d-Allulose Inhibits Ghrelin-Responsive, Glucose-Sensitive and Neuropeptide Y Neurons in the Arcuate Nucleus and Central Injection Suppresses Appetite-Associated Food Intake in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Cannula Implantation and Icv Injection
2.4. Preparation of Single Neurons from ARC
2.5. Measurements of [Ca2+]i in Single ARC Neurons
2.6. Statistical Analysis
3. Results
3.1. Effects of d-Allulose on Ghrelin-Induced [Ca2+]i Increases in ARC Neurons
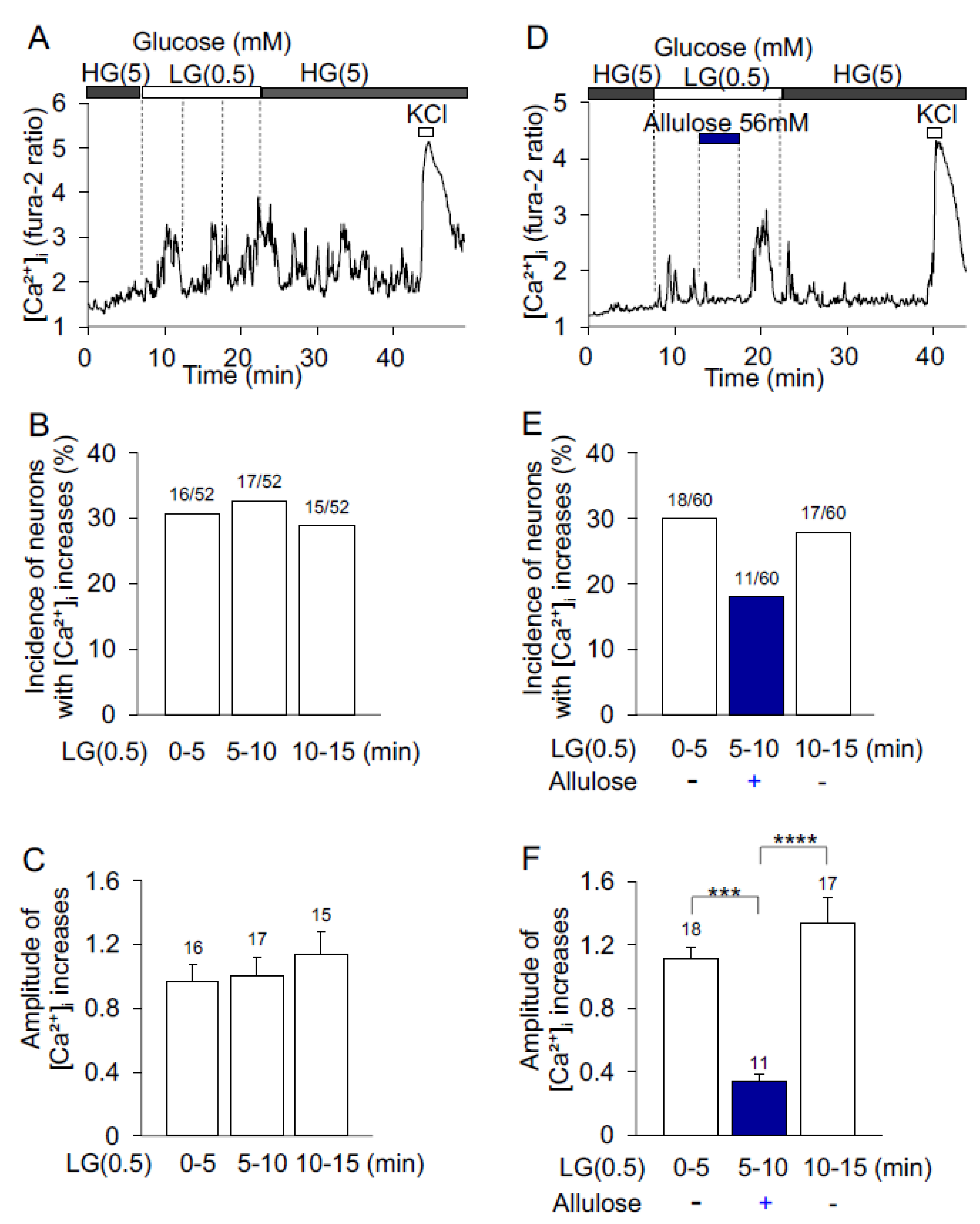
3.2. d-Allulose Effect on Low-Glucose-Induced [Ca2+]i Increases in ARC Neurons
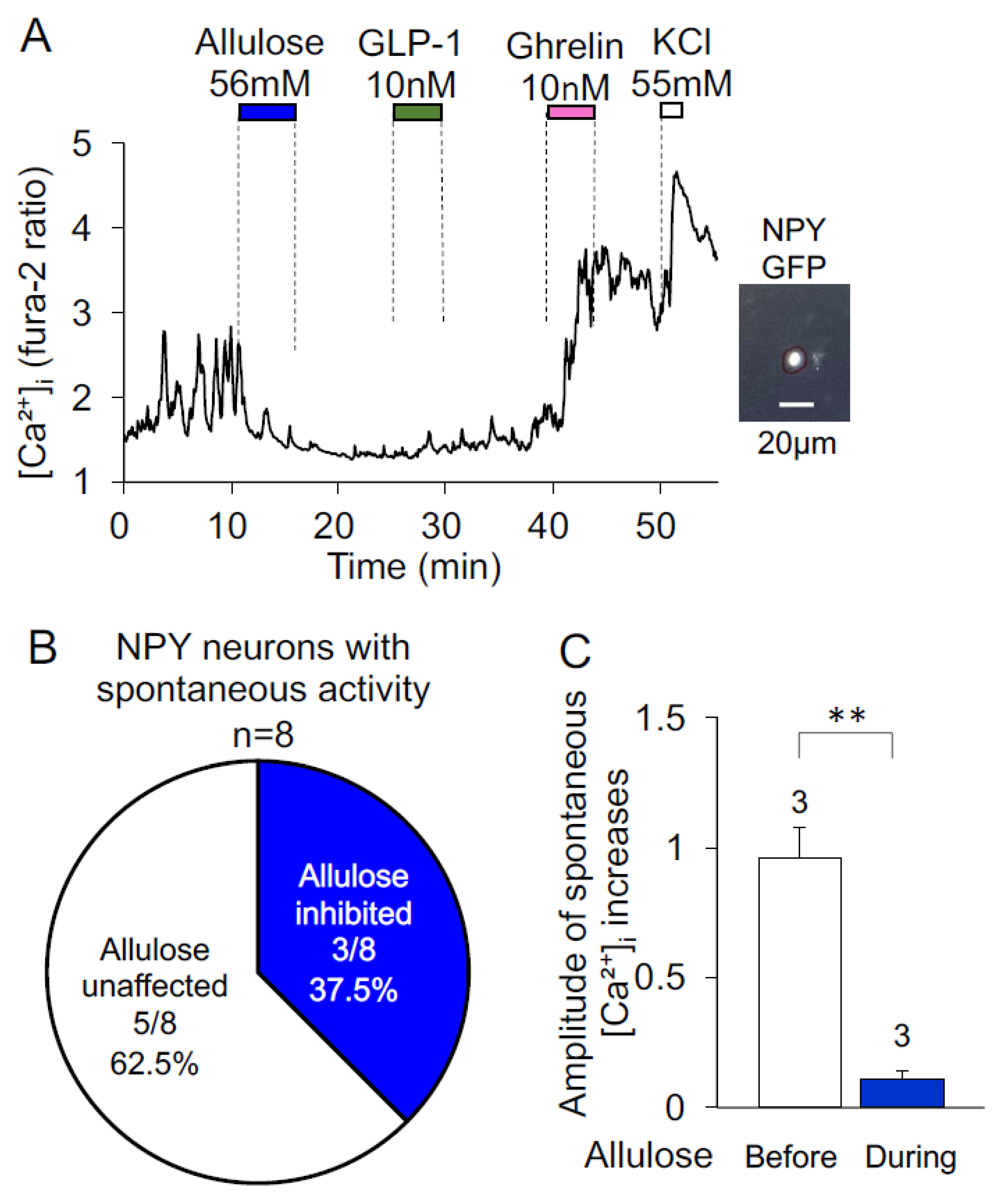
