Micronutrients and Renal Outcomes: A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Characteristics
2.2. Sample Collection
2.3. Chemicals and Reagents for Micronutrients Measurement
2.4. Sample Preparation
2.5. Inductively Coupled Plasma Mass Spectrometry (ICP-MS) Analysis
2.6. Statistical Analysis
3. Results
3.1. Study Design and Subject Characteristics
3.2. Demographics and Clinical Characteristics Comparisons among Various CKD Stages
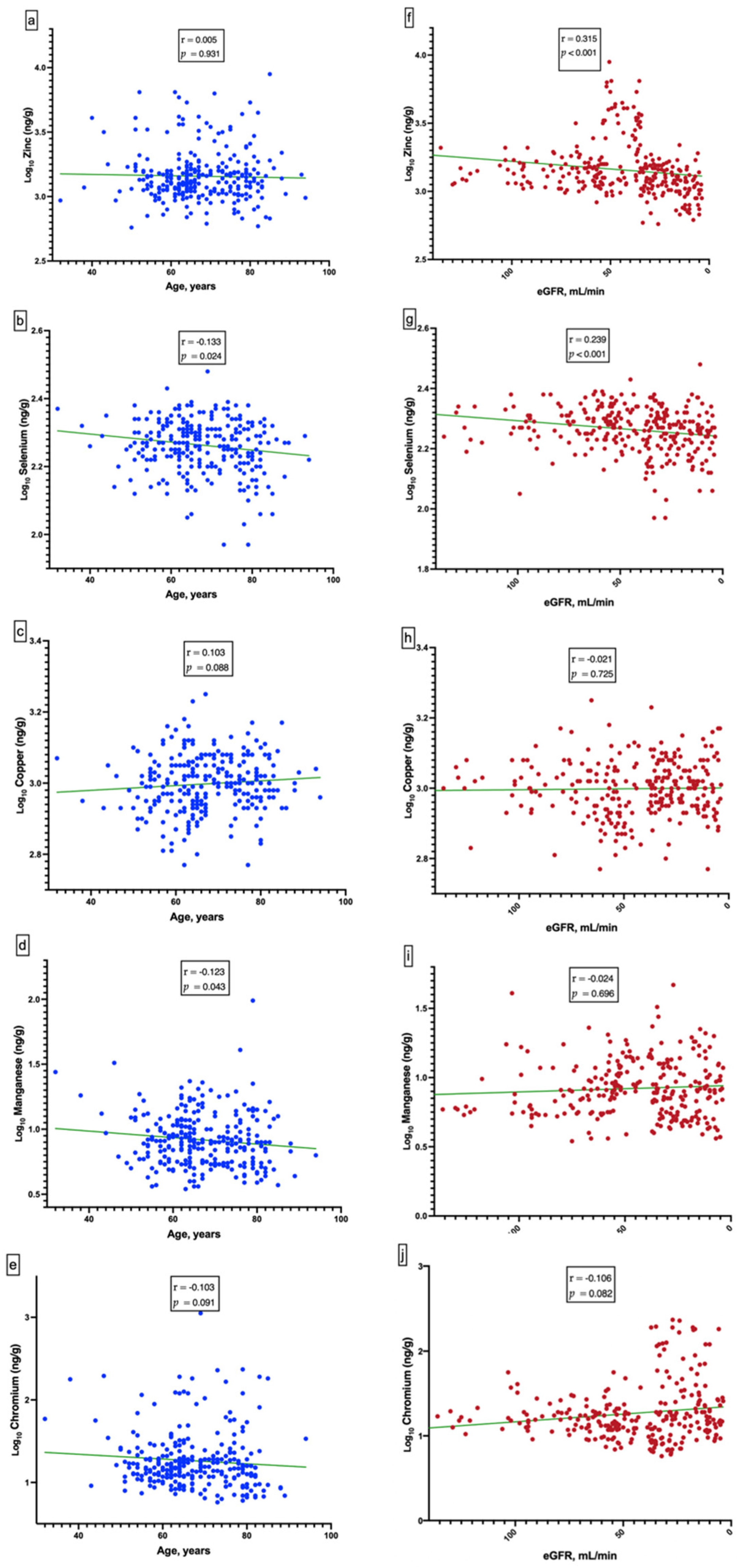
3.3. Kidney Function and Independent Variables
3.4. Relationship of Renal Outcome and Independent Variables
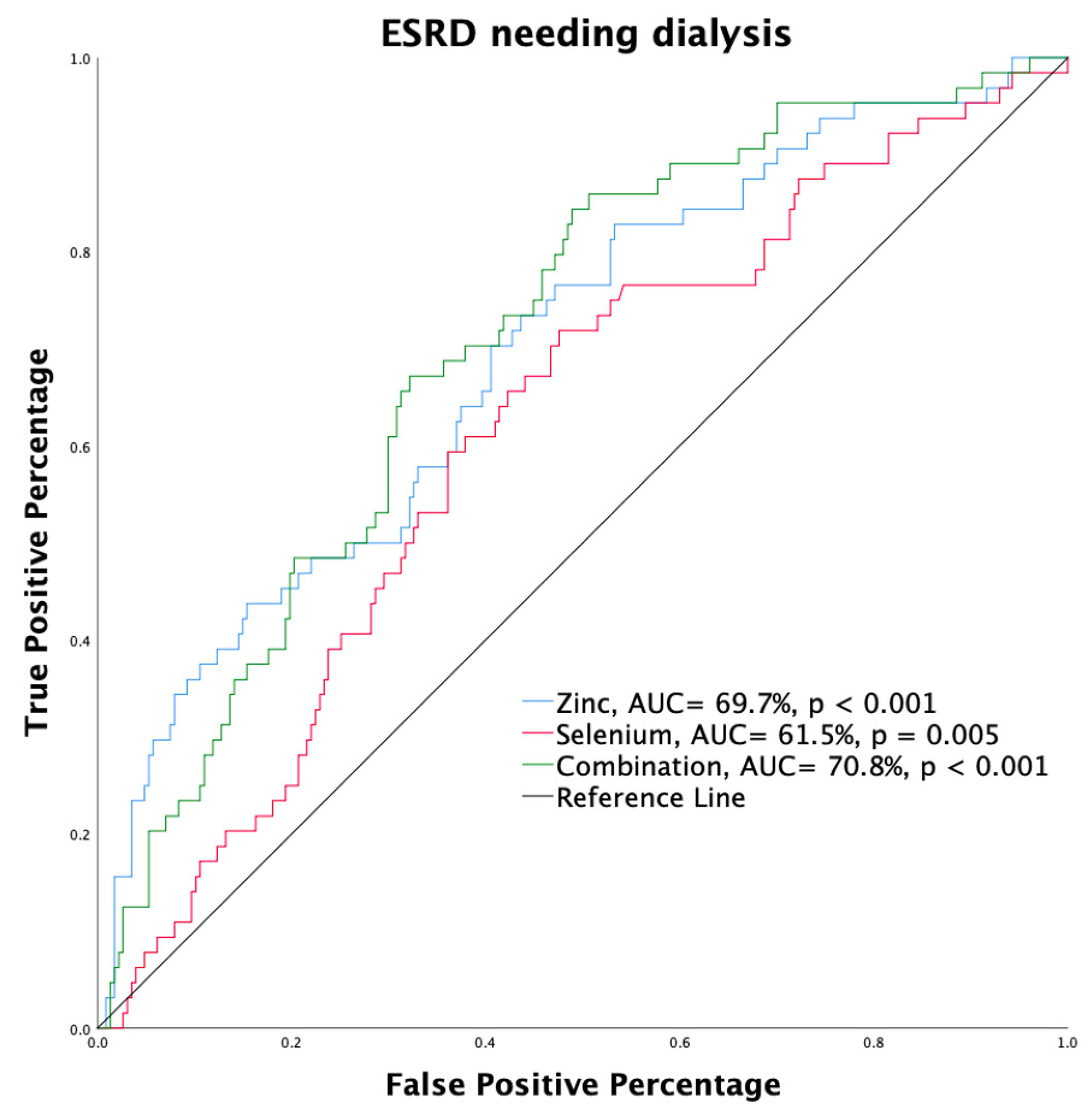
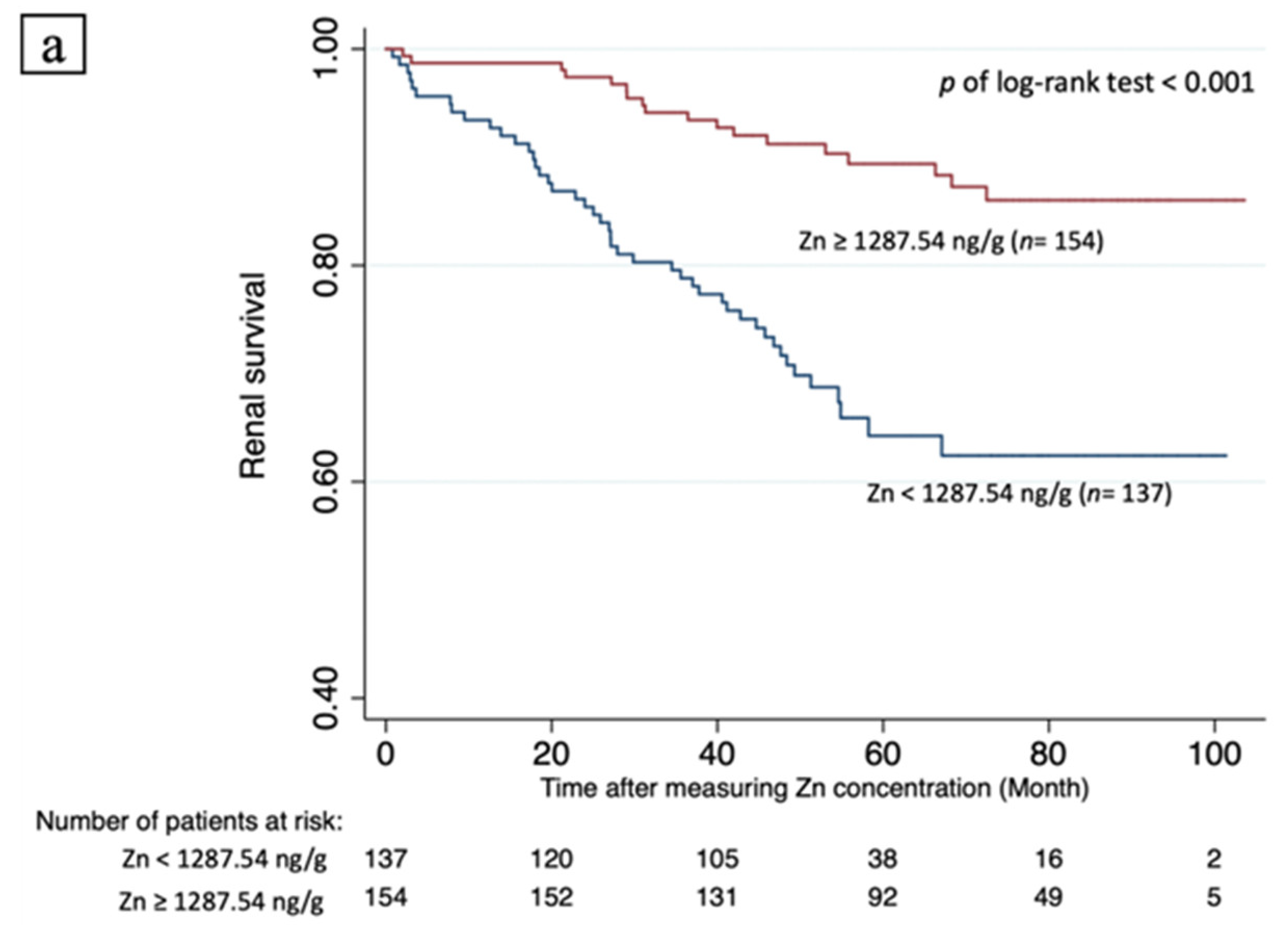
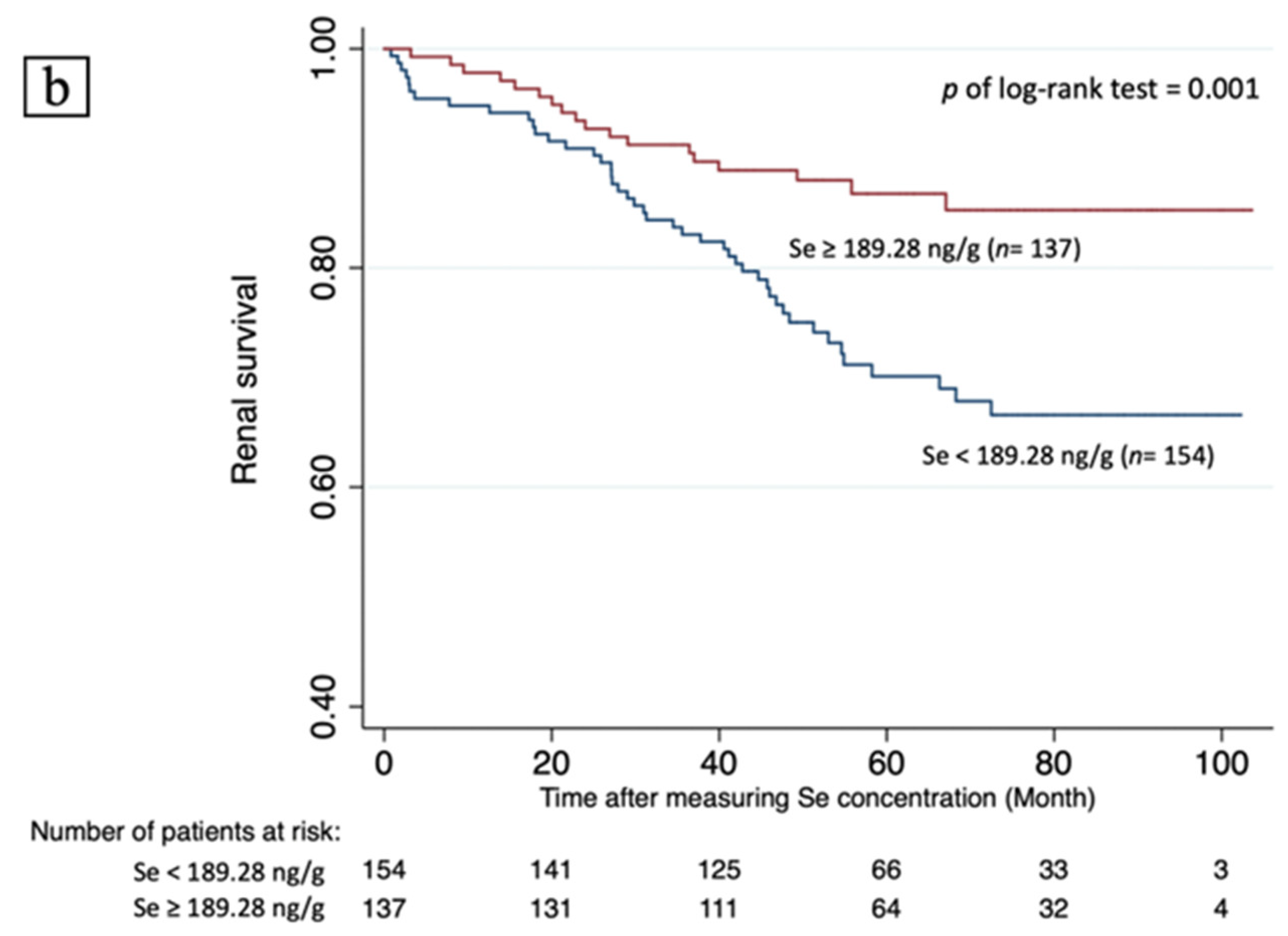
3.5. Renal Survival and Predicting Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakatani, S.; Mori, K.; Shoji, T.; Emoto, M. Association of Zinc Deficiency with Development of CVD Events in Patients with CKD. Nutrients 2021, 13, 1680. [Google Scholar] [CrossRef] [PubMed]
- Gromadzka, G.; Tarnacka, B.; Flaga, A.; Adamczyk, A. Copper Dyshomeostasis in Neurodegenerative Diseases-Therapeutic Implications. Int. J. Mol. Sci. 2020, 21, 9259. [Google Scholar] [CrossRef]
- Friedman, B.J.; Freeland-Graves, J.H.; Bales, C.W.; Behmardi, F.; Shorey-Kutschke, R.L.; Willis, R.A.; Crosby, J.B.; Trickett, P.C.; Houston, S.D. Manganese balance and clinical observations in young men fed a manganese-deficient diet. J. Nutr. 1987, 117, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Finley, J.W.; Penland, J.G. Adequacy or deprivation of dietary selenium in healthy men: Clinical and psychological findings. J. Trace Elem. Exp. Med. Off. Publ. Int. Soc. Trace Elem. Res. Hum. 1998, 11, 11–27. [Google Scholar] [CrossRef]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavesi, T.; Moreira, J.C. Mechanisms and individuality in chromium toxicity in humans. J. Appl. Toxicol. 2020, 40, 1183–1197. [Google Scholar] [CrossRef]
- Nastoulis, E.; Karakasi, M.V.; Couvaris, C.M.; Kapetanakis, S.; Fiska, A.; Pavlidis, P. Greenish-blue gastric content: Literature review and case report on acute copper sulphate poisoning. Forensic Sci. Rev. 2017, 29, 77–91. [Google Scholar]
- O’Neal, S.L.; Zheng, W. Manganese Toxicity Upon Overexposure: A Decade in Review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.C.; Shih, C.T.; Lee, C.H.; Huang, Y.L. Changes in Trace Elements During Early Stages of Chronic Kidney Disease in Type 2 Diabetic Patients. Biol. Trace Elem. Res. 2018, 186, 330–336. [Google Scholar] [CrossRef]
- Abbasi, A.A.; Prasad, A.S.; Rabbani, P.; DuMouchelle, E. Experimental zinc deficiency in man. Effect on testicular function. J. Lab. Clin. Med. 1980, 96, 544–550. [Google Scholar]
- Shankar, A.H.; Prasad, A.S. Zinc and immune function: The biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998, 68, 447–463. [Google Scholar] [CrossRef] [Green Version]
