Valorization of Grape Pomace and Berries as a New and Sustainable Dietary Supplement: Development, Characterization, and Antioxidant Activity Testing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Raw Material Analysis
2.3.1. Particle Size Distribution
2.3.2. Powder Flow
2.3.3. Phytochemical Extraction
2.3.4. Phytochemical Analysis
Total Polyphenolic Content (TPC) Assay
HPLC-DAD Phenolic Profile Assessment
Antioxidant Activity Assay
2.4. Encapsulation
2.5. Analysis of Capsules
2.5.1. Average Mass and Uniformity of Mass
2.5.2. Disintegration
2.5.3. Uniformity of Content
3. Results
3.1. Raw Material analysis
3.1.1. Particle Size Distribution
3.1.2. Powder Flow
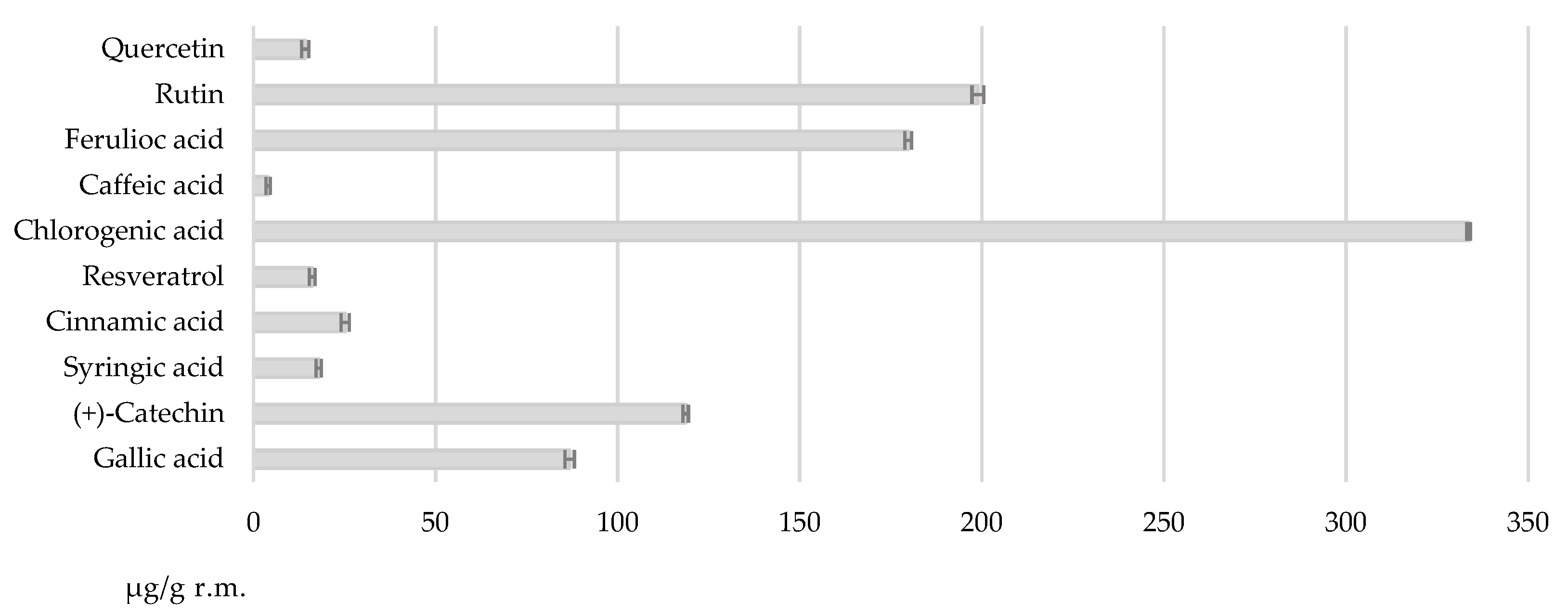
3.1.3. Phytochemical Analysis
3.2. Analysis of Capsules
3.2.1. Average Mass and Uniformity of Mass
3.2.2. Disintegration
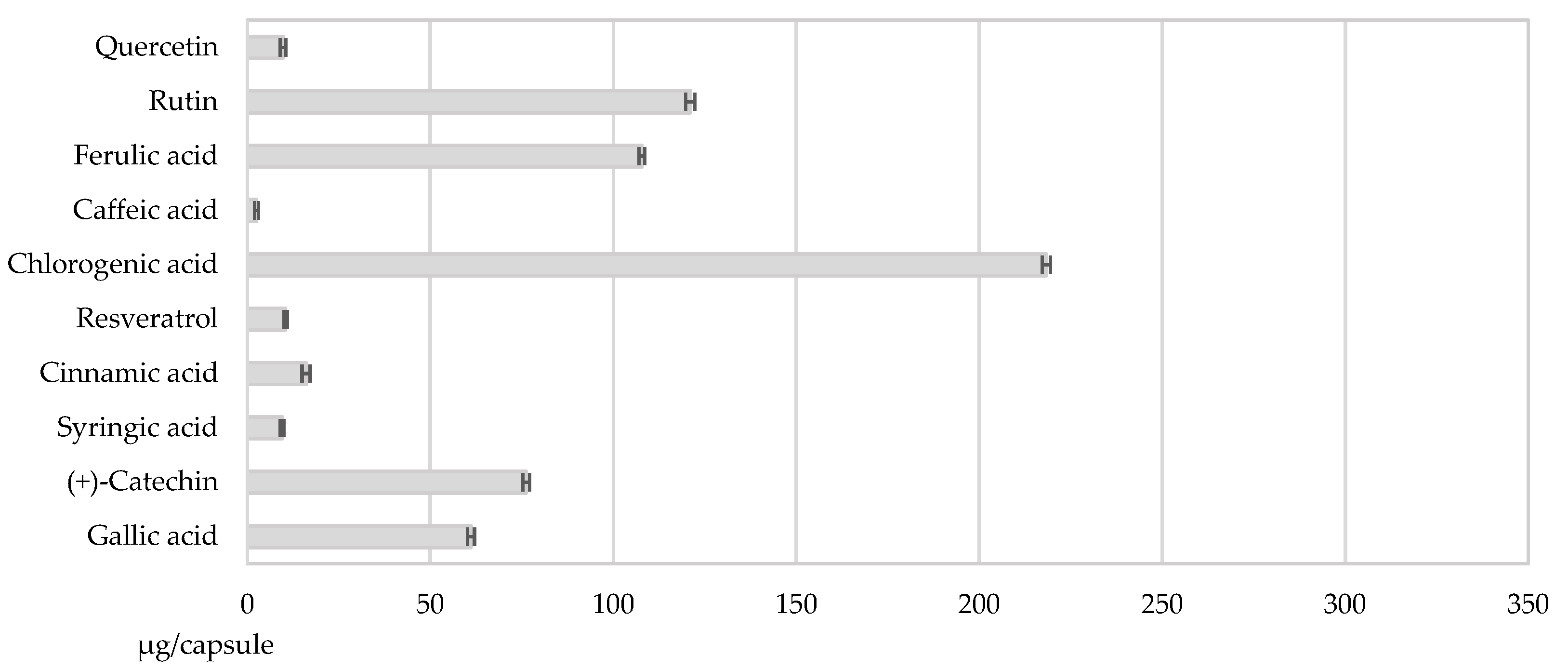
3.2.3. Uniformity of Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CS | Cabernet Sauvignon |
| d.w. | dry weight |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| FN | Feteasca Neagra |
| GAE | gallic acid equivalents |
| OS | oxidative stress |
| r.m. | raw material |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| RSA | radical scavenging assay |
| SD | standard deviation |
| TPC | total phenolic content |
| V0 | apparent volume |
| Vf | final volume |
References
