Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics
Abstract
1. Introduction
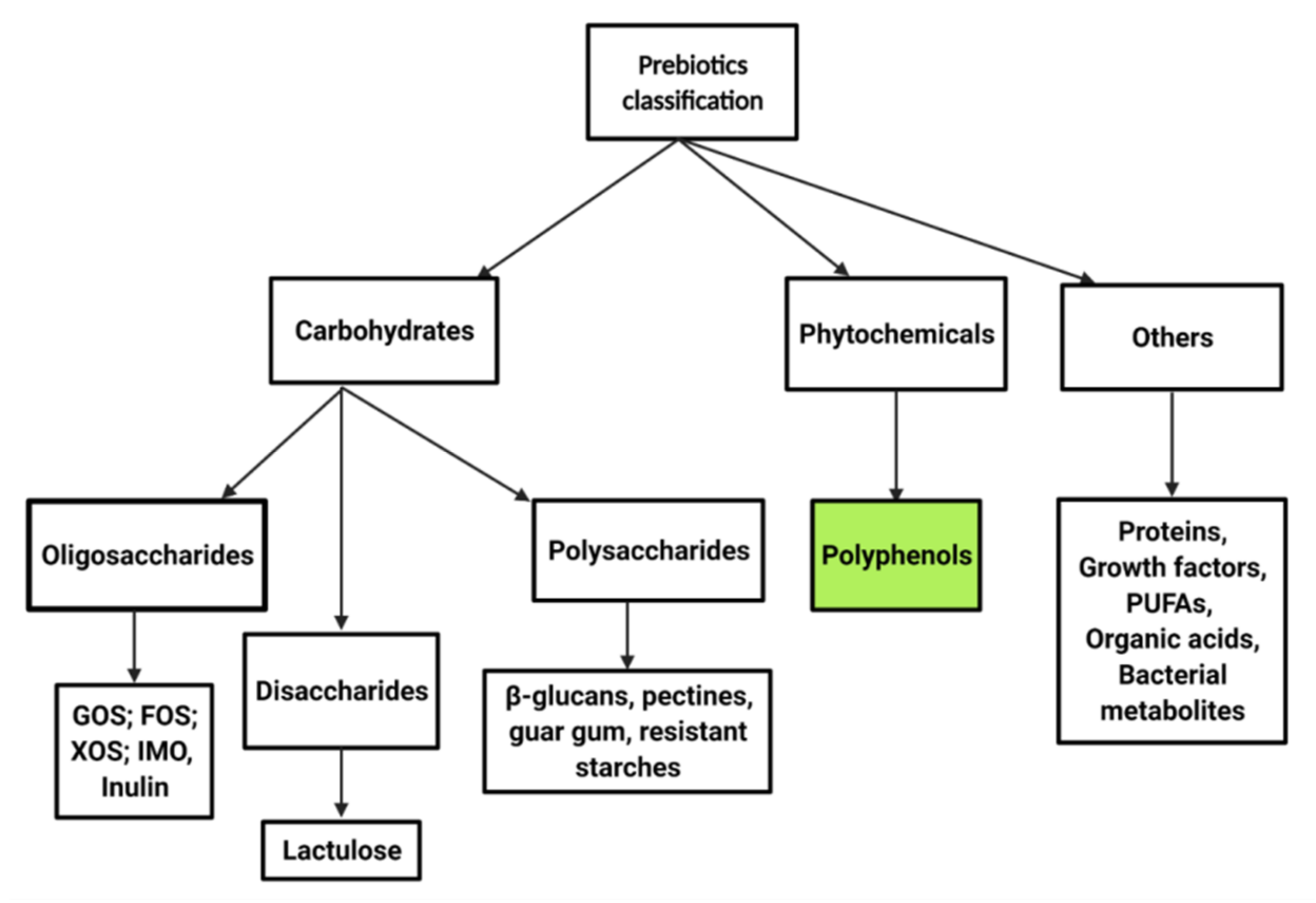
2. Prebiotics and Polyphenol Classification
3. The Concept of Prebiotics
3.1. Polyphenols as a Prebiotic Substrate
3.2. Types of Polyphenols Found in Food and Their Effects on Host Health
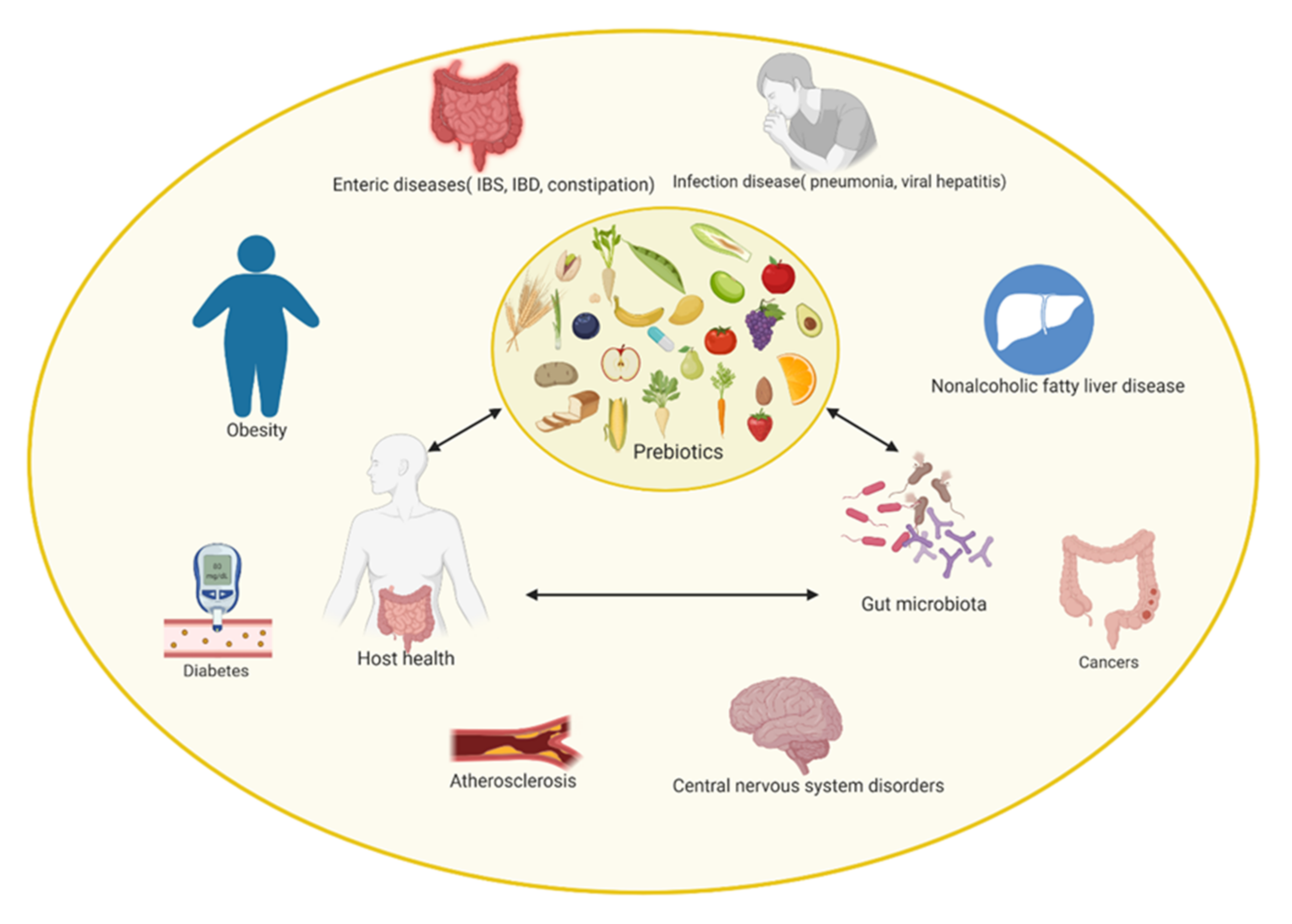
4. Prebiotics as a Nutritional Substrate for Human Gut Microbiota
5. In Vitro Modulation of Gut Microbiota through Polyphenol Consumption
6. In Vivo Modulation of Gut Microbiota through Polyphenol Consumption
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fava, F.; Rizzetto, L.; Tuohy, K.M. Gut microbiota and health: Connecting actors across the metabolic system. Proc. Nutr. Soc. 2018, 78, 177–188. [Google Scholar] [CrossRef]
- Lane, N. The unseen world: Reflections on Leeuwenhoek (1677) ‘Concerning little animals’. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140344. [Google Scholar] [CrossRef]
- Falk, P.G.; Hooper, L.V.; Midtvedt, T.; Gordon, J.I. Creating and Maintaining the Gastrointestinal Ecosystem: What We Know and Need To Know from Gnotobiology. Microbiol. Mol. Biol. Rev. 1998, 62, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Varin, T.V.; Le Barz, M.; Desjardins, Y.; Levy, E.; Roy, D.; Marette, A. Gut Microbiota Dysbiosis in Obesity-Linked Metabolic Diseases and Prebiotic Potential of Polyphenol-Rich Extracts. Curr. Obes. Rep. 2015, 4, 389–400. [Google Scholar] [CrossRef]
- Salvucci, E. The human-microbiome superorganism and its modulation to restore health. Int. J. Food Sci. Nutr. 2019, 70, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.; Ley, R.; Hamady, M.; Fraser, C.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Martín, M.Á. Impact of diet on gut microbiota. Curr. Opin. Food Sci. 2021, 37, 83–90. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Ogunrinola, G.A.; Oyewale, J.O.; Oshamika, O.O.; Olasehinde, G.I. The Human Microbiome and Its Impacts on Health. Int. J. Microbiol. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Wen, L.; Duffy, A. Factors Influencing the Gut Microbiota, Inflammation, and Type 2 Diabetes. J. Nutr. 2017, 147, 1468S–1475S. [Google Scholar] [CrossRef]
- Ishiguro, E.; Haskey, H.; Campbell, K. Gut Microbiota-Interactive Effects on Nutrition and Health; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Arboleya, S.; Binetti, A.; Salazar, N.; Fernández, N.; Solís, G.; Barranco, A.H.; Margolles, A.; Reyes-Gavilan, C.D.L.; Gueimonde, M. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol. Ecol. 2011, 79, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; Gaerlan, S.C.; De Leoz, M.L.A.; Dimapasoc, L.M.; Kalanetra, K.M.; Lemay, D.; German, J.B.; Mills, D.A.; Lebrilla, C.B. Human milk oligosaccharides in premature infants: Absorption, excretion, and influence on the intestinal microbiota. Pediatr. Res. 2015, 78, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Sevelsted, A.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H. Cesarean Section and Chronic Immune Disorders. Pediatrics 2015, 135, e92–e98. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; Brandt, P.A.V.D.; Stobberingh, E.E. Factors Influencing the Composition of the Intestinal Microbiota in Early Infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Shi, J.; Mehwish, H.M.; Zhu, J.; Li, Q.; Shao, D.; Huang, Q.; Yang, H. Interaction between diet composition and gut microbiota and its impact on gastrointestinal tract health. Food Sci. Hum. Wellness 2017, 6, 121–130. [Google Scholar] [CrossRef]
- Valdes, A.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Z. Ernährungswissenschaft 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Cremon, C.; Barbaro, M.R.; Ventura, M.; Barbara, G. Pre- and probiotic overview. Curr. Opin. Pharmacol. 2018, 43, 87–92. [Google Scholar] [CrossRef]
- Hutkins, R.W.; A Krumbeck, J.; Bindels, L.B.; Cani, P.D.; Fahey, G.; Goh, Y.J.; Hamaker, B.; Martens, E.C.; Mills, D.; A Rastal, R.; et al. Prebiotics: Why definitions matter. Curr. Opin. Biotechnol. 2016, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Sharma, S.; Agarwal, N.; Verma, P. Miraculous Health Benefits of Prebiotics. Int. J. Pharm. Sci. Res. 2012, 3, 1544–1553. [Google Scholar]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef]
- Vitetta, L.; Briskey, D.; Alford, H.; Hall, S.; Coulson, S. Probiotics, prebiotics and the gastrointestinal tract in health and disease. Inflammopharmacology 2014, 22, 135–154. [Google Scholar] [CrossRef]
- Shi, L.H.; Balakrishnan, K.; Thiagarajah, K.; Ismail, N.I.M.; Yin, O.S. Beneficial Properties of Probiotics. Trop. Life Sci. Res. 2016, 27, 73–90. [Google Scholar] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.; Roberfroid, M. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Moorthy, M.; Chaiyakunapruk, N.; Jacob, S.A.; Palanisamy, U.D. Prebiotic potential of polyphenols, its effect on gut microbiota and anthropometric/clinical markers: A systematic review of randomised controlled trials. Trends Food Sci. Technol. 2020, 99, 634–649. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Feo, V.; Battistelli, A.; Da Cruz, A.G.; Coppola, R. Polyphenols, the new frontiers of prebiotics. Adv. Food Nutr. Res. 2020, 94, 35–89. [Google Scholar] [CrossRef]
- Pandey, K.; Rizvi, S. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef]
- Hussain, M.B.; Hassan, S.; Waheed, M.; Javed, A.; Farooq, M.A.; Tahir, A. Bioavailability and Metabolic Pathway of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019. [Google Scholar]
- Neri-Numa, I.A.; Pastore, G.M. Novel insights into prebiotic properties on human health: A review. Food Res. Int. 2020, 131, 108973. [Google Scholar] [CrossRef]
- Farias, D.D.P.; de Araújo, F.F.; Neri-Numa, I.A.; Pastore, G.M. Prebiotics: Trends in food, health and technological applications. Trends Food Sci. Technol. 2019, 93, 23–35. [Google Scholar] [CrossRef]
- Hurtado-Romero, A.; Del Toro-Barbosa, M.; Garcia-Amezquita, L.E.; García-Cayuela, T. Innovative technologies for the production of food ingredients with prebiotic potential: Modifications, applications, and validation methods. Trends Food Sci. Technol. 2020, 104, 117–131. [Google Scholar] [CrossRef]
- Singh, A.K.; Cabral, C.; Kumar, R.; Ganguly, R.; Rana, H.K.; Gupta, A.; Lauro, M.R.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Gębalski, J.; Mazurek, E.; Podhorecka, M.; Śniegocki, M.; Szychta, P.; Sawicka, E.; Malinowski, B. The Influence of Polyphenol Compounds on Human Gastrointestinal Tract Microbiota. Nutrients 2020, 12, 350. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An Important Class of Phytochemicals. In Phytochemical—Isolation, Characterisation and Role in Human Health; BoD–Books on Demand: Norderstedt, Germany, 2015; pp. 533–538. [Google Scholar]
