Brown Seaweed Food Supplementation: Effects on Allergy and Inflammation and Its Consequences
Abstract
1. Introduction
2. Approach to Systematic Search
2.1. Databases and Search Strategy
2.2. Eligibility Criteria
3. Whole Seaweed or Crude Extract Supplementation
3.1. Whole Seaweed or Crude Extract: Effects in Steady State
3.2. Whole Seaweed or Crude Extract: Effects on Allergy
3.3. Whole Seaweed or Crude Extract: Effects in Acute and Chronic Inflammation
3.4. Whole Seaweed or Crude Extract: Late Consequences of Inflammation and Sequels
4. Brown Seaweed Polysaccharide Supplementation
4.1. Brown Seaweed Polysaccharide Effects in Steady State
4.2. Effects of Polysaccharides from Brown Seaweed in Allergy, Acute Inflammation and in the Modulation of Immune Responses
4.2.1. Anti-Allergic Effects of Brown Seaweed-Derived Polysaccharides
4.2.2. Effects of Brown Seaweed Polysaccharides on Innate and Adaptive Immune System (Production Animals)
4.2.3. Effects of Brown Seaweed Polysaccharides on Innate and Adaptive Immune System (Mouse Models)
4.2.4. Anti-Inflammatory Effects of Fucoidan in Animal Models and Clinical Trials
4.3. Fucoidan Ingestion and Atherosclerosis in Animal Models
5. Phenolic Compounds and Phytosterols
5.1. Phytosterols
5.2. Polyphenols
5.2.1. Polyphenols: Effects in Steady State
5.2.2. Polyphenols: Effects on Allergy
5.2.3. Polyphenols: Effects in Acute and Chronic Inflammation
5.2.4. Polyphenols: Late Consequences of Inflammation and Sequels
6. Fucoxanthin(ol) and Meroterpenoids
6.1. Fucoxanthin(ol) and Meroterpenoids: Effects in Acute and Chronic Inflammation
6.2. Fucoxanthin(ol) and Meroterpenoids: Late Consequences of Inflammation and Sequels
7. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Compound/Format | Effective Concentrations | Algal Source | Experimental Model | Inflammatory Phase 1 | Significant Findings 2 | Reference |
|---|---|---|---|---|---|---|
| EtOH extract | 300–2000–5000 mg/kg; single dose | Sargassum micracanthum | BALB/c mouse; toxicity; follow-up 2 weeks | steady state | non-toxic up to 5000 mg/kg | [3] |
| EtOH-H2O extracts | single dose LD50 = 500–2000 mg/kg; oxidative stress reduction: 200 mg/kg/day, 4 weeks | Fucus vesiculosus | Swiss mouse; Sprague Dawley rat; toxicity: acute anti-oxidant activity: 4 weeks | steady state | LD50 acute toxic > 750 mg/kg 4 week supplementation: ↓ 13% food intake in 4 week 30–50% ↓ WBC but no change in differential counts 20–25% ↑ in liver and kidney weight ↑ PON-1 activity (protects against ox-LDL) ↑ SOD | [5] |
| MeOH-, hexane- or chloroform-extract | >2000 mg/kg, single dose | Cystoseira compressa (Esper) | male and female albino mouse; toxicity | steady state | no lethality in oral testing (2000 mg/kg) | [4] |
| EtOH extract, polyphenol-rich ultrafiltrate | 100 mg/day, 8 weeks | Ascophyllum nodosum | human, BMI ≥ 25 | steady state | marginal ↓ lymphocyte DNA damage in only obese =CRP =cholesterol, HDL, LDL, TG anti-oxidant =in vitro LPS-/TPA-induced pro-inflammatory cytokines in monocytes, lymphocytes =total plasma peroxide | [7] |
| whole, dried | 4.8 g/dayay, 4 weeks | Sargassum muticum | human | steady state | ↓ oxLDL (14%); correlated to ↑ total antioxidant status ↑ NK count; =lymphocyte count ↓ fatigue ↑ liver function (↓ AST, ALT) | [6] |
| commercial extract | 2% in feed, 3 weeks | Ascophyllum nodosum | Spanish and Boer × Spanish goat; stress by transport and feed withholding | steady state | =cortisol =WBC and subset counts, but ↓ Eosinophil =phagocytosis ↓ lipid peroxidation; ↓ SOD (strain diff.) ↑ glutathione peroxidase (10–15%) | [8] |
| commercial extract | 0.5% in feed; 41 weeks | Ascophyllum nodosum | Lohmann LSL-Lite and Lohmann Brown-Lite hens, heat stress | steady state | short-term↓ feed intake; ↑ feed/egg efficiency strain-dependent ↑ production, ↑ feed efficiency, ↑ heat stress resistance, improved ALP, ALT, GGT liver parameters | [161] |
| seaweed powder + anti-bacterial peptides (cecropin) | 1–5% of basal diet | Laminaria japonica | Arbor Acres broiler chicks | steady state | synergistic effect of seaweed + cecropin: ↑ antibodies, ↑ lymphocytes, microbiota: ↑ Lactobacillus, ↓ E.coli ↑ feed conversion ratio | [9] |
| hot water extract | 100–500 mg/kg in feed, 12 weeks | Sargassum oligocystum | fish, Pangasius (Pangasianodon hypophthalmus) | steady state | ↑ weight, daily growth rate, feed conversion ratio ↑ WBC, RBC, Hb, Hc, platelets | [10] |
| seaweed meal | 3–6–9% in feed, 6 weeks | Sargassum ilicifolium | fish, Asian sea bass (Lates calcarifer) | steady state | ↑ growth; ↑ pancreatic enzyme activities ↑ serum Ig; (alternative) complement pathway components, lysozyme ↑ Ig in skin mucus ↑ liver SOD, IL-1β mRNA | [11] |
| whole seaweed | 10% in feed, 8 weeks | Sargassum ilicifolium | fish, great sturgeon (Huso huso) | steady state | ↑ growth ↑ serum protein, lysozyme, IgM, respiratory burst, complement ↑ Hb, RBC, WBC =TG, LDH, AST, ALT ↓ blood cholesterol ↑ survival upon Yersinia ruckeri infection (14 days infection) | [12] |
| aqueous extract | 400 mg/kg, 8 weeks | Sargassum angustifolium | fish, rainbow trout (Oncohrynchus mykiss) | steady state | ↑ weight gain ↑ Hb, Hc, RBC, WBC, total protein, albumin↑ survival and immune response to Yersinia ruckeri infection | [13] |
| EtOH extract | 200 mg/kg, 31 days | Sargassum horneri | male C57BL/6 mouse; in vivo anti-DNP-IgE i.v. + DNP i.n. challenge | allergy | ↓ nasal rubbing ↓ mast cell degranulation Proposed mechanism: Chlorophyll-C2 →↓ PI-3K + ↓ Btk, ↓ Syk active upon FcεR trigger →↓ Ca-mobilization | [15] |
| EtOH extract | 10, 50, 100 mg/kg, 1×/day, 3 weeks | Sargassum horneri | female NC/Nga mouse; house dust mite/dayNCB skin sensibilization DNCB skin challenge (2×/week) | allergy | ↓ atopic dermatitis symptoms ↓ epidermal hyperplasia, hyperkeratosis, skin dryness ↓ mast cell + eosinophil skin infiltrates ↓ IL-25, IL-33 → ↓ Rantes (CCL5), Eotaxin (CCL11), TARC (CCL17) in skin ↓ IL-4, -5, -13, ↓ IL-6, -10, IFN-γ in serum ↓ spleen size increase ↓ IgG1, IgG2a increase | [16] |
| EtOH extract | 100–300 mg/kg/day in diet, 5 weeks | Costaria costata | male NC/Nga mouse; DNCB-induced atopic dermatitis skin allergy | allergy | ↓ inflammatory cell and mast cell infiltration, epidermal thickness, erythema, hemorrhage, dead skin cell layers, skin dehydration ↓ Eotaxin and TARC (CCL17)↓ serum IgE and histamine normalization of spleen lymphocyte proliferation and cytokine production | [17] |
| water extract | 100–300 mg/kg/day, 4 weeks | Laminaria japonica | female NC/Nga mouse; DNCB-sensitized and challenged dorsal skin | allergy | ↓ dermatitis severity; ↓ inflammatory mediators ↑ skin moisture in vitro: ↓ p38 MAPK,↑ ERK, ↓ STAT1 in HaCaT human keratinocyte cell line | [18] |
| dried powder | 1–5–10%, 6 weeks | Eisenia (=Ecklonia) arborea | female Brown Norway rat; oral OVA immunization | allergy | ↓ OVA-specific IgE, ↓ total IgE (n.s. with 10% diet) ↓ serum histamine ↑ IFN-γ in spleen and MLN ↓ IL-10 in spleen and MLN | [14] |
| EtOH extract | 0.1–0.3 g/kg/day, 3 days prior to experiment | Laminaria japonica | male SD rat; carrageenan-induced paw inflammation | 1,2 | ↓ paw swelling, leukocyte infiltration in vitro: ↓ IκB phosphorylation → ↓ iNOS, COX2, TNF-α, IL-1β, IL-6 | [19] |
| diethylether fraction | 0.1–1 mg/mouse, 2× | Sargassum fusiforme | ICR mouse; ear-swelling after irritant | 1,2 | ↓ ear swelling by arachidonic acid, TPA, oxazolone in vitro: ↓ mast cell degranulation by inhibiting PLA2, COX2, LOX, HA | [20] |
| ethyl acetate extract | 200 mg/kg, 5 weeks | Eisenia bicyclis = Ecklonia bicyclis | male SD rat; isolated peritoneal Mϕ | 1,2 | ↓ LPS-induced iNOS expression and NO production ↓ NF-κB nuclear translocation with and without LPS =(LPS-induced) tumoricidal activity against B16 melanoma | [21] |
| EtOH extract +/− enzyme-treatment | 50–200 mg/kg, 3 weeks | Hizikia. = Sargassum fusiforme | male C57BL mouse; LPS-stimulation of isolated peritoneal Mϕ conA-induced splenic lymphocyte proliferation | 1,2 | ↑ LPS-stimulated IL-1β, TNF-α, IL-6 by peritoneal Mϕ ↑ conA-induced splenocyte proliferation | [22] |
| EtOH extract | 6–24 mg/kg/day, 4 days | Sargassum serratifolium | male ICR mouse; LPS i.p. (2 mg/kg), blood sampling after 2 h | 1 | ↓ TNF, IL-1β, IL-6 confirming in vitro findings | [23] |
| enzymatic extract | 50–200 μg/mL, 1 h prior to and during exp | Sargassum polycystum Chnoospora minima | zebrafish embryos; in vivo 24 h challenge w/H2O2 (10 μg/mL) or LPS (10 μg/mL), monitored 5d | 1,2 | ↓ H2O2-induced ROS levels and cell death ↓ LPS-induced ROS, NO and cell death | [24] |
| whole seaweed | 0–4% feed, 40 days | Sargassum latifolium | Barki sheep (Ovis aries); i.v. LPS challenge (1.25 μg/kg), after day.28 + day.35 | 2 | ↓ body temperature, respiration rate ↓ leukocytosis, ESR, HSP70 ↑ blood anti-oxidant capacity (CAT, SOD) ↓ damage-related molecules: malondialdehyde (MDA; lipid peroxidation product); ALAT, LDH | [25] |
| whole seaweed | 0–4% feed, 40 days | Sargassum latifolium | Barki sheep (Ovis aries); heat stress (solar experiment 8–17) vs. mild temperature without solar exposure | 2,3 | ↓ Δ leukocytosis, Δ ESR ↓ proinflammatory cytokines, HSP70 ↑ body weight gain, kidney function, blood anti-oxidant function | [26] |