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Ciulca, S.; Roma, G.; Alexa, E.; Radulov, I.; Cocan, I.; Madosa, E.; Ciulca, A. Variation of Polyphenol Content and Antioxidant Activity in Some Bilberry (Vaccinium myrtillus L.) Populations from Romania. Agronomy 2021, 11, 2557. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, C.; Kapoor, H.C. Antioxidant Activity of Some Fruits in Indian Diet. Acta Hortic. 2005, 696, 563–565. [Google Scholar] [CrossRef]
- Chan, S.W.; Tomlinson, B. Effects of Bilberry Supplementation on Metabolic and Cardiovascular Disease Risk. Molecules 2020, 25, 1653. [Google Scholar] [CrossRef] [Green Version]
- Laczkó-zöld, E.; Komlósi, R.; Ülkei, T.; Fogarasi, E.; Croitoru, M.; Fülöp, I.; Domokos, E.; Ştefănescu, R.; Varga, E. Extractability of Polyphenols from Black Currant, Red Currant and Gooseberry and Their Antioxidant Activity. Acta Biol. Hung. 2018, 69, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Geybels, M.S.; Verhage, B.A.J.; Arts, I.C.W.; Van Schooten, F.J.; Alexandra Goldbohm, R.; Van Den Brandt, P.A. Dietary Flavonoid Intake, Black Tea Consumption, and Risk of Overall and Advanced Stage Prostate Cancer. Am. J. Epidemiol. 2013, 177, 1388–1398. [Google Scholar] [CrossRef] [Green Version]
- Ghibu, S.; Craciun, C.E.; Rusu, R.; Morgovan, C.; Mogosan, C.; Rochette, L.; Gal, A.F.; Dronca, M. Impact of Alpha-Lipoic Acid Chronic Discontinuous Treatment in Cardiometabolic Disorders and Oxidative Stress Induced by Fructose Intake in Rats. Antioxidants 2019, 8, 636. [Google Scholar] [CrossRef] [Green Version]