3.3. Effects of d-Allulose on Spontaneous [Ca2+]i Increases in NPY Neurons
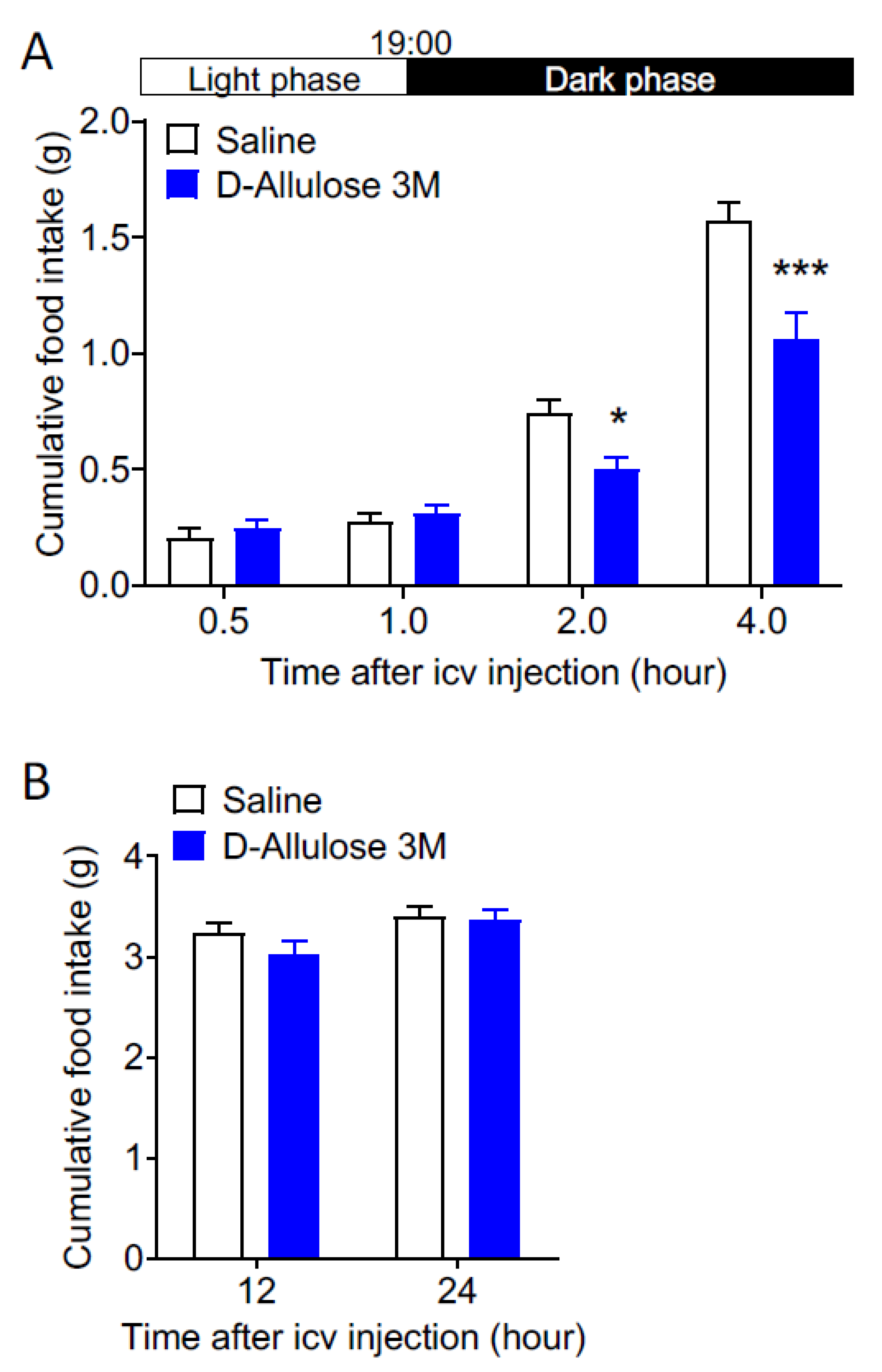
3.4. Effects of Central Injection of d-Allulose on Feeding
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Fact Sheet 2017. N311. Available online: https://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 21 February 2022).