- Egan, D.; O’Kennedy, R.; Moran, E.; Cox, D.; Prosser, E.; Thornes, R.D. The Pharmacology, Metabolism, Analysis, and Applications of Coumarin and Coumarin-Related Compounds. Drug Metab. Rev. 1990, 22, 503–529. [Google Scholar] [CrossRef]
- Sieniawska, E.; Baj, T. Chapter 10—Tannins. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 199–232. [Google Scholar]
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.M. Factors affecting intake, metabolism and health benefits of phenolic acids: Do we understand individual variability? Eur. J. Nutr. 2020, 59, 1275–1293. [Google Scholar] [CrossRef] [PubMed]
- Panche, N.A.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I.; Meririnne, E.; Alfthan, G.; Aro, A. Plasma Kinetics and Urinary Excretion of the Flavanones Naringenin and Hesperetin in Humans after Ingestion of Orange Juice and Grapefruit Juice. J. Nutr. 2001, 131, 235–241. [Google Scholar] [CrossRef]
- Gadkari, P.V.; Balaraman, M. Catechins: Sources, extraction and encapsulation: A review. Food Bioprod. Process. 2015, 93, 122–138. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, L.G.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicine 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Dwyer, J.; Adlercreutz, H.; Scalbert, A.; Jacques, P.; McCullough, M.L. Dietary lignans: Physiology and potential for cardiovascular disease risk reduction. Nutr. Rev. 2010, 68, 571–603. [Google Scholar] [CrossRef]
- Nelson, D.L. Functional Foods: Surces, Health Effects and Future Perspectives; Nova Science Publishers, Incorporated: Hauppauge, NY, USA, 2017. [Google Scholar]
- Martel, J.; Ojcius, D.M.; Ko, Y.-F.; Young, J.D. Phytochemicals as Prebiotics and Biological Stress Inducers. Trends Biochem. Sci. 2020, 45, 462–471. [Google Scholar] [CrossRef]
- Dingeo, G.; Brito, A.; Samouda, H.; Iddir, M.; La Frano, M.R.; Bohn, T. Phytochemicals as modifiers of gut microbial communities. Food Funct. 2020, 11, 8444–8471. [Google Scholar] [CrossRef]
- Aravind, S.M.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.; Pereira, C.B.; Pérez-Gregorio, R.; Mateus, N.; Freitas, V. Recent advances on dietary polyphenol’s potential roles in Celiac Disease. Trends Food Sci. Technol. 2021, 107, 213–225. [Google Scholar] [CrossRef]
- Espin, C.J.; Gonzalez-Sarrias, A.; Tomas-Barberan, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef] [PubMed]
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm Activity of Plant Polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef]
- Morais, C.A.; de Rosso, V.V.; Estadella, D.; Pisani, L.P. Anthocyanins as inflammatory modulators and the role of the gut microbiota. J. Nutr. Biochem. 2016, 33, 1–7. [Google Scholar] [CrossRef]
- Gowd, V.; Karim, N.; Shishir, M.R.I.; Xie, L.; Chen, W. Dietary polyphenols to combat the metabolic diseases via altering gut microbiota. Trends Food Sci. Technol. 2019, 93, 81–93. [Google Scholar] [CrossRef]
- Nash, V.; Ranadheera, C.S.; Georgousopoulou, E.N.; Mellor, D.D.; Panagiotakos, D.B.; McKune, A.J.; Kellett, J.; Naumovski, N. The effects of grape and red wine polyphenols on gut microbiota—A systematic review. Food Res. Int. 2018, 113, 277–287. [Google Scholar] [CrossRef]
- Cueva, C.; Gil-Sánchez, I.; Ayuda-Durán, B.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C.; Bartolomé, B.; Moreno-Arribas, M.V. An Integrated View of the Effects of Wine Polyphenols and Their Relevant Metabolites on Gut and Host Health. Molecules 2017, 22, 99. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Li, X.Y.; Shen, L. Modulation effect of tea consumption on gut microbiota. Appl. Microbiol. Biotechnol. 2020, 104, 981–987. [Google Scholar] [CrossRef]
- Lavefve, L.; Howard, L.R.; Carbonero, F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020, 11, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Pozuelo, M.J.; Agis-Torres, A.; Hervert-Hernández, D.; López-Oliva, M.E.; Muñoz-Martínez, E.; Rotger, R.; Goñi, I. Grape Antioxidant Dietary Fiber Stimulates Lactobacillus Growth in Rat Cecum. J. Food Sci. 2012, 77, H59–H62. [Google Scholar] [CrossRef]
- Liu, S.; Jia, M.; Chen, J.; Wan, H.; Dong, R.; Nie, S.; Xie, M.; Yu, Q. Removal of bound polyphenols and its effect on antioxidant and prebiotics properties of carrot dietary fiber. Food Hydrocoll. 2019, 93, 284–292. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Joudu, I.; Bhat, R. Bioactives From Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef]
- Barroso, E.; Muñoz-González, I.; Jiménez, E.; Bartolomé, B.; Moreno-Arribas, M.; Peláez, C.; Martínez-Cuesta, M.C.; Requena, T. Phylogenetic profile of gut microbiota in healthy adults after moderate intake of red wine. Mol. Nutr. Food Res. 2017, 61, 1600620. [Google Scholar] [CrossRef]
- Koch, W. Theaflavins, Thearubigins, and Theasinensins. In Handbook of Dietary Phytochemicals; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–29. [Google Scholar]
- Van Dorsten, F.A.; Peters, S.; Gross, G.; Gomez-Roldan, V.; Klinkenberg, M.; de Vos, R.; Vaughan, E.; van Duynhoven, J.P.; Possemiers, S.; van de Wiele, T.; et al. Gut Microbial Metabolism of Polyphenols from Black Tea and Red Wine/Grape Juice Is Source-Specific and Colon-Region Dependent. J. Agric. Food Chem. 2012, 60, 11331–11342. [Google Scholar] [CrossRef]
- Van Duynhoven, J.; Vaughan, E.E.; van Dorsten, F.; Gomez-Roldan, V.; de Vos, R.; Vervoort, J.; van der Hooft, J.J.J.; Roger, L.; Draijer, R.; Jacobs, D.M. Interactions of black tea polyphenols with human gut microbiota: Implications for gut and cardiovascular health. Am. J. Clin. Nutr. 2013, 98 (Suppl. S6), 1631S–1641S. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, G.; Nzekoue, F.K.; Giusti, F.; Vittori, S.; Sagratini, G. Optimization of an extraction method for the simultaneous quantification of sixteen polyphenols in thirty-one pulse samples by using HPLC-MS/MS dynamic-MRM triple quadrupole. Food Chem. 2018, 266, 490–497. [Google Scholar] [CrossRef]
- Herbello-Hermelo, P.; Lamas, J.P.; Lores, M.; González, M.R.D.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Polyphenol bioavailability in nuts and seeds by an in vitro dialyzability approach. Food Chem. 2018, 254, 20–25. [Google Scholar] [CrossRef]
- Alasalvar, C.; Bolling, B.W. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br. J. Nutr. 2015, 113 (Suppl. S2), 68–78. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Coman, V.; Vodnar, D.C. Hydroxycinnamic acids and human health: Recent advances. J. Sci. Food Agric. 2020, 100, 483–499. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; Taher, E.A.; Sheikh, B.Y.; Anjum, S.; Saeed, A.; AlAjmi, M.F.; Moustafa, M.S.; Al-Mousawi, S.M.; Farag, M.A.; Hegazy, M.-E.F.; et al. Hydroxycinnamic Acids: Natural Sources, Biosynthesis, Possible Biological Activities, and Roles in Islamic Medicine; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 269–292. [Google Scholar]