- Ghibu, S.; Decea, N.; Morgovan, C.; Mogosan, C. An Experimental Model to Induce Metabolic Syndrome in Rats. The Fructose-Enriched Diet. Farmacia 2013, 61, 420–426. [Google Scholar]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and Vegetable Intake and the Risk of Cardiovascular Disease, Total Cancer and All-Cause Mortality-A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Pap, N.; Fidelis, M.; Azevedo, L.; do Carmo, M.A.V.; Wang, D.; Mocan, A.; Pereira, E.P.R.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B.; et al. Berry Polyphenols and Human Health: Evidence of Antioxidant, Anti-Inflammatory, Microbiota Modulation, and Cell-Protecting Effects. Curr. Opin. Food Sci. 2021, 42, 167–186. [Google Scholar] [CrossRef]
- Golovinskaia, O.; Wang, C.K. Review of Functional and Pharmacological Activities of Berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L.; Määttä-Riihinen, K.; Kärenlampi, S.; Hohtola, A. Activation of Flavonoid Biosynthesis by Solar Radiation in Bilberry (Vaccinium myrtillus L.) Leaves. Planta 2004, 218, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.S.P.; Caleja, C.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C.F.R. Vaccinium myrtillus L. Fruits as a Novel Source of Phenolic Compounds with Health Benefits and Industrial Applications—A Review. Curr. Pharm. Des. 2020, 26, 1917–1928. [Google Scholar] [CrossRef]
- Graff, A.; Petrone, C.; Associate, R.; Ba, D.S.; Upton, R.; Pizzorno, J.; Myrtillus, V. American Herbal PharmacopoeiaTM and Herapeutic Ompendium. Bilberry Fruit. 2001. Available online: http://naturamedica.lt/wp-content/uploads/2015/07/AHP-Bilberry.pdf (accessed on 29 June 2022).
