Protein Intake and Frailty in Older Adults: A Systematic Review and Meta-Analysis of Observational Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy and Selection Criteria
2.3. Data extraction, Quality Assessment, and Risk of Bias
2.4. Statistical Analysis
3. Results
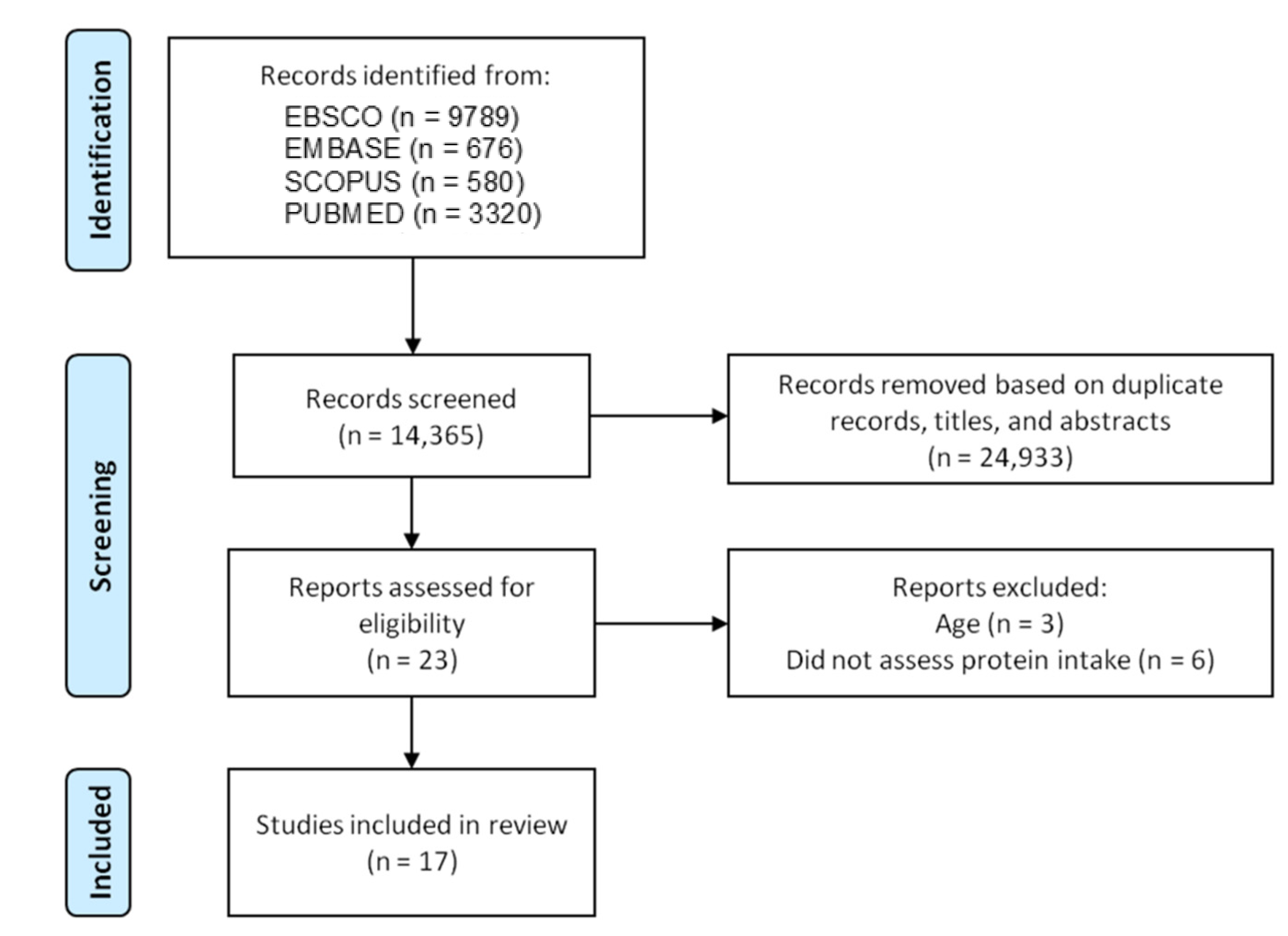
3.1. Literature Search
3.2. Main Characteristics of the Included Studies
3.3. Quality Assessment
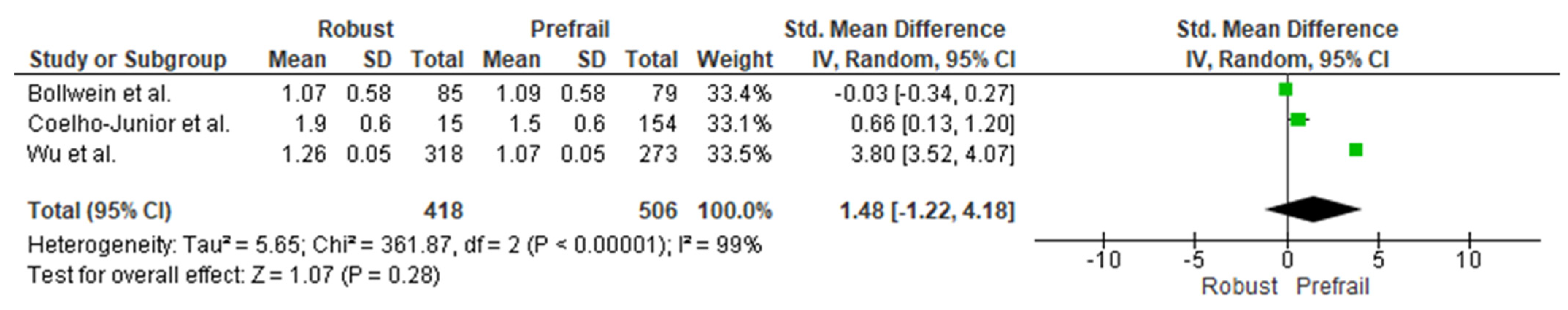
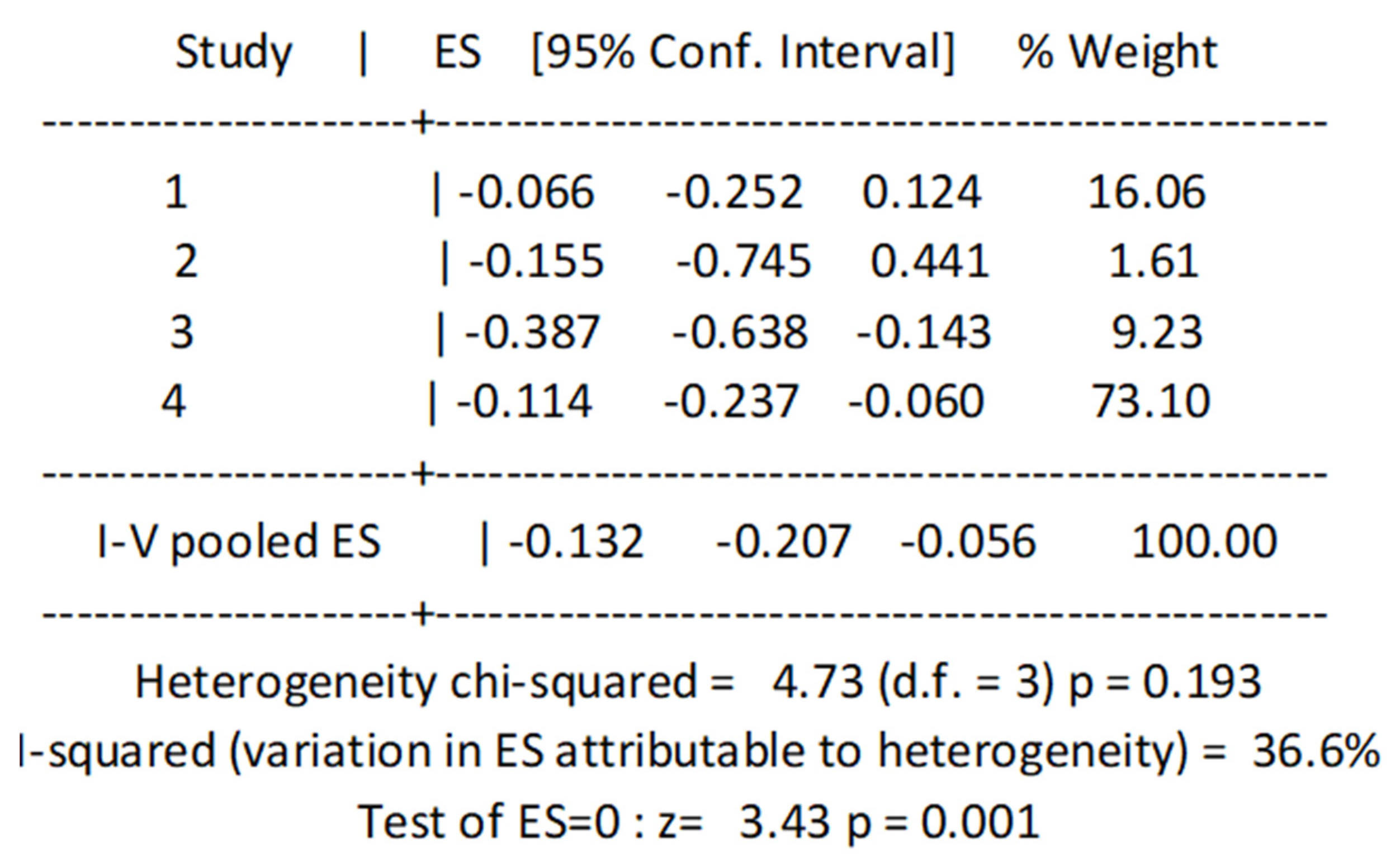
3.4. Cross-Sectional Associations between Protein Intake and Prefrailty
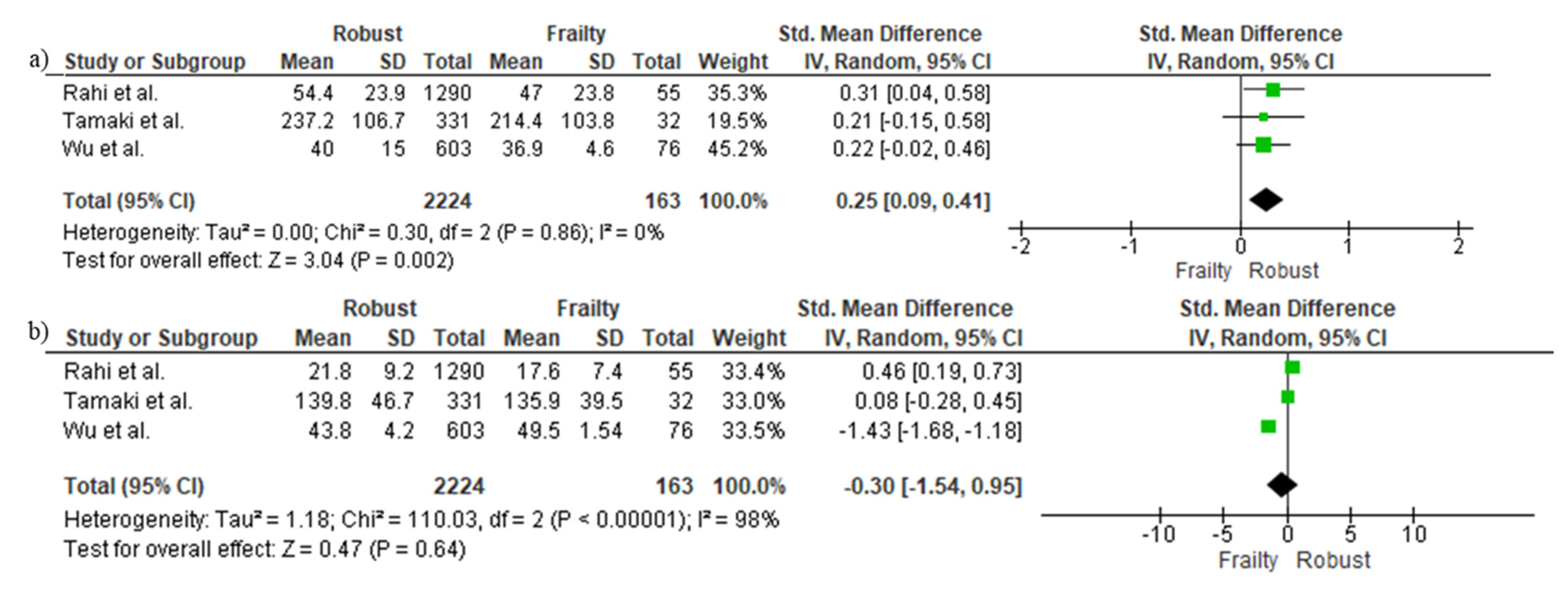
3.5. Cross-Sectional Associations between Protein Intake and Frailty Using Continuous Data
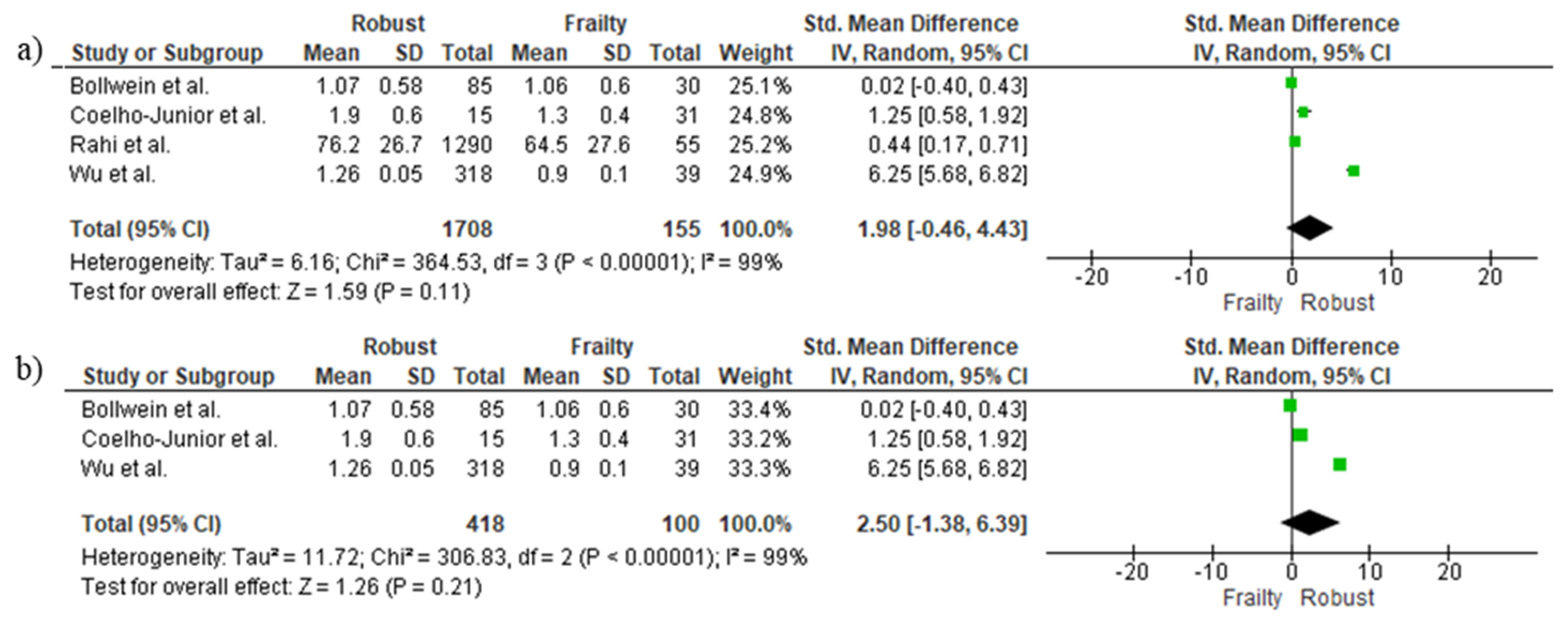
3.6. Cross-Sectional Associations between Protein Intake and Frailty Using Binary Data
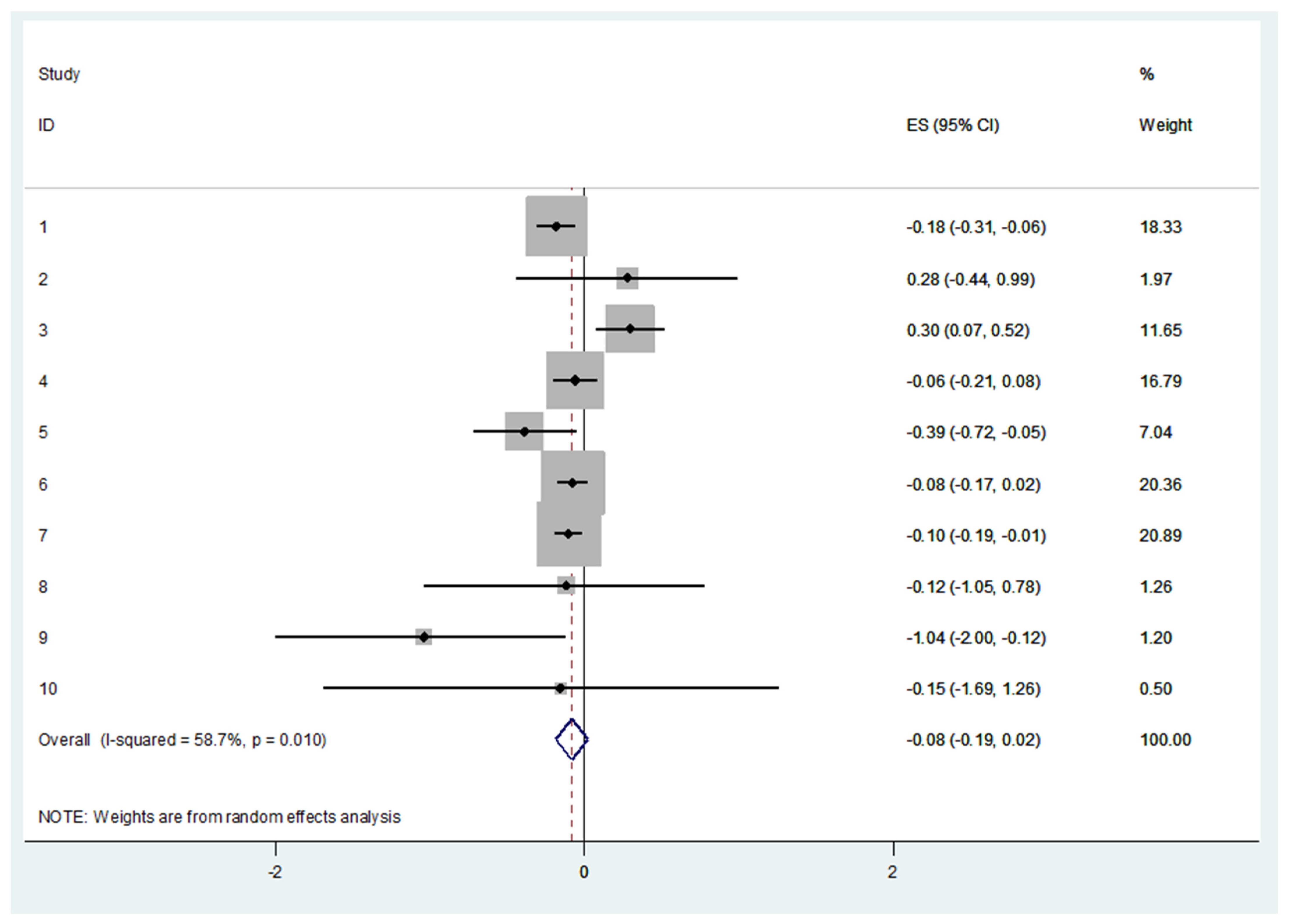
3.7. Cross-Sectional Differences in Protein Sources between Frail and Robust People
3.8. Longitudinal Associations between Protein Intake and Incidence of Frailty
