Anti-Periodontitis Effect of Ethanol Extracts of Alpinia Katsumadai Seeds
Abstract
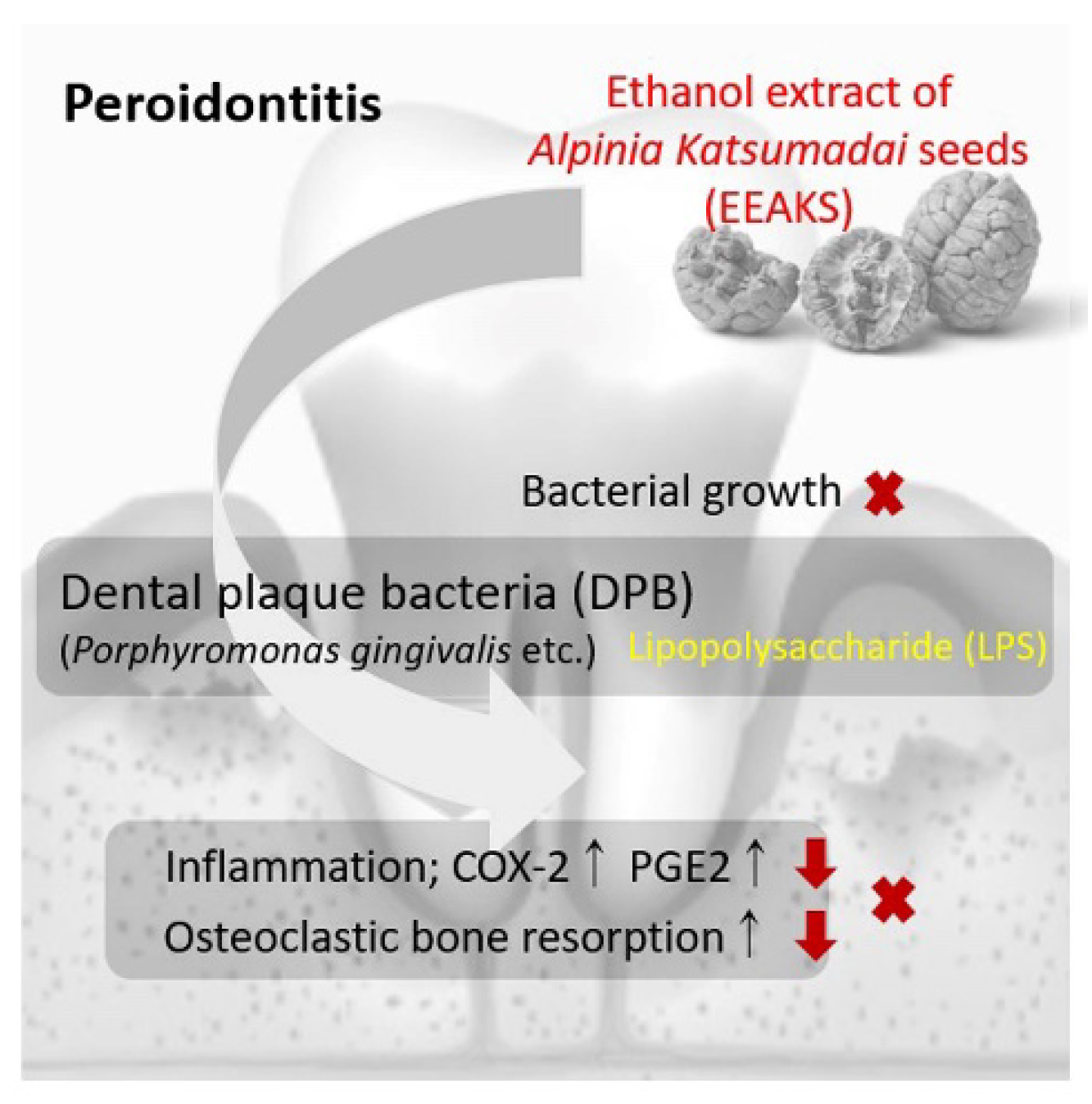
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Media
2.2. Bacterial Culture
2.3. Ethanol Extracts of Alpinia Katsumadai Seeds
2.4. Lipopolysaccharide Extractions from Dental Plaque Bacteria
2.5. Enzyme-Linked Immunosorbent Assay
2.6. Cytotoxicity Assay
2.7. Protease Antibody Array
2.8. Osteoclast Formation
2.9. Pit Formation
2.10. Statistical Analysis
3. Results
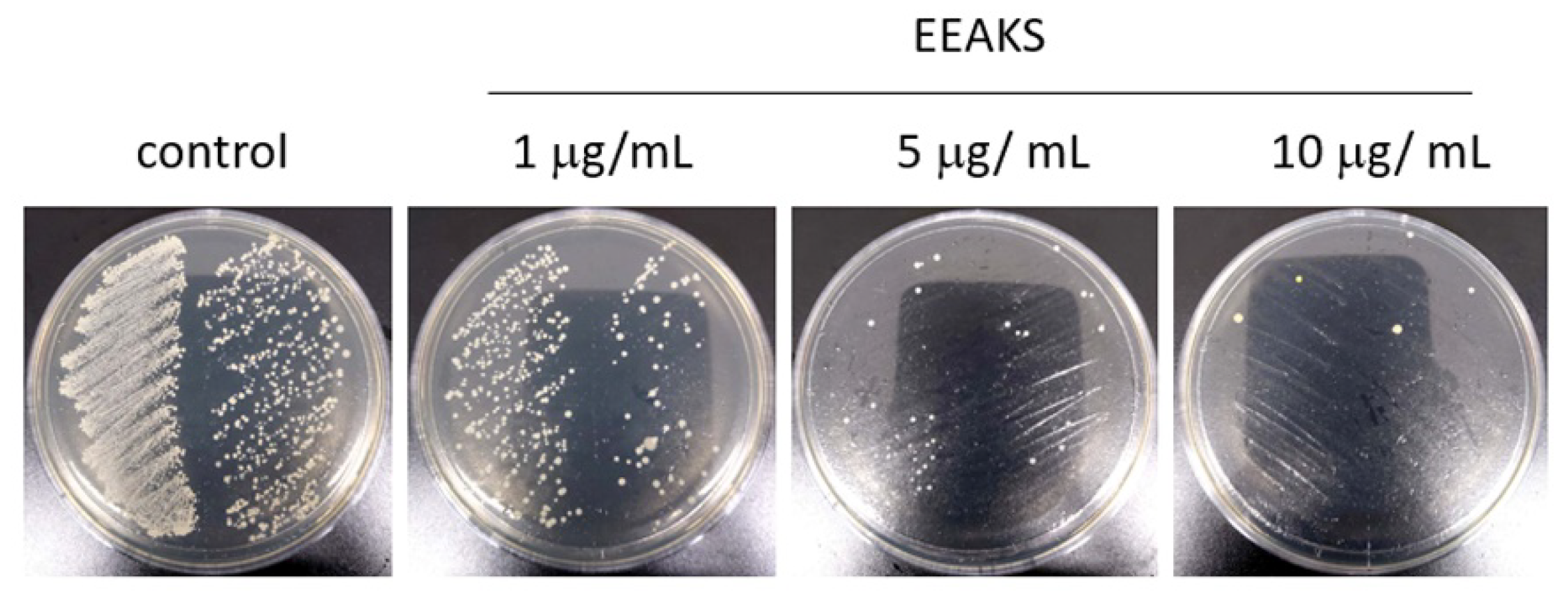
3.1. Effects of EEAKSs on P. gingivalis Growth
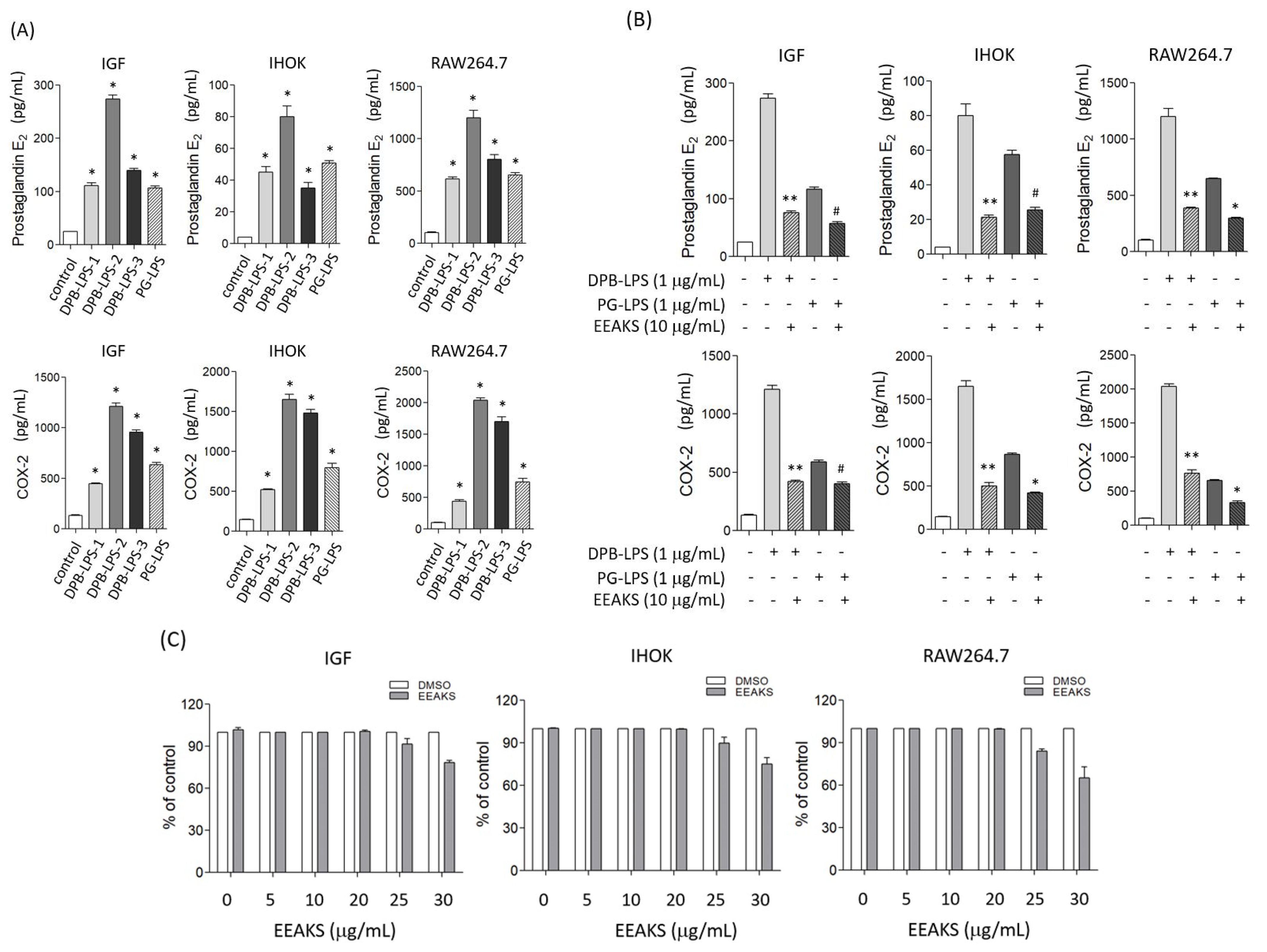
3.2. Effect of EEAKSs on Bacterial LPS-Induced PGE2 and COX-2 Levels
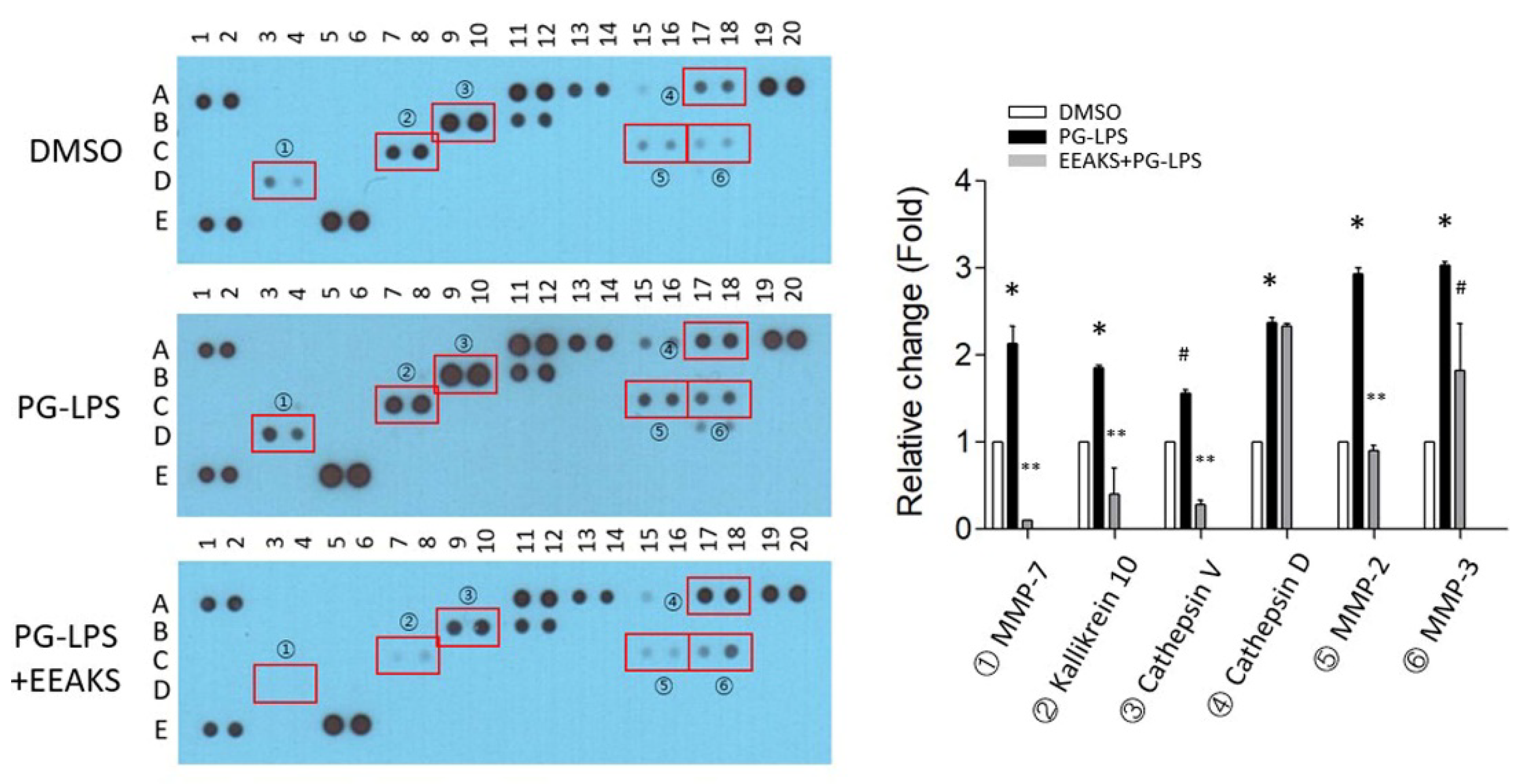
3.3. Identify the Proteases Involved in PG-LPS-Induced Inflammation
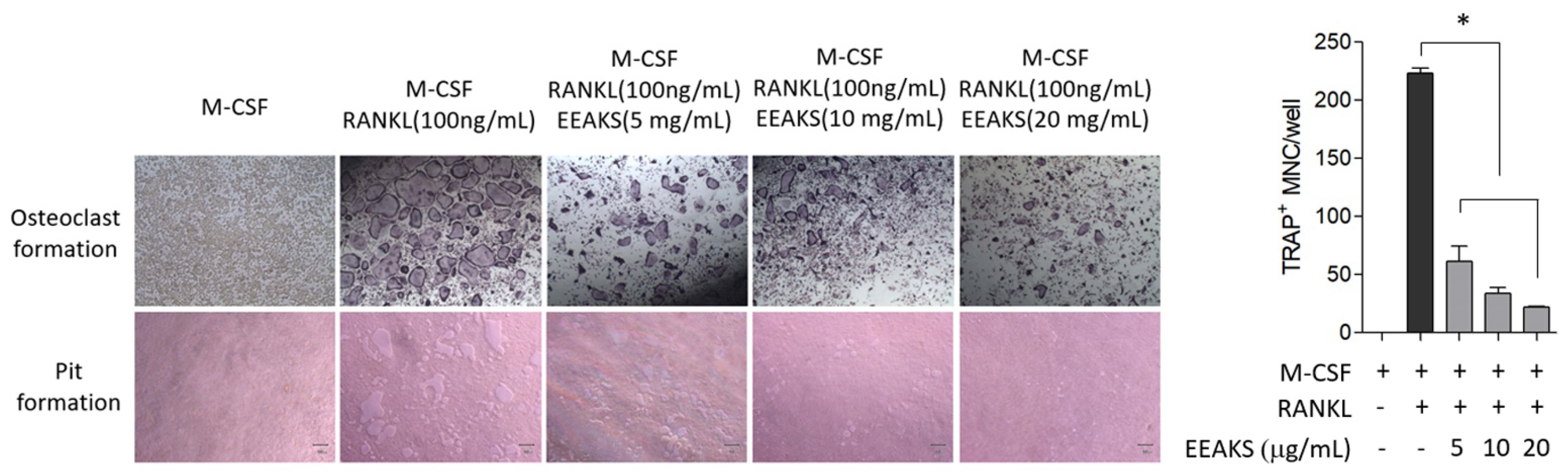
3.4. Effects of EEAKSs on RANKL-Induced Osteoclast Formation and Bone Resorption
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Yoneda, M.; Hirofuji, T. Mixed Red-Complex Bacterial Infection in Periodontitis. Int. J. Dent. 2013, 2013, 587279. [Google Scholar] [CrossRef]
- Sampath, V. Bacterial endotoxin-lipopolysaccharide; structure, function and its role in immunity in vertebrates and invertebrates. Agric. Nat. Resour. 2018, 52, 115–120. [Google Scholar] [CrossRef]