- Chu, W.K.; Cheung, S.C.M.; Lau, R.A.W. Bilberry (Vaccinium myrtillus L.). Herb. Med. Biomol. Clin. Asp. 2011, 20115386, 55–71. [Google Scholar] [CrossRef]
- Šumić, Z.; Vakula, A.; Tepić, A.; Čakarević, J.; Vitas, J.; Pavlić, B. Modeling and Optimization of Red Currants Vacuum Drying Process by Response Surface Methodology (RSM). Food Chem. 2016, 203, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Nour, V.; Trandafir, I.; Ionica, M.E. Ascorbic Acid, Anthocyanins, Organic Acids and Mineral Content of Some Black and Red Currant Cultivars. Fruits 2011, 66, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Zdunić, G.; Šavikin, K.; Pljevljakušić, D.; Djordjević, B. Black (Ribes nigrum L.) and Red Currant (Ribes rubrum L.) Cultivars; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780124081178. [Google Scholar]
- Vesela Shopska, A.; Georgieva, R.; Nedyalkov, P.; Shopska, V.; Kaneva, M. Effect of Blueberries Addition during Beer Maturation on Yeast Metabolism. Food Sci. Appl. Biotechnol. 2021, 4, 105–110. [Google Scholar] [CrossRef]
- Nedyalkov, P.; Bakardzhiyski, I.; Dinkova, R.; Shopska, V.; Kaneva, M. Influence of the Time of Bilberry (Vaccinium myrtillus L.) Addition on the Phenolic and Protein Profile of Beer. Acta Sci. Pol. Technol. Aliment. 2022, 21, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Berry Phenolic Antioxidants—Implications for Human Health? Front. Pharmacol. 2018, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape Pomace as a Sustainable Source of Bioactive Compounds: Extraction, Characterization, and Biotechnological Applications of Phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef] [PubMed]
- Grassi, F.; De Lorenzis, G. Back to the Origins: Background and Perspectives of Grapevine Domestication. Int. J. Mol. Sci. 2021, 22, 4518. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, R.; Tarafdar, A.; Singh, S.; Negi, T.; Gaur, V.K.; Gnansounou, E.; Bharathiraja, B. Green Processing and Biotechnological Potential of Grape Pomace: Current Trends and Opportunities for Sustainable Biorefinery. Bioresour. Technol. 2020, 314, 123771. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Bin, S.; Vallini, V.; Fava, F.; Michelini, E.; Roda, A.; Minnucci, G.; Bucchi, G.; Tassoni, A. Recovery of Polyphenols from Red Grape Pomace and Assessment of Their Antioxidant and Anti-Cholesterol Activities. N. Biotechnol. 2016, 33, 338–344. [Google Scholar] [CrossRef] [PubMed]
- García-Lomillo, J.; González-SanJosé, M.L. Applications of Wine Pomace in the Food Industry: Approaches and Functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef]
- Theagarajan, R.; Malur Narayanaswamy, L.; Dutta, S.; Moses, J.A.; Chinnaswamy, A. Valorisation of Grape Pomace (Cv. Muscat) for Development of Functional Cookies. Int. J. Food Sci. Technol. 2019, 54, 1299–1305. [Google Scholar] [CrossRef]
- Chowdhary, P.; Gupta, A.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Current Trends and Possibilities for Exploitation of Grape Pomace as a Potential Source for Value Addition. Environ. Pollut. 2021, 278, 116796. [Google Scholar] [CrossRef]
- Ping, L.; Pizzi, A.; Guo, Z.D.; Brosse, N. Condensed Tannins Extraction from Grape Pomace: Characterization and Utilization as Wood Adhesives for Wood Particleboard. Ind. Crops Prod. 2011, 34, 907–914. [Google Scholar] [CrossRef] [Green Version]
- Averilla, J.N.; Oh, J.; Kim, H.J.; Kim, J.S.; Kim, J.S. Potential Health Benefits of Phenolic Compounds in Grape Processing By-Products. Food Sci. Biotechnol. 2019, 28, 1607–1615. [Google Scholar] [CrossRef]
- Venkitasamy, C.; Zhao, L.; Zhang, R.; Pan, Z. Grapes; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128141397. [Google Scholar]
- Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the Approximation of the Laws of the Member States Relating to Food Supplements. Off. J. Eur. Communities 2002, 183, 51–57.
