Multiple Direct Effects of the Dietary Protoalkaloid N-Methyltyramine in Human Adipocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Adipose Tisue Samples
2.2. Adipocyte Preparation, Glucose Transport and Lipolysis Assays
2.3. Tyramine and Benzylamine Oxidation by Human Adipose Tissue
2.4. Reagents
2.5. Statistical Analyses
3. Results
3.1. N-Methyltyramine Reproduces the Stimulatory Effect of Tyramine on Glucose Uptake in Human Adipocytes
3.2. Dose Dependency of Glucose Uptake Activation by NMT and Related Phenethylamines
3.3. Sensitivity of NMT Activation of Hexose Uptake to Amine Oxidase Inhibitors
3.4. Oxidation of Radiolabelled Tyramine and Benzylamine by Adipose Tissue: Competition by NMT and Various Substrates or Inhibitors
3.5. Lipolytic and Antilipolytic Effects of NMT in Human Adipocytes
3.6. Effect of a Combination of Insulin and NMT on Glycerol Release and Glucose Uptake by Human Adipocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| 2-DG | 2-DG: 2-[1, 2-3H]-D-deoxyglucose |
| ANOVA | analysis of variance |
| AOC | copper-containing amine oxidases |
| BFI | 2-benzofuranyl-2-imidazoline |
| BMI | body mass index |
| BTT 2052 | indane hydrazino alcohol |
| DAO | diamine oxidase, identical to AOC1 gene product |
| hAT | human adipose tissue |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| MAO | monoamine oxidase |
| ND | not determined |
| NMT | N-methyltyramine |
| NS | no significant difference |
| SEM | standard error of the mean |
| SSAO | semicarbazide-sensitive amine oxidase, identical to VAP-1 and to AOC3 gene product |
| TAAR | trace amine-associated receptor |
| VAP-1 | vascular adhesion protein-1, identical to SSAO and to AOC3 gene product |
References
- Broadley, K.J. The vascular effects of trace amines and amphetamines. Pharmacol. Ther. 2010, 125, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Gainetdinov, R.R.; Hoener, M.C.; Berry, M.D. Trace amines and their receptors. Pharmacol. Rev. 2018, 70, 549–620. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.D.; Gainetdinov, R.R.; Hoener, M.C.; Shahid, M. Pharmacology of human trace amine-associated receptors: Therapeutic opportunities and challenges. Pharmacol. Ther. 2017, 180, 161–180. [Google Scholar] [CrossRef]