- Barry, M.; Stephen, W.C. Proteolytic and hydrolytic enzymes from putative periodontal pathogens: Characterization, molecular genetics, effects on host defenses and tissues and detection in gingival crevice fluid. Periodontology 2003, 31, 105–124. [Google Scholar]
- Kim, H.H.; Kwon, H.J.; Ryu, Y.B.; Chang, J.S.; Cho, K.O.; Hosmillo, M.D.; Rho, M.C.; Park, S.J.; Lee, W.S. Antiviral activity of Alpinia katsumadai extracts against rotaviruses. Res. Vet. Sci. 2012, 92, 320–323. [Google Scholar] [CrossRef]
- Lee, M.Y.; Lee, N.H.; Seo, C.S.; Lee, J.A.; Jung, D.; Kim, J.H.; Shin, H.K. Alpinia katsumadai seed extract attenuate oxidative stress and asthmatic activity in a mouse model of allergic asthma. Food Chem. Toxicol. 2010, 48, 1746–1752. [Google Scholar] [CrossRef]
- Nam, J.-W.; Seo, E.-K. Structural Characterization and Biological Effects of Constituents of the Seeds of Alpinia katsumadai (Alpina Katsumadai Seed). Nat. Prod. Commun. 2012, 7, 795–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, Y.S.; Chung, W.Y.; Kim, J.; Park, H.J.; Kim, E.C.; Park, K.K. Buddlejasaponin IV induces cell cycle arrest at G2/M phase and apoptosis in immortalized human oral keratinocytes. Phytother. Res. 2011, 25, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Mysak, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duskova, J. Porphyromonas gingivalis: Major Periodontopathic Pathogen Overview. J. Immunol. Res. 2014, 2014, 