- Morgovan, C.; Ghibu, S.; Juncan, A.M.; Rus, L.L.; Butucă, A.; Vonica, L.; Muntean, A.; Moş, L.; Gligor, F.; Olah, N.K. Nutrivigilance: A New Activity in the Field of Dietary Supplements. Farmacia 2019, 67, 537–544. [Google Scholar] [CrossRef]
- Chiba, T.; Sato, Y.; Kobayashi, E.; Ide, K.; Yamada, H.; Umegaki, K. Behaviors of Consumers, Physicians and Pharmacists in Response to Adverse Events Associated with Dietary Supplement Use. Nutr. J. 2017, 16, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Council of Europe. European Pharmacopoeia 10th Edition|EDQM—European Directorate for the Quality of Medicines. Available online: https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-10th-edition- (accessed on 29 June 2022).
- Frum, A.; Georgescu, C.; Gligor, F.G.; Stegarus, D.I.; Dobrea, C.M.; Tita, O.; Blaga, L.; Sciences, A.; Industry, F.; Street, I.R.; et al. Identification and Quantification of Phenolic Compounds from Red Grape Pomace. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2018, 19, 45–52. [Google Scholar]
- Georgescu, C.; Bratu, I. Tamas M Studiul Unor Polifenoli Din Rhododendron Kotschyi. Rev. Chim. 2005, 56, 779–780. [Google Scholar]
- Gligor, F.G.; Frum, A.; Vicaș, L.G.; Totan, M.; Roman-Filip, C.; Dobrea, C.M. Determination of a Mixture of Plantago lanceolata L. and Salvia officinalis L. by High-Performance Liquid Chromatography with Ultraviolet Detection (HPLC-UV). Anal. Lett. 2020, 53, 1391–1406. [Google Scholar] [CrossRef]
- Craciun, V.I.; Gligor, F.G.; Juncan, A.M.; Chis, A.A.; Rus, L.L. A New, Rapid and Efficient HPLC Method to Assay Resveratrol in Food Supplements. Rev. Chim. 2019, 70, 3202–3205. [Google Scholar] [CrossRef]
- Popovici, C.; Saykova, I.; Tylkowski, B. Evaluation de l’activité Antioxydant Des Composés Phénoliques Par La Réactivité Avec Le Radical Libre DPPH. Rev. Génie Ind. 2009, 4, 25–39. [Google Scholar]
- Tița, O.; Constantinescu, M.A.; Tița, M.A.; Georgescu, C. Use of Yoghurt Enhanced with Volatile Plant Oils Encapsulated in Sodium Alginate to Increase the Human Body’s Immunity in the Present Fight Against Stress. Int. J. Environ. Res. Public Health 2020, 17, 7588. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M.; Arlorio, M.; Coïsson, J.D. Spent Grape Pomace as a Still Potential By-Product. Int. J. Food Sci. Technol. 2015, 50, 2022–2031. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. The Potential of Grape Pomace Varieties as a Dietary Source of Pectic Substances. Foods 2021, 10, 867. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Schulz, P.; Rizvi, S.S.H. Valorization of Bioactive Compounds in Fruit Pomace from Agro-Fruit Industries: Present Insights and Future Challenges. Food Biosci. 2021, 44, 101384. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.M.; Santos, L.; Serratosa, M.P.; Angeles, M.; Santos, V.; Morikawa, T.; Ferreira, S.M.; Santos, L. A Potential Valorization Strategy of Wine Industry By-Products and Their Application in Cosmetics—Case Study: Grape Pomace and Grapeseed. Molecules 2022, 27, 969. [Google Scholar] [CrossRef]
- Gligor, F.G.; Dobrea, C.M.; Georgescu, C.; Vonica Gligor, L.A.; Frum, A.; Totan, M. Silymarin Food Supplements—Oral Solid Dosage Forms. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2016, 17, 349–354. [Google Scholar]
- Mititelu, M.; Moroșan, E.; Nicoară, A.C.; Secăreanu, A.A.; Musuc, A.M.; Atkinson, I.; Cusu, J.P.; Nițulescu, G.M.; Ozon, E.A.; Sarbu, I.; et al. Development of Immediate Release Tablets Containing Calcium Lactate Synthetized from Black Sea Mussel Shells. Mar. Drugs 2022, 20, 45. [Google Scholar] [CrossRef]