| commercial extract | 1% feed, 27 days prior to and during exp (10 days) | Ascophyllum nodosum | crossbred wether lamb (Ovis aries); heat stress | 1,2 | ↓ heat stress-induced reduction of phagocyte oxidative burst ↑ SOD ↓ heat stress-induced changes in GSH-peroxidase activity ↓ lipid peroxidation ↑ leukocyte phagocytosis | [27] |
| EtOH and organic-purified fraction | 15 mg/kg/day, 6 weeks | Turbinaria ornata | female C57BL/6J mouse; DSS-induced colitis | 1,2 | ↓ disease activity index ↓ histopathology incl. length reduction, neutrophil infiltrate ↓ TNF ↑ FoxP3, Treg, but = Th17 ↑ IL-10 | [28] |
| aqueous extract (AE) + probiotic mix | 100–300 mg/kg, 2×/day for 7 days | Laminaria japonica | male BALB/c mouse; DSS-induced colitis | 1,2 | AE alone: ↓ colitis, incl. ↓ colonic IL-1. IL-6 AE + probiotics: synergistic ↓ colitis, ↓ IL-1, -6, -12p40; not IFN-γ, IL-10, IL-12p70 | [29] |
| EtOH-extract | 50–200 mg/kg/day, d.28–98 | Sargassum muticum | male DBA/1J mouse; collagen-induced arthritis | 1,2,3 | ↓ arthritis and edema ↓ IL-6, TNF, IFN-γ in serum ↓ joint degradation, inflammatory cytokines in joints possibly explained by apo-9′ fucoxanthinone (sim. effects) | [30] |
| GFS = hot water extract with galactofucan sulfate | therapy: 4 × 560 mg/day, 10 days, 1–24 mo. maintenance: 2 × 560 mg/day | Undaria pinnatifida | human; response in patients with active or latent Herpes infection | 2 | 15/15 patients with active disease: ↓ symptoms or full clearance of infection no side effects inhibition of relapse in pts with latent disease | [31] |
| hot water extract, and HCl-EtOH extract | 100 mg/kg/day (heat extract), 10 mg/kg/day (HCl-EtOH extract), for 3–7 days | Laminaria japonica | kuruma shrimp (Marsupenaeus japonicas) in vivo WSSV infection in vitro hemocyte analysis | 1,2 | ↑ survival upon WSSV infectionhemocyte fMet-Leu-Phe stimulation: ↑ chemotaxis ↑ superoxide production ↑ phenol oxidase activity ↑ phagocytosis | [32] |
| hot water-EtOH extract | 20–200 mg/kg; 7 weeks | Hizikia. = Sargassum fusiforme | male SD rat; ligature-induced periodontitis | 3 | ↓ alveolar bone loss due to inflammation | [33] |
| hot water extract of 10 different herbs incl. S. fusiforme | 10 mg/kg; 10 weeks | Sargassum fusiforme | female SD rat; induced autoimmune thyroiditis by CFA-IFA thyroglobulin immunization | 3 | ↓ AI-thyroiditis (cellular infiltrate), ↓ auto antibodies | [34] |
| whole seaweed, freeze-dried, powdered | 5% in chow, 8 weeks | Undaria pinnatifida | male C57BL/6J mouse, HFD+/− seaweed supplement | 3 | ↑ total plasma cholesterol (cf. HFD) fecal cholesterol excretion ↓ ↓ MCP-1 induction obese phenotype not prevented no glycemic improvement in i.p. glucose tolerance test microbiome composition (HFD + seaweed) is closer to LFD than to HFD | [38] |
| whole seaweed, freeze-dried | 5% in chow, 16 weeks | Undaria pinnatifida (Up), Laminaria japonica (Lj), Sargassum fulvellum (Sf), Hizikia = (Sargassum) fusiforme (Hf) | male C57BL/6N mouse, HFD+/− seaweed supplement | 3 | body weight or adiposity: no change cf. HFD, except HFD+Up →↑ weight + ↑ subcutaneous adipose tissue adiponectin: ↓ with HFD+Up leptin: ↓ with HFD+Lj, +Sf, +Hf insulin resistance: ↓ w/HFD+Lj blood glucose: ↓ HFD+Lj or +Hf = LFD; HFD+Up = HFD crown-like structures in adipose tissue: ↓ ↓ with all; =LFD LPS-induced pro-inflammatory cytokines by BMDM: ↓ with all | [36] |
| MeOH extract + carob pod | 0.1%/0.9%, 4 weeks | Undaria pinnatifida | male Wistar rat, MetS after 8 weeks western diet | 2,3 | =body weight, fat mass or muscle =food or energy intake ↓ systolic blood pressure at low dose; ↑ at high dose =CRP ↑ insulin, =glucose ↓ non-esterified fatty acids | [37] |
| hot aqueous extract | 500 mg/kg/day started after 2 weeks, for 2 weeks | Ecklonia cava | female Sprague Dawley rat, letrozole-induced PCOS | 2,3 | ↓ vaginal leukocyte infiltration restore normal estrous cycle restore normal plasma hormonal levelsnormalize expression of gonadotropin- and steroid hormone-related genes = weight gain upon PCOS induction | [39] |
Appendix B
| Compound/Format | Effective Concentrations | Algal Source | Experimental Model | Inflammatory Phase 1 | Significant Findings 2 | Reference |
|---|---|---|---|---|---|---|
| Fucoidan | 0.2%, 2% fucoidan chow for 8 weeks | Cladosiphon okamuranus | Rats | steady state | Uptake through intestinal tract | [46] |
| Fudoidan 7 kDa (LMW) | 2000 mg/kg, Single dose | Ascophyllum nodusum | rats | steady state | No toxicity | [50] |
| Sodium-Alginate (SA) | 5% SA containing chow for 6 weeks | Unknown | Male C57BL/6, Non-alcoholic steatosis model | steady state | ↑ Intestinal barrier function in small intestine ↓ Hepatic lipid accumulationLiver: ↓ TNFα, ↓ Collagen-1α1 Liver: ↓ Macrophage infiltration | [162] |
| Fucoidan-rich extract | 50–100–150 mg/kg for 14 days | Undaria pinnatifada | In vitro: RAW 264.7 In vivo: C57BL/6 mice | 1,2 | In vitro: ↑ NO, ↑ TNF-α, IL-1α, IL-1β, IL-6 In vivo: ↑ CD3+, CD4+ ↑ TNF-α, IFN-γ ↑ IgM | [163] |
| Fucoidan | 10–100 mg/kg for 21 days | Cladosiphon tokida | In vitro: RAW 264.7 + S180 tumor cells In vivo: S180 tumor bearing mice | 2 | ↑ NO-production by macrophages↑ NK-cell mediated cytotoxicity ↑ NF-κβ translocation in macrophages | [52] |
| Fucoidan-rich extract | 50–100–150 mg/kg for 14 days | Undaria pinnatifada | Cyclophosmamide-immunosuppressed male C57BL/6 mice | 1,2 | All doses: ↑ NK cytotoxicity 100–150 mg/kg: ↑ proliferation of T cells 150 mg/kg: ↑ TNF-α, IgM and total IgG | [93] |
| Extracted polysaccharides (SFP) | 100–200–300 mg/kg, 14 days | Sargassum fusiforme | Cyclophosmamide-immunosuppressed ICR mice | 1,2 | Compared to the cy model group: ↑ Thymus and spleen indices Liver: ↑ SOD, ↓ MDA, ↑ GSH ↑ villus height in normal mice, = in CY-treated mice ↑ intestinal epithelial lymphocyte and Goblet cells in normal and CY-treated mice | [164] |
| Fucoidan | Buccal LPS injection on 3 separate days followed by fucoidan ingestion P. gingivalis infection day 10–25, followed Fucoidan ingestion day 29–49 | Sargassum wightii | Mouse–Buccal LPS and bacterial (P. gingivalis) induced inflammation In vitro: RAW 264.7—LPS induced inflammation | 2 | ↓ TNF-α, IL-1β, IL-6 ↓ monocyte and dendritic cell recruitment =lymphocyte numbers ↓ IL-17, ↑ IL-10 ↓ inhibited antigen-specific immune response No inhibition of bacterial induced periodontitis In vitro: ↓ iNOS, COX-2 ↓ NO, PGE2 ↓ TNF-α, IL-6, IFN-γ | [55] |
| Sulfated polysaccharide >30 kDa | 25–50–100 μg/mL | Sargassum horneri | Zebrafish embryos—LPS induced inflammation In vitro: RAW 264.7—LPS induced inflammation | 1,2 | ↓ LPS-induced NO production, toxicity, cell death In vitro: ↓ p-IKβ ↓ iNOS, COX-2 ↓ NO, PGE2 ↓ TNF-α, IL-6 | [56] |
| Fucoidan | 25–50–100 μg/mL | Fucus vesiculosus | Zebrafish embryos—LPS induced inflammation In vitro: RAW 264.7—LPS induced inflammation | 1,2 | ↓ NO, ROS production ↓ neutrophil and macrophage recruitment dose dependent activity In vitro: ↓ iNOS, COX-2 ↓ NO, PGE2 ↓ TNF-α, IL-1β | [103] |
| Fucoidan | 12.5–25–50 μg/mL | Turbinaria ornata | Zebrafish embryos—LPS induced inflammation In vitro: RAW 264.7—LPS induced inflammation | 1,2 | ↓ NO, ROS production ↑ Cell viability dose dependent activity In vitro: ↓ iNOS, COX-2 ↓ NO, PGE2 ↓ TNF-α, IL-1β | [53] |
| Fucoidan | 12.5–25–50 μg/mL | Laminaria japonica | Zebrafish embryos—LPS induced inflammation In vitro: RAW 264.7—LPS induced inflammation | 1,2 | ↓ NO, ROS production ↓ cell death rate dose dependent activity In vitro: ↓ MAPK, NF-κβ ↓ NO, ↓ TNF-α, IL-1β, IL-6 | [54] |
| Laminarin, fucoidan and ash | Laminarin 1 g, fucoidan 0.8 g, day 107 of gestation until weaning (day 26) | Laminaria | Pregnant + Lactating sows | 2 | ↑ Colostrum IgA ↑ Piglets serum IgG | [64] |
| Fucoidan | 6 g/kg feed for 21 days | Laminaria japonica | Immunosuppressed African catfish | 1,2 | Macrophages: ↑ oxidative burst, ↑ phagocytic activity Lymphocytes: ↑ transformation index Serum: ↑ lysozyme, NO and bactericidal activity ↑ Survival rate in challenge test | [70] |
| Fucoidan-rich extract | 2% inclusion, 45 days feeding trial | Sargassum wightii | Sutchi Catfish | 1,2 | Macrophages: ↑ oxidative burst, ↑ phagocytic activity ↑ Total lymphocyte count ↓ Albumin/Globulin ratio ↑ IFN-γ ↑ Survival rate in challenge test | [73] |
| Laminarin | 0.2 g/kg/day for 21 days | Laminaria digitata | Rainbow trout | 1,2 | ↑ TNF-α, IL-8 | [72] |
| Fucoidan | 0.05%, 0.1% and 0.2% per kilogram feed | Sargassum horneri | Yellow catfish | 1,2 | Macrophages: ↑ oxidative burst, ↑ phagocytic activity Serum: ↑ lysozyme ↑ CAT ↑ SOD, ↓ MDA↑ Survival rate in challenge test | [75] |
| Fucoidan-rich seaweed extract (FRSE) + Methionine | 2% FRSE + 0.3 methionine | Sargassum wightii | Carp (Labeo rohita) | 1,2 | ↑ respiratory burst activity, phagocytic activity, ↑ MPO activity, lysozyme activity, ↑ total immunoglobulin and TLC | [71] |
| Laminarin | 0.5–1.0% inclusion in diet for 48 days | Commercially sourced | Grouper (Epinephelus coioides) | 1,2 | ↑ IL-1β, IL-8 and TLR2 ↑ lysozyme, CAT and SOD ↑ growth rate and the feed efficiency | [76] |