- Raab, S.; Wang, H.; Uhles, S.; Cole, N.; Alvarez-Sanchez, R.; Künnecke, B.; Ullmer, C.; Matile, H.; Bedoucha, M.; Norcross, R.D.; et al. Incretin-like effects of small molecule trace amine-associated receptor 1 agonists. Mol. Metab. 2016, 5, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.F.; Sabino, V.; Cottone, P. Trace amine associated receptor 1 (TAAR1) modulation of food reward. Front. Pharmacol. 2018, 9, 129. [Google Scholar] [CrossRef]
- Yamada, M.; Yasuhara, H. Clinical pharmacology of MAO inhibitors: Safety and future. Neurotoxicology 2004, 25, 215–221. [Google Scholar] [CrossRef]
- VanDenBerg, C.M.; Blob, L.F.; Kemper, E.M.; Azzaro, A.J. Tyramine pharmacokinetics and reduced bioavailability with food. J. Clin. Pharmacol. 2003, 43, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Varma, D.R.; Deng, X.F.; Chemtob, S.; Nantel, F.; Bouvier, M. Characterization of the vasorelaxant activity of tyramine and other phenylethylamines in rat aorta. Can. J. Physiol. Pharmacol. 1995, 73, 742–746. [Google Scholar] [CrossRef]
- Fujimoto, S.; Mori, M.; Tsushima, H. Mechanisms underlying the hydrogen peroxide-induced, endothelium-independent relaxation of the norepinephrine-contraction in guinea-pig aorta. Eur. J. Pharmacol. 2003, 459, 65–73. [Google Scholar] [CrossRef]
- Adams, F.; Boschmann, M.; Schaller, K.; Franke, G.; Gorzelniak, K.; Janke, J.; Klaus, S.; Luft, F.C.; Heer, M.; Jordan, J. Tyramine in the assessment of regional adrenergic function. Biochem. Pharmacol. 2006, 72, 1724–1729. [Google Scholar] [CrossRef]
- Carpéné, C.; Galitzky, J.; Belles, C.; Zakaroff-Girard, A. Mechanisms of the antilipolytic response of human adipocytes to tyramine, a trace amine present in food. J. Physiol. Biochem. 2018, 74, 623–633. [Google Scholar] [CrossRef]
- Carpéné, C.; Les, F.; Mercader-Barceló, J.; Boulet, N.; Briot, A.; Grolleau, J.L. High doses of tyramine stimulate glucose transport in human fat cells. J. Physiol. Biochem. 2022, 78, 543–556. [Google Scholar] [CrossRef]
- Kim, H.J.; Kwak, B.-M.; Ahn, J.-H.; Park, J.-S. Simultaneous determination of synephrine and N-methyltyramine in orange fruit and juice from korean market by UPLC-FLD. Korean J. Food Sci. Technol. 2014, 46, 276–282. [Google Scholar] [CrossRef][Green Version]
- Sommer, T.; Dlugash, G.; Hübner, H.; Gmeiner, P.; Pischetsrieder, M. Monitoring of the dopamine D2 receptor agonists hordenine and N-methyltyramine during the brewing process and in commercial beer samples. Food Chem. 2019, 276, 745–753. [Google Scholar] [CrossRef]