- Frum, A.; Lengyel, E.; Georgescu, C.; Gligor, F.; Tita, O. Analysis of Phenolic Compounds Extracted from Three Types of Berries. In Proceedings of the 6th BIOATLAS Conference, Brașov, Romania, 27–28 May 2016; Volume 12, pp. 5–9. [Google Scholar]
- Costa, C.; Tsatsakis, A.; Mamoulakis, C.; Teodoro, M.; Briguglio, G.; Caruso, E.; Tsoukalas, D.; Margina, D.; Dardiotis, E.; Kouretas, D.; et al. Current Evidence on the Effect of Dietary Polyphenols Intake on Chronic Diseases. Food Chem. Toxicol. 2017, 110, 286–299. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [Green Version]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative Stress in Chronic Kidney Disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Beetch, M.; Harandi-Zadeh, S.; Shen, K.; Lubecka, K.; Kitts, D.D.; O’Hagan, H.M.; Stefanska, B. Dietary Antioxidants Remodel DNA Methylation Patterns in Chronic Disease. Br. J. Pharmacol. 2020, 177, 1382–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicero, A.F.G.; Colletti, A. Polyphenols Effect on Circulating Lipids and Lipoproteins: From Biochemistry to Clinical Evidence. Curr. Pharm. Des. 2018, 24, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding Oxidants and Antioxidants: Classical Team with New Players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiñones, M.; Miguel, M.; Aleixandre, A. Beneficial Effects of Polyphenols on Cardiovascular Disease. Pharmacol. Res. 2013, 68, 125–131. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in Cancer Prevention: New Insights (Review). Int. J. Funct. Nutr. 2020, 1, 1–11. [Google Scholar] [CrossRef]
- Meresman, G.F.; Götte, M.; Laschke, M.W. Plants as Source of New Therapies for Endometriosis: A Review of Preclinical and Clinical Studies. Hum. Reprod. Update 2021, 27, 367–392. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative Stress and Inflammation in Osteoarthritis Pathogenesis: Role of Polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef] [PubMed]
- Valsamidou, E.; Gioxari, A.; Amerikanou, C.; Zoumpoulakis, P.; Skarpas, G.; Kaliora, A.C. Dietary Interventions with Polyphenols in Osteoarthritis: A Systematic Review Directed from the Preclinical Data to Randomized Clinical Studies. Nutrients 2021, 13, 1420. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Tripathi, P.; Yadawa, A.K.; Singh, S. Promising Polyphenols in Parkinson’s Disease Therapeutics. Neurochem. Res. 2020, 45, 1731–1745. [Google Scholar] [CrossRef] [PubMed]
- Szczechowiak, K.; Diniz, B.S.; Leszek, J. Diet and Alzheimer’s Dementia—Nutritional Approach to Modulate Inflammation. Pharmacol. Biochem. Behav. 2019, 184, 172743. [Google Scholar] [CrossRef] [PubMed]
- Sathya, S.; Devi, K.P. The Use of Polyphenols for the Treatment of Alzheimer’s Disease. In Role of the Mediterranean Diet in the Brain and Neurodegerative Diseases; Farooqui, T., Farooqui, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 239–252. [Google Scholar] [CrossRef]