| Fucoidan | 250–500 mg/kg feed | Sargassum, Padina and Turbinaria | White shrimp (Litopenaeus vannamei) | 1,2 | ↑ THC, PA and SOD ↑ LGBP, Toll and lectin | [74] |
| Laminarin, fucoidan and ash | Lam 1 g, Fuc 0.8 g, d 83 of gestation until weaning (day 28) | Laminaria | Pregnant + Lactating sows | 1,2 | ↑ Piglets villus height in the jejunum and ileum | [67] |
| Extract | D 83 of gestation until weaning (day 28) | Ascophyllum nodosum and Fucus | Pregnant + Lactating sows | 1,2 | ↑ Piglets CD4+ and CD8+ T cells | [66] |
| Laminarin | 1 g/day Pre-weaning (0–62 days) and post-weaning (63–93 days) | Laminaria | Holstein Friesian bull calves | 1,2 | ↓ Growth ↑ serum haptoglobin ↓ lymphocyte levels ↓ stimulated IFN-γ (in vitro challenges) | [68] |
| Sodium alginate oligosaccharides | 0, 0.04 and 0.2% of diet | Unknown | broiler chickens Salmonella Enteritis challenge | 1,2 | in unchallenged animals: ↑ IFN-γ, IL-10, IL-1β | [69] |
| Highly viscous polysaccharide extract (HVPE) | 10–30–100 mg/kg/day for 14 days | Kjellmania crassifolia | C57BL/6 mice | 1,2 | ConA-stimulated spleen cells: ↑ IFN-γ, IL-12, IL-6, IgA secretion ConA-stimulated Peyer’s patch cells: ↑ IgA ↑ Peritoneal macrophage phagocytic activity | [77] |
| Fucoidan (LMWF) | 200–400–1000 mg/kg/day (6 weeks) | Laminaria japonica | Sprague Dawley rats, Mycoplasma pneumoniae antigen stimulation | 1,2 | ↑ Spleen weight, splenocyte proliferation potential ↑ NK cell activity, ↑ Phagocytic activity ↑ IFN-γ, IL-2 IL-4 ↑ IgG, IgA, ↓ IgE ↑ Antigen-specific antibodies | [61] |
| Fucoidan containing product (88.3% purity) | 410–1025 mg/kg | Cladosiphon okamuramus | Balb/c mice, OVA-immunized | 1,2 | ↑ Immune cell proliferation, IL-2, ↑ macrophage phagocytic activity ↑ serum IgM, IgG, IgA ↓ IgE ↓ IL-4, -5 | [62] |
| Fucoidan (LMWF, MMWF, HMWF) | Unclear | Cladosiphon okamuranus | Pathogen free Male Balb/c mice | 1,2 | HMWF: ↑ Proportion CD8+ T-cells in spleen, ↓ CD4/CD8 ratio | [78] |
| water-soluble polysaccharides | 50–100–200 for 10 days | Sargassum fusiforme | Cyclophosmamide-immunusuppressed ICR mice | 1,2 | Compared to the cy model group: ↑ spleen index ↑ splenic lymphocyte proliferation potential ↑ splenocyte produced IL-2, Il-6, IFN-γPeritoneal macrophages: ↑ phagocytic activity, ↑ produced IL-2, Il-6, IFN-γ | [92] |
| Fucoidan | 150 mg/kg daily for 2 weeks | Fucus vesiculosus | mice | 1,2 | increased cytolytic activity of NK cells | [89] |
| Laminarin | 500–1000 mg/kg/day for 10 days | Unknown, commercially sourced | Cyclophosmamide-immunusuppressed male Balb/c mice | 1,2 | Compared to the cy model group: ↑ cytotoxicity of NK cells ↑ serum IL-12 and IFN-γ | [94] |
| Fucoidan | 0.1–0.5 mg/day for 14 days | Undaria pinnatifada | HSV-1 infected mice/5 fluorouracil immunosuppressed mice | 2 | Protection against herpes infection ↑ Macrophage NO production ↑ CTL activity ↑ B-cell blastogenesis↑ Antibody titers | [82] |
| Fucoidan | 25–250 mg/kg/day, 3 times weekly for 4 weeks | Unknown | Leishmania infection, Balb/c mice | 3 | In vitro: 93% reduction Amastigote multiplication in macrophagesIn vivo: complete elimination parasite in liver and spleen Th2 ◊ Th1 response Splenocytes: ↑ superoxide and NO production | [79] |
| Fucoidan | 500 mg/kg every 2 days, for 6 weeks | Fucus vesiculosus | Schistosomiasis Japonica infeced C57BL/6 mice | 3 | ↓ Granuloma size, ↓ hepatic inflammation↓ IL-6, IL-12, TNF-α ↑ IL-4, IL-13 ↑ IL-10, TGF-β ↑ Th2 response ↑ Treg infiltration in liver | [106] |
| Fucoidan | 0.034 g/mouse/day 10 days before, 40 days after tumor inoculation | Undaria pinnatifada | A20-tumor bearing Male Balb/c mice/Do-11-10-Tg mice (transgenic for TCR) | 3 | ↑ NK cell activity ↑ CTL activity ↑ IFN-γ, IL-12 | [84] |
| Ascophyllan | 500 mg/kg. 4 days before–10 days after tumor implantation | Ascophyllum nodosum | S-180 sarcoma bearing SPF male ddY mice | 3 | Oral route stronger anti-tumor effects than intraperitoneal injection, effects via interaction with intestinal immune system ↓ 69% tumor size reduction ↑ TNF-α, IL-12 | [91] |
| Polysaccharides | 100–200 mg/kg for 28 days | Sargassum fusiforme | A549 adenolungcarcinoma- bearing Balb/c nuce mice | 3 | ↑ Serum TNF-α ↑ Peritoneal macrophage production of TNF-α, IL-1β ↑ Splenocyte lymphocyte proliferation Liver and Kidney: ↑ SOD, ↓ MDA | [87] |
| Fucoidan (LMWF/IMWF/HMWF) | 5 g/kg/day | Cladosiphon okamuramus | Colon 26 tumor bearing mice; Myd-88−/− mice | 3 | No intestinal absorption Effects via interaction with intestinal immune system All: ↑ Median survival timeIMWF ↓ Tumor weight, ↓ Cell divisions, ↑ Apoptosis HMWF: ↑ Splenic NK cell numbers | [85] |
| Polysaccharide | 100–200–400 mg/kg for 28 days | Sargassum fusiforme | HepG2-tumor-bearing mice | 3 | ↑ Serum TNF-α, NO, IL-1β, IgM ↑ Peritoneal macrophage production of TNF-α, IL-1β ↑ Apoptosis in HepG2-tumor cells | [86] |
| Polysaccharides | 50–100–200 mg/kg | Sargassum fusiforme | CNE- tumor- bearing mice | 3 | ↑ Serum NO, IL-1β, IgM ↑ Peritoneal macrophage production of NO, IL-1β↑ Splenocyte lymphocyte proliferation ↑ IgM production Activity is, in part, mediated via TLR2 and TLR4 | [88] |
| Fucoidan | 200–400 mg/kg (6×/week) for 4 months | Fucus vesiculosus | DMBA-induced mammary carcinogenesis in female Sprague Dawley rats | 3 | ↓ Tumor incidence, ↓ Tumor weight ↑ Serum IL-6, IL-12p40, IFN-γ ↓ IL-10 TGF-β ↓ MRNA expression levels of FOXP3, TGF-β, ↑ IFN-γ in tumors ↓ Foxp3, PD1, =PDL1/PDL2 protein levels in tumors ↓ p-PI3K, p-AKT protein levels in tumors | [90] |
| Fucoidan | 5 mg/day for 10 days (start direct after infection) | Undaria pinnatifada | Influenza infected mice | 3 | ↑ Survival ↑ Inhibition of viral replication ↑ Mucosal antibody levels (IgA) ↑ Serum antibody titer (IgM, IgG) | [80] |
| Fucoidan | 6 g/day for 6–13 months | Unknown, commercially obtained | Human, HTLV-infected patients | 3 | ↓ HTLV proviral load =CD4+, CD8+ frequencies =NK-cell, mDC and pDC frequencies | [81] |
| Oligo Fucoidan | 4400 mg/day for 48 weeks | Unknown, commercially sourced | Patients with chronic Hepatitis B virus 2193 | 3 | vitamin D dependent activity ↓ HBV DNA ↑ CD4+CD45RO+ and CD8+CD45RO+ | [109] |
| Fucoidan | 3 g/day for 6 months | Cladosiphon okamuranus | Human, male cancer survivors | 3 | ↑ NK-cell activity | [165] |
| Fucoidan | 100–1000 mg/day for 4 weeks | Fucus vesiculosus (85%), Macrocystis pyrifera (10%), Laminaria japonica (5%) | Human volunteers | 3 | ↑ Cytotoxic T cell numbers ↑ Phagocytic capacity monocytes ↓ IL-6No dose response | [110] |
| Fudoidan (75% pure) | 3 g/day for 12 days | Undaria pinnatifada | Human volunteers | 3 | ↑ CD34+ cells, CD34+ cells expressing CXCR4 (45% ◊ 90%)↑ serum SDF-1 and IFN-γ | [166] |
| Fucoidan | 300 mg/day for 4 weeks | Mekabu fucoidan | Human, Influenza vaccinated elderly | 3 | ↑ Antibody titers | [167] |
| Sulphated polysaccharide | 20–40–80 mg/kg/day for 5 days | Sargassum hemiphyllum | Mouse–arachidonic acid induced ear-inflammation | 2 | dose dependent activity ↓ Ear swelling and erythema ↓ TNF-α, IL-1β, IL-6 ↓ neutrophil infiltration | [95] |
| Combined preparation including fucoidan | Fucoidan: 18–54 mg/kg/day for 7 days | Laminaria japonica | Mouse–Carrageenan induced pouch inflammation | 2 | ↓ NO, PGE2 ↓ neutrophil and macrophage recruitment | [96] |
| Fucoidan | 0.02 g/kg for 14 days | Commercially sourced from Sigma | Mouse–aspirin induced ulcer | 2,3 | Protection against ulceration ↓ AST, ALT ↓ PGE2 ↓ IFN-γ, IL-6, IL-10 ↓ stomach glycogen | [97] |
| Fucoidan | 0.05% w/w in chow | Cladosiphon okamuranus | Mouse–DSS-induced colitis | 2 | ↓ IFN-γ, IL-6 ↑ IL-10, TGF-β | [98] |
| Fucoidan & Fucoidan-polyphenol complex (Synergy) | 10 mg/mouse, 400 mg/kg/day | Fucus vesiculosus | Mouse–DSS-induced colitis | 2,3 | Oral synergy best results ↑ colon length ↓ colon weight/body weight, spleen weight ↓ Histology damage score↓ TNF-α, IL-12 IP fucoidan actually worsened colitis symptoms | [99] |
| Fucoidan | 200 mg/kg | Laminaria japonica | Mouse–myocardial ischemia-reperfusion injury | 2,3 | ↓ myocardial infarct size ↓ HMGB1, p-IκB-a and NF-κB ↓ TNF-a and IL-6, ↑ IL-10 ↓ infiltration PMNs, ↓ MPO activity ↓ histopathological damages in myocardium | [104] |
| Fucoidan | 200 mg/kg/day for 14 days | Sargassum hemiphyllum | C57BL/6 mice, irradiation induced pneumonitis and lung fibrosis | 3 | ↓ Lung fibrosis ↓ Neutrophil and macrophage infiltration ↓ TIMP-1, CXCL-1, MCP-1, MIP-2, IL-Ra ↓ Procollagen-1α | [105] |
| Fucoidan (LMWF: 1 ± 0.2 kDa MMWF: 3.5 ± 0.3 kDa HMWF: 100 ± 4 kDa) | 300 mg/kg dissolved in water, one day after booster immunization until the end of the experiment (day 22–47) | Undaria pinnatifada | Male DBA/1J mice, Collagen-induced arthritis | 2,3 | LMWF: ↓ reduced severity of inflammation↓ IFN-γ, TNF-α ↓ IgG2a, HMWF: ↑ Increased severity of inflammation↑ IFN-γ, TNF-α ↑ IgG2a ↑ adhesion molecules and migration potential of macrophages | [102] |
| Fucoidan | 4 g/day for 4 at least weeks | Cladosiphon novae Caledoniae | Advanced cancer patients | 3 | Responsiveness of IL-1β independent prognostic factor for QOL-scores ↓ TNF-α, IL-1β, IL-6 = QOL-scores | [111] |