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of Frailty: Opportunities, Challenges, and Future Directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for Clinical Practice and Public Health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Marzetti, E.; Picca, A.; Calvani, R.; Cesari, M.; Uchida, M. Prevalence of Prefrailty and Frailty in South America: A Systematic Review of Observational Studies. J. Frailty Aging 2020, 9, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Ofori-Asenso, R.; Chin, K.L.; Mazidi, M.; Zomer, E.; Ilomaki, J.; Zullo, A.R.; Gasevic, D.; Ademi, Z.; Korhonen, M.J.; LoGiudice, D.; et al. Global Incidence of Frailty and Prefrailty Among Community-Dwelling Older Adults. JAMA Netw. Open 2019, 2, e198398. [Google Scholar] [CrossRef] [Green Version]
- Kojima, G. Frailty as a Predictor of Hospitalisation among Community-Dwelling Older People: A Systematic Review and Meta-Analysis. J. Epidemiol. Community Health 2016, 70, 722–729. [Google Scholar] [CrossRef]
- Kojima, G. Frailty Significantly Increases the Risk of Fractures among Middle-Aged and Older People. Evid. Based Nurs. 2017, 20, 119–120. [Google Scholar] [CrossRef]
- Vermeiren, S.; Vella-Azzopardi, R.; Beckwée, D.; Habbig, A.-K.; Scafoglieri, A.; Jansen, B.; Bautmans, I.; Bautmans, I.; Verté, D.; Beyer, I.; et al. Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1163.e1–1163.e17. [Google Scholar] [CrossRef]
- Hajek, A.; Bock, J.-O.; Saum, K.-U.; Matschinger, H.; Brenner, H.; Holleczek, B.; Haefeli, W.E.; Heider, D.; König, H.-H. Frailty and Healthcare Costs-Longitudinal Results of a Prospective Cohort Study. Age Ageing 2018, 47, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo-López, L.; Maseda, A.; de Labra, C.; Regueiro-Folgueira, L.; Rodríguez-Villamil, J.L.; Millán-Calenti, J.C. Nutritional Determinants of Frailty in Older Adults: A Systematic Review. BMC Geriatr. 2017, 17, 108. [Google Scholar] [CrossRef] [Green Version]
- Ward, R.E.; Orkaby, A.R.; Chen, J.; Hshieh, T.T.; Driver, J.A.; Gaziano, J.M.; Djousse, L. Association between Diet Quality and Frailty Prevalence in the Physicians’ Health Study. J. Am. Geriatr. Soc. 2019, 68, 770–776. [Google Scholar] [CrossRef]
- Coelho-Junior, H.J.; Marzetti, E.; Picca, A.; Cesari, M.; Uchida, M.C.; Calvani, R. Protein Intake and Frailty: A Matter of Quantity, Quality, and Timing. Nutrients 2020, 12, 2915. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Asakura, K.; Suga, H.; Sasaki, S. Three-generation Study of Women on Diets and Health Study Group High Protein Intake Is Associated with Low Prevalence of Frailty among Old Japanese Women: A Multicenter Cross-Sectional Study. Nutr. J. 2013, 12, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaimoto, K.; Yamashita, M.; Suzuki, T.; Makizako, H.; Koriyama, C.; Kubozono, T.; Takenaka, T.; Ohishi, M.; Kanouchi, H. Association of protein and magnesium intake with prevalence of prefrailty and frailty in community-dwelling older Japanese women. J. Nutr. Sci. Vitaminol. 2021, 67, 39–47. [Google Scholar] [CrossRef]
- Rahi, B.; Colombet, Z.; Gonzalez-Colaço Harmand, M.; Dartigues, J.F.; Boirie, Y.; Letenneur, L.; Feart, C. Higher Protein but Not Energy Intake Is Associated With a Lower Prevalence of Frailty Among Community-Dwelling Older Adults in the French Three-City Cohort. J. Am. Med. Dir. Assoc. 2016, 17, 672.e7–672.e11. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Júnior, H.J.; Rodrigues, B.; Uchida, M.; Marzetti, E. Low Protein Intake Is Associated with Frailty in Older Adults: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2018, 10, 1334. [Google Scholar] [CrossRef] [Green Version]
- Coelho-Júnior, H.J.; Calvani, R.; Picca, A.; Gonçalves, I.O.; Landi, F.; Bernabei, R.; Cesari, M.; Uchida, M.C.; Marzetti, E. Protein-Related Dietary Parameters and Frailty Status in Older Community-Dwellers across Different Frailty Instruments. Nutrients 2020, 12, 508. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, T.; Fukuda, Y.; Sato, R.; Ogasawara, M.; Tamura, K. Association of Physical Prefrailty with Prevalence of Inadequate Nutrient Intake in Community-Dwelling Japanese Elderly Women: A Cross-Sectional Study. Asia Pac. J. Clin. Nutr. 2021, 30, 263–274. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-Analysis of Observational Studies in Epidemiology: A Proposal for Reporting. Meta-Analysis Of Observational Studies in Epidemiology (MOOSE) Group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Green, S.; Higgins, J. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Study Quality Assessment Tools|NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 3 February 2022).