- Pisano, M.; Hilas, O. Zinc and Taste Disturbances in Older Adults: A Review of the Literature. Consult. Pharm. 2016, 31, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Rucker, D.; Thadhani, R.; Tonelli, M. Trace element status in hemodialysis patients. Semin. Dial. 2010, 23, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Dvornik, S.; Cuk, M.; Racki, S.; Zaputovic, L. Serum zinc concentrations in the maintenance hemodialysis patients. Coll. Antropol. 2006, 30, 125–129. [Google Scholar] [PubMed]
- Lobo, J.C.; Stockler-Pinto, M.B.; Farage, N.E.; Faulin Tdo, E.; Abdalla, D.S.; Torres, J.P.; Velarde, L.G.; Mafra, D. Reduced plasma zinc levels, lipid peroxidation, and inflammation biomarkers levels in hemodialysis patients: Implications to cardiovascular mortality. Ren. Fail. 2013, 35, 680–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashidi, A.A.; Salehi, M.; Piroozmand, A.; Sagheb, M.M. Effects of zinc supplementation on serum zinc and C-reactive protein concentrations in hemodialysis patients. J. Ren. Nutr. 2009, 19, 475–478. [Google Scholar] [CrossRef]
- Wang, L.J.; Wang, M.Q.; Hu, R.; Yang, Y.; Huang, Y.S.; Xian, S.X.; Lu, L. Effect of Zinc Supplementation on Maintenance Hemodialysis Patients: A Systematic Review and Meta-Analysis of 15 Randomized Controlled Trials. Biomed Res. Int. 2017, 2017, 1024769. [Google Scholar] [CrossRef]
- Zachara, B.A. Selenium and selenium-dependent antioxidants in chronic kidney disease. Adv. Clin. Chem. 2015, 68, 131–151. [Google Scholar] [CrossRef]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [Green Version]
- Vogt, T.M.; Ziegler, R.G.; Patterson, B.H.; Graubard, B.I. Racial differences in serum selenium concentration: Analysis of US population data from the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 2007, 166, 280–288. [Google Scholar] [CrossRef]
- Kudva, A.K.; Shay, A.E.; Prabhu, K.S. Selenium and inflammatory bowel disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, B.K.; Alfulaij, N.; Seale, L.A. The Impact of Selenium Deficiency on Cardiovascular Function. Int. J. Mol. Sci. 2021, 22, 10713. [Google Scholar] [CrossRef] [PubMed]
- Marti del Moral, L.; Agil, A.; Navarro-Alarcon, M.; Lopez-Ga de la Serrana, H.; Palomares-Bayo, M.; Oliveras-Lopez, M.J. Altered serum selenium and uric acid levels and dyslipidemia in hemodialysis patients could be associated with enhanced cardiovascular risk. Biol. Trace Elem. Res. 2011, 144, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, Y.; Ohsawa, M.; Itai, K.; Kato, K.; Tanno, K.; Turin, T.C.; Onoda, T.; Endo, S.; Okayama, A.; Fujioka, T. Serum selenium levels in hemodialysis patients are significantly lower than those in healthy controls. Blood Purif. 2011, 32, 43–47. [Google Scholar] [CrossRef]