| Fucoidan | 400 mg/kg for 22 weeks | Fucus vesiculosus | Mice, colorectal carcinogenesis model | 2,3 | ↑ Beneficial Microbiome modulation ↓ IL-17 and IL-23 ↑ IFN-γ, IL-4 and IL-10 ↑ NK cells, CD4+ T cells in blood | [101] |
| Fucoidan | 300–600 mg/kg/day for 5 weeks | Fucus vesiculosus | Non-obese diabetic mice (autoimmune diabetes model) | 2 | ↑ serum insulin levels Delayed onset and ↓ incidence of diabetes ↓ Th1 cytokines ↑ Th2 cytokines Dendritic cells: ↓ MHC-II, ↓ CD86 Pancreatic cells: ↓ TLR4 expression Microbiota: ↑ Akkermansia, ↑ Lactobaccillus, ↓ Bacteroides | [108] |
| Fucoidan | 400 mg/kg | Ascophyllum nodosum | Colonic inflammation- induced SPF- C57BL/6 mice (antibiotics treated) | 3 | ↓ Colonic inflammation↓ Dysbiosis ↓ Infiltration of inflammatory cells ↓ Space between mucosa and submucosa ↑ Crypt depth ↓ TNF-α, IL-1β, IL-6, ↑ IL-10 | [100] |
| Fucoidan | 200 mg/kg | Laminaria japonica/Ascophyllum nodosum | SPF C57BL/6 J mice, High fat diet | 3 | ↓ Body weight gain, ↓ Fat mass ↓ Insulin resistance ↓ Endotoxemia (↓ LBP) ↓ Systemic inflammation (↓ TNF-α, IL-1β, MCP-1) Microbiome: ↑ Akkermansia, Alloprevotella, Blautia, Bacteroides | [47] |
| Fucoidan and Fucoxanthin | 275 mg LMF | Unknown, commercially obtained | Clinical trial, NAFLD patients | ↓ AST =adiponectin | [168] | |
| Fucoidan | 50 mg/kg for 14 days | Fucus vesiculosus | Balb/C mice, ConA-induced acute liver injury | 3 | ↓ AST, ALT ↓ Histopathological changes ↓ IFN-γ, TNF-α ↓ Apoptosis inhibition | [107] |
| Fucoidan | 1 or 5% inclusion in diet (12 weeks) | Cladosiphon okamuranus | Diet-induced dyslipidemia in ApoE−/− mice | 3 | ↓ Tissue weight (liver and WAT), hepatic steatosis ↓ Blood lipids (TC, TG, non-HDL-C)↓ Blood glucose ↑ Plasma LPL activity, HDL-C ↑ Insulin-sensitivity ↑ Pparα, ↓ Srebf1 | [169] |
| LJP12 | 50–100–200 mg/kg/day | Laminaria japonica | Atherogenic diet fed LDLR−/− mice | 3 | Dose-dependent activity ↓ Atherosclerotic plaque formation ↓ Plasma lipids (TC, TG, LDL-C), ↑ HDL-C/LDL-C ↓ Systemic inflammation (↓ TNF-α, IL-1β, IL-6, MCP-1, ↑ IL-10) ↑ SOD, ↓ MDA ↓ p-p 65, p-Iκβ, p-ERK, p-JNK, p-P38 | [112] |
| Fucoidan (LMWF) | 200 mg/kg/day | Laminaria japonica | Diet-induced dyslipidemia in ApoE−/− mice | 3 | ↓ TG, OX-LDL ↓ p-JNK, cyclin-D1 ↓ IL-6, ↑ IL-10 ↓ Macrophages ◊ foam cells ↓ Migration of SMCs into intimal layer | [114] |
| Fucoidan | Unknown | Fucus vesiculosus | Rats | 3 | ↓ Endothelial hyperplasia, ↓ vascular modulation ↓ α-actin+ cells ↓ Vessel inflammation↓ Macrophage infiltration ↓ Apoptosis in vessel wall | [115] |
| Fucoidan (LMWF) | 200 mg/kg/day | Laminaria japonica | Diet-induced dyslipidemia in ApoE−/− mice | 3 | ↓ Inflammatory infiltration ↓ Limit enlargement of AAA ↓ Maximal aortic diameter Preserved elastin ↓ TNF-α, IL-1β, MCP-1 ↓ MMP | [116] |
| Fucoidan | 50–100 mg/kg/day | Laminaria japonica | In vitro: oxLDL treatment RAW264.7 In vivo: Atherogenic diet fed LDLR−/− mice | 3 | In vitro: ↓ oxLDL-induced LOX-1, ↓ TNF-α, IL-1β, IL-6 ↓ ICAM-1, VCAM-1 ↓ ROS In vivo: ↓ Atherosclerotic plaque formation ↑ Plaque stability↓ Macrophage infiltration ↓ Plasma lipids (TC, TG, non-HDL-C) ↓ ROS | [113] |
| Fucoidan (LMWF) <3 kDa | 5 mg/day. With or without added probiotics | Undaria pinnatifada | Male Balb/c mice. | 2 | Fucoidan enhances effects of probiotic strain ↑ IFN-γ, TNF-α, IL-6, IL-12 OVA-immunization: ↓ lgE, IL-4, ↑ IFN-γ Desulphated Fucoidan no effects | [170] |
| Extracted polysaccharide (not fucoidan) | 50 mg/kg/day for 2 weeks | Laminaria japonica | SPF Kunming mice, OVA-immunized asthma model | 2,3 | ↓ Eosinophils in BALF ↓ lung inflammation↓ Serum IgE ↑ IL-12, ↓ TGF-β, IL-13 in BALF and lung | Lin et al. 2015. Multidiscip Respir Med. PMID: 26110056 [58] |
| Fucoidans | 100–400 mg/kg/day | Undaria pinnatifada | OVA-immunized female Balb/c mice, exacerbated allergic astma | 2,3 | ↓ Infiltration of macrophages, neutrophils, CD4+ lymphocytes ↓ Lipid peroxidation ↓ IL-4, ↓ IgE ↓ Mast cell activation, degranulation ↓ Mucus hypersecretion, ↓ Goblet cell hyperplasia | Herath et al. 2020. Molecules. PMID: 32580518 [57] |
| Fucoidan | 100–400 μg/mouse/day for 4 days | Laminaria japonica | Female Balb/c mice, passive cutaneous anaphylaxis model | 2,3 | ↓ allergic symptoms (Edema) Oral fucoidan anti-allergy effects, IP-fucoidan no effects =Serum IgE, IgG1 ↑ Galectin-9 secretion by intestinal epithelial cells | [59] |
| Fucoidan | 60 μg/mouse/day for 17 days after OVA-immunization | Laminaria japonica | Female Balb/c mice, OVA-immunized | 2,3 | ↓ allergic symptoms (Rectal temperature reduction) =Serum IgE ↑ Serum galectin-9 ↓ Degranulation of mast cells ↓ IgE-attachment on mast cells | [60] |
| Alginate, 108 kDa | 2 mg alginate one day before every OVA-challenge | Laminaria japonica | OVA-immunized female Balb/c mice, food allergy model | 2,3 | ↓ IL-4, ↓ IgE ↓ Mast cell degranulation, ↓ Histamine ↑ Number of Tregs in spleen ↓ Th0 ◊ Th2 | [63] |
Appendix C
| Compound/Format | Effective Concentrations | Algal Source | Experimental Model | Inflammatory Phase 1 | Significant Findings 2 | Reference |
|---|---|---|---|---|---|---|
| Seaweed extract containing fucosterol Enzyme-modified extract | NH: 200 mg/kg EH: 50, 100 and 200 mg/kg; 3 weeks | Sargassum fusiforme = Hijiki | Male C57BL/6 mice; ConA activation induced splenocyte proliferation | 1 | ↑ splenocyte proliferation In peritoneal Macrophages:↓ IL-6 ↓ IL-1β ↓ TNF-α (EH > NH) | [22] |
| Lipid extract containing 24(S)-Saringo-sterol | 50% (w/w), 10 weeks | Sargassum fusiforme | APPswePS1ΔE9 Mice, Alzheimer’s disease +/− seaweed supplement | 3 | ↑ LXR-responsive gene expression in CNS ↑ ApoE | [118] |
| Fucosterol extract | 25, 50 or 100 mg/kg; 3 days | Eisenia bicyclis | Male BALB/c mice, ConA- induced acute liver injury after Fucosterol pretreatment | 1 | ↓ TNF-a ↓ IL-6 ↓ IL-1β ↓ NF-κB p65 ↑ PPARγ expression ↑ p-P38 MAPK levels | [117] |
| ethyl acetate extract => Fucosterol | 200 mg/kg 2 weeks | Sargassum fusiforme | NC/N ga male mice, DNCB induced AD-like dermatitis | allergy | ↓ Scratching ↓ epidermal thickness of dorsal skin ↓ mast cells ↓ serum level of IgE In cultured splenocytes: ↓ IL-4 ↓ TNF-α ↑ IFN-γ | [171] |
Appendix D
| Compound/Format | Effective Concentrations | Algal Source | Experimental Model | Inflammatory Phase 1 | Significant Findings 2 | Reference |
|---|---|---|---|---|---|---|
| (poly)phenol-rich seaweed extract | 400 mg; 8 weeks | Ascophyllum nodosum | 80 participants; 30–65 years old with a body mass index (in kg/m2) ≥ 25. | Steady state | ↓ DNA damage in obese people No significant differences in CRP, antioxidant status and inflammatory cytokines | [7] |
| MeOH–EtAc extract => phlorotannin | 100–200 mg/kg/day 3 days before experiment | Eisenia bicyclis = Ecklonia bicyclis | Sprague Dawley rat, platelet aggregation + activation | Steady state | Eisenia bicyclis extract strongly inhibits in vivo platelet aggregation. Mechanism in vitro: downregulated ADP-induced platelet activation (Ca-mobilization, fibrinogen binding, granule release—mediated via decreased Src, PI3K, PLCgamma2, MAPK signaling) | [122] |
| MeOH extract=> Eckol | 25, 50, 100 μg/mL, pretreatment 2 h prior to anaphylaxis induction 25, 50, 100 μg/mL, 24 h | Ecklonia cava | BALB/c mice; anti-DNP-IgE induced PCA C57BL/6 male mice; anti-DNP-IgE and IgE-BSA induced allergic | allergy | ↑ binding of IgE to FcɛRI ↓ FcɛRI expression ↓ mRNA level of IL-1β, IL-6, TNF-α, IFN-γ ↓ NF-κB nuclear translocation ↓ IκB degradation ↓ secretion of inflammatory mediators, such as histamine, β-hexosaminidase, leukotrienes and prostaglandins ↓ production of Th2-type cytokines, such as IL-4, IL-5, IL-13 (not 25 ug/mL) | [123] |
| MeOH-ethyl acetate extract (crude (72–74%) phlorotannins = phloroglucinol oligomer); | oral 1× or 2×; 0.1, 1 mg/ear | Ecklonia kurome Ecklonia arborea | male ICR mouse; AA induced ear oedema | allergy | inhibitory effects are comparable to known anti-allergic agents presumed mechanisms: inhibition of MC degranulation + inhibition of COX-2 and LOX, and to lesser extent PLA2, activities | [125] |
| (poly)phenol-rich brown algae extract | 100 mg/kg 500 mg/kg; 12 weeks | Ecklonia cava | C57BL/6 male mice; HFD+/− seaweed supplement | 1,3 | ↓ MCP1 ↓ TNF-α ↓ IL-1 ↓ COX-2 (slightly decreased) protein level ↓ NF-κB protein level↓ Body weight ↓ adipose tissue weight ↓ deposition in the liver ↓ TG and TC levels ↓ leptin and adiponectin ratio ↓ Glucose Tolerance Test ↓ Insulin Resistance ↑ protein levels of P-AMPK ↑ SIRT1 protein levels ↑ PGC1α protein levels in the nucleus | [128] |
| A single compound Pyrogallol-Phloroglucinol-6,6-Bieckol from the brown algae ethanoic extract | 2 mg/kg; 4 weeks | Ecklonia cava | C57Bl/6N mice; HFD +/− seaweed supplement | 1,2,3 | In visceral fat: ↓ TNF-a mRNA levels ↓ IL-1β mRNA levels ↓ Macrophage infiltration ↓ M1/M2 ratio (CD86 and CD80 lower, CD163 and CD206 higher) ↓ RAGE ↓ RAGE-RAGE Ligand Bonding ↓ Body weight ↓ size of visceral adipocytes ↓ fat mass ↓ serum TG and TC ↓ AGEs, HMGB1 and S100beta secret by adipocytes | [137] |