- Yokoo, Y.; Kohda, H.; Kusumoto, A.; Naoki, H.; Matsumoto, N.; Amachi, T.; Suwa, Y.; Fukazawa, H.; Ishida, H.; Tsuji, K.; et al. Isolation from beer and structural determination of a potent stimulant of gastrin release. Alcohol Alcohol. 1999, 34, 161–168. [Google Scholar] [CrossRef]
- Mercader, J.; Wanecq, E.; Chen, J.; Carpéné, C. Isopropylnorsynephrine is a stronger lipolytic agent in human adipocytes than synephrine and other amines present in Citrus aurantium. J. Physiol. Biochem. 2011, 67, 443–452. [Google Scholar] [CrossRef]
- Leroux, M.; Lemery, T.; Boulet, N.; Briot, A.; Zakaroff, A.; Bouloumié, A.; Andrade, F.; Pérez-Matute, P.; Arbones-Mainar, J.M.; Carpéné, C. Effects of the amino acid derivatives, β-hydroxy-β-methylbutyrate, taurine, and N-methyltyramine, on triacylglycerol breakdown in fat cells. J. Physiol. Biochem. 2019, 75, 263–273. [Google Scholar] [CrossRef]
- Visentin, V.; Morin, N.; Fontana, E.; Prevot, D.; Boucher, J.; Castan, I.; Valet, P.; Grujic, D.; Carpéné, C. Dual action of octopamine on glucose transport into adipocytes: Inhibition via beta3-adrenoceptor activation and stimulation via oxidation by amine oxidases. J. Pharmacol. Exp. Ther. 2001, 299, 96–104. [Google Scholar]
- Marti, L.; Morin, N.; Enrique-Tarancon, G.; Prevot, D.; Lafontan, M.; Testar, X.; Zorzano, A.; Carpéné, C. Tyramine and vanadate synergistically stimulate glucose transport in rat adipocytes by amine oxidase-dependent generation of hydrogen peroxide. J. Pharmacol. Exp. Ther. 1998, 285, 342–349. [Google Scholar]
- Carpéné, C.; Les, F.; Hasnaoui, M.; Biron, S.; Marceau, P.; Richard, D.; Galitzky, J.; Joanisse, D.R.; Mauriège, P. Anatomical distribution of primary amine oxidase activity in four adipose depots and plasma of severely obese women with or without a dysmetabolic profile. J. Physiol. Biochem. 2016, 73, 475–486. [Google Scholar] [CrossRef]
- Haj Ahmed, W.; Peiro, C.; Fontaine, J.; Ryan, B.J.; Kinsella, G.K.; O’Sullivan, J.; Grolleau, J.L.; Henehan, G.T.M.; Carpéné, C. Methylxanthines inhibit primary amine oxidase and monoamine oxidase activities of human adipose tissue. Medicines 2020, 7, 18. [Google Scholar] [CrossRef]
- Les, F.; Deleruyelle, S.; Cassagnes, L.E.; Boutin, J.A.; Balogh, B.; Arbones-Mainar, J.M.; Biron, S.; Marceau, P.; Richard, D.; Nepveu, F.; et al. Piceatannol and resveratrol share inhibitory effects on hydrogen peroxide release, monoamine oxidase and lipogenic activities in adipose tissue, but differ in their antilipolytic properties. Chem. Biol. Interact. 2016, 258, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Visentin, V.; Prevot, D.; De Saint Front, V.D.; Morin-Cussac, N.; Thalamas, C.; Galitzky, J.; Valet, P.; Zorzano, A.; Carpéné, C. Alteration of amine oxidase activity in the adipose tissue of obese subjects. Obes. Res. 2004, 12, 547–555. [Google Scholar] [CrossRef]