476068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 2013, 35, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.R.; Kumar, P.M.; Reddy, V.S.; Kumar, A.K.; Babu, M.; Chavan, C.V. Estimation of prostaglandin E2levels in gingival crevicular fluid in periodontal health, disease and after treatment. Contemp. Clin. Dent. 2013, 4, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of Bone Resorption in Periodontitis. J. Immunol. Res. 2015, 2015, 615486. [Google Scholar] [CrossRef] [Green Version]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Ioannidou, E.; Swede, H.; Fares, G.; Himmelfarb, J. Tooth loss strongly associates with malnutrition in chronic kidney disease. J. Periodontol. 2014, 85, 899–907. [Google Scholar] [CrossRef] [Green Version]
- Ara, T.; Nakatani, S.; Kobata, K.; Sogawa, N.; Sogawa, C. The Biological Efficacy of Natural Products against Acute and Chronic Inflammatory Diseases in the Oral Region. Medicines 2018, 5, 122. [Google Scholar] [CrossRef] [Green Version]
- Bedran, T.B.L.; Morin, M.P.; Spolidorio, D.P.; Grenier, D. Black tea extract and its theaflavin derivatives inhibit the growth of periodontopathogens and modulate interleukin-8 and beta-defensin secretion in oral epithelial cells. PLoS ONE 2015, 10, e0143158. [Google Scholar]