- Martini, D.; Chiavaroli, L.; González-Sarrías, A.; Bresciani, L.; Palma-Duran, S.A.; Dall’Asta, M.; Deligiannidou, G.E.; Massaro, M.; Scoditti, E.; Combet, E.; et al. Impact of Foods and Dietary Supplements Containing Hydroxycinnamic Acids on Cardiometabolic Biomarkers: A Systematic Review to Explore Inter-Individual Variability. Nutrients 2019, 11, 1805. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of Gallic Acid on Gut Health: Focus on the Gut Microbiome, Immune Response, and Mechanisms of Action. Front. Immunol. 2020, 11, 580208. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in Food and Their Health Benefits. Mater. Veg. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Aherne, S.A.; O’Brien, N.M. Dietary flavonols: Chemistry, food content, and metabolism. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef]
- Kawabata, K.; Sugiyama, Y.; Sakano, T.; Ohigashi, H. Flavonols enhanced production of anti-inflammatory substance(s) byBifidobacterium adolescentis: Prebiotic actions of galangin, quercetin, and fisetin. BioFactors 2013, 39, 422–429. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Caro, G.P.; Oliver, C.M.; Weerakkody, R.; Singh, T.; Conlon, M.; Borges, G.; Sanguansri, L.; Lockett, T.; Roberts, S.A.; Crozier, A.; et al. Chronic administration of a microencapsulated probiotic enhances the bioavailability of orange juice flavanones in humans. Free. Radic. Biol. Med. 2015, 84, 206–214. [Google Scholar] [CrossRef]
- Stevens, Y.; Van Rymenant, E.; Grootaert, C.; Van Camp, J.; Possemiers, S.; Masclee, A.; Jonkers, D. The Intestinal Fate of Citrus Flavanones and Their Effects on Gastrointestinal Health. Nutrients 2019, 11, 1464. [Google Scholar] [CrossRef]
- Vlachojannis, J.; Erne, P.; Zimmermann, B.; Chrubasik-Hausmann, S. The Impact of Cocoa Flavanols on Cardiovascular Health. Phytotherapy Res. 2016, 30, 1641–1657. [Google Scholar] [CrossRef] [PubMed]
- Selmi, C.; Cocchi, C.A.; Lanfredini, M.; Keen, C.L.; Gershwin, M.E. Chocolate at heart: The anti-inflammatory impact of cocoa flavanols. Mol. Nutr. Food Res. 2008, 52, 1340–1348. [Google Scholar] [CrossRef]
- Sokolov, A.N.; Pavlova, M.A.; Klosterhalfen, S.; Enck, P. Chocolate and the brain: Neurobiological impact of cocoa flavanols on cognition and behavior. Neurosci. Biobehav. Rev. 2013, 37, 2445–2453. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F. Soya isoflavones: A new and promising ingredient for the health foods sector. Food Res. Int. 2002, 35, 187–193. [Google Scholar] [CrossRef]
- Zaheer, K.; Humayoun Akhtar, M. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Fernandes, I.; Norberto, S.; Mateus, N.; Calhau, C. Interplay between Anthocyanins and Gut Microbiota. J. Agric. Food Chem. 2014, 62, 6898–6902. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. Int. Rev. J. 2019, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, B.; Gul, K.; Wani, A.A.; Singh, P. Health Benefits of Anthocyanins and Their Encapsulation for Potential Use in Food Systems: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2223–2230. [Google Scholar] [CrossRef]
- Hidalgo, M.; Concha, M.J.O.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.E.; Gibson, G.R.; De Pascual-Teresa, S. Metabolism of Anthocyanins by Human Gut Microflora and Their Influence on Gut Bacterial Growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef]
- Hester, S.N.; Mastaloudis, A.; Gray, R.; Antony, J.M.; Evans, M.; Wood, S.M. Efficacy of an Anthocyanin and Prebiotic Blend on Intestinal Environment in Obese Male and Female Subjects. J. Nutr. Metab. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Janssen, K.; Mensink, R.P.; Cox, F.J.; Harryvan, J.L.; Hovenier, R.; Hollman, P.C.; Katan, M.B. Effects of the flavonoids quercetin and apigenin on hemostasis in healthy volunteers: Results from an in vitro and a dietary supplement study. Am. J. Clin. Nutr. 1998, 67, 255–262. [Google Scholar] [CrossRef]
- Verbeek, R.; Plomp, A.C.; van Tol, E.; van Noort, J. The flavones luteolin and apigenin inhibit in vitro antigen-specific proliferation and interferon-gamma production by murine and human autoimmune T cells. Biochem. Pharmacol. 2004, 68, 621–629. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Kasiri, N.; Rahmati, M.; Ahmadi, L.; Eskandari, N. The significant impact of apigenin on different aspects of autoimmune disease. Inflammopharmacology 2018, 26, 1359–1373. [Google Scholar] [CrossRef] [PubMed]
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of Environmental Factors on Stilbene Biosynthesis. Plants 2021, 10, 90. [Google Scholar] [CrossRef]
- Mompeo, O.; Spector, T.D.; Hernandez, M.M.; Le Roy, C.; Istas, G.; Le Sayec, M.; Mangino, M.; Jennings, A.; Rodriguez-Mateos, A.; Valdes, A.M.; et al. Consumption of Stilbenes and Flavonoids is Linked to Reduced Risk of Obesity Independently of Fiber Intake. Nutrients 2020, 12, 1871. [Google Scholar] [CrossRef]
- Larrosa, M.; Yañéz-Gascón, M.J.; Selma, M.V.; González-Sarrías, A.; Toti, S.; Ceron, J.; Tomas-Barberan, F.; Dolara, P.; Espín, J.C. Effect of a Low Dose of Dietary Resveratrol on Colon Microbiota, Inflammation and Tissue Damage in a DSS-Induced Colitis Rat Model. J. Agric. Food Chem. 2009, 57, 2211–2220. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.; Portillo, M.; Martínez, J.; Milagro, F. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Corona, G.; Kreimes, A.; Barone, M.; Turroni, S.; Brigidi, P.; Keleszade, E.; Costabile, A. Impact of lignans in oilseed mix on gut microbiome composition and enterolignan production in younger healthy and premenopausal women: An in vitro pilot study. Microb. Cell Factories 2020, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Touré, A.; Xueming, X. Flaxseed Lignans: Source, Biosynthesis, Metabolism, Antioxidant Activity, Bio-Active Components, and Health Benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef]
- Schogor, A.L.B.; Huws, S.; DOS Santos, G.T.; Scollan, N.D.; Hauck, B.D.; Winters, A.L.; Kim, E.J.; Petit, H.V. Ruminal Prevotella spp. May Play an Important Role in the Conversion of Plant Lignans into Human Health Beneficial Antioxidants. PLoS ONE 2014, 9, e87949. [Google Scholar] [CrossRef] [PubMed]
- Lampe, W.J.; Atkinson, C.; Hullar, M.A.J. Assessing Exposure to Lignans and Their Metabolites in Humans. J. AOAC Int. 2006, 89, 1174–1181. [Google Scholar] [CrossRef]