- Morin, N.; Lizcano, J.M.; Fontana, E.; Marti, L.; Smih, F.; Rouet, P.; Prévot, D.; Zorzano, A.; Unzeta, M.; Carpéné, C. Semicarbazide-sensitive amine oxidase substrates stimulate glucose transport and inhibit lipolysis in human adipocytes. J. Pharmacol. Exp. Ther. 2001, 297, 563–572. [Google Scholar]
- Carpéné, C.; Mauriège, P.; Boulet, N.; Biron, S.; Grolleau, J.L.; Garcia-Barrado, M.J.; Iglesias-Osma, M.C. Methylamine activates glucose uptake in human adipocytes without overpassing action of insulin or stimulating its secretion in pancreatic islets. Medicines 2019, 6, 89. [Google Scholar] [CrossRef]
- Vakal, S.; Jalkanen, S.; Dahlström, K.M.; Salminen, T.A. Human copper-containing amine oxidases in drug design and development. Molecules 2020, 25, 1293. [Google Scholar] [CrossRef]
- Bour, S.; Iglesias-Osma, M.C.; Marti, L.; Duro, P.; Garcia-Barrado, M.J.; Pastor, M.F.; Prevot, D.; Visentin, V.; Valet, P.; Moratinos, J.; et al. The imidazoline I2-site ligands BU 224 and 2-BFI inhibit MAO-A and MAO-B activities, hydrogen peroxide production, and lipolysis in rodent and human adipocytes. Eur. J. Pharmacol. 2006, 552, 20–30. [Google Scholar] [CrossRef]
- Foley, J.E.; Kashiwagi, A.; Chang, H.; Huecksteadt, T.P.; Lillioja, S.; Verso, M.A.; Reaven, G. Sex difference in insulin-stimulated glucose transport in rat and human adipocytes. Am. J. Physiol. 1984, 246, E211–E215. [Google Scholar] [CrossRef] [PubMed]
- Mercier, N.; Moldes, M.; El Hadri, K.; Feve, B. Regulation of semicarbazide-sensitive amine oxidase expression by tumor necrosis factor-alpha in adipocytes: Functional consequences on glucose transport. J. Pharmacol. Exp. Ther. 2003, 304, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Reese, E.A.; Bunzow, J.R.; Arttamangkul, S.; Sonders, M.S.; Grandy, D.K. Trace amine-associated receptor 1 displays species-dependent stereoselectivity for isomers of methamphetamine, amphetamine, and para-hydroxyamphetamine. J. Pharmacol. Exp. Ther. 2007, 321, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Enrique-Tarancon, G.; Castan, I.; Morin, N.; Marti, L.; Abella, A.; Camps, M.; Casamitjana, R.; Palacin, M.; Testar, X.; Degerman, E.; et al. Substrates of semicarbazide-sensitive amine oxidase co-operate with vanadate to stimulate tyrosine phosphorylation of insulin-receptor-substrate proteins, phosphoinositide 3-kinase activity and GLUT4 translocation in adipose cells. Biochem. J. 2000, 350 Pt 1, 171–180. [Google Scholar] [CrossRef]