- Qin, X.; Lu, Y.; Peng, Z.; Fan, S.; Yao, Y. Systematic Chemical Analysis Approach Reveals Superior Antioxidant Capacity via the Synergistic Effect of Flavonoid Compounds in Red Vegetative Tissues. Front. Chem. 2018, 6, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollica, A.; Scioli, G.; Valle, A.D.; Cichelli, A.; Novellino, E.; Bauer, M.; Kamysz, W.; Llorent-Martínez, E.J.; Córdova, M.L.F.; Castillo-López, R.; et al. Phenolic Analysis and In Vitro Biological Activity of Red Wine, Pomace and Grape Seeds Oil Derived from Vitis Vinifera l. Cv. Montepulciano d’abruzzo. Antioxidants 2021, 10, 1704. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, A.; Balaha, M.; Acquaviva, A.; Ferrante, C.; Cataldi, A.; Menghini, L.; Rapino, M.; Orlando, G.; Brunetti, L.; Leone, S.; et al. Phenolic Characterization and Neuroprotective Properties of Grape Pomace Extracts. Molecules 2021, 26, 6216. [Google Scholar] [CrossRef] [PubMed]
- Sri Harsha, P.S.C.; Lavelli, V. Use of Grape Pomace Phenolics to Counteract Endogenous and Exogenous Formation of Advanced Glycation End-Products. Nutrients 2019, 11, 1917. [Google Scholar] [CrossRef] [Green Version]
- León-González, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-Oxidant Activity of Polyphenols and Its Implication on Cancer Chemoprevention and Chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Seck, I.; Hosu, A.; Cimpoiu, C.; Ndoye, S.F.; Ba, L.A.; Sall, C.; Seck, M. Phytochemicals Content, Screening and Antioxidant/pro-Oxidant Activities of Carapa Procera (Barks) (Meliaceae). S. Afr. J. Bot. 2021, 137, 369–376. [Google Scholar] [CrossRef]
- Dlamini, L.M.; Tata, C.M.; Djuidje, M.C.F.; Ikhile, M.I.; Nikolova, G.D.; Karamalakova, Y.D.; Gadjeva, V.G.; Zheleva, A.M.; Njobeh, P.B.; Ndinteh, D.T. Antioxidant and Prooxidant Effects of Piptadeniastrum Africanum as the Possible Rationale behind Its Broad Scale Application in African Ethnomedicine. J. Ethnopharmacol. 2019, 231, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Fernando, W.; Rupasinghe, H.P.V.; Hoskin, D.W. Dietary Phytochemicals with Anti-Oxidant and pro-Oxidant Activities: A Double-Edged Sword in Relation to Adjuvant Chemotherapy and Radiotherapy? Cancer Lett. 2019, 452, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.R.; Appel, C.L. Polyphenols as Dietary Supplements: A Double-Edged Sword. Nutr. Diet. Suppl. 2010, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Canning, A.D.; Death, R.G.; Waltham, N.J. Pharmaceutical Companies Should Pay for Raiding Nature’s Medicine Cabinet. Lancet 2021, 398, 840–841. [Google Scholar] [CrossRef]
- European Medicines Agency. Good Manufacturing Practice. 2018. Available online: https://www.ema.europa.eu/en/human-regulatory/research-development/compliance/good-manufacturing-practice (accessed on 21 June 2022).
- Adnan, M.; Azad, M.O.K.; Ju, H.S.; Son, J.M.; Park, C.H.; Shin, M.H.; Alle, M.; Cho, D.H. Development of Biopolymer-Mediated Nanocomposites Using Hot-Melt Extrusion to Enhance the Bio-Accessibility and Antioxidant Capacity of Kenaf Seed Flour. Appl. Nanosci. 2020, 10, 1305–1317. [Google Scholar] [CrossRef]
- Tang, X.; Zakhvatayeva, A.; Zhang, L.; Wu, Z.F.; Sun, P.; Wu, C.Y. Flow Behaviour of Pharmaceutical Powders during Rotary Die Filling with a Paddle Feeder. Int. J. Pharm. 2020, 585, 119547. [Google Scholar] [CrossRef] [PubMed]
- Ngeacharernkul, P.; Stamatis, S.D.; Kirsch, L.E. Particle Size Distribution Equivalency as Novel Predictors for Bioequivalence. AAPS PharmSciTech 2018, 19, 2787–2800. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on Process Validation for Finished Products-Information and Data to be Provided in Regulatory Submissions. 2016. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-process-validation-finished-products-information-data-be-provided-regulatory-submissions_en.pdf (accessed on 21 June 2022).