- Hosokawa, Y.; Hosokawa, I.; Ozaki, K.; Nakanishi, T.; Nakae, H.; Matsuo, T. Tea polyphenols inhibit IL-6 production in tumor necrosis factor superfamily 14-stimulated human gingival fibroblasts. Mol. Nutr. Food Res. 2010, 54, S151–S158. [Google Scholar] [CrossRef]
- Guimaraes, M.; Coimbra, L.S.; de Aquino, S.; Spolidorio, L.C.; Kirkwood, K.; Rossa, C. Potent anti-inflammatory effects of systemically administered curcumin modulate periodontal disease in vivo. J. Periodontal Res. 2011, 46, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, M.; de Aquino, S.; Coimbra, L.S.; Spolidorio, L.C.; Kirkwood, K.; Rossa, J.C. Curcumin modulates the immune response associated with LPS-induced periodontal disease in rats. Innate Immun. 2011, 18, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.H.; Lee, H.M.; Raja, V.; Hou, W.; Iacono, V.J.; Scaduto, J.; Johnson, F.; Golub, L.M.; Gu, Y. Enhanced efficacy of chemically modified curcumin in experimental periodontitis: Systemic implications. J. Exp. Pharmacol. 2019, 11, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saglam, M.; Köseoğlu, S.; Hatipoglu, M.; Esen, H.H.; Köksal, E. Effect of sumac extract on serum oxidative status, RANKL/OPG system and alveolar bone loss in experimental periodontitis in rats. J. Appl. Oral Sci. 2015, 23, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, K.; Hirata, M.; Grundler, F.M.W.; Inada, M.; Miyaura, C. Effects of O-methylated (-)-epigallocatechin gallate (EGCG) on LPS-induced osteoclastogenesis, bone resorption, and alveolar bone loss in mice. FEBS Open Bio 2017, 7, 1972–1981. [Google Scholar]