- Fujishima, Y.; Ohsawa, M.; Itai, K.; Kato, K.; Tanno, K.; Turin, T.C.; Onoda, T.; Endo, S.; Okayama, A.; Fujioka, T. Serum selenium levels are inversely associated with death risk among hemodialysis patients. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2011, 26, 3331–3338. [Google Scholar] [CrossRef] [Green Version]
- Tonelli, M.; Wiebe, N.; Bello, A.; Field, C.J.; Gill, J.S.; Hemmelgarn, B.R.; Holmes, D.T.; Jindal, K.; Klarenbach, S.W.; Manns, B.J.; et al. Concentrations of Trace Elements and Clinical Outcomes in Hemodialysis Patients: A Prospective Cohort Study. Clin. J. Am. Soc. Nephrol. CJASN 2018, 13, 907–915. [Google Scholar] [CrossRef]
- Salehi, M.; Sohrabi, Z.; Ekramzadeh, M.; Fallahzadeh, M.K.; Ayatollahi, M.; Geramizadeh, B.; Hassanzadeh, J.; Sagheb, M.M. Selenium supplementation improves the nutritional status of hemodialysis patients: A randomized, double-blind, placebo-controlled trial. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2013, 28, 716–723. [Google Scholar] [CrossRef] [Green Version]
- Lask, B.; Fosson, A.; Rolfe, U.; Thomas, S. Zinc deficiency and childhood-onset anorexia nervosa. J. Clin. Psychiatry 1993, 54, 63–66. [Google Scholar]
- Wessels, I.; Rolles, B.; Slusarenko, A.J.; Rink, L. Zinc deficiency as a possible risk factor for increased susceptibility and severe progression of Corona Virus Disease 19. Br. J. Nutr. 2022, 127, 214–232. [Google Scholar] [CrossRef]
- Weyh, C.; Kruger, K.; Peeling, P.; Castell, L. The Role of Minerals in the Optimal Functioning of the Immune System. Nutrients 2022, 14, 644. [Google Scholar] [CrossRef] [PubMed]
- Beigi Harchegani, A.; Dahan, H.; Tahmasbpour, E.; Bakhtiari Kaboutaraki, H.; Shahriary, A. Effects of zinc deficiency on impaired spermatogenesis and male infertility: The role of oxidative stress, inflammation and apoptosis. Hum. Fertil. 2020, 23, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; Hemmelgarn, B.; Klarenbach, S.; Field, C.; Manns, B.; Thadhani, R.; Gill, J. Trace elements in hemodialysis patients: A systematic review and meta-analysis. BMC Med. 2009, 7, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Management of protein-energy wasting in non-dialysis-dependent chronic kidney disease: Reconciling low protein intake with nutritional therapy. Am. J. Clin. Nutr. 2013, 97, 1163–1177. [Google Scholar] [CrossRef] [Green Version]
- Kazancioglu, R. Risk factors for chronic kidney disease: An update. Kidney Int. Suppl. 2013, 3, 368–371. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Nyunt, M.S.Z.; Gao, Q.; Gwee, X.; Chua, D.Q.; Yap, K.B.; Pan, F.; Ng, T.P. Malnutrition Risk and Kidney Function and Decline in Community-Dwelling Older Adults. J. Ren. Nutr. 2022, 21, S1051-2276. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, A.; Kanda, E.; Itano, S.; Kondo, M.; Wada, Y.; Kadoya, H.; Kidokoro, K.; Nagasu, H.; Sasaki, T.; Kashihara, N. Effect of zinc deficiency on chronic kidney disease progression and effect modification by hypoalbuminemia. PLoS ONE 2021, 16, e0251554. [Google Scholar] [CrossRef] [PubMed]