- Molino, S.; Casanova, N.A.; Henares, J.; Ángel, R.; Miyakawa, M.E.F. Natural Tannin Wood Extracts as a Potential Food Ingredient in the Food Industry. J. Agric. Food Chem. 2020, 68, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Macáková, K.; Kolečkář, V.; Cahlíková, L.; Chlebek, J.; Hošt’álková, A.; Kuča, K.; Jun, D.; Opletal, L. Chapter 6—Tannins and their Influence on Health. In Recent Advances in Medicinal Chemistry; Attaur, R., Choudhary, M.I., Perry, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 159–208. [Google Scholar]
- Sharma, K.; Kumar, V.; Kaur, J.; Tanwar, B.; Goyal, A.; Sharma, R.; Gat, Y.; Kumar, A. Health effects, sources, utilization and safety of tannins: A critical review. Toxin Rev. 2019, 40, 1–13. [Google Scholar] [CrossRef]
- Raman, M.; Ambalam, P.; Doble, M. 9—Probiotics, prebiotics, and fibers in nutritive and functional beverages. In Nutrients in Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 315–367. [Google Scholar]
- Nazhand, A.; Souto, E.B.; Lucarini, M.; Souto, S.B.; Durazzo, A.; Santini, A. Ready to Use Therapeutical Beverages: Focus on Functional Beverages Containing Probiotics, Prebiotics and Synbiotics. Beverages 2020, 6, 26. [Google Scholar] [CrossRef]
- Hussain, M.I.; Syed, Q.A.; Khattak, M.N.K.; Hafez, B.; Reigosa, M.J.; El-Keblawy, A. Natural product coumarins: Biological and pharmacological perspectives. Biologia 2019, 74, 863–888. [Google Scholar] [CrossRef]
- Lončar, M.; Jakovljević, M.; Šubarić, D.; Pavlić, M.; Služek, V.B.; Cindrić, I.; Molnar, M. Coumarins in Food and Methods of Their Determination. Foods 2020, 9, 645. [Google Scholar] [CrossRef]
- Krautkramer, A.K.; Fan, J.; Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 2020, 19, 77–94. [Google Scholar] [CrossRef]
- Bolca, S.; van de Wiele, T.; Possemiers, S. Gut metabotypes govern health effects of dietary polyphenols. Curr. Opin. Biotechnol. 2013, 24, 220–225. [Google Scholar] [CrossRef]
- Selma, M.V.; Tomás-Barberán, F.A.; Romo-Vaquero, M.; Cortés-Martín, A.; Espín, J.C. Understanding Polyphenols’ Health Effects Through the Gut Microbiota. In Dietary Polyphenols; Wiley: Hoboken, NJ, USA, 2020; pp. 497–531. [Google Scholar]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Popa, D.E.; Dragoi, C.M.; Arsene, A.L.; Dumitrescu, I.B.; Nicolae, A.C.; Burcea-Dragomiroiu, B.S.V.A.G.T. The Relationship Between Phenolic Compounds from Diet and Microbiota. In Phenolic Compounds—Biological Activity; IntechOpen: London, UK, 2017. [Google Scholar]
- Li, Z.; Summanen, P.H.; Komoriya, T.; Henning, S.M.; Lee, R.-P.; Carlson, E.; Heber, D.; Finegold, S.M. Pomegranate ellagitannins stimulate growth of gut bacteria in vitro: Implications for prebiotic and metabolic effects. Anaerobe 2015, 34, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.K.; Piwowarski, J.P. Ellagitannins, Gallotannins and their Metabolites—The Contribution to the Anti-Inflammatory Effect of Food Products and Medicinal Plants. Curr. Med. Chem. 2018, 25, 4946–4967. [Google Scholar] [CrossRef]
- Ito, H. Metabolites of the Ellagitannin Geraniin and Their Antioxidant Activities. Planta Med. 2011, 77, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Kosmala, M.; Jurgoński, A.; Juśkiewicz, J.; Karlińska, E.; Macierzyński, J.; Rój, E.; Zduńczyk, Z. Chemical Composition of Blackberry Press Cake, Polyphenolic Extract, and Defatted Seeds, and Their Effects on Cecal Fermentation, Bacterial Metabolites, and Blood Lipid Profile in Rats. J. Agric. Food Chem. 2017, 65, 5470–5479. [Google Scholar] [CrossRef] [PubMed]
- Catalkaya, G.; Venema, K.; Lucini, L.; Rocchetti, G.; Delmas, D.; Daglia, M.; De Filippis, A.; Xiao, H.; Quiles, J.L.; Xiao, J.; et al. Interaction of dietary polyphenols and gut microbiota: Microbial metabolism of polyphenols, influence on the gut microbiota, and implications on host health. Food Front. 2020, 1, 109–133. [Google Scholar] [CrossRef]
- Selma, M.V.; Beltrán, D.; García-Villalba, R.; Espín, J.C.; Tomás-Barberán, F.A. Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species. Food Funct. 2014, 5, 1779–1784. [Google Scholar] [CrossRef]
- Garcia-Villalba, R.; Espin, J.C.; Tomas-Barberan, F.A. Chromatographic and spectroscopic characterization of urolithins for their determination in biological samples after the intake of foods containing ellagitannins and ellagic acid. J. Chromatogr. A 2016, 1428, 162–175. [Google Scholar] [CrossRef]
- Stojanov, S.; Kreft, S. Gut Microbiota and the Metabolism of Phytoestrogens. Rev. Bras. Farmacogn. 2020, 30, 145–154. [Google Scholar] [CrossRef]
- Yoder, S.C.; Lancaster, S.M.; Hullar, M.A.; Lampe, J.W. Chapter 7—Gut Microbial Metabolism of Plant Lignans: Influence on Human Health. In Diet-Microbe Interactions in the Gut; Tuohy, K., Del Rio, D., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 103–117. [Google Scholar]
- Giuliani, C.; Marzorati, M.; Innocenti, M.; Vilchez-Vargas, R.; Vital, M.; Pieper, D.H.; Van de Wiele, T.; Mulinacci, N. Dietary supplement based on stilbenes: A focus on gut microbial metabolism by the in vitro simulator M-SHIME(R). Food Funct. 2016, 7, 4564–4575. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.-S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.A.P.; E Kulling, S. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Rubió, L.; Macià, A.; Romero, M.P. Hydroxycinnamates. Dietary Polyphenols; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 129–162. [Google Scholar]
- Gómez-Juaristi, M.; Martínez-López, S.; Sarria, B.; Bravo, L.; Mateos, R. Bioavailability of hydroxycinnamates in an instant green/roasted coffee blend in humans. Identification of novel colonic metabolites. Food Funct. 2018, 9, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Nuñez-Gómez, V.; Navarro-González, I.; Sánchez-Martínez, L.; García-Alonso, J.; Periago, M.J.; González-Barrio, R. Raspberry dietary fibre: Chemical properties, functional evaluation and prebiotic in vitro effect. LWT 2020, 134, 110140. [Google Scholar] [CrossRef]
- Rodríguez-Costa, S.; Cardelle-Cobas, A.; Roca-Saavedra, P.; Porto-Arias, J.J.; Miranda, J.; Cepeda, A. In vitro evaluation of the prebiotic effect of red and white grape polyphenolic extracts. J. Physiol. Biochem. 2017, 74, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Gil-Sánchez, I.; Cueva, C.; Sanz-Buenhombre, M.; Guadarrama, A.; Moreno-Arribas, M.V.; Bartolomé, B. Dynamic gastrointestinal digestion of grape pomace extracts: Bioaccessible phenolic metabolites and impact on human gut microbiota. J. Food Compos. Anal. 2018, 68, 41–52. [Google Scholar] [CrossRef]
- Costa, J.R.; Amorim, M.; Vilas-Boas, A.; Tonon, R.V.; Cabral, L.M.C.; Pastrana, L.; Pintado, M. Impact of in vitro gastrointestinal digestion on the chemical composition, bioactive properties, and cytotoxicity of Vitis vinifera L. cv. Syrah grape pomace extract. Food Funct. 2019, 10, 1856–1869. [Google Scholar] [CrossRef]