- Wu, Y.-H.; Kuraji, R.; Taya, Y.; Ito, H.; Numabe, Y. Effects of theaflavins on tissue inflammation and bone resorption on experimental periodontitis in rats. J. Periodontal Res. 2018, 53, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Thomas, B.; Shetty, V.; Bhandary, R.; Shetty, R.M. An in-vitro evaluation of the efficacy of garlic extract as an antimicrobial agent on periodontal pathogens: A microbiological study. Arch. Oral. Biol. 2013, 34, 445–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakri, I.; Douglas, C. Inhibitory effect of garlic extract on oral bacteria. Arch. Oral Biol. 2005, 50, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Bachrach, G.; Jamil, A.; Naor, R.; Tal, G.; Ludmer, Z.; Steinberg, D. Garlic Allicin as a Potential Agent for Controlling Oral Pathogens. J. Med. Food 2011, 14, 1338–1343. [Google Scholar] [CrossRef]
- Velliyagounder, K.; Ganeshnarayan, K.; Velusamy, S.K.; Fine, D.H. In VitroEfficacy of Diallyl Sulfides against the Periodontopathogen Aggregatibacter actinomycetemcomitans. Antimicrob. Agents Chemother. 2012, 56, 2397–2407. [Google Scholar] [CrossRef] [Green Version]
- Giannobile, W.V. Host-Response Therapeutics for Periodontal Diseases. J. Periodontol. 2008, 79, 1592–1600. [Google Scholar] [CrossRef]