- Damianaki, K.; Lourenco, J.M.; Braconnier, P.; Ghobril, J.P.; Devuyst, O.; Burnier, M.; Lenglet, S.; Augsburger, M.; Thomas, A.; Pruijm, M. Renal handling of zinc in chronic kidney disease patients and the role of circulating zinc levels in renal function decline. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2020, 35, 1163–1170. [Google Scholar] [CrossRef]
- Xie, C.; Zeng, M.; Shi, Z.; Li, S.; Jiang, K.; Zhao, Y. Association between Selenium Status and Chronic Kidney Disease in Middle-Aged and Older Chinese Based on CHNS Data. Nutrients 2022, 14, 2695. [Google Scholar] [CrossRef]
- Granata, S.; Zaza, G.; Simone, S.; Villani, G.; Latorre, D.; Pontrelli, P.; Carella, M.; Schena, F.P.; Grandaliano, G.; Pertosa, G. Mitochondrial dysregulation and oxidative stress in patients with chronic kidney disease. BMC Genomics 2009, 10, 388. [Google Scholar] [CrossRef] [Green Version]
- Ceballos-Picot, I.; Witko-Sarsat, V.; Merad-Boudia, M.; Nguyen, A.T.; Thevenin, M.; Jaudon, M.C.; Zingraff, J.; Verger, C.; Jungers, P.; Descamps-Latscha, B. Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic. Biol. Med. 1996, 21, 845–853. [Google Scholar] [CrossRef]
- Avissar, N.; Ornt, D.B.; Yagil, Y.; Horowitz, S.; Watkins, R.H.; Kerl, E.A.; Takahashi, K.; Palmer, I.S.; Cohen, H.J. Human kidney proximal tubules are the main source of plasma glutathione peroxidase. Am. J. Physiol. 1994, 266, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Birringer, M.; Pilawa, S.; Flohe, L. Trends in selenium biochemistry. Nat. Prod. Rep. 2002, 19, 693–718. [Google Scholar] [CrossRef]
- Yoshimura, S.; Suemizu, H.; Nomoto, Y.; Sakai, H.; Katsuoka, Y.; Kawamura, N.; Moriuchi, T. Plasma glutathione peroxidase deficiency caused by renal dysfunction. Nephron 1996, 73, 207–211. [Google Scholar] [CrossRef]
- Bellisola, G.; Perona, G.; Galassini, S.; Moschini, G.; Guidi, G.C. Plasma selenium and glutathione peroxidase activities in individuals living in the Veneto region of Italy. J. Trace Elem. Electrolytes Health Dis. 1993, 7, 242–244. [Google Scholar] [PubMed]
- Alehagen, U.; Aaseth, J.; Alexander, J.; Brismar, K.; Larsson, A. Selenium and Coenzyme Q10 Supplementation Improves Renal Function in Elderly Deficient in Selenium: Observational Results and Results from a Subgroup Analysis of a Prospective Randomised Double-Blind Placebo-Controlled Trial. Nutrients 2020, 12, 3780. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Bao, B. Molecular Mechanisms of Zinc as a Pro-Antioxidant Mediator: Clinical Therapeutic Implications. Antioxidants 2019, 8, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Zhang, X.; Yang, L.; Wang, J.; Hu, Y.; Bian, A.; Liu, J.; Ma, J. Zinc inhibits high glucose-induced NLRP3 inflammasome activation in human peritoneal mesothelial cells. Mol. Med. Rep. 2017, 16, 5195–5202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Cui, W.; Tan, Y.; Luo, P.; Chen, Q.; Zhang, C.; Qu, W.; Miao, L.; Cai, L. Zinc is essential for the transcription function of Nrf2 in human renal tubule cells in vitro and mouse kidney in vivo under the diabetic condition. J. Cell. Mol. Med. 2014, 18, 895–906. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Pedruzzi, L.M.; Cardozo, L.F.; Daleprane, J.B.; Stockler-Pinto, M.B.; Monteiro, E.B.; Leite, M., Jr.; Vaziri, N.D.; Mafra, D. Systemic inflammation and oxidative stress in hemodialysis patients are associated with down-regulation of Nrf2. J. Nephrol. 2015, 28, 495–501. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.A.; Draves, S.O.; Rosca, M. Mitochondria in Diabetic Kidney Disease. Cells 2021, 10, 2945. [Google Scholar] [CrossRef]
- Escobedo-Monge, M.F.; Ayala-Macedo, G.; Sakihara, G.; Peralta, S.; Almaraz-Gomez, A.; Barrado, E.; Marugan-Miguelsanz, J.M. Effects of Zinc Supplementation on Nutritional Status in Children with Chronic Kidney Disease: A Randomized Trial. Nutrients 2019, 11, 2671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voelkl, J.; Tuffaha, R.; Luong, T.T.D.; Zickler, D.; Masyout, J.; Feger, M.; Verheyen, N.; Blaschke, F.; Kuro, O.M.; Tomaschitz, A.; et al. Zinc Inhibits Phosphate-Induced Vascular Calcification through TNFAIP3-Mediated Suppression of NF-kappaB. J. Am. Soc. Nephrol. 2018, 29, 1636–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Eisenberg, R.; Mowrey, W.B.; Wylie-Rosett, J.; Abramowitz, M.K.; Bushinsky, D.A.; Melamed, M.L. Association between dietary zinc intake and abdominal aortic calcification in US adults. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2020, 35, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
| Renal Survival, n = 227 | Non-Renal Survival, n = 64 | p Value | |
|---|---|---|---|
| Age, year | 67.25 ± 10.23 | 67.47 ± 10.30 | 0.878 |
| Observation period, month | 67.78 (49.45–83.90) | 29.03 (17.80–45.73) | < 0.001 * |
| Male, n (%) | 117 (51.5) | 31 (48.4) | 0.661 |
| Creatinine, mg/dL | 1.73 ± 1.24 | 4.16 ± 2.40 | < 0.001 * |
| eGFR, mL/min/1.73 m2 | 51.17 ± 28.39 | 20.09 ± 15.53 | < 0.001 * |
| Albumin, g/dL | 4.34 ± 0.43 | 4.09 ± 0.42 | < 0.001 * |
| Hemoglobin, g/dL | 12.37 ± 1.95 | 10.52 ± 1.58 | < 0.001 * |
| Ca, mg/dL | 9.27 ± 0.60 | 8.98 ± 0.58 | 0.001 |
| P, mg/dL | 3.84 ± 0.65 | 4.52 ± 1.13 | < 0.001 * |
| Body mass index | 26.06 ± 4.19 | 25.82 ± 5.17 | 0.707 |
| Zinc, ng/g $ | 1416.91 (1145.87–1701.87) | 1169.17 (943.05–1380.41) | < 0.001 &* |
| Selenium, ng/g $ | 190.45 (170.76–209.61) | 176.40 (161.81–193.15) | 0.005 &* |
| Copper, ng/g$ | 996.19 (883.11–1120.38) | 1017.17 (880.04–1154.45) | 0.680 & |
| Manganese, ng/g $ | 7.99 (5.77–11.72) | 7.97 (5.11–9.76) | 0.174 & |
| Chromium, ng/g$ | 15.09 (11.45–21.22) | 16.84 (12.18–30.12) | 0.074 & |
| Diabetes, n (%) | 104 (45.8) | 40 (62.5) | 0.023 * |