- Sayago-Ayerdi, G.S.; Zamora-Gasga, V.M.; Venema, K. Prebiotic effect of predigested mango peel on gut microbiota assessed in a dynamic in vitro model of the human colon (TIM-2). Food Res. Int. 2019, 118, 89–95. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.G.; Venema, K.; Tabernero, M.; Sarriá, B.; Bravo, L.L.; Mateos, R. Bioconversion by gut microbiota of predigested mango (Mangifera indica L.) ‘Ataulfo’ peel polyphenols assessed in a dynamic (TIM-2) in vitro model of the human colon. Food Res. Int. 2021, 139, 109963. [Google Scholar] [CrossRef]
- Bertha, C.-T.; Alberto, S.-B.J.; Tovar, J.; Sáyago-Ayerdi, S.G.; Zamora-Gasga, V.M. In vitro gastrointestinal digestion of mango by-product snacks: Potential absorption of polyphenols and antioxidant capacity. Int. J. Food Sci. Technol. 2019, 54, 3091–3098. [Google Scholar] [CrossRef]
- Sun, H.; Chen, Y.; Cheng, M.; Zhang, X.; Zheng, X.; Zhang, Z. The modulatory effect of polyphenols from green tea, oolong tea and black tea on human intestinal microbiota in vitro. J. Food Sci. Technol. 2017, 55, 399–407. [Google Scholar] [CrossRef]
- Xu, M.; Yang, K.; Zhu, J. Monitoring the Diversity and Metabolic Shift of Gut Microbes during Green Tea Feeding in an In Vitro Human Colonic Model. Molecules 2020, 25, 5101. [Google Scholar] [CrossRef]
- Kemperman, R.A.; Gross, G.; Mondot, S.; Possemiers, S.; Marzorati, M.; Van de Wiele, T.; Doré, J.; Vaughan, E.E. Impact of polyphenols from black tea and red wine/grape juice on a gut model microbiome. Food Res. Int. 2013, 53, 659–669. [Google Scholar] [CrossRef]
- Chen, H.; Sang, S. Biotransformation of tea polyphenols by gut microbiota. J. Funct. Foods 2014, 7, 26–42. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macià, A.; Romero-Fabregat, M.-P.; Motilva, M.J.; Rubió, L. Application of in vitro gastrointestinal digestion and colonic fermentation models to pomegranate products (juice, pulp and peel extract) to study the stability and catabolism of phenolic compounds. J. Funct. Foods 2015, 14, 529–540. [Google Scholar] [CrossRef]
- Chen, L.; Tai, W.C.S.; Hsiao, W.L.W. Dietary saponins from four popular herbal tea exert prebiotic-like effects on gut microbiota in C57BL/6 mice. J. Funct. Foods 2015, 17, 892–902. [Google Scholar] [CrossRef]
- Bialonska, D.; Ramnani, P.; Kasimsetty, S.G.; Muntha, K.R.; Gibson, G.R.; Ferreira, D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int. J. Food Microbiol. 2010, 140, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Del juncal-Guzmán, D.; Hernández-Maldonado, L.M.; Sánchez-Burgos, J.A.; González-Aguilar, G.A.; Ruiz-Valdiviezo, V.M.; Tovar, J.; Sáyago-Ayerdi, S.G. In vitro gastrointestinal digestion and colonic fermentation of phenolic compounds in UV-C irradiated pineapple (Ananas comosus) snack-bars. Lwt 2021, 138, 110636. [Google Scholar] [CrossRef]
- Campos, D.A.; Coscueta, E.R.; Vilas-Boas, A.A.; Silva, S.; Teixeira, J.A.; Pastrana, L.M.; Pintado, M.M. Impact of functional flours from pineapple by-products on human intestinal microbiota. J. Funct. Foods 2020, 67, 103830. [Google Scholar] [CrossRef]
- Ribeiro, T.; Costa, C.M.; Lopes, T.B.-; Silva, S.; Veiga, M.; Monforte, A.R.; Nunes, J.; Vicente, A.A.; Pintado, M. Prebiotic effects of olive pomace powders in the gut: In vitro evaluation of the inhibition of adhesion of pathogens, prebiotic and antioxidant effects. Food Hydrocoll. 2021, 112, 106312. [Google Scholar] [CrossRef]
- Coman, M.M.; Oancea, A.M.; Verdenelli, M.C.; Cecchini, C.; Bahrim, G.E.; Orpianesi, C.; Cresci, A.; Silvi, S. Polyphenol content and in vitro evaluation of antioxidant, antimicrobial and prebiotic properties of red fruit extracts. Eur. Food Res. Technol. 2017, 244, 735–745. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Xian, Y.; Fan, R.; Shao, J.; Toney, A.M.; Chung, S.; Ramer-Tait, A.E. Polyphenolic fractions isolated from red raspberry whole fruit, pulp, and seed differentially alter the gut microbiota of mice with diet-induced obesity. J. Funct. Foods 2021, 76, 104288. [Google Scholar] [CrossRef]
- Mayta-Apaza, A.C.; Pottgen, E.; De Bodt, J.; Papp, N.; Marasini, D.; Howard, L.; Abranko, L.; Van de Wiele, T.; Lee, S.-O.; Carbonero, E. Impact of tart cherries polyphenols on the human gut microbiota and phenolic metabolites in vitro and in vivo. J. Nutr. Biochem. 2018, 59, 160–172. [Google Scholar] [CrossRef]
- Allaw, M.; Manca, M.L.; Caddeo, C.; Recio, M.C.; Pérez-Brocal, V.; Moya, A.; Fernàndez-Busquets, X.; Manconi, M. Advanced strategy to exploit wine-making waste by manufacturing antioxidant and prebiotic fibre-enriched vesicles for intestinal health. Colloids Surf. B Biointerfaces 2020, 193, 111146. [Google Scholar] [CrossRef]
- Boto-Ordóñez, M.; Urpi-Sarda, M.; Queipo-Ortuño, M.I.; Tulipani, S.; Tinahones, F.J.; Andres-Lacueva, C. High levels of Bifidobacteria are associated with increased levels of anthocyanin microbial metabolites: A randomized clinical trial. Food Funct. 2014, 5, 1932–1938. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Sánchez-Alcoholado, L.; Pérez-Martínez, P.; Andrés-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuño, M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Zorraquín, I.; Sánchez-Hernández, E.; Ayuda-Durán, B.; Silva, M.; González-Paramás, A.M.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. Current and future experimental approaches in the study of grape and wine polyphenols interacting gut microbiota. J. Sci. Food Agric. 2020, 100, 3789–3802. [Google Scholar] [CrossRef]
- Dueñas, M.; Cueva, C.; Muñoz-González, I.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. Studies on Modulation of Gut Microbiota by Wine Polyphenols: From Isolated Cultures to Omic Approaches. Antioxidants 2015, 4, 1–21. [Google Scholar] [CrossRef]
- Fidelis, M.; Santos, J.S.; Escher, G.B.; Rocha, R.S.; Cruz, A.G.; Cruz, T.M.; Marques, M.B.; Nunes, J.B.; Carmo, M.A.V.D.; de Almeida, L.A.; et al. Polyphenols of jabuticaba [Myrciaria jaboticaba (Vell.) O.Berg] seeds incorporated in a yogurt model exert antioxidant activity and modulate gut microbiota of 1,2-dimethylhydrazine-induced colon cancer in rats. Food Chem. 2021, 334, 127565. [Google Scholar] [CrossRef]