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-Range, and/or Mid-Quartile Range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Luo, D.; Weng, H.; Zeng, X.-T.; Lin, L.; Chu, H.; Tong, T. Optimally Estimating the Sample Standard Deviation from the Five-Number Summary. Res. Synth. Methods 2020, 11, 641–654. [Google Scholar] [CrossRef]
- Bartali, B.; Frongillo, E.A.; Bandinelli, S.; Lauretani, F.; Semba, R.D.; Fried, L.P.; Ferrucci, L. Low Nutrient Intake Is an Essential Component of Frailty in Older Persons. J. Gerontol. Ser. A 2006, 61, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Bollwein, J.; Diekmann, R.; Kaiser, M.J.; Bauer, J.M.; Uter, W.; Sieber, C.C.; Volkert, D. Distribution but Not Amount of Protein Intake Is Associated with Frailty: A Cross-Sectional Investigation in the Region of Nürnberg. Nutr. J. 2013, 12, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castaneda-Gameros, D.; Redwood, S.; Thompson, J.L. Low Nutrient Intake and Frailty Among Overweight and Obese Migrant Women From Ethnically Diverse Backgrounds Ages 60 Years and Older: A Mixed-Methods Study. J. Nutr. Educ. Behav. 2017, 49, 3–10.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, S.; Suga, H.; Sasaki, S. Diet with a Combination of High Protein and High Total Antioxidant Capacity Is Strongly Associated with Low Prevalence of Frailty among Old Japanese Women: A Multicenter Cross-Sectional Study. Nutr. J. 2017, 16, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.Y.; Yeh, N.H.; Chang, H.Y.; Wang, C.F.; Hung, S.Y.; Wu, S.J.; Pan, W.H. Adequate Protein Intake in Older Adults in the Context of Frailty: Cross-Sectional Results of the Nutrition and Health Survey in Taiwan 2014–2017. Am. J. Clin. Nutr. 2021, 114, 649–660. [Google Scholar] [CrossRef]
- Tamaki, K.; Kusunoki, H.; Tsuji, S.; Wada, Y.; Nagai, K.; Itoh, M.; Sano, K.; Amano, M.; Maeda, H.; Hasegawa, Y.; et al. The Relationship between Dietary Habits and Frailty in Rural Japanese Community-Dwelling Older Adults: Cross-Sectional Observation Study Using a Brief Self-Administered Dietary History Questionnaire. Nutrients 2018, 10, 1982. [Google Scholar] [CrossRef] [Green Version]
- Smit, E.; Winters-Stone, K.M.; Loprinzi, P.D.; Tang, A.M.; Crespo, C.J. Lower Nutritional Status and Higher Food Insufficiency in Frail Older US Adults. Br. J. Nutr. 2013, 110, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Sewo Sampaio, P.Y.; Sampaio, R.A.C.; Yamada, M.; Arai, H. Systematic Review of the Kihon Checklist: Is It a Reliable Assessment of Frailty? Geriatr. Gerontol. Int. 2016, 16, 893–902. [Google Scholar] [CrossRef]
- Morley, J.E.; Malmstrom, T.K.; Miller, D.K. A Simple Frailty Questionnaire (FRAIL) Predicts Outcomes in Middle Aged African Americans. J. Nutr. Health Aging 2012, 16, 601–608. [Google Scholar] [CrossRef] [Green Version]
- Ensrud, K.E.; Ewing, S.K.; Taylor, B.C.; Fink, H.A.; Cawthon, P.M.; Stone, K.L.; Hillier, T.A.; Cauley, J.A.; Hochberg, M.C.; Rodondi, N.; et al. Comparison of 2 Frailty Indexes for Prediction of Falls, Disability, Fractures, and Death in Older Women. Arch. Intern. Med. 2008, 168, 382–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beasley, J.M.; Lacroix, A.Z.; Neuhouser, M.L.; Huang, Y.; Tinker, L.; Woods, N.; Michael, Y.; Curb, J.D.; Prentice, R.L. Protein Intake and Incident Frailty in the Women’s Health Initiative Observational Study. J. Am. Geriatr. Soc. 2010, 58, 1063–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.H.; Martins, B.A.; Okada, K.; Matsushita, E.; Uno, C.; Satake, S.; Kuzuya, M. A 3-Year Prospective Cohort Study of -Dietary Patterns and Frailty Risk among Community-Dwelling Older Adults. Clin. Nutr. 2021, 40, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, R.; Tange, C.; Tomida, M.; Nishita, Y.; Kato, Y.; Yuki, A.; Ando, F.; Shimokata, H.; Arai, H. Dietary Factors Associated with the Development of Physical Frailty in Community-Dwelling Older Adults. J. Nutr. Health Aging 2019, 23, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Insausti, H.; Perez-Tasigchana, R.F.; Lopez-Garcia, E.; Garcia-Esquinas, E.; Rodriguez-Artalejo, F.; Guallar-Castillon, P. Macronutrients Intake and Incident Frailty in Older Adults: A Prospective Cohort Study. J. Gerontol. Ser. A 2016, 71, 1329–1334. [Google Scholar] [CrossRef]
- Shikany, J.M.; Barrett-Connor, E.; Ensrud, K.E.; Cawthon, P.M.; Lewis, C.E.; Dam, T.T.L.; Shannon, J.; Redden, D.T. Macronutrients, Diet Quality, and Frailty in Older Men. J. Gerontol. Ser. A 2014, 69, 695–701. [Google Scholar] [CrossRef]
- Deer, R.R.; Volpi, E. Protein Intake and Muscle Function in Older Adults. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4394186/ (accessed on 8 November 2019).