| Hypertension, n (%) | 185 (81.5) | 57 (89.0) | 0.213 |
| ASCVD, n (%) | 65 (28.6) | 18 (28.1) | 0.897 |
| CAD, n (%) | 42 (18.5) | 13 (20.3) | 0.761 |
| CHF, n (%) | 21 (9.3) | 3 (4.7) | 0.236 |
| CVA, n (%) | 8 (3.5) | 2 (3.1) | 1.000 |
| PAD, n (%) | 3 (1.3) | 1 (1.6) | 1.000 |
| Stage 0–1 (n = 32) | Stage 2 (n = 44) | Stage 3 (n = 107) | Stage 4 (n = 65) | Stage 5 (n = 43) | p for Trend | |
|---|---|---|---|---|---|---|
| Age, year | 61.25 ± 7.55 | 65.53 ± 7.44 | 67.47 ± 10.80 | 70.31 ± 11.12 | 68.63 ± 9.69 | <0.001 &,* |
| Male, n (%) | 13 (40.6) | 24 (54.5) | 63 (58.9) | 32 (49.2) | 16 (37.2) | 0.434 @ |
| Creatinine, mg/dL | 0.68 ± 0.15 | 1.00 ± 0.19 | 1.56 ± 0.32 | 2.61 ± 0.69 | 5.94 ± 1.99 | <0.001 &,* |
| eGFR, mL/min/1.73 m2 | 103.81 ± 19.85 | 68.34 ± 8.32 | 43.66 ± 9.46 | 23.84 ± 6.38 | 9.06 ± 3.20 | <0.001 &,* |
| Albumin, g/dL | 4.56 ± 0.22 | 4.43 ± 0.38 | 4.35 ± 0.38 | 4.14 ± 0.44 | 4.03 ± 0.55 | <0.001 &,* |
| Hemoglobin, g/dL | 13.37 ± 1.11 | 13.50 ± 1.64 | 12.55 ± 1.66 | 10.89 ± 1.51 | 9.57 ± 1.29 | <0.001 &,* |
| Calcium, mg/dL | 9.30 ± 0.37 | 9.34 ± 0.36 | 9.35 ± 0.41 | 9.18 ± 0.84 | 8.67 ± 0.60 | <0.001 &,* |
| Phosphorus, mg/dL | 3.74 ± 0.52 | 3.73 ± 0.48 | 3.79 ± 0.59 | 3.95 ± 0.67 | 4.98 ± 1.19 | <0.001 &,* |
| Body mass index | 25.15 ± 2.87 | 26.42 ± 4.11 | 26.93 ± 4.81 | 25.04 ± 3.61 | 25.34 ± 5.30 | 0.354 & |
| Zinc, ng/g $ | 1387.14 (1159.11–1670.47) | 1433 (1173.62–1600.39) | 1511.59 (1156.25–3104.54) | 1285.86 (1093.10–1497.03) | 1015.07 (791.61–1191.06) | <0.001 $,* |
| Selenium, ng/g $ | 187.96 (175.75–206.95) | 196.07 (176.54–213.11) | 193.40 (169.88–216.15) | 177.66 (159.81–194.75) | 177.28 (150.98–192.67) | 0.001 $,* |
| Copper, ng/g $ | 1025.16 (967.95–1092.79) | 994.27 (846.29–1133.45) | 957.15 (851.16–1120.62) | 1024.51 (931.86–1125.91) | 973.56 (859.99–1191.79) | 0.817 $ |
| Manganese, ng/g $ | 5.96 (5.52–7.65) | 7.42 (5.49–9.48) | 8.72 (7.37–12.59) | 7.46 (5.14–11.85) | 8.24 (5.18–10.31) | 0.376 $ |
| Chromium, ng/g $ | 15.60 (12.87–19.37) | 16.44 (11.87–19.19) | 12.53 (9.14–19.32) | 18.62 (12.50–40.60) | 15.40 (12.43–26.34) | 0.013 $,* |
| Diabetes, n (%) | 18 (56.3) | 18 (40.9) | 53 (49.5) | 33 (50.8) | 22 (51.2) | 0.881 @ |
| Hypertension, n (%) | 20 (62.5) | 38 (86.4) | 90 (84.1) | 54 (83.1) | 40 (93) | 0.007 @,* |
| ASCVD, n (%) | 5 (15.6) | 12 (27.3) | 35 (32.7) | 23 (35.4) | 8 (18.6) | 0.648 @ |
| CAD, n (%) | 2 (6.3) | 7 (15.9) | 22 (20.6) | 19 (29.2) | 5 (11.6) | 0.211 @ |
| CHF, n (%) | 2 (6.3) | 3 (6.8) | 7 (6.5)) | 10 (15.4) | 2 (4.7) | 0.534 @ |
| CVA, n (%) | 0 (0) | 2 (4.5) | 5 (4.7) | 3 (4.6) | 0 (0) | 0.898 @ |
| PAD, n (%) | 1 (3.1) | 0 (0) | 2 (1.9) | 0 (0) | 1 (3.1) | 0.802 @ |
| Simple Linear Regression | Multiple Regression Analysis, Model 1 | Multiple Regression Analysis, Model 2 | ||||
|---|---|---|---|---|---|---|
| β ± SE | p | β ± SE | p | β ± SE | p | |