| Polyphenols-rich Ethanol extract | 200, 400 mg/kg; 7 days | Sargassum horneri | BALB/c female mice; PM induced airway inflammation in allergic asthma | 1,2 | ↓ granulocyte infiltration ↓ macrophage infiltration ↓ TLRs expression in lungs ↓ NF-κB pathway activation ↓ pro-inflammatory cytokines IL-1β, TNF-α, IL-6 expression in lung↑ IL-10 expression in lung ↓ IL-25, IL-33, IL-8, TGF-β in lung ↓ cytokines (IL-25, IL-33) in serum | [124] |
| phlorotannins-rich extract | PREC (75 and 150) or dieckol (50 and 100 mg/kg; 4 weeks Combination cisplatin (1, 3 or 5 μM) with PREC (35, 50, 75 or 100 μg/mL); 48 h | Ecklonia cava | BALB/c athymic female nude mice; SKOV3 cells induced ovarian carcinoma | 1 | PREC: ↓ Akt in ovarian cancer ↓ NF-κB in ovarian cancer ↑ intracellular ROS in ovarian cancer ↓ cisplatin-induced ROS in normal HEK293 ↑ ROS production in SKOV3 cells the combined treatment of PREC and cisplatin: ↓ ROS production in in SKOV3 cells and normal HEK 293cells Dieckol: ↑pathwayscisplatin-induced ROS production Dieckol: ↓pathwayscisplatin-induced ROS production (slightly) | [129] |
| phlorotannin derived from the brown alga | 0.5 and 1.0 mg/kg/day; Pretreatment for 7 days | Ecklonia stolonifera | Male Kunming mice; CCl4 induced acute liver injury | 1,2 | ↓ TNF-a ↓ IL-1β ↓ IL-6 ↓ ROS ↑ IL-10 ↑ CD11c+ | [135] |
| Ethyl acetate fraction containing diphlorethohydroxycarmalol | 6.25, 12.5, 25 μg/mL | Ishige okamurae | zebrafish embryo; FD induced ROS, NO production and cell death | 1,2 | ↓ ROS production ↓ NO production | [134] |
| polyphenol-rich brown algae extract | 25, 50 and 100 μg/mL; pretreatment for 1 h | Ecklonia cava | Zebrafish embryos | 1,2 | ↓ ROS expression ↓ NO expression ↓ iNOS | [133] |
| chloroform–methanol extract Apo-9′-fucoxanthinone (AF) | 25, 50 and 100 μg/mL; pretreatment for 1 h | Sargassum muticum | Zebrafish embryos | 1,2 | ↓ IL-1β, ↓ TNF-α ↓ ROS ↓ COX-2 ↓ NF-κB ↓ MAPK ↓ iNOS ↓ NO | [131] |
| Six purified phlorotannins ((eckol; 6,60-bieckol; 6,80-bieckol; 8,80-bieckol; phlorofucofuroeckol (PFF)-A and PFF-B) | 10, 100, 200 µM | Eisenia arborea | ICR mice; AA, TPA and OXA induced ear swelling | 1 | ↓ COX-2 mRNA expression ↓ COX-2 enzymatic activity | [136] |
| Ethanol extract including Dieckol, 2,7-phloroglucinol-6,6-bieckol (PHB), PFF-A and pyrogallol-phloroglucinol-6,6′-bieckol (PPB) | 2.5 mg/kg; 4 weeks | Ecklonia cava | 57BL/6N mice; HFD+/− seaweed supplement | 1,2,3 | ↓ TNF-α ↓ IL-6 ↓ TLR4 expression ↓ NF-kB expression ↓ CD11b ↓ CD86 ↑ CD206 ↓ body weight ↓ food intake ↓ fat mass ↓ leptin resistance ↑ leptin sensitivity ↓ PPAR expression ↓ CEBP expression ↓ FAS expression ↓ ACC expression ↓ ER | [126] |
| Ethanolic extract including phlorotannin | 70 mg/kg; 4 weeks | Ecklonia cava | male 57BL/6N mice, HFD+/− seaweed supplement | 1,2,3 | ↓ NF-kB ↓ TNF-α ↓ IL-6 ↓ TLR4 expression ↓ CD4 and CD8a ↓ CD11b ↑ CD206 ↓ CD80 ↑ IL-10 ↑ TGF-β mRNA ↓ Body weight ↓ Food intake ↓ Adipocyte size ↓ Leptin resistance ↓ RAGE | [127] |
| phlorotannins-rich ethanolic extract | 100, 200, 400 mg/kg/day; 8 weeks | Ecklonia cava | Male Sprague Dawley rats; Experimental periodontitis induced by placing a sterile 4-0 silk ligature around the gingival cervix of the right mandibular second molar teeth | 1,2 | ↓ COX-2 activity ↓ PGE2 ↓ NO ↓ iNOS | [130] |
| Ethanolic extract and PPB | ECE: 70 mg/kg PPB: 2.5 mg/kg; 4 weeks | Ecklonia cava | Male C57BL/6N mice; HFD+/− seaweed supplement | 1,2,3 | ↓ MCP-1 ↓ TNF-α ↓ IL-6 ↓ IL-10 ↓ iNOS ↓ CD80 ↑ CD206 ↓ ER stress ↓ size of white adipocytes ↑ PPAR expression | [138] |
| baicalein, luteolin, rosmarinic acid and cis-2-decenoic acid (10-HAD) | Test diet for mice:(baicalein (650 mg/kg diet), luteolin (300 mg/kg diet), rosmarinic acid (500 mg/kg diet), monolaurin (500 mg/kg diet), 10-HAD (500 mg/kg diet)); 4 weeks Capsule for human: (baicalein 250 mg/day, luteolin 75 mg/day, rosmarinic acid 100 mg/day, monolaurin 250 mg/day, 10-HAD 100 mg/day and iodine 0.15 mg/day); 3 times/day 6 months | Unknown | C3H/HeN inbred female mice; LD infection +/− test diet Adult human; with a history of acute LD | 1,2 | Animal study: ↓ IL-6 ↓ TNF-α ↓ INF-γ Human study: By administration of the composition, 17.7% had slight physical and psychological improvement, and 17.7% were none responsive ↓ IL-17 | [139] |
| commercial (30%) polyphenolic fraction (VNP)oral admin | Rat: 1 dose 2000 mg/kg Human: 2400 mg/day 8 weeks | Sargassum fusiformi, Ecklonia kurome, Ecklonia Stolonifera, Eisenia bicyclis and Ecklonia cava | Sprague Dawley rat human (men) | 1 | rat: serum ferric reducing antioxidant power (FRAP) significantly elevated 30 min after treatment, but declined quickly thereafterhuman: ↑ erectile function (significantly) =usefulness of these polyphenolic compounds as chemo preventive agents against vascular risk factors originating from oxidative stress | [132] |
| Compound 21 | 35 mg/kg; 7 days | Synthesized by the department of pharmaceutical chemistry | Female Lewis rats; MBP-induced EAE | 3 | Attenuates neurological deficits, immune infiltration and demyelination in EAE rats Reduces the population of Th1/Th17 cells and inhibits their infiltration into the CNS | [142] |
| phloroglucinol derivative Compound 21 | 1, 5, 10, 20, 50 mg/kg; 4 weeks | Unknown | Cuprizone induced intoxication in mice, | 3 | significantly improved neurological dysfunction and motor coordination impairment decreased microglia and astrocytes activities and the subsequent neuro-inflammatory response | [141] |
| EtOAc Fraction of seaweed Crude Extract | 200 mg/kg; 8 weeks | Ecklonia cava from Jeju or Gijang | C57BL/6 mice; HFD+/− seaweed supplement | 1,3 | ↓ TNF-a mRNA levels ↓ IL-1ß mRNA levels↓ body weights ↓ weight gain ↓ fat tissue mass ↓ Plasma ALT and cholesterol levels ↑ PPARγ2 mRNA expression ↑ C/EBPα, mRNA ↓ Blood glucose levels | [140] |
Appendix E
| Compound/Format | Effective Concentrations | Algal Source | Experimental Model | Inflammatory Phase 1 | Significant Findings 2 | Reference |
|---|---|---|---|---|---|---|
| Fucoxanthin-containing extract | 13, 26 and 65 mg/kg Fx for 4 weeks | Laminaria japonica | Male Sprague Dawley rats (5 weeks old; ±200 g), diabetes induced by i.p. administration of streptozotocin followed by nicotinamide | 1,3 | ↓ TNF-α mRNA expression in plasma and testis ↓ IL-6 mRNA expression in plasma and testis ↑ CAT, SOD and GPx activity in plasma ↑ SOD activity in testis ↓ H2O2 and O2− levels ↓ MDA level in plasma, testis and sperm ↓ Plasma glucose level ↓ Insulin level ↓ HOMA-IR ↓ SOCS-3 mRNA expression in hypothalamus | [150] |
| Fucoxanthin-rich brown algae extract | Colitis: 1, 2 or 5 g/kg bw/day for 7 days; CACC: 0.5, 1 or 2.5 g/kg bw/day for 11 weeks | Sargassum muticum | Male BALB/c mice (6–8 weeks old), 3% DSS-induced colitis for 14 days or CACC induction by a single i.p. injection of azoxymethane + 2% DSS for 7 consecutive days at weeks 3, 6 and 9 | 1,2 | DSS-induced colitis: ↓ MDA level in colonic tissue=MDA level in plasma ↑ SOD levels in plasma ↓ TNF-α in colonic tissue and plasma ↓ IL-6 in plasma =IL-6 in colonic tissue ↓ Total NO content in colonic tissue↓ NO release in colonic tissueCACC: ↓ MDA level in colonic tissue=MDA level in plasma ↑ SOD levels in plasma ↓ TNF-α in colonic tissue and plasma =IL-6 in colonic tissue and plasma ↑ proliferation T and B cells ↓ Total NO content in colonic tissue↓ NO release in colonic tissue | [151] |
| Fucoxanthin-rich extract | 100, 200 or 500 mg/kg Fx, dissolved in olive oil and administered for 5 days | Sargassum glaucescens | Male Syrian hamsters (7 weeks old; 80–90 g), Cisplatin induced testicular damage group with CP i.p. injection before Fx treatment | 1,2,3 | ↑ CAT and GPx activity in testis ↑ SOD activity in plasma ↓ MDA level in testicular tissue, sperm and plasma ↓ NO levels =Body weight | [152] |
| Fucoxanthin | 0.4% and 0.6% Fx for 5 weeks | Unknown | Kunming strain mice (20–22 g) fed regular chow or HFD for 9 weeks + 5 weeks with Fx added to diet | 1,2,3 | ↓ Mammary gland inflammation↓ MPO activity ↓ IL-1β in the blood ↓ TNF-α in the blood ↓ MDA level ↓ COX-2 and iNOS mRNA expression ↓ Body weight gain | [155] |
| Fucoxanthin-rich wakame lipids (WLs) | WLs with 1.06% Fx; WLs with 2.22% Fx, administered for 5 weeks | Undaria pinnatifida | Male C57BL/6J mice (8 weeks old), normal-fat or HFD for 10 weeks followed by normal-fat or HFD with Fx for 5 weeks | 2,3 | ↓ MCP-1 mRNA expression in WAT =TNF-alfa mRNA expression in WAT ↓ Body weight gain =Food intake ↓ WAT weight gain ↓ LDL cholesterol ↓ Plasma leptin ↓ Plasma insulin ↓ blood glucose ↓ Leptin mRNA expression in WAT ↑ ADRB3 mRNA expression in WAT ↑ GLUT4 mRNA expression in skeletal muscle | [156] |
| Fucoxanthin and fucoxanthinol | 150 nmol/mouse, before induction of ear swelling | Unknown | Male ICR-strain mice (4 weeks old), AA-, TPA- or OXA-induced ear swelling | 1 | ↓ Inflammation in mouse ear swelling ↓ PLA2 and COX-2 enzymatic activities | [157] |
| Lipid extracts containing fucoxanthin | 0.10% Fx, 1 g Fx/kg of diet for 27 days | Undaria pinnatifida | Male KK-Ay mice (3 weeks old), fed an experimental diet with or without Fx | 1,2,3 | ↓ TNF-α mRNA expression in WAT ↓ MCP-1 mRNA expression in WAT ↓ Blood glucose level ↑ Glucose intolerance | [145] |