- Ohshiba, T.; Miyaura, C.; Inada, M.; Ito, A. Role of RANKL-induced osteoclast formation and MMP-dependent matrix degradation in bone destruction by breast cancer metastasis. Br. J. Cancer 2003, 88, 1318–1326. [Google Scholar] [CrossRef] [Green Version]
- Lerner, U.H. Stimulation of bone resorption by the kallikrein-kinin system and the coagulation cascade. Acta Orthop. Scand. 1995, 66, 45–50. [Google Scholar] [CrossRef]
- Fasanya, H.O.; Siemann, D.W. The Role of Cathepsins in the Growth of Primary and Secondary Neoplasia in the Bone. Osteology 2020, 1, 3–28. [Google Scholar] [CrossRef]
- Kusano, K.; Miyaura, C.; Inada, M.; Tamura, T.; Ito, A.; Nagase, H.; Kamoi, K.; Suda, T. Regulation of Matrix Metalloproteinases (MMP-2, -3, -9, and -13) by Interleukin-1 and Interleukin-6 in Mouse Calvaria: Association of MMP Induction with Bone Resorption. Endocrinology 1998, 139, 1338–1345. [Google Scholar] [CrossRef]
- Lynch, C.C.; Hikosaka, A.; Acuff, H.B.; Martin, M.D.; Kawai, N.; Singh, R.K.; Vargo-Gogola, T.C.; Begtrup, J.L.; Peterson, T.; Fingleton, B.; et al. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell 2005, 7, 485–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, L.Y.; Katsaros, D.; Scorilas, A.; Fracchioli, S.; Piccinno, R.; Longrais, I.A.R.D.L.; Howarth, D.J.; Diamandis, E. Prognostic value of human kallikrein 10 expression in epithelial ovarian carcinoma. Clin. Cancer Res. 2001, 7, 2372–2379. [Google Scholar]