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, A.E.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, A.J.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Mu, W.; Jiang, B.; Zhang, T. Characterization of D-tagatose-3-epimerasefrom Rhodobacter sphaeroides that converts D-fructose into D-psicose. Biotechnol. Lett. 2009, 31, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Poonperm, W.; Takata, G.; Ando, Y.; Sahachaisaree, V.; Lumyong, P.; Lumyong, S.; Izumori, K. Efficient conversion of allitol to D-psicose by Bacillus pallidus Y25. J. Biosci. Bioeng. 2007, 103, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Van Opstal, A.M.; Hafkemeijer, A.; van den Berg-Huysmans, A.A.; Hoeksma, M.; Mulder, P.J.T.; Pijl, H.; Rombouts, A.R.B.S.; van der Ground, J. Brain activity and connectivity changes in response to nutritive natural sugars, non-nutritive natural sugar replacements and artificial sweeteners. Nutr. Neurosci. 2021, 5, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, N.; Iida, T.; Yamada, T.; Okuma, K.; Takehara, I.; Yamamoto, T.; Yamada, K.; Tokuda, M. Study on the postprandial blood glucose suppression effect of D-psicose in borderline diabetes and the safety of long-term ingestion by normal human subjects. Biosci. Biotechnol. Biochem. 2010, 74, 510–519. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, Y.; Sendo, M.; Dezaki, K.; Hira, T.; Sato, T.; Nakata, M.; Goswami, G.; Aoki, R.; Arai, T.; Kumari, P.; et al. GLP-1 release and vagal afferent activation mediate the beneficial metabolic and chronotherapeutic effects of D-allulose. Nat. Commun. 2018, 9, 113. [Google Scholar] [CrossRef]
- Hossain, M.A.; Kitagaki, S.; Nakano, D.; Nishiyama, A.; Funamoto, Y.; Matsunaga, T.; Tsukamoto, I.; Yamaguchi, F.; Kamitori, K.; Dong, Y.; et al. Rare sugar d-psicose improves insulin sensitivity and glucose tolerance in type 2 diabetes (OLETF) rats. Biochem. Biophys. Res. Commun. 2011, 405, 7–12. [Google Scholar] [CrossRef]
- Hossain, M.A.; Yamaguchi, F.; Matsuo, T.; Tsukamoto, I.; Toyoda, Y.; Ogawa, M.; Nagata, Y.; Tokuda, M. Rare sugar d-allulose: Potential role and therapeutic monitoring in maintaining obesity and type 2 diabetes mellitus. Pharmacol. Ther. 2015, 155, 49–59. [Google Scholar] [CrossRef]
- Shintani, T.; Yamada, T.; Hayashi, N.; Iida, T.; Nagata, Y.; Ozaki, N.; Toyoda, Y. Rare Sugar Syrup Containing d-Allulose but Not High-Fructose Corn Syrup Maintains Glucose Tolerance and Insulin Sensitivity Partly via Hepatic Glucokinase Translocation in Wistar Rats. Agric. Food Chem. 2017, 65, 2888–2894. [Google Scholar] [CrossRef]
- Han, Y.; Han, H.J.; Kim, A.-H.; Choi, J.-Y.; Cho, S.-J.; Park, Y.B.; Jung, U.J.; Choi, M.-S. D-Allulose supplementation normalized the body weight and fat-pad mass in diet-induced obese mice via the regulation of lipid metabolism under isocaloric fed condition. Mol. Nutr. Food Res. 2016, 60, 1695–1706. [Google Scholar] [CrossRef]
- Han, Y.; Kwon, E.; Yu, M.K.; Lee, S.J.; Kim, H.-J.; Kim, S.-B.; Kim, Y.H.; Choi, M.-S. A preliminary study for evaluating the dose-dependent effect of D-allulose for fat mass reduction in adult humans: A randomized, double blind, placebo-controlled trial. Nutrients 2018, 10, 160. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, T.; Baba, Y.; Hashiguchi, M.; Takeshita, K.; Izumori, K.; Suzuki, H. Dietary D-psicose, a C-3 epimer of D-fructose, suppresses the activity of hepatic lipogenic enzymes in rats. Asia. Pac. J. Clin. Nutr. 2001, 10, 233–237. [Google Scholar] [CrossRef]