- Trindade, P.L.; Soares, E.D.R.; Inada, K.O.P.; Martins, F.F.; Rudnicki, M.; Perrone, D.; Monteiro, M.; Souza-Mello, V.; Daleprane, J.B. Consumption of phenolic-rich jabuticaba (Myrciaria jaboticaba) powder ameliorates obesity-related disorders in mice. Br. J. Nutr. 2021, 1–9. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, B.; Hu, Y.; Wang, J.; Liu, J.-M.; Qin, R.; Lv, S.; Wang, S. Correlation Analysis of Intestinal Redox State with the Gut Microbiota Reveals the Positive Intervention of Tea Polyphenols on Hyperlipidemia in High Fat Diet Fed Mice. J. Agric. Food Chem. 2019, 67, 7325–7335. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, L.; Shen, C.-L.; Wang, J.-S. Green tea polyphenols modify gut-microbiota dependent metabolisms of energy, bile constituents and micronutrients in female Sprague–Dawley rats. J. Nutr. Biochem. 2018, 61, 68–81. [Google Scholar] [CrossRef]
- Song, D.; Ho, C.T.; Zhang, X.; Wu, Z.; Cao, J. Modulatory effect of Cyclocarya paliurus flavonoids on the intestinal microbiota and liver clock genes of circadian rhythm disorder mice model. Food Res. Int. 2020, 138 Pt A, 109769. [Google Scholar] [CrossRef]
- Wu, T.; Shen, M.; Yu, Q.; Chen, Y.; Chen, X.; Yang, J.; Huang, L.; Guo, X.; Xie, J. Cyclocarya paliurus polysaccharide improves metabolic function of gut microbiota by regulating short-chain fatty acids and gut microbiota composition. Food Res. Int. 2021, 141, 110119. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, Q.; Huang, H.; Hou, K.; Dong, R.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. The effect of bound polyphenols on the fermentation and antioxidant properties of carrot dietary fiber in vivo and in vitro. Food Funct. 2020, 11, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Bouranis, J.A.; Beaver, L.M.; Choi, J.; Wong, C.P.; Jiang, D.; Sharpton, T.J.; Stevens, J.F.; Ho, E. Composition of the Gut Microbiome Influences Production of Sulforaphane-Nitrile and Iberin-Nitrile from Glucosinolates in Broccoli Sprouts. Nutr. 2021, 13, 3013. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Huang, L.; Lei, P.; Liu, X.; Li, B.; Shan, Y. Sulforaphane Normalizes Intestinal Flora and Enhances Gut Barrier in Mice with BBN-Induced Bladder Cancer. Mol. Nutr. Food Res. 2018, 62, 1800427. [Google Scholar] [CrossRef] [PubMed]
- Ștefănescu, B.-E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. Chemical Composition and Biological Activities of the Nord-West Romanian Wild Bilberry (Vaccinium myrtillus L.) and Lingonberry (Vaccinium vitis-idaea L.) Leaves. Antioxidants 2020, 9, 495. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, L.; Li, C.; Wu, H.; Ran, D.; Zhang, Z. Sulforaphane alter the microbiota and mitigate colitis severity on mice ulcerative colitis induced by DSS. AMB Expr. 2020, 10, 119. [Google Scholar] [CrossRef]
- Mitrea, L.; Călinoiu, L.-F.; Precup, G.; Bindea, M.; Rusu, B.; Trif, M.; Ferenczi, L.-J.; Ştefănescu, B.-E.; Vodnar, D.-C. Inhibitory Potential of Lactobacillus plantarum on Escherichia coli. Bull. Uasvm Food Sci. Technol. 2017, 74, 1–4. [Google Scholar] [CrossRef]

| Class | Subclass | Examples of Compounds | Source | References |
|---|---|---|---|---|
| Coumarin | Simple coumarins Furanocoumarins Dihydrofuranocoumarins Pyranocoumarins Phenylcoumariuns Bicoumaurins | Esculetin Psoralen Anthogenol Grandivittin Pseudocordatolide Isodispar B Dicoumarol | Seeds Roots Leaves Tonka bean | [42,43] |
| Tannins | Complex tannins Condensed tannins Ellagitannins Gallotannins | Tannic acid Chinese gallotannin Hexahydroxydiphenic acid | Bark Wood Leaves Fruit rRoots Plant galls Seeds | [44] |
| Phenolic acids | Hydroxycinnamic acids | Curcumin Caffeic acid Ferulic acid | Fruits Cereals | [45] |
| Hydroxybenzoic acids | Gallic acid Protocatechuic acid Vanillic acid | Onion Raspberry Blackberry Strawberry | [45] | |
| Flavonoids | Flavonols | Kaempferol Quercitin Myricetin | Onions Tea Lettuce Broccoli Apples | [46] |
| Flavanones | Naringenin Hesperetin | Oranges Grapefruits | [47] | |
| Flavanols | Gallocatechin Catechins | Tea Red wine Chocolate | [48] | |
| Isoflavones | Genistein Glycitein Daidzein | Soybeans Legumes | [49] | |
| Anthocyanins | Pelargonidin Delphinidin Malvidin | Blackcurrant Strawberries Red wine Chokeberry | [50] | |
| Flavones | Apigenin Luteolin | Parsley Celery Red pepper Lemon Thyme | [51] | |
| Stilbenes | Resveratrol | Red wine | [52] | |
| Lignans | Pinoresinol | Flaxseed Sesame seed Red wine | [53] | |
| Lariciresinol Secoisolariciresinol Sesamin | ||||
| Polyphenol Source | Strains (spp) | Conditions | Method | Time (Fermentation/Incubation/Exposure) | Materials | Main Metabolites | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|
| Raspberry | N.S. 1 | In vitro gastrointestinal digestion with heat-stable α-amylase at 25 °C, 30 min protease, at 95 °C, 35 min and with α-amyloglucosidase, 60 °C, 35 min) | In vitro fermentation | 48 h fermentation | Fecal samples (healthy volunteers) | Propionic acid, butyric acid, acetic acid, isobutyric acid, isovaleric acid, valeric acid, isocaproic acid, caproic acid, and heptanoic acid | Polyphenols had a better prebiotic-like effect, in comparison with the fiber fractions ↑ 3 Bifodobacteria | [137] |
| Olive pomace | Firmicutes, Lactobacillus spp., Enterococcus spp., Clostridium leptum, Bacteroidetes, Bacteroides spp., Prevotella spp., Bifidobacterium spp. | In vitro simulations of gastrointestinal digestion A portion of the non-absorbable sample was lyophilized→ 2 exposed to fecal fermentation (fresh fecal inoculum) | In vitro simulated gastrointestinal digestion In vitro fecal fermentation | Samples were collected after 0, 12, 24, and 48 h of incubation | Feces (healthy volunteers) | Acetate, propionate, and butyrate | ↑ SCFAs, potential antioxidant, and antimicrobial activity. Beneficial modifications were observed in Firmicutes and Bacteroidetes groups (after intervention) | [153] |
| Red and white grapes | Lactobacillus, Bifidobacterium for pure cultures; B. longum, L. reuteri, B. vulgatus, Clostridium perfringens, Enterobacter cloacae for mixed cultures | In vitro GI digestion (Infogest protocol) In vitro colonic fermentation assays The DNA extraction-with 1 mL of the sample using Realpure Microspin Real kit | In vitro GI digestion | 48 h fermentation | Feces (healthy volunteers) | N.S. | ↑ Lactobacillus and Bifidobacterium White grape polyphenolic extracts → ↑ for total bacteria and Bifidobacterium spp. Red grape polyphenolic extracts which showed significant changes for all the analyzed bacterial groups, without Bacteroides spp. ↑ Firmicutes and Proteobacteria from 0 to 48 h, both substrates | [138] |
| Predigested mango peel | Bifidobacterium, Lactobacillus, Dorea, Lactococcus | In vitro model of the colon (TIM-2) using human fecal microbiota and sampled after 0, 24, 48, and 72 h A carbohydrate mixture of Standard Ileal Effluent Medium (SIEM)—control | Dynamic in vitro model of the human colon | 72 h experimental period | Fecal samples (healthy donors) | Acetic acid, propionic acid, butyric acid, valeric acid, formic acid, iso-valeric acid, ammonia | Mango peel fermentation → 80 bacterial genera identified ↑ Bifidobacterium with a maximum at 24 h fermentation; at 72 h mango peel favored ↑ Bifidobacterium and Lactobacillus | [141] |