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
| Sieve (µm) | Weight (g) | Weight (%) |
|---|---|---|
| 710 | 4.25 | 4.27 |
| 224 | 94.93 | 95.36 |
| 125 | 0.37 | 0.37 |
| 90 | 0.00 | 0.00 |
| 63 | 0.00 | 0.00 |
| Number of Beats | Volume (mL) | Compressibility Index | Hausner Ratio |
|---|---|---|---|
| 0 | 208 | 14.71% | 1.17 |
| 10 | 184 | ||
| 500 | 174 | ||
| 1000 | 174 |
| Capsule No. | Full Capsule (mg) | Content (mg) |
|---|---|---|
| 1 | 820 | 703 |
| 2 | 827 | 707 |
| 3 | 817 | 701 |
| 4 | 815 | 700 |
| 5 | 823 | 702 |
| 6 | 820 | 701 |
| 7 | 835 | 713 |
| 8 | 827 | 712 |
| 9 | 822 | 702 |
| 10 | 827 | 707 |
| 11 | 831 | 713 |
| 12 | 817 | 698 |
| 13 | 826 | 707 |
| 14 | 830 | 709 |
| 15 | 815 | 700 |
| 16 | 826 | 708 |
| 17 | 819 | 699 |
| 18 | 824 | 707 |
| 19 | 815 | 696 |
| 20 | 823 | 703 |
| Average | 822.95 | 704.4 |
| Basket No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Time (Minutes:Seconds) | 2:10 | 2:03 | 1:20 | 2:13 | 2:35 | 1:59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frum, A.; Dobrea, C.M.; Rus, L.L.; Virchea, L.-I.; Morgovan, C.; Chis, A.A.; Arseniu, A.M.; Butuca, A.; Gligor, F.G.; Vicas, L.G.; et al. Valorization of Grape Pomace and Berries as a New and Sustainable Dietary Supplement: Development, Characterization, and Antioxidant Activity Testing. Nutrients 2022, 14, 3065. https://doi.org/10.3390/nu14153065
Frum A, Dobrea CM, Rus LL, Virchea L-I, Morgovan C, Chis AA, Arseniu AM, Butuca A, Gligor FG, Vicas LG, et al. Valorization of Grape Pomace and Berries as a New and Sustainable Dietary Supplement: Development, Characterization, and Antioxidant Activity Testing. Nutrients. 2022; 14(15):3065. https://doi.org/10.3390/nu14153065
Chicago/Turabian StyleFrum, Adina, Carmen Maximiliana Dobrea, Luca Liviu Rus, Lidia-Ioana Virchea, Claudiu Morgovan, Adriana Aurelia Chis, Anca Maria Arseniu, Anca Butuca, Felicia Gabriela Gligor, Laura Gratiela Vicas, and et al. 2022. "Valorization of Grape Pomace and Berries as a New and Sustainable Dietary Supplement: Development, Characterization, and Antioxidant Activity Testing" Nutrients 14, no. 15: 3065. https://doi.org/10.3390/nu14153065
APA StyleFrum, A., Dobrea, C. M., Rus, L. L., Virchea, L.-I., Morgovan, C., Chis, A. A., Arseniu, A. M., Butuca, A., Gligor, F. G., Vicas, L. G., Tita, O., & Georgescu, C. (2022). Valorization of Grape Pomace and Berries as a New and Sustainable Dietary Supplement: Development, Characterization, and Antioxidant Activity Testing. Nutrients, 14(15), 3065. https://doi.org/10.3390/nu14153065