- El Hadri, K.; Moldes, M.; Mercier, N.; Andreani, M.; Pairault, J.; Fève, B. Semicarbazide-sensitive amine oxidase in vascular smooth muscle cells: Differentiation-dependent expression and role in glucose uptake. Arter. Thromb. Vasc. Biol. 2002, 22, 89–94. [Google Scholar] [CrossRef]
- Ma, M.; Quan, Y.; Li, Y.; He, X.; Xiao, J.; Zhan, M.; Zhao, W.; Xin, Y.; Lu, L.; Luo, L. Bidirectional modulation of insulin action by reactive oxygen species in 3T3-L1 adipocytes. Mol. Med. Rep. 2018, 18, 807–814. [Google Scholar] [CrossRef]
- Iglesias-Osma, M.C.; Bour, S.; Garcia-Barrado, M.J.; Visentin, V.; Pastor, M.F.; Testar, X.; Marti, L.; Enrique-Tarancon, G.; Valet, P.; Moratinos, J.; et al. Methylamine but not mafenide mimics insulin-like activity of the semicarbazide-sensitive amine oxidase-substrate benzylamine on glucose tolerance and on human adipocyte metabolism. Pharmacol. Res. 2005, 52, 475–484. [Google Scholar] [CrossRef]
- Mercader, J.; Iffiu-Soltesz, Z.; Brenachot, X.; Foldi, A.; Dunkel, P.; Balogh, B.; Attané, C.; Valet, P.; Matyus, P.; Carpéné, C. SSAO substrates exhibiting insulin-like effects in adipocytes as a promising treatment option for metabolic disorders. Future Med. Chem. 2010, 2, 1735–1749. [Google Scholar] [CrossRef]
- Visentin, V.; Prevot, D.; Marti, L.; Carpéné, C. Inhibition of rat fat cell lipolysis by monoamine oxidase and semicarbazide-sensitive amine oxidase substrates. Eur. J. Pharmacol. 2003, 466, 235–243. [Google Scholar] [CrossRef]
- Morin, N.; Visentin, V.; Calise, D.; Marti, L.; Zorzano, A.; Testar, X.; Valet, P.; Fischer, Y.; Carpene, C. Tyramine stimulates glucose uptake in insulin-sensitive tissues in vitro and in vivo via its oxidation by amine oxidases. J. Pharmacol. Exp. Ther. 2002, 303, 1238–1247. [Google Scholar] [CrossRef]
- Saponaro, C.; Gaggini, M.; Carli, F.; Gastaldelli, A. The Subtle balance between Lipolysis and Lipogenesis: A critical point in metabolic homeostasis. Nutrients 2015, 7, 9453–9474. [Google Scholar] [CrossRef]
- Carpéné, C.; Collon, P.; Remaury, A.; Cordi, A.; Hudson, A.; Nutt, D.; Lafontan, M. Inhibition of amine oxidase activity by derivatives that recognize imidazoline I2 sites. J. Pharmacol. Exp. Ther. 1995, 272, 681–688. [Google Scholar]