| Meroterpenoid | 90 and 180 mg MES/kg BW for 10 weeks | Sargassum serratifolium | Male C57BL/6J mice (6-weeks old), HCD | 2 | ↓ MCP-1, KC concentration in serum ↓ COX-2, ICAM-1, VCAM-1, MMP-9, MCP-1, beta-actin expression in aortic tissues | [148] |
| Meroterpenoid | 60 and 120 mg MES/kg BW/day for 8 weeks | Sargassum serratifolium | Male C57BL/6J mice (7-weeks old), HFD-induced obesity | 2,3 | ↓ F4/80, MCP-1 expression in epididymal tissue ↓ Body, liver and epididymal issue weight ↓ ALT, AST ↓ TG, glucose, free fatty acid in plasma ↑ HDL cholesterol ↑ UCP-1, ADRB3 expression in subcutaneous fat | [158] |
| Fucoxanthin and fucoidan | 275 mg LMF and 275 mg HSFx in 1 capsule, 3 capsules twice daily for 12 weeks | Unknown | Patients with nonalcoholic fatty liver disease (20–75 y/o) | 3 | ↓ BMI ↓ ALT =liver steatosis (=CAP) =adiponectin =fasting insulin =insulin resistance | [159] |
References
- Cherry, P.; O’Hara, C.; Magee, P.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [PubMed]
- Bramer, W.; Milic, J.; Mast, F. Reviewing retrieved references for inclusion in systematic reviews using EndNote. J. Med. Libr. Assoc. 2017, 105, 84–87. [Google Scholar] [CrossRef]
- Jeong, D.-H.; Kim, K.-B.; Kim, M.-J.; Kang, B.-K.; Ahn, D.-H. Anti-inflammatory activity of ethanolic extract of Sargassum micracanthum. J. Microbiol. Biotechnol. 2013, 23, 1691–1698. [Google Scholar] [CrossRef]
- Güner, A.; Köksal, C.; Erel, B.; Kayalar, H.; Nalbantsoy, A.; Sukatar, A.; Yavaşoğlu, N.K. Antimicrobial and antioxidant activities with acute toxicity, cytotoxicity and mutagenicity of Cystoseira compressa (Esper) Gerloff & Nizamuddin from the coast of Urla (Izmir, Turkey). Cytotechnology 2013, 67, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Zaragozá, M.C.; López, D.; Sáiz, M.P.; Poquet, M.; Pérez, J.; Puig-Parellada, P.; Màrmol, F.; Simonetti, P.; Gardana, C.; Lerat, Y.; et al. Toxicity and Antioxidant Activity in Vitro and in Vivo of Two Fucus vesiculosus Extracts. J. Agric. Food Chem. 2008, 56, 7773–7780. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Seo, I.S.; Lee, S.J.; Lee, S.P. Study on the Health Benefits of Brown Algae (Sargassum muticum) in Volunteers. J. Food Nutr. Res. 2015, 3, 126–130. [Google Scholar] [CrossRef][Green Version]
- Baldrick, F.R.; McFadden, K.; Ibars, M.; Sung, C.; Moffatt, T.; Megarry, K.; Thomas, K.; Mitchell, P.; Wallace, J.M.W.; Pourshahidi, L.K.; et al. Impact of a (poly)phenol-rich extract from the brown algae Ascophyllum nodosum on DNA damage and antioxidant activity in an overweight or obese population: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 688–700. [Google Scholar] [CrossRef]
- Kannan, G.; Saker, K.; Terrill, T.; Kouakou, B.; Galipalli, S.; Gelaye, S. Effect of seaweed extract supplementation in goats exposed to simulated preslaughter stress. Small Rumin. Res. 2007, 73, 221–227. [Google Scholar] [CrossRef]
- Bai, J.; Wang, R.; Yan, L.; Feng, J. Co-Supplementation of Dietary Seaweed Powder and Antibacterial Peptides Improves Broiler Growth Performance and Immune Function. Braz. J. Poult. Sci. 2019, 21. [Google Scholar] [CrossRef]
- Baleta, F.N.; Bolanos, J.M. Growth and immune response of Pangasius hypophthalmus fed diets containing seaweed extracts as immunostimulant. Braz. Arch. Biol. Technol. 2019, 62. [Google Scholar] [CrossRef]
- Zeynali, M.; Bahabadi, M.N.; Morshedi, V.; Ghasemi, A.; Mozanzadeh, M.T. Replacement of dietary fishmeal with Sargassum ilicifolium meal on growth, innate immunity and immune gene mRNA transcript abundance in Lates calcarifer juveniles. Aquacult. Nutr. 2020, 26, 1657–1668. [Google Scholar] [CrossRef]
- Yeganeh, S.; Adel, M. Effects of dietary algae (Sargassum ilicifolium) as immunomodulator and growth promoter of juvenile great sturgeon (Huso huso Linnaeus, 1758). J. Appl. Phycol. 2018, 31, 2093–2102. [Google Scholar] [CrossRef]
- Zeraatpisheh, F.; Firouzbakhsh, F.; Khalili, K.J. Effects of the macroalga Sargassum angustifolium hot water extract on hematological parameters and immune responses in rainbow trout (Oncohrynchus mykiss) infected with Yersinia rukeri. Environ. Boil. Fishes 2018, 30, 2029–2037. [Google Scholar] [CrossRef]
- Sugiura, Y.; Matsuda, K.; Okamoto, T.; Kakinuma, M.; Amano, H. Anti-allergic effects of the brown alga Eisenia arborea on Brown Norway rats. Fish. Sci. 2008, 74, 180–186. [Google Scholar] [CrossRef]
- Yoshioka, H.; Ishida, M.; Nishi, K.; Oda, H.; Toyohara, H.; Sugahara, T. Studies on anti-allergic activity of Sargassum horneri extract. J. Funct. Foods 2014, 10, 154–160. [Google Scholar] [CrossRef]
- Han, E.J.; Fernando, I.P.S.; Kim, H.-S.; Jeon, Y.-J.; Madusanka, D.M.D.; Dias, M.K.H.M.; Jee, Y.; Ahn, G. Oral Administration of Sargassum horneri Improves the HDM/DNCB-Induced Atopic Dermatitis in NC/Nga Mice. Nutr. 2020, 12, 2482. [Google Scholar] [CrossRef]
- Kim, O.-K.; Lee, M.; Kwon, H.O.; Lee, D.; Park, J.; Kim, E.; You, Y.; Lim, Y.T.; Jun, W.; Lee, J. Costaria costata Extract Suppresses Development of Atopic Dermatitis in chloro-2,4-dinitrobenzene-treated NC/Nga Mice. Skin Pharmacol. Physiol. 2018, 31, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-H.; Song, H.-K.; Lee, A.; Ha, H.; Kim, T. Laminaria japonica Suppresses the Atopic Dermatitis-Like Responses in NC/Nga Mice and Inflamed HaCaT Keratinocytes via the Downregulation of STAT1. Nutrients 2020, 12, 3238. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Park, S.J.; Park, S.M.; Cho, I.J.; Park, C.I.; Kim, Y.W.; Kim, S.C. Inhibition of acute phase inflammation by Laminaria japonica through regulation of inos-Nf- B pathway. Evid. Based Complement Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Kinoshita, Y.; Abe, M.; Murase, N.; Tanaka, R.; Matsushita, T.; Usui, M.; Hanaoka, K.-I.; Miyata, M. Suppressive effects of the diethyl ether fraction from a brown alga Sargassum fusiforme on allergic and inflammatory reactions. Fish. Sci. 2016, 82, 369–377. [Google Scholar] [CrossRef]
- Namkoong, S.; Kang, S.-C.; Do, H.; Jang, K.-H.; Jang, S.-A.; Choung, M.-G.; Sohn, E.-H. Immunomodulatory Effects of Supplementation with Extracts from the Marine Brown Alga Eisenia bicyclis on Macrophages. Korean J. Plant Resour. 2011, 24, 298–303. [Google Scholar] [CrossRef]
- Park, S.Y.; Hwang, E.; Shin, Y.-K.; Lee, D.-G.; Yang, J.-E.; Park, J.-H.; Yi, T.-H. Immunostimulatory effect of enzyme-modified Hizikia fusiforme in a mouse model in vitro and ex vivo. Mar. Biotechnol. 2017, 19, 65–75. [Google Scholar] [CrossRef]
- Joung, E.J.; Gwon, W.-G.; Shin, T.-S.; Jung, B.-M.; Choi, J.; Kim, H.-R. Anti-inflammatory action of the ethanolic extract from Sargassum serratifolium on lipopolysaccharide-stimulated mouse peritoneal macrophages and identification of active components. J. Appl. Phycol. 2017, 29, 563–573. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.-S.; Ranasinghe, P.; Gunasekara, U.K.D.S.S.; Jeon, Y.-J. Antioxidant and anti-inflammatory functionality of ten Sri Lankan seaweed extracts obtained by carbohydrase assisted extraction. Food Sci. Biotechnol. 2018, 27, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, G.; Fouda, W.A.; Ellamie, A.M.; Ibrahim, W.M. Dietary supplementation of Sargassum latifolium modulates thermo-respiratory response, inflammation, and oxidative stress in bacterial endotoxin-challenged male Barki sheep. Environ. Sci. Pollut. Res. 2020, 27, 33863–33871. [Google Scholar] [CrossRef] [PubMed]
- Ellamie, A.M.; Fouda, W.A.; Ibrahim, W.M.; Ramadan, G. Dietary supplementation of brown seaweed (Sargassum latifolium) alleviates the environmental heat stress-induced toxicity in male Barki sheep (Ovis aries). J. Therm. Biol. 2020, 89, 102561. [Google Scholar] [CrossRef]
- Saker, K.E.; Fike, J.H.; Veit, H.; Ward, D.L. Brown seaweed- (TascoTM) treated conserved forage enhances antioxidant status and immune function in heat-stressed wether lambs. J. Anim. Physiol. Anim. Nutr. 2004, 88, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-H.; Lee, S.M.; Na Kim, Y.; Jeon, Y.-J.; Heo, J.-D.; Jeong, E.J.; Rho, J.-R. Standardized Fraction of Turbinaria ornata Alleviates Dextran Sulfate Sodium-Induced Chronic Colitis in C57BL/6 Mice via Upregulation of FOXP3+ Regulatory T Cells. Biomolecules 2020, 10, 1463. [Google Scholar] [CrossRef]
- Ko, S.J.; Bu, Y.; Bae, J.; Bang, Y.-m.; Kim, J.; Lee, H.; Lee, B.-J.; Hyun, Y.H.; Park, J.-W. Protective effect of Laminaria japonica with probiotics on murine colitis. Mediat. Inflamm. 2014, 2014, 417814. [Google Scholar] [CrossRef]
- Jeon, H.; Yoon, W.-J.; Ham, Y.-M.; Yoon, S.-A.; Kang, S.C. Anti-Arthritis Effect through the Anti-Inflammatory Effect of Sargassum muticum Extract in Collagen-Induced Arthritic (CIA) Mice. Molecules 2019, 24, 276. [Google Scholar] [CrossRef]
- Cooper, R.; Dragar, C.; Elliot, K.; Fitton, J.; Godwin, J.; Thompson, K. GFS, a preparation of Tasmanian Undaria pinnatifida is associated with healing and inhibition of reactivation of Herpes. BMC Complement. Altern. Med. 2002, 2, 11. [Google Scholar] [CrossRef]