| Age | −0.769 ± 0.163 | <0.001 * | - | - | −0.591 ± 0.178 | 0.001 * |
| Gender & | 1.382 ± 3.461 | 0.690 | - | - | −0.161 ± 3.572 | 0.964 |
| Diabetes & | −1.093 ± 3.459 | 0.752 | −1.069 ± 3.357 | 0.750 | −2.954 ± 3.469 | 0.395 |
| Albumin | 16.474 ± 3.333 | <0.001 * | 19.824 ± 3.703 | <0.001 * | 17.554 ± 4.228 | <0.001 * |
| ASCVD & | −5.489 ± 3.798 | 0.150 | −2.719 ± 3.739 | 0.468 | −3.480 ± 3.782 | 0.359 |
| Body mass index | 0.195 ± 0.395 | 0.622 | 0.075 ± 0.385 | 0.847 | 0.043 ± 0.391 | 0.912 |
| Chromium $ | −13.131 ± 5.122 | 0.011 * | −15.218 ± 5.011 | 0.003 * | −9.380 ± 5.782 | 0.106 |
| Manganese $ | −9.305 ± 8.631 | 0.282 | −14.604 ± 8.605 | 0.091 | −16.034 ± 9.953 | 0.108 |
| Copper $ | −7.661 ± 22.862 | 0.738 | 1.408 ± 23.135 | 0.952 | 0.744 ± 22.280 | 0.973 |
| Zinc $ | 25.139 ± 8.692 | 0.004 * | 24.298 ± 8.616 | 0.005 * | 16.959 ± 10.982 | 0.124 |
| Selenium $ | 77.571 ± 22.031 | 0.001 * | 60.316 ± 21.875 | 0.006 * | 23.257 ± 23.220 | 0.318 |
| Crude | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| Variable | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Continuous variables | ||||||
| Zn, ng/g | 0.999 (0.998–1.000) | 0.002 * | 0.999 (0.998–1.000) | 0.002 * | 0.999 (0.998–1.000) & | 0.027 * |
| Se, ng/g | 0.991 (0.982–1.000) | 0.043 * | 0.990 (0.981–0.999) | 0.042 * | 0.998 (0.988–1.009) & | 0.729 |
| Cu, ng/g | 1.000 (0.999 –1.002) | 0.810 | 1.000 (0.999–1.002) | 0.847 | 1.000 (0.999–1.002) & | 0.738 |
| Mn, ng/g | 0.967 (0.911–1.027) | 0.276 | 0.973 (0.916–1.033) | 0.371 | 0.955 (0.881–1.035) & | 0.261 |
| Cr, ng/g | 1.005 (0.999–1.011) | 0.113 | 1.005 (0.999–1.012) | 0.102 | 1.008 (0.999–1.017) & | 0.083 |
| Albumin, g/dL | 0.290 (0.152–0.553) | <0.001 * | 0.255 (0.130–0.502) | <0.001 * | 0.381 (0.174–0.833) & | 0.016 * |
| Body mass index, kg/m2 | 0.991 (0.930–1.057) | 0.794 | 0.990 (0.928–1.057) | 0.766 | 1.012 (0.944–0.084) & | 0.741 |
| Categorical variables | ||||||
| Zn > 1511.5396 ng/g | 1 | 1 | 1 | |||
| Zn = 1167.5252–1511.5396 ng/g | 5.000 (2.233–11.194) | <0.001 * | 5.012 (2.211–11.359) | <0.001 * | 4.105 (1.697–9.930) # | 0.002 * |
| Zn < 1167.5252 ng/g | 2.973 (1.291–6.847) | 0.010 * | 2.987 (1.291–6.912) | 0.011 * | 2.808 (1.161–6.788) # | 0.022 * |
| Se > 198.454 ng/g | 1 | 1 | 1 | |||
| Se = 174.2536–198.4540 ng/g | 2.306 (1.141–4.662) | 0.018 * | 2.331 (1.142–4.759) | 0.020 * | 1.396 (0.637–3.060) # | 0.405 |
| Se < 174.2536 ng/g | 1.474 (0.705–3.082) | 0.303 | 1.445 (0.690–3.026) | 0.329 | 1.388 (0.623–3.092) # | 0.423 |
| Albumin < 4 g/dL | 3.930 (2.108–7.327) | <0.001 * | 4.257 (2.243–8.077) | <0.001 * | 3.751 (1.861–7.561) # | <0.001 * |
| ASCVD (yes vs. no) | 0.893 (0.477–1.673) | 0.724 | 0.903 (0.477–1.708) | 0.753 | 0.843 (0.407–1.745) # | 0.646 |
| Diabetes (yes vs. no) | 1.857 (1.048–3.290) | <0.001 * | 1.848 (1.040–3.282) | 0.036 * | 2.525 (1.308–4.875) # | 0.006 * |