- Nagata, Y.; Kanasaki, A.; Tamaru, S.; Tanaka, K. D-Psicose, an epimer of D-fructose, favorably alters lipid metabolism in sprague-dawley rats. J. Agric. Food Chem. 2015, 63, 3168–3176. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, M.; Nakanishi, Y.; Yamada, T.; Iida, T.; Matsuo, T. Inhibition by dietary D-psicose of body fat accumulation in adult rats fed a high-sucrose diet. Biosci. Biotechnol. Biochem. 2013, 77, 1123–1126. [Google Scholar] [CrossRef] [Green Version]
- Ochiai, M.; Onishi, K.; Yamada, T.; Iida, T.; Matsuo, T. D-Psicose increases energy expenditure and decreases body fat accumulation in rats fed a high-sucrose diet. Int. J. Food Sci. Nutr. 2014, 65, 245–250. [Google Scholar] [CrossRef]
- Han, Y.; Park, H.; Choi, B.-R.; Ji, Y.; Kwon, E.-Y.; Choi, M.-S. Alteration of Microbiome Profile by D-Allulose in Amelioration of High-Fat-Diet-Induced Obesity in Mice. Nutrients 2020, 12, 352. [Google Scholar] [CrossRef] [Green Version]
- Rakhat, Y.; Wang, L.; Kaneko, K.; Han, W.; Seino, Y.; Yabe, D.; Yada, T. D-Allulose cooperates with glucagon-like peptide-1 and activates proopiomelanocortin neurons in the arcuate nucleus and central injection inhibits feeding in mice. Biochem. Biophys. Res. Commun. 2022, 613, 159–165. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Woods, D.; Porte, S.C., Jr.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef]
- Kohno, D.; Yada, T. Arcuate NPY neurons sense and integrate peripheral metabolic signals to control feeding. Neuropeptides 2012, 46, 315–319. [Google Scholar] [CrossRef]
- Hahn, T.M.; Breininger, J.F.; Baskin, D.G.; Schwartz, M.W. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat. Neurosci. 1998, 1, 271–272. [Google Scholar] [CrossRef]
- Takahashi, K.A.; Cone, R.D. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology 2005, 146, 1043–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Murakami, M.; Hasegawa, S.; Yoshizawa, F.; Sugahara, K. Neuropeptide Y content in the hypothalamic paraventricular nucleus responds to fasting and refeeding in broiler chickens. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2005, 141, 146–152. [Google Scholar] [CrossRef]
- Kohno, D.; Sone, H.; Tanaka, S.; Kurita, H.; Gantulda, D.; Yada, T. AMP-activated protein kinase activates neuropeptide Y neurons in the hypothalamic arcuate nucleus to increase food intake in rats. Neurosci. Lett. 2011, 499, 194–198. [Google Scholar] [CrossRef]
- Gropp, E.; Shanabrough, M.; Borok, E.; Xu, A.W.; Janoschek, R.; Buch, T.; Plum, L.; Balthazar, N.; Hampel, B.; Waisman, A.; et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat. Neurosci. 2005, 8, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Luquet, S.; Perez, F.A.; Hnasko, T.S.; Palmiter, R.D. NPY/AgRP neurons are essentials for feeding in adult mice but can be ablated in neonates. Science 2005, 310, 683–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakazato, M.; Murakami, N.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukara, S. A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Muroya, S.; Yada, T.; Shioda, S.; Takigawa, M. Glucose-sensitive neurons in the rat arcuate nucleus contain neuropeptide Y. Neurosci. Lett. 1999, 264, 113–116. [Google Scholar] [CrossRef]
- Aponte, Y.; Atasoy, D.; Sternson, S.M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 2011, 14, 351–355. [Google Scholar] [CrossRef]
- Cowley, M.A.; Smith, R.G.; Diano, S.; Tshop, M.; Pronchuk, N.; Grove, K.L.; Strasburger, C.J.; Bidlingmaier, M.; Esterman, M.; Heiman, M.L. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003, 37, 649–661. [Google Scholar] [CrossRef] [Green Version]
- Kohno, D.H.; Gao, Z.; Muroya, S.; Kikuyama, S.; Yada, T. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca²⁺ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes 2003, 52, 948–956. [Google Scholar] [CrossRef] [Green Version]