- Yasuda, Y.; Li, Z.; Greenbaum, D.; Bogyo, M.; Weber, E.; Brömme, D.; Humphries, J.E.; Kimber, M.J.; Barton, Y.-W.; Hsu, W.; et al. Cathepsin V, a Novel and Potent Elastolytic Activity Expressed in Activated Macrophages. J. Biol. Chem. 2004, 279, 36761–36770. [Google Scholar] [CrossRef] [Green Version]
- Itoh, K.; Udagawa, N.; Katagiri, T.; Iemura, S.; Ueno, N.; Yasuda, H.; Higashio, K.; Quinn, J.M.; Gillespie, M.T.; Martin, T.J.; et al. Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factor-kappaB ligand. Endocrinology 2001, 142, 3656–3662. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.W.; Hwang, Y.S. Anti-Periodontitis Effect of Ethanol Extracts of Alpinia Katsumadai Seeds. Nutrients 2022, 14, 136. https://doi.org/10.3390/nu14010136
Shin SW, Hwang YS. Anti-Periodontitis Effect of Ethanol Extracts of Alpinia Katsumadai Seeds. Nutrients. 2022; 14(1):136. https://doi.org/10.3390/nu14010136
Chicago/Turabian StyleShin, Seo Woo, and Young Sun Hwang. 2022. "Anti-Periodontitis Effect of Ethanol Extracts of Alpinia Katsumadai Seeds" Nutrients 14, no. 1: 136. https://doi.org/10.3390/nu14010136
APA StyleShin, S. W., & Hwang, Y. S. (2022). Anti-Periodontitis Effect of Ethanol Extracts of Alpinia Katsumadai Seeds. Nutrients, 14(1), 136. https://doi.org/10.3390/nu14010136