| Univariate, Crude | Multivariable, Model 1 | Multivariable, Model 2 | Multivariable, Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Zn ≥ 1287.24 ng/g # | 0.263 (0.152–0.455) | <0.001 * | 0.259 (0.148–0.454) | <0.001 * | 0.241 (0.128–0.453) | <0.001 * | 0.450 (0.231–0.878) | 0.019 * |
| Se ≥ 189.28 ng/g # | 0.472 (0.273–0.816) | 0.007 * | 0.454 (0.260–0.795) | 0.006 * | 0.473 (0.258–0.866) | 0.015 * | 0.641 (0.346–1.187) | 0.157 |
| Male | 0.802 (0.488–1.320) | 0.385 | - | - | 1.236 (0.716–2.135) | 0.446 | 2.532 (1.340–1.784) | 0.004 * |
| Age | 0.979 (0.959–1.000) | 0.052 | - | - | 0.980 (0.956–1.004) | 0.105 | 0.979 (0.955–1.005) | 0.107 |
| Albumin | 0.646 (0.378–1.102) | 0.109 | 0.567 (0.328–0.982) | 0.043 * | 1.210 (0.657–2.228) | 0.540 | 1.462 (0.736–2.905) | 0.278 |
| eGFR | 0.927 (0.908–0.946) | <0.001 * | 0.919 (0.899–0.940) | <0.001 * | - | - | 0.921 (0.897–0.946) | <0.001 * |
| Body mass index | 1.005 (0.951–1.062) | 0.865 | 0.984 (0.929–1.043) | 0.595 | 1.016 (0.964–1.071) | 0.544 | 1.031 (0.976–1.089) | 0.272 |
| Diabetes | 1.638 (0.984–2.729) | 0.058 | - | - | 1.521 (0.866–2.673) | 0.145 | 1.901 (1.041–3.473) | 0.037 * |
| Hypertension | 1.506 (0.686–3.307) | 0.307 | 1.463 (0.655–3.269) | 0.353 | 1.264 (0.567–2.833) | 0.570 | 0.970 (0.432–2.178) | 0.942 |
| Coronary artery disease | 0.662 (0.352–1.248) | 0.202 | 0.696 (0.368–1.316) | 0.265 | 0.598 (0.306–1.168) | 0.132 | 0.606 (0.305–1.203) | 0.152 |
| Congestive heart failure | 0.419 (0.131–1.337) | 0.142 | 0.437 (0.136–1.403) | 0.164 | 0.360 (0.108–1.198) | 0.096 | 0.176 (0.050–0.618) | 0.176 |
| Cerebrovascular disease | 0.704 (0.172–2.881) | 0.625 | 0.727 (0.176–3.003) | 0.107 | 1.200 (0.279–5.160) | 0.806 | 1.786 (0.406–7.862) | 0.443 |
| Peripheral artery disease | 1.719 (0.236–12.534) | 0.593 | 1.862 (0.252–13.754) | 0.542 | 1.803 (0.235–13.818) | 0.570 | 1.576 (0.202–12.320) | 0.664 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-Y.; Chiu, C.-H.; Wu, I.-W.; Hsu, H.-J.; Chen, Y.-T.; Hsu, C.-K.; Pan, H.-C.; Lee, C.-C.; Sun, C.-Y. Micronutrients and Renal Outcomes: A Prospective Cohort Study. Nutrients 2022, 14, 3063. https://doi.org/10.3390/nu14153063
Chen C-Y, Chiu C-H, Wu I-W, Hsu H-J, Chen Y-T, Hsu C-K, Pan H-C, Lee C-C, Sun C-Y. Micronutrients and Renal Outcomes: A Prospective Cohort Study. Nutrients. 2022; 14(15):3063. https://doi.org/10.3390/nu14153063
Chicago/Turabian StyleChen, Chun-Yu, Chun-Hui Chiu, I-Wen Wu, Heng-Jung Hsu, Yih-Ting Chen, Cheng-Kai Hsu, Heng-Chih Pan, Chin-Chan Lee, and Chiao-Yin Sun. 2022. "Micronutrients and Renal Outcomes: A Prospective Cohort Study" Nutrients 14, no. 15: 3063. https://doi.org/10.3390/nu14153063
APA StyleChen, C.-Y., Chiu, C.-H., Wu, I.-W., Hsu, H.-J., Chen, Y.-T., Hsu, C.-K., Pan, H.-C., Lee, C.-C., & Sun, C.-Y. (2022). Micronutrients and Renal Outcomes: A Prospective Cohort Study. Nutrients, 14(15), 3063. https://doi.org/10.3390/nu14153063