- Goswami, C.; Dezaki, K.; Wang, L.; Inui, A.; Seino, Y.; Yada, T. Ninjin-yoeito activates ghrelin-responsive and unresponsive NPY neurons in the arcuate nucleus and counteracts cisplatin-induced Anorexia. Neuropeptides 2019, 75, 58–64. [Google Scholar] [CrossRef]
- Wang, L.; Han, W.; Iwasaki, Y.; Rakhat, Y.; Sharp, G.W.G.; Seino, Y.; Yada, T. Onion component, isoalliin, stimulates feeding and activates the arcuate nucleus neuropeptide Y, ghrelin- and Ninjin’yoeito-responsive neurons. Neuropeptides 2021, 89, 102180. [Google Scholar] [CrossRef]
- Brini, M.; Calì, T.; Ottolini, D.; Carafoli, E. Neuronal calcium signaling: Function and dysfunction. Cell Mol. Life Sci. 2014, 71, 2787–2814. [Google Scholar] [CrossRef]
- Puri, B.K. Calcium Signaling and Gene Expression. Adv. Exp. Med. Biol. 2020, 1131, 537–545. [Google Scholar] [CrossRef]
- Yada, T.; Sakurada, M.; Ihida, K.; Nakata, M.; Murata, F.; Arimura, A.; Kikuchi, M. Pituitary adenylate cyclase activating polypeptide is an extraordinarily potent intra-pancreatic regulator of insulin secretion from islet beta-cells. J. Biol. Chem. 1994, 269, 1290–1293. [Google Scholar] [CrossRef]
- Kohno, D.; Sone, H.; Minokoshi, Y.; Yada, T. Ghrelin raises [Ca2+]i via AMPK in hypothalamic arcuate nucleus NPY neurons. Biochem. Biophys. Res. Commun. 2008, 366, 388–392. [Google Scholar] [CrossRef]
- Kurita, H.; Xu, K.Y.; Maejima, Y.; Nakata, M.; Dezaki, K.; Santoso, P.; Yang, Y.; Arai, T.; Gantulga, D.; Muroya, S.; et al. Arcuate Na+,K+-ATPase senses systemic energy states and regulates feeding behavior through glucose-inhibited neurons. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E320–E333. [Google Scholar] [CrossRef] [Green Version]
- Kohno, D.; Koike, M.; Ninomiya, Y.; Kojima, I.; Kitamura, T.; Yada, T. Sweet Taste Receptor Serves to Activate Glucose and Leptin-Responsive Neurons in the Hypothalamic Arcuate Nucleus and Participates in Glucose Responsiveness. Front. Neurosci. 2016, 10, 502. [Google Scholar] [CrossRef]
- Teysseire, F.; Bordier, V.; Budzinska, A.; Weltens, N.; Rehfeld, J.F.; Holst, J.J.; Hartmann, B.; Beglinger, C.; Oudenhove, L.V.; Wölnerhanssen, B.K.; et al. The Role of D-allulose and Erythritol on the Activity of the Gut Sweet Taste Receptor and Gastrointestinal Satiation Hormone Release in Humans: A Randomized, Controlled Trial. J. Nutr. 2022, 152, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, M.; Hira, T.; Nakamura, M.; Iida, T.; Kishimoto, Y.; Hara, H. Secretion of GLP-1 but not GIP is potently stimulated by luminal d-Allulose (d-Psicose) in rats. Biochem. Biophys. Res. Commun. 2018, 496, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, I.; Hossain, M.A.; Yamaguchi, F.; Hirata, Y.; Dong, Y.; Kamitori, K.; Sui, L.; Nonaka, M.; Ueno, M.; Nishimoto, K.; et al. Intestinal absorption, organ distribution, and urinary excretion of the rare sugar D-psicose. Drug Des. Dev. Ther. 2014, 8, 1955–1964. [Google Scholar] [CrossRef]
- Langlet, F.; Levin, B.E.; Luquet, S.; Mazzone, M.; Messina, A.; Dunn-Meynell, A.A.; Balland, E.; Lacombe, A.; Mazur, D.; Carmeliet, P.; et al. Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab. 2013, 17, 607–617. [Google Scholar] [CrossRef] [Green Version]
