
| Trans fat | g | – | – | – | 0.16 | – |
| Vitamin A | µg | 910 | 1852 | 1000 | – | – |
| mg RE | – | – | – | 1.25 | 1.18 | |
| Vitamin C | mg | 53 | 139 | 329 | 323 | 87 |
| Vitamin D | µg | 16 | 14 | 14 | 19.2 | 18.7 |
| Vitamin E | mg | 20 | 139 | 40.7 | 39 | 35 |
| Thiamin (Vitamin B1) | mg | 0.6 | 1.4 | 1.4 | 1.28 | 0.97 |
| Riboflavin (Vitamin B2) | mg | 1.8 | 1.9 | 1.9 | 1.63 | 3.20 |
| Niacin (Vitamin B3) | mg | 5.3 | 19 | 19 | 7.54 | 7.6 |
| Pantothenic acid (Vitamin B5) | mg | 3.1 | 8.3 | 8.3 | 5.36 | 4.5 |
| Pyridoxine (Vitamin B6) | mg | 0.6 | 1.4 | 1.4 | 0.99 | 0.66 |
| Biotin (Vitamin B7) | µg | 65 | 56 | 56 | 86 | 80 |
| Folates (Vitamin B9) | µg | 210 | 370 | 370 | 210 | 268 |
| Cobalamin (Vitamin B12) | µg | 1.8 | 2.3 | 4.3 | 2.5 | 3.2 |
| Vitamin K | µg | 21 | 14 | 14 | 26 | 22 |
| Choline | mg | – | – | – | 90 | – |
| Calcium | mg | 315 | 463 | 437.8 | 571 | 434 |
| Phosphorus | mg | 370 | 380 | 446.0 | 503 | 351 |
| Magnesium | mg | 86 | 74 | 74 | 104 | 97 |
| Sodium | mg | – | – | – | 87.3 | 131.4 |
| Potassium | mg | 1140 | 704 | 1155.8 | 991 | 1125 |
| Copper | mg | 1.7 | 0.9 | 0.9 | 1.48 | 1.60 |
| Iodine | µg | 100 | 417 | 417 | 100 | 85 |
| Iron | mg | 12 | 52.5 | 43.8 | 35.1 | 10.5 |
| Zinc | mg | 11.1 | 18.5 | 18.5 | 19.5 | 11.1 |
| Selenium | µg | – | – | – | 26 | 27 |
| Manganese | mg | – | – | – | 1.71 | – |
| Phytic acid | mg | 255 | 475 | 420 | 465 | 251 |
| Phytic acid/zinc ratio | – | 2.2 | 2.5 | 2.0 | 2.36 | 2.24 |
| Phytic acid/iron ratio | – | 1.9 | 0.8 | 0.8 | 1.12 | 2.02 |
| Ascorbic acid/iron molar ratio | – | 1.4 | 0.8 | 2.4 | 2.93 | 2.64 |
| Ascorbic acid/iron weight ratio | – | 4.4 | 2.6 | 7.5 | 9.20 | 8.29 |
| Calcium/phosphorus weight ratio | – | 0.9 | 1.2 | 1.0 | 1.14 | 1.24 |
| Zinc/copper weight ratio | – | 6.5 | 20.6 | 20.6 | 13.18 | 6.94 |
| Zinc/iron weight ratio | – | 0.9 | 0.4 | 0.4 | 0.56 | 1.06 |
| Certainty Assessment | No. of Patients | Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | High Iron Content | WHO Standard Iron Content | Relative Risk (95% CI) | Absolute (95% CI) | |
| Blood Hemoglobin (mg/dL) | |||||||||||
| 2 | RCTs | serious a | not serious b | not serious | serious c | none | 219 | 232 | - | MD 0.33 mg/dL higher (0.02 higher to 0.64 higher) | ⨁⨁◯◯ Low |
| Any Anemia (Blood Hemoglobin < 11 mg/dL) | |||||||||||
| 2 | RCTs | serious d | not serious | not serious | serious e | none | 59/219 (26.9%) | 98/232 (42.2%) | RR 0.66 (0.48 to 0.91) | 144 fewer per 1000 (from 220 fewer to 38 fewer) | ⨁⨁◯◯ Low |
| Iron Deficiency Anemia | |||||||||||
| 1 | RCT | serious f | not serious | not serious | serious g | none | 5/63 (7.9%) | 17/83 (20.5%) | RR 0.39 (0.15 to 0.99) | 125 fewer per 1000 (from 174 fewer to 2 fewer) | ⨁⨁◯◯ Low |
| Severe Anemia (Blood Hemoglobin < 9 mg/dL) | |||||||||||
| 2 | RCTs | serious d | not serious | not serious | serious h | none | 6/126 (4.8%) | 18/232 (7.8%) | RR 0.88 (0.30 to 2.56) | 9 fewer per 1000 (from 54 fewer to 121 more) | ⨁⨁◯◯ Low |
| Recovery from SAM | |||||||||||
| 3 | RCTs | not serious | serious i | not serious | serious j | none | 1096/1696 (64.6%) | 1386/1985 (69.8%) | RR 0.91 (0.84 to 0.99) | 63 fewer per 1000 (from 112 fewer to 7 fewer) | ⨁⨁◯◯ Low |
| All-cause mortality | |||||||||||
| 3 | RCTs | not serious | not serious | not serious | serious k | none | 135/1696 (8.0%) | 149/1990 (7.5%) | RR 1.30 (0.87 to 1.95) | 22 more per 1000 (from 10 fewer to 71 more) | ⨁⨁⨁◯ Moderate |