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Collamati, A.; D’Angelo, E.; Pahor, M.; Bernabei, R.; et al. Sarcopenia: An Overview. Aging Clin. Exp. Res. 2017, 29, 11–17. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Picca, A.; Coelho-Junior, H.J.; Calvani, R.; Marzetti, E.; Vetrano, D.L. Biomarkers Shared by Frailty and Sarcopenia in Older Adults: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2022, 73, 101530. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; D’Angelo, E.; Sisto, A.; Marzetti, E. Protein Intake and Muscle Health in Old Age: From Biological Plausibility to Clinical Evidence. Nutrients 2016, 8, 295. [Google Scholar] [CrossRef]
- Morley, J.E.; von Haehling, S.; Anker, S.D.; Vellas, B. From Sarcopenia to Frailty: A Road Less Traveled. J. Cachexia Sarcopenia Muscle 2014, 5, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Atherton, P.J.; Etheridge, T.; Watt, P.W.; Wilkinson, D.; Selby, A.; Rankin, D.; Smith, K.; Rennie, M.J. Muscle Full Effect after Oral Protein: Time-Dependent Concordance and Discordance between Human Muscle Protein Synthesis and MTORC1 Signaling. Am. J. Clin. Nutr. 2010, 92, 1080–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohé, J.; Low, A.; Wolfe, R.R.; Rennie, M.J. Human Muscle Protein Synthesis Is Modulated by Extracellular, Not Intramuscular Amino Acid Availability: A Dose-Response Study. J. Physiol. 2003, 552, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of Whey Hydrolysate, Casein, or Soy Protein Isolate: Effects on Mixed Muscle Protein Synthesis at Rest and Following Resistance Exercise in Young Men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- Greenhaff, P.L.; Karagounis, L.G.; Peirce, N.; Simpson, E.J.; Hazell, M.; Layfield, R.; Wackerhage, H.; Smith, K.; Atherton, P.; Selby, A.; et al. Disassociation between the Effects of Amino Acids and Insulin on Signaling, Ubiquitin Ligases, and Protein Turnover in Human Muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E595–E604. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of Leucine and Its Metabolite β-Hydroxy-β-Methylbutyrate on Human Skeletal Muscle Protein Metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Piasecki, M.; Atherton, P.J. The Age-Related Loss of Skeletal Muscle Mass and Function: Measurement and Physiology of Muscle Fibre Atrophy and Muscle Fibre Loss in Humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef]
- Lexell, J.; Taylor, C.C.; Sjöström, M. What is the cause of the ageing atrophy?: Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J. Neurol. Sci. 1988, 84, 275–294. [Google Scholar] [CrossRef]
- Nilwik, R.; Snijders, T.; Leenders, M.; Groen, B.B.L.; van Kranenburg, J.; Verdijk, L.B.; Van Loon, L.J.C. The Decline in Skeletal Muscle Mass with Aging Is Mainly Attributed to a Reduction in Type II Muscle Fiber Size. Exp. Gerontol. 2013, 48, 492–498. [Google Scholar] [CrossRef]
- Scott, W.; Stevens, J.; Binder-Macleod, S. Human Skeletal Muscle Fiber Type Classifications. Phys. Ther. 2001, 81, 1810–1816. [Google Scholar] [CrossRef]
- van Vliet, S.; Burd, N.A.; van Loon, L.J. The Skeletal Muscle Anabolic Response to Plant- versus Animal-Based Protein Consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorissen, S.H.M.; Witard, O.C. Characterising the Muscle Anabolic Potential of Dairy, Meat and Plant-Based Protein Sources in Older Adults. Proc. Nutr. Soc. 2018, 77, 20–31. Available online: https://pubmed.ncbi.nlm.nih.gov/28847314/ (accessed on 16 June 2022). [CrossRef] [PubMed]
- Li, C.-Y.; Fang, A.-P.; Ma, W.-J.; Wu, S.-L.; Li, C.-L.; Chen, Y.-M.; Zhu, H.-L. Amount Rather than Animal vs Plant Protein Intake Is Associated with Skeletal Muscle Mass in Community-Dwelling Middle-Aged and Older Chinese Adults: Results from the Guangzhou Nutrition and Health Study. J. Acad. Nutr. Diet. 2019, 119, 1501–1510. [Google Scholar] [CrossRef] [Green Version]
- Borack, M.S.; Volpi, E. Efficacy and Safety of Leucine Supplementation in the Elderly. J. Nutr. 2016, 146, 2625S–2629S. [Google Scholar] [CrossRef] [Green Version]
- Bolster, D.R.; Vary, T.C.; Kimball, S.R.; Jefferson, L.S. Leucine Regulates Translation Initiation in Rat Skeletal Muscle Via Enhanced EIF4G Phosphorylation. J. Nutr. 2004, 134, 1704–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dardevet, D.; Sornet, C.; Balage, M.; Grizard, J. Stimulation of in Vitro Rat Muscle Protein Synthesis by Leucine Decreases with Age. J. Nutr. 2000, 130, 2630–2635. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Junior, H.J.; Calvani, R.; Gonçalves, I.O.; Rodrigues, B.; Picca, A.; Landi, F.; Bernabei, R.; Uchida, M.C.; Marzetti, E. High Relative Consumption of Vegetable Protein Is Associated with Faster Walking Speed in Well-Functioning Older Adults. Aging Clin. Exp. Res. 2019, 31, 837–844. [Google Scholar] [CrossRef]
- Schoufour, J.D.; Franco, O.H.; Kiefte-De Jong, J.C.; Trajanoska, K.; Stricker, B.; Brusselle, G.; Rivadeneira, F.; Lahousse, L.; Voortman, T. The Association between Dietary Protein Intake, Energy Intake and Physical Frailty: Results from the Rotterdam Study. Br. J. Nutr. 2019, 121, 393–401. [Google Scholar] [CrossRef] [Green Version]