- Imai, T.; Takahashi, Y. Chemotaxis Assay for Marsupenaeus japonicas Hemocytes and Application for the Development of an Oral Immunostimulant Against White Spot Syndrome Virus. Front. Cell Dev. Biol. 2020, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-G.; Shin, Y.-K.; Park, J.-H.; Park, S.-Y.; Hwang, E.; Yang, J.-E.; Jo, H.; Kim, K.-Y.; Mavlonov, G.T.; Yi, T.-H. Alveolar Bone Protective Effect of Hiziki Extracts on the Progression of Periodontitis. Mar. Biotechnol. 2018, 20, 313–323. [Google Scholar] [CrossRef]
- Song, X.-H.; Zan, R.-Z.; Yu, C.-H.; Wang, F. Effects of modified Haizao Yuhu Decoction in experimental autoimmune thyroiditis rats. J. Ethnopharmacol. 2011, 135, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Oh, J.-H.; Kim, J.; Lee, Y. Anti-inflammatory and anti-diabetic effects of brown seaweeds in high-fat diet-induced obese mice. Nutr. Res. Pract. 2016, 10, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Villaluenga, C.; Peñas, E.; Rico, D.; Martin-Diana, A.B.; Portillo, M.P.; Macarulla, M.T.; De Luis, D.A.; Miranda, J. Potential Usefulness of a Wakame/Carob Functional Snack for the Treatment of Several Aspects of Metabolic Syndrome: From In Vitro to In Vivo Studies. Mar. Drugs 2018, 16, 512. [Google Scholar] [CrossRef]
- Mendez, R.L.; Miranda, C.; Armour, C.R.; Sharpton, T.J.; Stevens, J.F.; Kwon, J.Y. Supplementation with Sea Vegetables Palmaria mollis and Undaria pinnatifida Exerts Metabolic Benefits in Diet-Induced Obesity in Mice. Curr. Dev. Nutr. 2020, 4, nzaa072. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lee, S.Y.; Lee, S.R.; Pyun, B.-J.; Kim, H.J.; Lee, Y.H.; Kwon, S.W.; Suh, D.H.; Lee, C.H.; Hong, E.-J.; et al. Therapeutic Effect of Ecklonia cava Extract in Letrozole-Induced Polycystic Ovary Syndrome Rats. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. Environ. Boil. Fishes 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Van Weelden, G.; Bobinski, M.; Okla, K.; van Weelden, W.J.; Romano, A.; Pijnenborg, A.M.A. Fucoidan Structure and Activity in Relation to Anti-Cancer Mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Laurienzo, P. Marine Polysaccharides in Pharmaceutical Applications: An Overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef]
- Zhao, X.; Xue, C.-H.; Li, B.-F. Study of antioxidant activities of sulfated polysaccharides from Laminaria japonica. J. Appl. Phycol. 2007, 20, 431–436. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from Fucoidan: An Update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, T.; Nakazato, K.; Tomioka, S.; Iha, M.; Nakajima, K. Intestinal Absorption of Fucoidan Extracted from the Brown Seaweed, Cladosiphon okamuranus. Mar. Drugs 2014, 13, 48–64. [Google Scholar] [CrossRef]
- Shang, Q.; Song, G.; Zhang, M.; Shi, J.; Xu, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan improves metabolic syndrome in association with increased Akkermansia population in the gut microbiota of high-fat diet-fed mice. J. Funct. Foods 2017, 28, 138–146. [Google Scholar] [CrossRef]
- Kadena, K.; Tomori, M.; Iha, M.; Nagamine, T. Absorption Study of Mozuku Fucoidan in Japanese Volunteers. Mar. Drugs 2018, 16, 254. [Google Scholar] [CrossRef]
- Iraha, A. Fucoidan enhances intestinal barrier function by upregulating the expression of claudin-1. World J. Gastroenterol. 2013, 19, 5500–5507. [Google Scholar] [CrossRef]
- Chauvierre, C.; Aid-Launais, R.; Aerts, J.; Chaubet, F.; Maire, M.; Chollet, L.; Rolland, L.; Bonafé, R.; Rossi, S.; Bussi, S.; et al. Pharmaceutical Development and Safety Evaluation of a GMP-Grade Fucoidan for Molecular Diagnosis of Cardiovascular Diseases. Mar. Drugs 2019, 17, 699. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Q.; Song, J. Toxicological evaluation of fucoidan extracted from Laminaria japonica in Wistar rats. Food Chem. Toxicol. 2005, 43, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Takeda, K.; Tomimori, K.; Kimura, R.; Ishikawa, C.; Nowling, T.K. Anti-tumor activity of fucoidan is mediated by nitric oxide released from macrophages. Int. J. Oncol. 2011, 40, 251–260. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Fernando, I.P.S.; Lee, W.W.; Sanjeewa, K.K.A.; Kim, H.-S.; Lee, D.-S.; Jeon, Y.-J. Isolation and purification of fucoidan fraction in Turbinaria ornata from the Maldives; Inflammation inhibitory potential under LPS stimulated conditions in in-vitro and in-vivo models. Int. J. Biol. Macromol. 2019, 131, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Wang, L.; Fu, X.; Duan, D.; Jeon, Y.-J.; Xu, J.; Gao, X. In vitro and in vivo anti-inflammatory activities of a fucose-rich fucoidan isolated from Saccharina japonica. Int. J. Biol. Macromol. 2020, 156, 717–729. [Google Scholar] [CrossRef]
- Park, J.; Cha, J.-D.; Choi, K.-M.; Lee, K.-Y.; Han, K.M.; Jang, Y.-S. Fucoidan inhibits LPS-induced inflammation in vitro and during the acute response in vivo. Int. Immunopharmacol. 2017, 43, 91–98. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Fernando, I.; Kim, S.-Y.; Kim, H.-S.; Ahn, G.; Jee, Y.; Jeon, Y.-J. In vitro and in vivo anti-inflammatory activities of high molecular weight sulfated polysaccharide; containing fucose separated from Sargassum horneri: Short communication. Int. J. Biol. Macromol. 2018, 107, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Herath, K.H.I.N.M.; Kim, H.J.; Kim, A.; Sook, C.E.; Lee, B.-Y.; Jee, Y. The Role of Fucoidans Isolated from the Sporophylls of Undaria pinnatifida against Particulate-Matter-Induced Allergic Airway Inflammation: Evidence of the Attenuation of Oxidative Stress and Inflammatory Responses. Molecules 2020, 25, 2869. [Google Scholar] [CrossRef]
- Lin, R.; Liu, X.; Meng, Y.; Xu, M.; Guo, J. Effects of Laminaria japonica polysaccharides on airway inflammation of lungs in an asthma mouse model. Multidiscip. Respir. Med. 2015, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Tanino, Y.; Hashimoto, T.; Ojima, T.; Mizuno, M. F-fucoidan from Saccharina japonica is a novel inducer of galectin-9 and exhibits anti-allergic activity. J. Clin. Biochem. Nutr. 2016, 59, 25–30. [Google Scholar] [CrossRef]
- Mizuno, M.; Sakaguchi, K.; Sakane, I. Oral Administration of Fucoidan Can Exert Anti-Allergic Activity after Allergen Sensitization by Enhancement of Galectin-9 Secretion in Blood. Biomolecules 2020, 10, 258. [Google Scholar] [CrossRef]
- Hwang, P.-A.; Lin, H.-T.V.; Lin, H.-Y.; Lo, S.-K. Dietary Supplementation with Low-Molecular-Weight Fucoidan Enhances Innate and Adaptive Immune Responses and Protects against Mycoplasma pneumoniae Antigen Stimulation. Mar. Drugs 2019, 17, 175. [Google Scholar] [CrossRef] [PubMed]
- Tomori, M.; Nagamine, T.; Miyamoto, T.; Iha, M. Evaluation of the Immunomodulatory Effects of Fucoidan Derived from Cladosiphon Okamuranus Tokida in Mice. Mar. Drugs 2019, 17, 547. [Google Scholar] [CrossRef]
- Yu, B.; Bi, D.; Yao, L.; Li, T.; Gu, L.; Xu, H.; Li, X.; Li, H.; Hu, Z.-L.; Xu, X. The inhibitory activity of alginate against allergic reactions in an ovalbumin-induced mouse model. Food Funct. 2020, 11, 2704–2713. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.G.; Sweeney, T.; Bahar, B.; O’Doherty, J.V. Effect of maternal seaweed extract supplementation on suckling piglet growth, humoral immunity, selected microflora, and immune response after an ex vivo lipopolysaccharide challenge1. J. Anim. Sci. 2012, 90, 505–514. [Google Scholar] [CrossRef]
- Walsh, A.M.; Sweeney, T.; O’Shea, C.J.; Doyle, D.N.; O’Doherty, J.V. Effects of supplementing dietary laminarin and fucoidan on intestinal morphology and the immune gene expression in the weaned pig. J. Anim. Sci. 2012, 90, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Azizi, A.F.N.; Miyazaki, R.; Yumito, T.; Ohashi, Y.; Uno, S.; Miyajima, U.; Kumamoto, M.; Uchiyama, S.; Yasuda, M. Effect of maternal supplementation with seaweed powder on immune status of liver and lymphoid organs of piglets. J. Vet. Med. Sci. 2018, 80, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Heim, G.; O’Doherty, J.V.; O’Shea, C.; Doyle, D.N.; Egan, A.M.; Thornton, K.; Sweeney, T. Maternal supplementation of seaweed-derived polysaccharides improves intestinal health and immune status of suckling piglets. J. Nutr. Sci. 2015, 4, e27. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, R.P.; Doherty, J.V.O.; Earley, B.; Clarke, A.M.; Kenny, D.A. Effect of supplementation with n-3 polyunsaturated fatty acids and/or β-glucans on performance, feeding behaviour and immune status of Holstein Friesian bull calves during the pre- and post-weaning periods. J. Anim. Sci. Biotechnol. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Yan, G.L.; Guo, Y.M.; Yuan, J.M.; Liu, D.; Zhang, B.K. Sodium alginate oligosaccharides from brown algae inhibit Salmonella Enteritidis colonization in broiler chickens. Poult. Sci. 2011, 90, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- El-Boshy, M.; El-Ashram, A.; Risha, E.; Abdelhamid, F.; Zahran, E.; Gab-Alla, A. Dietary fucoidan enhance the non-specific immune response and disease resistance in African catfish, Clarias gariepinus, immunosuppressed by cadmium chloride. Vet. Immunol. Immunopathol. 2014, 162, 168–173. [Google Scholar] [CrossRef]