- Cioni, L.; De Siena, G.; Ghelardini, C.; Sernissi, O.; Alfarano, C.; Pirisino, R.; Raimondi, L. Activity and expression of semicarbazide-sensitive benzylamine oxidase in a rodent model of diabetes: Interactive effects with methylamine and alpha-aminoguanidine. Eur. J. Pharmacol. 2006, 529, 179–187. [Google Scholar] [CrossRef]
- Basile, L.; Pappalardo, M.; Guccione, S.; Milardi, D.; Ramsay, R.R. Computational comparison of imidazoline association with the I2 binding site in human monoamine oxidases. J. Chem. Inf. Model. 2014, 54, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Singer, T.P.; Von Korff, R.W.; Murphy, D.L. Monoamine Oxidase: Structure, Function and Altered Functions; Academic Press: Cambridge, MA, USA, 1979; pp. 1–557. ISBN 012646880X. [Google Scholar]
- Fontaine, J.; Tavernier, G.; Morin, N.; Carpéné, C. Vanadium-dependent activation of glucose transport in adipocytes by catecholamines is not mediated via adrenoceptor stimulation or monoamine oxidase activity. World J. Diabetes 2020, 11, 622–643. [Google Scholar] [CrossRef] [PubMed]
- Carpéné, C.; Daviaud, D.; Boucher, J.; Bour, S.; Visentin, V.; Gres, S.; Duffaut, C.; Fontana, E.; Testar, X.; Saulnier-Blache, J.S.; et al. Short- and long-term insulin-like effects of monoamine oxidases and semicarbazide-sensitive amine oxidase substrates in cultured adipocytes. Metabolism 2006, 55, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Lafontan, M.; Berlan, M. Fat cell alpha 2-adrenoceptors: The regulation of fat cell function and lipolysis. Endocr. Rev. 1995, 16, 716–738. [Google Scholar] [CrossRef]
- Koda, H.; Yokoo, Y.; Matsumoto, N.; Suwa, Y.; Fukazawa, H.; Ishida, H.; Tsuji, K.; Nukaya, H.; Kuriyama, K. Antagonistic effect of N-methyltyramine on alpha2-adrenoceptor in mice. Jpn. J. Pharmacol. 1999, 81, 313–315. [Google Scholar] [CrossRef]
- Cripps, M.J.; Bagnati, M.; Jones, T.A.; Ogunkolade, B.W.; Sayers, S.R.; Caton, P.W.; Hanna, K.; Billacura, M.P.; Fair, K.; Nelson, C.; et al. Identification of a subset of trace amine-associated receptors and ligands as potential modulators of insulin secretion. Biochem. Pharmacol. 2020, 171, 113685. [Google Scholar] [CrossRef]
- Stohs, S.J.; Preuss, H.G.; Shara, M. The safety of Citrus aurantium (bitter orange) and its primary protoalkaloid p-synephrine. Phytother. Res. 2011, 25, 1421–1428. [Google Scholar] [CrossRef]