- Ten Haaf, D.S.; Van Dongen, E.J.; Nuijten, M.A.; Eijsvogels, T.M.; De Groot, L.C.; Hopman, M.T. Protein Intake and Distribution in Relation to Physical Functioning and Quality of Life in Community-Dwelling Elderly People: Acknowledging the Role of Physical Activity. Nutrients 2018, 10, 506. [Google Scholar] [CrossRef] [Green Version]
- Loenneke, J.P.; Loprinzi, P.D.; Murphy, C.H.; Phillips, S.M. Per Meal Dose and Frequency of Protein Consumption Is Associated with Lean Mass and Muscle Performance. Clin. Nutr. 2016, 35, 1506–1511. [Google Scholar] [CrossRef]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein Ingestion to Stimulate Myofibrillar Protein Synthesis Requires Greater Relative Protein Intakes in Healthy Older versus Younger Men. J. Gerontol. Ser. A 2015, 70, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, Y.; Hirano, H.; Arai, H.; Morishita, S.; Ohara, Y.; Edahiro, A.; Murakami, M.; Shimada, H.; Kikutani, T.; Suzuki, T. Relationship Between Frailty and Oral Function in Community-Dwelling Elderly Adults. J. Am. Geriatr. Soc. 2017, 65, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Sisto, A.; Marzetti, E. Anorexia of Aging: Risk Factors, Consequences, and Potential Treatments. Nutrients 2016, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Bernabei, R.; Landi, F.; Calvani, R.; Cesari, M.; Del Signore, S.; Anker, S.D.; Bejuit, R.; Bordes, P.; Cherubini, A.; Cruz-Jentoft, A.J.; et al. Multicomponent Intervention to Prevent Mobility Disability in Frail Older Adults: Randomised Controlled Trial (SPRINTT Project). BMJ 2022, 377, e068788. [Google Scholar] [CrossRef] [PubMed]
| Year | Author | Country | Sample Characteristics | Sample Size (n) | Mean (Years) | Protein Intake | Dietary Intake Assessment Method | Frailty Assessment Tool |
|---|---|---|---|---|---|---|---|---|
| 2006 | Bartali et al. | Italy | Community-dwelling older adults | 802 | 74.0 | — | Food frequency questionnaire | Frailty phenotype |
| 2013 | Bollwein et al. | Germany | Community-dwelling older adults | 195 | 83 | 76.6 g | Food frequency questionnaire | Frailty phenotype |
| 2017 | Castaneda-Gameros et al. | United Kingdom | Community-dwelling women | 76 | 70.5 | — | 24 h dietary recall | Frailty phenotype |
| 2020 | Coelho-Junior et al. | Brazil | Community-dwelling older adults | 200 | ~67.4 | ~1.6 g/d/kg body weigh | 24 h dietary recall | Frailty phenotype, FRAIL scale, SOF |
| 2021 | Hayashi et al. | Japan | Community-dwelling older adults | 120 | 73 | 69.4 g | Food frequency questionnaire | Frailty phenotype |
| 2021 | Kaimoto et al. | Japan | Community-dwelling older men | 815 | 74.9 | ~79.9 g | Self-administered diet history questionnaire | Frailty phenotype |
| 2013 | Kobayashi et al. | Japan | Community-dwelling women | 481 | 74.7 | 74.0 g | Self | Frailty phenotype |
| 2017 | Kobayashi et al. | Japan | Community-dwelling women | 2108 | 74 | 74.0 g | Self | Frailty phenotype |
| 2016 | Rahi et al. | France | Community-dwelling women | 1345 | ~75,6 | ~70.3 g | 24 h dietary recall | Frailty phenotype |
| 2013 | Smit et al. | USA | Community-dwelling older adults | 4731 | 60+ | ~66.9 g | 24 h dietary recall | Frailty phenotype |
| 2018 | Tamaki et al. | Japan | Community-dwelling older adults | 800 | 72.6 | — | Self-administered diet history questionnaire | KCL and frailty phenotype |
| 2021 | Wu et al. | Taiwan | Community-dwelling older adults | 1920 | ~74 | — | 24 h dietary recall | Frailty phenotype |
| Year | Author | Follow-Up Period (Years) | Country | Sample Characteristics | Sample Size (n) | Mean Age (Years) | Protein Intake | Dietary Intake Assessment Method | Frailty |
|---|---|---|---|---|---|---|---|---|---|
| 2010 | Beasley et al. | 3.0 | USA | Community-dwelling older adults | 24417 | 65–79 | ~1.1 g/d/kg body weight | Food frequency questionnaire | Frailty phenotype |
| 2020 | Huang et al. | 3.0 | Japan | Community-dwelling older adults | 429 | 69.4 | 1.1 g/d/kg body weight | Food frequency questionnaire | Social frailty |
| 2019 | Otsuka et al. | 2.0 | Japan | Community-dwelling women | 283 | ~72 | ~77.2 g | 3-day food record | Frailty phenotype |
| 2016 | Sandoval-Insausti et al. | 3.5 | Spain | Community-dwelling older adults | 1822 | 68.7 | — | Diet history | Frailty phenotype |
| 2014 | Shikany et al. | 4.6 | USA | Community-dwelling older men | 5925 | 65+ | — | Food frequency questionnaire | Frailty phenotype |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho-Junior, H.J.; Calvani, R.; Picca, A.; Tosato, M.; Landi, F.; Marzetti, E. Protein Intake and Frailty in Older Adults: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2022, 14, 2767. https://doi.org/10.3390/nu14132767
Coelho-Junior HJ, Calvani R, Picca A, Tosato M, Landi F, Marzetti E. Protein Intake and Frailty in Older Adults: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients. 2022; 14(13):2767. https://doi.org/10.3390/nu14132767
Chicago/Turabian StyleCoelho-Junior, Hélio José, Riccardo Calvani, Anna Picca, Matteo Tosato, Francesco Landi, and Emanuele Marzetti. 2022. "Protein Intake and Frailty in Older Adults: A Systematic Review and Meta-Analysis of Observational Studies" Nutrients 14, no. 13: 2767. https://doi.org/10.3390/nu14132767
APA StyleCoelho-Junior, H. J., Calvani, R., Picca, A., Tosato, M., Landi, F., & Marzetti, E. (2022). Protein Intake and Frailty in Older Adults: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients, 14(13), 2767. https://doi.org/10.3390/nu14132767