| Withdrawal from the study | |||||||||||
| 3 | RCTs | not serious | serious l | not serious | serious m | none | 371/1696 (21.9%) | 381/1985 (19.2%) | RR 1.25 (0.98 to 1.60) | 48 more per 1000 (from 4 fewer to 115 more) | ⨁⨁◯◯ Low |
| Author | Side-Effect Criterion | Unit | Value in High-Iron RUTF Intervention Group | Value in WHO Standard-Iron RUTF Comparison Group | p-Value | Notes |
|---|---|---|---|---|---|---|
| Irena 2015 [25] | Percentage of children who reported at least one episode of diarrhea | % (n) | 20.0 (9) | 15.6 (7) | 0.6 | All of the children on P-RUTF who reported skin rash were from the same health center, and the rash was not specific to certain body parts; it was itchy and appeared as mild with no pustules or vesicles. |
| Percentage of children who reported vomiting | % (n) | 4.4 (2) | 6.7 (3) | 1.0 | ||
| Percentage occurrence of skin rash | % (n) | – | 13.3 (6) | – | ||
| Bahwere 2016 [12] | Percentage of children with side-effects related to RUTF intake | % (n/N) | 2.74 (2/73) | 2.22 (2/45) | 0.862 | No serious side-effects were detected, and no reasons for interrupting the study were identified. No difference was noted in rates of diarrhea, fever, or abdominal pain, and data were the same for children <24 months and >24 months. |
| Akomo 2019 [4] | Percentage of children with inflammation—adjusted plasma ferritin at discharge > 100 μg/L, indicative of excess iron reserve | % (n/N) | 1.6 (1/64) | 4.8 (4/84) | 0.559 | There was no effect of iron content on risk of iron overload or gut inflammation. Complaints of fever, diarrhea, or cough were rare in all study arms in both age groups, with a comparison of median values showing no statistical differences. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imdad, A.; Rogner, J.L.; François, M.; Ahmed, S.; Smith, A.; Tsistinas, O.J.; Tanner-Smith, E.; Das, J.K.; Chen, F.F.; Bhutta, Z.A. Increased vs. Standard Dose of Iron in Ready-to-Use Therapeutic Foods for the Treatment of Severe Acute Malnutrition in a Community Setting: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3116. https://doi.org/10.3390/nu14153116
Imdad A, Rogner JL, François M, Ahmed S, Smith A, Tsistinas OJ, Tanner-Smith E, Das JK, Chen FF, Bhutta ZA. Increased vs. Standard Dose of Iron in Ready-to-Use Therapeutic Foods for the Treatment of Severe Acute Malnutrition in a Community Setting: A Systematic Review and Meta-Analysis. Nutrients. 2022; 14(15):3116. https://doi.org/10.3390/nu14153116
Chicago/Turabian StyleImdad, Aamer, Jaimie L. Rogner, Melissa François, Shehzad Ahmed, Abigail Smith, Olivia J. Tsistinas, Emily Tanner-Smith, Jai K. Das, Fanny F. Chen, and Zulfiqar Ahmed Bhutta. 2022. "Increased vs. Standard Dose of Iron in Ready-to-Use Therapeutic Foods for the Treatment of Severe Acute Malnutrition in a Community Setting: A Systematic Review and Meta-Analysis" Nutrients 14, no. 15: 3116. https://doi.org/10.3390/nu14153116
APA StyleImdad, A., Rogner, J. L., François, M., Ahmed, S., Smith, A., Tsistinas, O. J., Tanner-Smith, E., Das, J. K., Chen, F. F., & Bhutta, Z. A. (2022). Increased vs. Standard Dose of Iron in Ready-to-Use Therapeutic Foods for the Treatment of Severe Acute Malnutrition in a Community Setting: A Systematic Review and Meta-Analysis. Nutrients, 14(15), 3116. https://doi.org/10.3390/nu14153116