- Carpéné, M.A.; Testar, X.; Carpéné, C. High Doses of Synephrine and Octopamine Activate Lipolysis in Human Adipocytes, Indicating that Amines from Citrus Might Influence Adiposity; Citrus, K.H., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2014; Chapter 8; pp. 141–168. [Google Scholar]
- Inchiosa, M.A. Experience (Mostly Negative) with the Use of Sympathomimetic Agents for Weight Loss. J. Obes. 2011, 2011, 764584. [Google Scholar] [CrossRef]
- Ratamess, N.A.; Bush, J.A.; Kang, J.; Kraemer, W.J.; Stohs, S.J.; Nocera, V.G.; Leise, M.D.; Diamond, K.B.; Campbell, S.C.; Miller, H.B.; et al. The effects of supplementation with p-synephrine alone and in combination with caffeine on metabolic, lipolytic, and cardiovascular responses during resistance exercise. J. Am. Coll. Nutr. 2016, 35, 657–669. [Google Scholar] [CrossRef]
- Ruiz-Moreno, C.; Del Coso, J.; Giráldez-Costas, V.; González-García, J.; Gutiérrez-Hellín, J. Effects of p-Synephrine during exercise: A brief narrative review. Nutrients 2021, 13, 233. [Google Scholar] [CrossRef]
- Pellati, F.; Benvenuti, S. Chromatographic and electrophoretic methods for the analysis of phenethylamine alkaloids in Citrus aurantium. J. Chromatogr. A 2007, 1161, 71–88. [Google Scholar] [CrossRef]
- Carpéné, C.; Iffiú-Soltész, Z. Monoaminergic systems and anti-obesity drug discovery: Chronic administration of sympathicomimetic amines, re-uptake inhibitors, or amine oxidase inhibitors? In Anti-Obesity Drug Discovery and Development; Atta-ur-Rahman, Iqbal Choudhary, M., Eds.; Bentham Sciences Publishers: Sharjah, United Arab Emirates, 2011; Volume 1, pp. 114–130. [Google Scholar]
- Viana, C.; Zemolin, G.M.; Müller, L.S.; Dal Molin, T.R.; Seiffert, H.; de Carvalho, L.M. Liquid chromatographic determination of caffeine and adrenergic stimulants in food supplements sold in Brazilian e-commerce for weight loss and physical fitness. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess 2016, 33, 1–9. [Google Scholar] [CrossRef]
- Lee, D.; Lee, J.H.; Kim, B.H.; Lee, S.; Kim, D.W.; Kang, K.S. Phytochemical combination (p-Synephrine, p-octopamine hydrochloride, and hispidulin) for improving obesity in obese mice induced by high-fat diet. Nutrients 2022, 14, 2164. [Google Scholar] [CrossRef]
- Shanahan, P. Modulation of Primary Amine Oxidase by Phytochemicals. Master’s Thesis, MA Technological University Dublin, Dublin, Ireland, 2018. [Google Scholar] [CrossRef]