- Duquenne, M.; Folgueira, C.; Bourouh, C.; Millet, M.; Silva, A.; Jerome, C.; Imbernon, M.; Fernandois, D.; Martinez-Coral, I.; Kusumakshi, S. Leptin brain entry via a tanycytic LepR EGFR shuttle controls lipid metabolism and pancreas function. Nat. Metab. 2021, 3, 1071–1090. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, A.; Lazcano, I.; Sánchez-Jaramillo, E.; Uribe, R.M.; Jaimes-Hoy, L.; Joseph-Bravo, P.; Charli, J.-L. Tanycytes and the Control of Thyrotropin-Releasing Hormone Flux Into Portal Capillaries. Front. Endocrinol. 2019, 10, 401. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, E.M.; Blázquez, J.L.; Pastor, F.E.; Pelaez, B.; Pena, P.; Peruzzo, B.; Amat, P. Hypothalamic Tanycytes: A Key Component of Brain-Endocrine Interaction. Int. Rev. Cytol. 2005, 247, 89–164. [Google Scholar] [CrossRef]
- Kumar, M.P.; Cremer, A.L.; Klemm, P.; Steuernagel, L.; Sundaram, S.; Jais, A.; Hausen, A.C.; Tao, J.; Secher, A.; Pedersen, T.A.; et al. Insulin signalling in tanycytes gates hypothalamic insulin uptake and regulation of AgRP neuron activity. Nat. Metab. 2021, 3, 1662–1679. [Google Scholar] [CrossRef]
- Cui, Q.N.; Stein, L.M.; Fortin, S.M.; Hayes, M.R. The role of glia in the physiology and pharmacology of GLP-1: Implications for obesity, diabetes, and neurodegenerative processes including glaucoma. Br. J. Pharmacol. 2022, 179, 715–726. [Google Scholar] [CrossRef]
- Chiba, Y.; Murakami, R.; Matsumoto, K.; Wakamatsu, K.; Nonaka, W.; Uemura, N.; Yanase, K.; Kamada, M.; Ueno, M. Glucose, fructose, and urate transporters in the choroid plexus epithelium. Int. J. Mol. Sci. 2020, 21, 7230. [Google Scholar] [CrossRef]
- Kojo, A.; Yamada, K.; Yamamoto, T. Glucose transporter 5 (GLUT5)-like immunoreactivity is localized in subsets of neurons and glia in the rat brain. J. Chem. Neuroanat. 2016, 74, 55–70. [Google Scholar] [CrossRef]
- Hill, J.W.; Elias, C.F.; Fukuda, M.; Williams, K.W.; Berglund, E.D.; Holland, W.L.; Cho, Y.-R.; Chuang, J.-C.; Xu, Y.; Choi, M.; et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010, 11, 286–297. [Google Scholar] [CrossRef] [Green Version]
- Kohno, D.; Nakata, M.; Maekawa, F.; Gijiwara, K.; Maejima, Y.; Kuramochi, M.; Shimazaki, T.; Okano, H.; Onaka, T.; Yada, T. Leptin suppresses ghrelin-induced activation of neuropeptide Y neurons in the arcuate nucleus via phosphatidylinositol 3-kinase- and phosphodiesterase 3-mediated pathway. Endocrinology 2007, 148, 2251–2263. [Google Scholar] [CrossRef] [PubMed]
- Maejima, Y.; Kohno, D.; Iwasaki, Y.; Yada, T. Insulin suppresses ghrelin-induced calcium signaling in neuropeptide Y neurons of the hypothalamic arcuate nucleus. Aging 2011, 3, 1092–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakhat, Y.; Kaneko, K.; Wang, L.; Han, W.; Seino, Y.; Yabe, D.; Yada, T. d-Allulose Inhibits Ghrelin-Responsive, Glucose-Sensitive and Neuropeptide Y Neurons in the Arcuate Nucleus and Central Injection Suppresses Appetite-Associated Food Intake in Mice. Nutrients 2022, 14, 3117. https://doi.org/10.3390/nu14153117
Rakhat Y, Kaneko K, Wang L, Han W, Seino Y, Yabe D, Yada T. d-Allulose Inhibits Ghrelin-Responsive, Glucose-Sensitive and Neuropeptide Y Neurons in the Arcuate Nucleus and Central Injection Suppresses Appetite-Associated Food Intake in Mice. Nutrients. 2022; 14(15):3117. https://doi.org/10.3390/nu14153117
Chicago/Turabian StyleRakhat, Yermek, Kentaro Kaneko, Lei Wang, Wanxin Han, Yutaka Seino, Daisuke Yabe, and Toshihiko Yada. 2022. "d-Allulose Inhibits Ghrelin-Responsive, Glucose-Sensitive and Neuropeptide Y Neurons in the Arcuate Nucleus and Central Injection Suppresses Appetite-Associated Food Intake in Mice" Nutrients 14, no. 15: 3117. https://doi.org/10.3390/nu14153117
APA StyleRakhat, Y., Kaneko, K., Wang, L., Han, W., Seino, Y., Yabe, D., & Yada, T. (2022). d-Allulose Inhibits Ghrelin-Responsive, Glucose-Sensitive and Neuropeptide Y Neurons in the Arcuate Nucleus and Central Injection Suppresses Appetite-Associated Food Intake in Mice. Nutrients, 14(15), 3117. https://doi.org/10.3390/nu14153117