| Green tea, oolong tea, and black tea | Bifidobacterium, Lactobacillus/Enterococcus, Bacteroides-Prevotella, Clostridium histolyticum | To obtain the fecal slurries it was necessary to mix fresh fecal + autoclaved phosphate buffered saline to yield 10% suspensions Green tea polyphenols, oolong tea polyphenols, black tea polyphenols, and fructooligosaccharides as the control group Fermentation—150 μL of fecal slurry to 1350 μL of culture medium | In vitro fermentation Intestinal absorption | 72 h | Fecal samples (healthy volunteers) | Formic acid, acetic acid, propionic acid, butyric acid | ↑ Bifidobacterium spp., oolong tea, and black tea had better effects than green tea Proliferation of Lactobacillus/Enterococcus spp. ↓ 4 Firmicutes/Bacteroidetes ratio and Clostridium histolyticum | [144] |
| Grape pomace (GP) | Bifidobacteria, Lactobacillus | Simulation of the effect of digestive tract was performed by dissolving 900 mg of the lyophilized GP extract into 20 mL of ultra-pure water In vitro fermentation was assessed using only 2 of the previous strains Samples of the fermentation broth were prelevated at 0, 4, 8, 24, and 48 h for metabolites’ analysis | In vitro stimulated gastrointestinal digestion | 48 h fermentation | Syrah grape pomace | Acetic acid, butyric acid, formic acid, propionic acid | Until GI digestion, grape pomace extract proved to have antimicrobial activity against pathogenic bacteria | [140] |
| Pomegranate juice, pomegranate pulp, pomegranate peel extract | N.S. | In vitro digestion procedure applied The method consisted of a continuous-flow dialysis system performed with a dialysis tube | In vitro GI digestion In vitro fermentation | 0, 2, 8, 24, 48, 72 h | Fresh fecal samples (three healthy adults) | Urolithin A, urolithin B, gallic acid, catechol, protocatechuic acid, coumaric acid | Pomegranate peel extract→ the best source of microbial substrates at the colonic level The use of pomegranate peel extracts obtained as a sub-product of the pomegranate juice industry → strategy to enrich or fortify (pomegranate products, fruit-based products) → enhancement of the pomegranate`s therapeutic effect (subjects with a low capacity to produce urolithin) | [148] |
| Pineapple | Bifidobacteria, Lactobacillus, E. coli, Adlercreutzia equolifaciens, Asaccharobacter celatus, Slackia equolifaciens, Eubacterium limosum, Enterobacter, Escherichia | In vitro digestion They were hydrolyzed with pepsin → gastric fraction Intestinal digestion (simulated by hydrolysis with pancreatin and α-amylase) The samples were centrifuged → the supernatants were brought to a volume of 50 mL → dialysis | In vitro gastrointestinal digestion Colonic fermentation | Samples were incubated and collected at 0, 6, 12, 24, and 48 h | Fecal samples (3 healthy adults) | Propionic acid, acetic acid, p-hydroxybenzoic acid, 3-hydroxybenzoic acid, 4-hydroxyphenyl acetic acid, p-hydroxybenzoic acid | The consumption of pineapple snack bars → the regulation of the antioxidant and anti-inflammatory effects - the presence of 4-hydroxyphenyl acetic acid ↓ anxiety and depression - p-hydroxybenzoic acid → potential therapeutic compound (could potentiate the anticancer role of adriamycin-breast cancer) | [151] |
| Red fruit extracts | L. rhamnosus, L. paracasei, L. splantarum, Bacillus cereus, S. aureus, E. coli, Listeria monocytogenes | Potential mechanisms involved in the inhibition of pathogenic bacterial growth analyzed with a well diffusion assay The kinetics growth was performed by using a modified de Man, Rogosa, Sharpe broth fermentation with red fruit extracts | In vitro fermentation | Growth conditions between 24–48 h | Collected from culture collection, human intestinal tract, isolated from food, probiotic strains combination | N.S. | ↓ B. cereus, S. aureus, E. coli Almost all probiotics ↑ in the presence of red fruits extracts, except L. paracasei ↑ antioxidant potential of the probiotic-fruit extract combination | [154] |
| Pomegranate extract (POMx), pomegranate juice (POM juice) | Bifidobacterium, Lactobacillus, Enterobacteriaceae, Bacteroides gragilis group, clostridia, bifidobacteria, and lactobacilli | Aliquots of 10 μL of the homogenized stool specimens were inoculated into seven different test broths The test tubes were inoculated at 37° C for 6 days | In vitro culture tubes | Between 24 h and 7 days | Stool specimens from 8 healthy volunteers | Urolithins A and B, punicalagin A and B, punicalin, glycosyl ellagic acid | ↑ Bifidobacterium and Lactobacillus (POMx) ↓ B. fragilis group, clostridia, and Enterobacteriaceae | [124] |
| Polyphenol Source | Strains (spp) | Conditions | Method | Time (Fermenation/Incubation/Exposure) | Materials | Main Metabolites | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|
| Blueberry | Proteobacteria, Deferribacteres, Actinobacteria, Bifidobacterium, Desulfovibrio, Adlercreutzia, Helicobacter, Flexispira, Prevotella | Four groups: group A, a normal-fat diet, group B, a high-fat diet, group C, a high-fat diet supplement with polyphenol extract, and group D a high-fat diet supplemented with Orlistat, as a positive control The fecal DNA extraction using a DNA isolation kit | Administrated as a supplement (200 mg/kg body weight/day) | 12 weeks | C57BL/6 J mice of 4 weeks | N.S. 1 | Supplementation with polyphenol extract ↓ 2 the body weight of the high fat diet-fed mice by 6–7% ↑ 3 Bifidobacterium, Desulfovibrio, Adlercreutzia, Helicobacter, and Flexispira | [154] |
| Lyophilized jabuticaba seed extract (LJE) | Firmicutes Bacteroidetes Proteobacteria | Animals were treated to develop cancer (by administrating dimethylhydrazine dihydrochloride (DMH)) The non-induced animals received similar s.c. injections of EDTA solution The treatments: 10 mL/kg body weight, orally, by gavage | In vivo, experimental design | 2 weeks | Wistar rats | Castalagin Vescalagin Procyanidin A Ellagic acid | ↑ Bacteroidetes, ↓ Firmicutes (when DMH treated mice received the yogurt or the yogurt with LJE) | [162] |
| Grape extract | Lachnoclostridium Blautia Bacteroides Lactobacillus Vibrio | Divided in five groups and the samples were administered intragastrically three times/week The feces were collected at the moment 0 (before the treatment) and 28 days The microbiota comparison was determined using the analysis of the DNA of the fecal samples of the animal | Intragastrically administration | 4 weeks | 5 female BALB/c mice (5 weeks old) | N.S. 1 | The microbiota was not affected by the sample composition or time of treatment No significant differences in bacterial composition and relative abundance | [157] |