- Carpéné, C.; Boulet, N.; Grolleau, J.L.; Morin, N. High doses of catecholamines activate glucose transport in human adipocytes independently from adrenoceptor stimulation or vanadium addition. World J. Diabetes 2022, 13, 37–53. [Google Scholar] [CrossRef]
- Müller, T.D.; Blüher, M.; Tschöp, M.H.; DiMarchi, R.D. Anti-obesity drug discovery: Advances and challenges. Nat. Rev. Drug Discov. 2021, 21, 201–223. [Google Scholar] [CrossRef] [PubMed]
- Fischer, Y.; Rose, H.; Thomas, J.; Deuticke, B.; Kammermeier, H. Phenylarsine oxide and hydrogen peroxide stimulate glucose transport via different pathways in isolated cardiac myocytes. Biochim. Biophys. Acta 1993, 1153, 97–104. [Google Scholar] [CrossRef]
- Stohs, S.J.; Hartman, M.J. A review of the receptor binding and pharmacological effects of N-methyltyramine. Phytother. Res. 2015, 29, 14–16. [Google Scholar] [CrossRef]

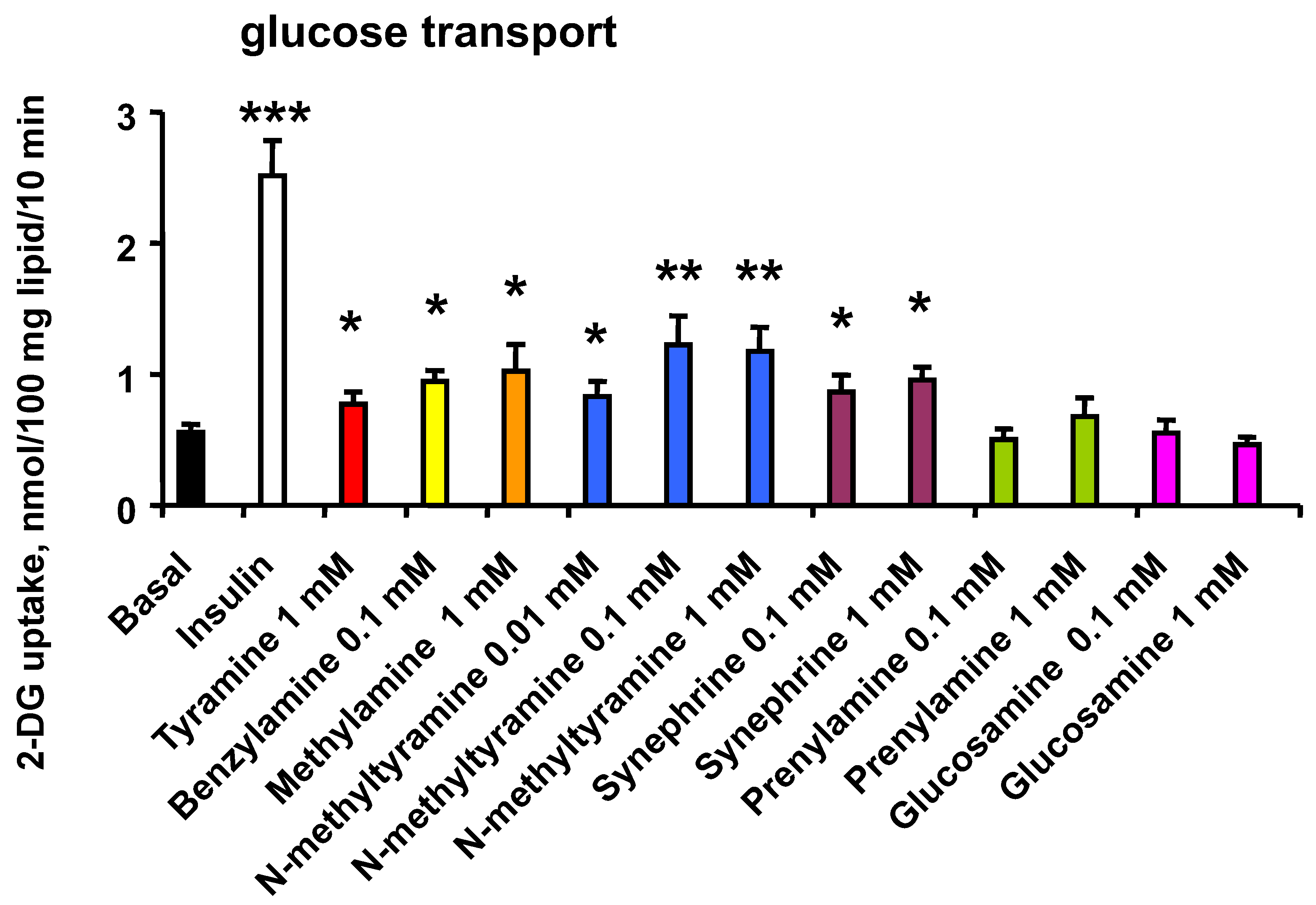
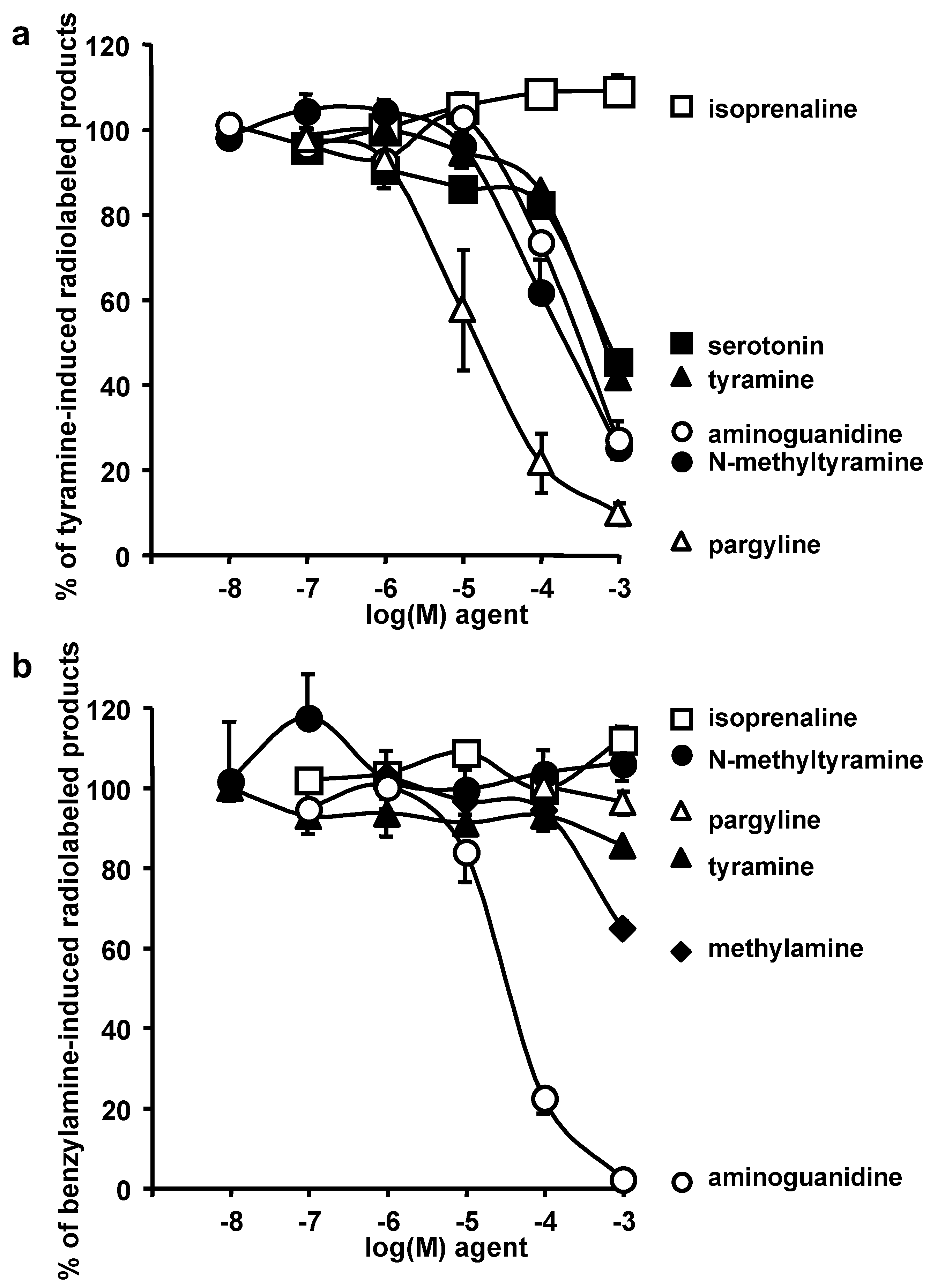
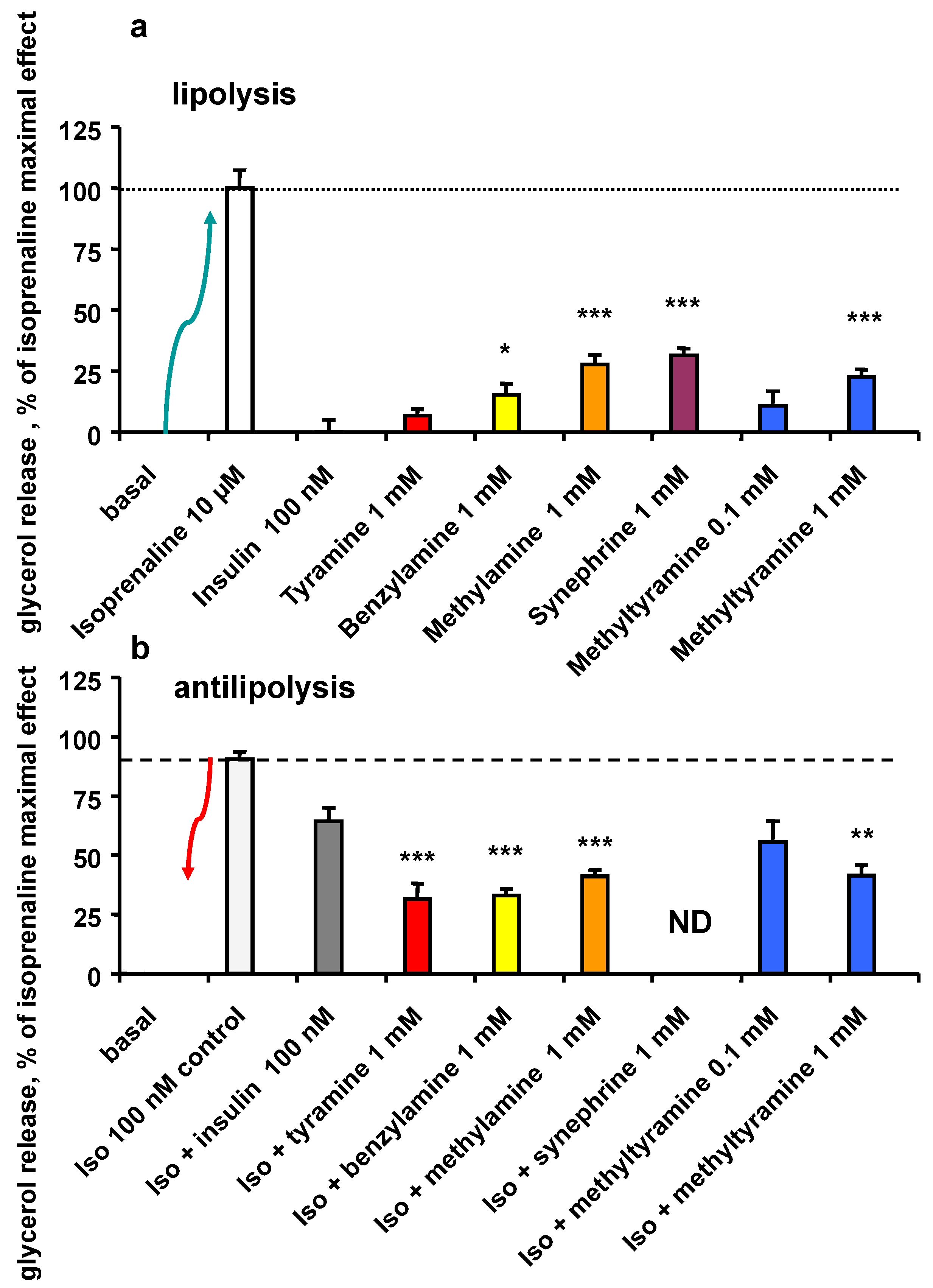

| 2-DG Uptake (% of Ins Max Effect) | Basal | Bza 1 mM | NMT 1 mM | Tyr 1 mM | |||
|---|---|---|---|---|---|---|---|
| control | 0 | 44.2 ± 9.9 | 33.1 ± 6.6 | 24.2 ± 4.6 | * | ||
| +0.1 mM pargyline | 1.3 ± 10.2 | 38.7 ± 5.3 | 20.2 ± 8.0 | 9.8 ± 2.0 | * | ||
| +1 mM aminoguanidine | 8.1 ± 6.1 | 7.7 ± 5.9 | ** | 2.5 ± 6.8 | ** | 6.9 ± 3.1 | * |
| +1 mM BTT 2052 | 5.7 ± 6.3 | 6.2 ± 5.1 | ** | 5.9 ± 6.3 | * | 8.7 ± 2.6 | * |
| +0.1 mM BFI | 11.2 ± 4.7 | ND | 6.2 ± 7.9 | * | 7.0 ± 5.0 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpéné, C.; Viana, P.; Fontaine, J.; Laurell, H.; Grolleau, J.-L. Multiple Direct Effects of the Dietary Protoalkaloid N-Methyltyramine in Human Adipocytes. Nutrients 2022, 14, 3118. https://doi.org/10.3390/nu14153118
Carpéné C, Viana P, Fontaine J, Laurell H, Grolleau J-L. Multiple Direct Effects of the Dietary Protoalkaloid N-Methyltyramine in Human Adipocytes. Nutrients. 2022; 14(15):3118. https://doi.org/10.3390/nu14153118
Chicago/Turabian StyleCarpéné, Christian, Pénélope Viana, Jessica Fontaine, Henrik Laurell, and Jean-Louis Grolleau. 2022. "Multiple Direct Effects of the Dietary Protoalkaloid N-Methyltyramine in Human Adipocytes" Nutrients 14, no. 15: 3118. https://doi.org/10.3390/nu14153118
APA StyleCarpéné, C., Viana, P., Fontaine, J., Laurell, H., & Grolleau, J.-L. (2022). Multiple Direct Effects of the Dietary Protoalkaloid N-Methyltyramine in Human Adipocytes. Nutrients, 14(15), 3118. https://doi.org/10.3390/nu14153118