| Tart cherries | Verrucomicrobia, Synergistes, Akkermansia, Cloacibacillus, Bifidobacterium, Bilophila, Firmicutes, Proteobacteria, Collinsella, Assacharobacter, Bacteroides, Parabacteroides | Participants consumed 237 mL of juice daily for 5 days Collection of stool sample before and a stool sample after the dietary intervention | In vivo human dietary intervention In vitro, fermentation | 5 days | 10 healthy participants (5 = male, 5 = female) | 4-hydroxyphenylpropionic acids, 4-hydroxyphenylacetic acid, quercetin-3-O-glucoride, quercetin-3-O-rutinoside, kaempferol-3-O-rutinoside, 3,4 and 4-hydroxybenzoic acid | Gut microbiota strongly influences polyphenol metabolites Polyphenols in tart cherries and concentrates were to a certain extent metabolized by Bifidobacterium | [156] |
| Herbal tea: ginseng (GS), red ginseng (RGS), notoginseng (NGS), Gynostemma pentaphyllum (jiaogulan- GpS) | Bacteroides, Lactobacillus, Bifidobacterium, Firmicutes, F. prasnitzii, Bacteroides | Eight-week old male mice, 5 experimental groups, daily single dose of herbal saponins at 500 mg/kg or Milli-Q H2O by gavage for 15 consecutive days Feces collected, 8:00 to 10:00 a.m. on day 0, day 5, day 10, and day 15 | In vivo-daily intake of herbal saponins | 15 days | 50 C5777BL/6 8 weeks old male mice | Butyrate | Ingested herbal saponins can increase the beneficial bacteria in the gut of the host ↓ Firmicutes on the GpS treatment group, ↑ Bacteroidetes the GpS and NGS group GpS, NGS, and GS ↑ Lactobacillus, whereas NGS and RGS ↑ Bifidobacterium ↑ F. prausnitzii in the GpS group | [149] |
| Red raspberry (polyphenolic extracts from whole fruit, seed, and pulp) | Ruminococcus, Mogibacteriaceae, Bifidobacterium, Coriobacteriaceae, Verrucomicrobia, Bacteroidetes, Actinobacteria, Proteobacteria, Akkermansia, Clostridiales, Dehdobacterium, Lachnospiraceae, Roseburia, Adlercreutzia | Five groups: a low-fat diet, high-fat diet, high-fat diet supplemented with 0.4% by weight-red raspberry (RR) whole fruit polyphenols, 0.1% by weight RR seed polyphenols, 0.3% by weight RR seed polyphenols Mice were fed for 16 weeks ad libitum | Administration of different types of diets | 16 weeks | C57BL/6 male mice | Butyrate, pentahydroxy-urolithin, tetrahydroxy-urolithin | High-fat diets with RR polyphenols have a prebiotic effect on the gut microbiota | [155] |
| Red wine polyphenols | Bifidobacterium, Enterococcus, Eggerthella lenta | Fecal, and 24 h urine samples (at baseline and after each intervention period) metabolites in urine were analyzed by UPLC-MS/MS Extraction of DNA was from 200 mg stools by using a QIAmp DNA Stool Mini Kit | Consumption of red wine, dealcoholized red wine, and gin | Three consecutive periods of 20 days each with an initial washout period | 9 adult men | Syringic acid, p-coumaric acid, 4-hydroxybenzoic acid, homovanillic acid, hydroxycinnamates, 3,4-dihydroxyphenylacetic acid | Bacterial changes after red wine consumption (±alcohol) have been associated with the excretion of phenolic metabolites Phenolic compounds are important in the maintenance of intestinal health | [158] |
| Red wine polyphenols | Bifidobacterium, Lactobacillus, F. prausnitzii, Roseburia, Escherichia coli, Enterobacter cloacae | Four periods: the participants were given a two-week washout period during which they did not consume any red wine, followed by two 30-day intervention periods during which they drank just red wine (272 mL/day) or dealcoholized red wine (272 mL/day), separated by a 5-day washout phase Three different fecal samples were provided by each participant, at baseline, after the washout period, and at the end DNA extraction from 200 mg of stools (performed with QIAamp DNA stool Mini Kit) | In vivo study (intake of red wine (RW) and dealcoholized red wine (DRW) polyphenols) | Two weeks washout period, 2 periods of 30 days each, and between a period of 15 days | Twenty adults (10 met the criteria for metabolic syndrome (MetS), and 10 healthy) | N.S. | ↑ Blautia coccoides, F. prausnitzii, Roseburia, and Lactobacillus, ↓ Clostridium histolyticum After RW and DRW intake ↓ Bacteroides, ↑ Prevotella, Bifidobacterium, and Eggerthella lenta ↓ Echerichia coli and Enterobacter cloacae in MetS group | [159] |
| Cyclorarya paliurus flavonoids | Prevotellaceae, Bacteroidaceae, Ruminococcaceae, Lachnospiraceae, Veillonellaceae, Enterobacteriaceae | To obtain a human intestinal microbial suspension, the supernatants prepared from each volunteer’s fecal sample were combined Three groups: control group (CONT group), the constant darkness group (CD group), and the constant darkness with flavonoid supplementation group (CPF group) Fecal samples were collected at baseline and 4 weeks after they were divided | In vivo, administration by gavage | Four weeks | Germ-free 6-week-old C57BL/6J male mice; 6 healthy volunteers | - | The diversity of the total bacterial community ↑ during CPF treatment ↓ Firmicutes/Bacteroidetes ratio in CPF treatment After 4 weeks CPF group: ↑Prevotellaceae, and Bacteroidaceae, and ↓ Ruminococcaceae, Lachnospiraceae, and Veillonellaceae In the CPF group ↑ Prevotella, and Bacteroides, and ↓ Faecalibacterium, Mitsuokella, Ruminococcus, Desulfovibrio, Megamonas | [166] |
| Carrot | Firmicutes, Bacteroidetes, Proteobacteria | Three groups: control group (CON), carrot dietary fiber (CDF), dephenolized carrot dietary fiber (CDF-DF) The CDF and CDF-DF groups, with daily intake of approximately 0.6 g in 200 μL of CDF and CDF-DF, by oral administration for 7 consecutive days Fecal slurries: homogenizing the fecal samples with pH 7.0, 0.1 M sodium phosphate buffer followed by filtration | In vivo, oral administration | Seven days | Male BALB/c mice; 3 healthy donors | Acetic acid, butyric acid, propionic acid, valeric acid | CDF-fed mice: ↑Bacteroides, and ↓ Proteobacteria The CDF group: ↓ Clostridiales, Coprococcus, Oscillospira, and Dehalobacterium, ↑ Lactobacillus compared to those in the CDF-DF, and CON groups | [168] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2022, 14, 137. https://doi.org/10.3390/nu14010137
Plamada D, Vodnar DC. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients. 2022; 14(1):137. https://doi.org/10.3390/nu14010137
Chicago/Turabian StylePlamada, Diana, and Dan Cristian Vodnar. 2022. "Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics" Nutrients 14, no. 1: 137. https://doi.org/10.3390/nu14010137
APA StylePlamada, D., & Vodnar, D. C. (2022). Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients, 14(1), 137. https://doi.org/10.3390/nu14010137