- Mir, I.N.; Sahu, N.P.; Pal, A.K.; Makesh, M. Synergistic effect of l-methionine and fucoidan rich extract in eliciting growth and non-specific immune re-sponse of Labeo rohita fingerlings against Aeromonas hydrophila. Aquaculture 2017, 479, 396–403. [Google Scholar] [CrossRef]
- Morales-Lange, B.; Bethke, J.; Schmitt, P.; Mercado, L. Phenotypical parameters as a tool to evaluate the immunostimulatory effects of laminarin in Oncorhynchus mykiss. Aquac. Res. 2014, 46, 2707–2715. [Google Scholar] [CrossRef]
- Prabu, D.L.; Sahu, N.P.; Pal, A.K.; Dasgupta, S.; Narendra, A. Immunomodulation and interferon gamma gene expression in sutchi cat fish, Pangasianodon hypophthalmus: Effect of dietary fucoidan rich seaweed extract (FRSE) on pre and post challenge period. Aquac. Res. 2016, 47, 199–218. [Google Scholar] [CrossRef]
- Setyawan, A.; Isnansetyo, A.; Murwantoko, M.; Soedarmanto, I.; Handayani, C.R. Comparative immune response of dietary fucoidan from three indonesian brown algae in white shrimp Litopenaeus vannamei. AACL Bioflux 2018, 11, 1707–1723. [Google Scholar]
- Yang, Q.; Yang, R.; Li, M.; Zhou, Q.; Liang, X.; Elmada, Z.C. Effects of dietary fucoidan on the blood constituents, anti-oxidation and innate immunity of juvenile yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2014, 41, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Li, W.; Lin, Q.; Lin, X.; Lin, J.; Zhu, Q.; Jiang, H.; Huang, Z. Dietary administration of laminarin improves the growth performance and immune responses in Epinephelus coioides. Fish Shellfish Immunol. 2014, 41, 402–406. [Google Scholar] [CrossRef]
- Katayama, S.; Nishio, T.; Kishimura, H.; Saeki, H. Immunomodulatory Properties of Highly Viscous Polysaccharide Extract from the Gagome Alga (Kjellmaniella crassifolia). Plant Foods Hum. Nutr. 2012, 67, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, J.; Wada-Funada, U.; Mano, H.; Matahira, Y.; Kawaguchi, M.; Wada, M. Proportion of Murine Cytotoxic T Cells is Increased by High Molecular-Weight Fucoidan Extracted from Okinawa mozuku (Cladosiphon okamuranus). J. Health Sci. 2005, 51, 394–397. [Google Scholar] [CrossRef]
- Kar, S.; Sharma, G.; Das, P.K. Fucoidan cures infection with both antimony-susceptible and -resistant strains of Leishmania donovani through Th1 response and macrophage-derived oxidants. J. Antimicrob. Chemother. 2011, 66, 618–625. [Google Scholar] [CrossRef]
- Hayashi, T.; Hayashi, K.; Kanekiyo, K.; Ohta, Y.; Lee, J.-B.; Hashimoto, M.; Nakano, T. Promising Antiviral Glyco-Molecules from an Edible Alga. In Combating the Threat of Pandemic Influenza: Drug Discovery Approaches; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 166–182. [Google Scholar] [CrossRef]
- Araya, N.; Takahashi, K.; Sato, T.; Nakamura, T.; Sawa, C.; Hasegawa, D.; Ando, H.; Aratani, S.; Yagishita, N.; Fujii, R.; et al. Fucoidan therapy decreases the proviral load in patients with human T-lymphotropic virus type-1-associated neurological disease. Antivir. Ther. 2011, 16, 89–98. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakano, T.; Hashimoto, M.; Kanekiyo, K.; Hayashi, T. Defensive effects of a fucoidan from brown alga Undaria pinnatifida against herpes simplex virus infection. Int. Immunopharmacol. 2008, 8, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Lee, J.-B.; Nakano, T.; Hayashi, T. Anti-influenza A virus characteristics of a fucoidan from sporophyll of Undaria pinnatifida in mice with normal and compromised immunity. Microbes Infect. 2013, 15, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Tamauchi, H.; Iizuka, M.; Nakano, T. The Role of NK cells in Antitumor Activity of Dietary Fucoidan from Undaria pinnatifida Sporophylls (Mekabu). Planta Med. 2006, 72, 1415–1417. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Ishihara, T.; Nakamoto, H.; Amaha, T.; Osaki, T.; Tsuka, T.; Imagawa, T.; Minami, S.; Takashima, O.; Ifuku, S.; et al. Effects of Oral Administration of Fucoidan Extracted from Cladosiphon okamuranus on Tumor Growth and Survival Time in a Tumor-Bearing Mouse Model. Mar. Drugs 2012, 10, 2337–2348. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zhang, J.; Nie, W.; Zhou, W.; Jin, L.; Chen, X.; Lu, J. Antitumor effects of polysaccharide from Sargassum fusiforme against human hepatocellular carcinoma HepG2 cells. Food Chem. Toxicol. 2017, 102, 53–62. [Google Scholar] [CrossRef]
- Chen, X.; Nie, W.; Yu, G.; Li, Y.; Hu, Y.; Lu, J.; Jin, L. Antitumor and immunomodulatory activity of polysaccharides from Sargassum fusiforme. Food Chem. Toxicol. 2012, 50, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Yu, G.; Nie, W.; Jin, J.; Chen, L.; Chen, X. Antitumor activity and underlying mechanism of Sargassum fusiforme polysaccharides in CNE-bearing mice. Int. J. Biol. Macromol. 2018, 112, 516–522. [Google Scholar] [CrossRef]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.; Holloway, A.; Karpiniec, S.S.; Dickinson, J.L. Fucoidan Suppresses the Growth of Human Acute Promyelocytic Leukemia Cells In Vitro and In Vivo. J. Cell. Physiol. 2015, 231, 688–697. [Google Scholar] [CrossRef]
- Xue, M.; Liang, H.; Tang, Q.; Xue, C.; He, X.; Zhang, L.; Zhang, Z.; Liang, Z.; Bian, K.; Zhang, L.; et al. The Protective and Immunomodulatory Effects of Fucoidan Against 7,12-Dimethyl benz[a]anthracene-Induced Experimental Mammary Carcinogenesis Through the PD1/PDL1 Signaling Pathway in Rats. Nutr. Cancer 2017, 69, 1234–1244. [Google Scholar] [CrossRef]
- Jiang, Z.; Abu, R.; Isaka, S.; Nakazono, S.; Ueno, M.; Okimura, T.; Yamaguchi, K.; Oda, T. Inhibitory effect of orally-administered sulfated polysaccharide ascophyllan isolated from ascophyllum nodosum on the growth of sarcoma-180 solid tumor in mice. Anticancer Res. 2014, 34, 1663–1671. [Google Scholar] [PubMed]
- Chen, X.; Nie, W.; Fan, S.; Zhang, J.; Wang, Y.; Lu, J.; Jin, L. A polysaccharide from Sargassum fusiforme protects against immunosuppression in cyclophospha-mide-treated mice. Carbohydr. Polym. 2012, 90, 1114–1119. [Google Scholar] [CrossRef]
- Lee, H.H.; Cho, Y.; Kim, G.-H.; Cho, H. Undaria pinnatifida Fucoidan-Rich Extract Recovers Immunity of Immunosuppressed Mice. J. Microbiol. Biotechnol. 2020, 30, 439–447. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, R.; Jian, Z.; Yu, H. Laminarin enhances the activity of natural killer cells in immunosuppressed mice. Cent. Eur. J. Immunol. 2019, 44, 357–363. [Google Scholar] [CrossRef]
- Hwang, P.-A.; Hung, Y.-L.; Chien, S.-Y. Inhibitory activity of Sargassum hemiphyllum sulfated polysaccharide in arachidonic acid-induced animal models of inflammation. J. Food Drug Anal. 2015, 23, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Kyung, J.; Kim, D.; Park, D.; Yang, Y.-H.; Choi, E.-K.; Lee, S.-P.; Kim, T.-S.; Lee, Y.-B.; Kim, Y.-B. Synergistic anti-inflammatory effects of Laminaria japonica fucoidan and Cistanche tubulosa extract. Lab. Anim. Res. 2012, 28, 91–97. [Google Scholar] [CrossRef]
- Choi, J.-I.; Raghavendran, H.R.B.; Sung, N.-Y.; Kim, J.-H.; Chun, B.S.; Ahn, D.H.; Choi, H.-S.; Kang, K.-W.; Lee, J.-W. Effect of fucoidan on aspirin-induced stomach ulceration in rats. Chem. Interact. 2010, 183, 249–254. [Google Scholar] [CrossRef]
- Matsumoto, S.; Nagaoka, M.; Hara, T.; Kimura-Takagi, I.; Mistuyama, K.; Ueyama, S. Fucoidan derived from Cladosiphon okamuranus Tokida ameliorates murine chronic colitis through the down-regulation of interleukin-6 production on colonic epithelial cells. Clin. Exp. Immunol. 2004, 136, 432–439. [Google Scholar] [CrossRef]
- Lean, Q.Y.; Eri, R.D.; Fitton, J.H.; Patel, R.P.; Gueven, N. Fucoidan Extracts Ameliorate Acute Colitis. PLoS ONE 2015, 10, e0128453. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ai, C.; Wen, C.; Qin, Y.; Liu, Z.; Wang, L.; Gong, Y.; Su, C.; Wang, Z.; Song, S. Fucoidan isolated from Ascophyllum nodosum alleviates gut microbiota dysbiosis and colonic inflammation in antibiotic-treated mice. Food Funct. 2020, 11, 5595–5606. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Liang, H.; Ji, X.; Zhou, Z.; Liu, Y.; Sun, T.; Zhang, L. Effects of fucoidan on gut flora and tumor prevention in 1,2-dimethylhydrazine-induced colorectal carcino-genesis. J. Nutr. Biochem. 2020, 82, 108396. [Google Scholar] [CrossRef]
- Park, S.-B.; Chun, K.-R.; Kim, J.-K.; Suk, K.; Jung, Y.-M.; Lee, W.-H. The differential effect of high and low molecular weight fucoidans on the severity of collagen-induced arthritis in mice. Phytother. Res. 2010, 24, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-W.; Hwang, S.J.; Han, M.H.; Lee, D.-S.; Yoo, J.S.; Choi, I.-W.; Cha, H.-J.; Kim, S.; Kim, H.-S.; Kim, G.-Y.; et al. Fucoidan inhibits lipopolysaccharide-induced inflammatory responses in RAW 264.7 macrophages and zebrafish larvae. Mol. Cell. Toxicol. 2017, 13, 405–417. [Google Scholar] [CrossRef]
- Li, C.; Gao, Y.; Xing, Y.; Zhu, H.; Shen, J.; Tian, J. Fucoidan, a sulfated polysaccharide from brown algae, against myocardial ischemia-reperfusion injury in rats via regulating the inflammation response. Food Chem. Toxicol. 2011, 49, 2090–2095. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-H.; Chengchuan Ko, E.; Chang, C.-L.; Yuan, K.S.-P.; Wu, A.T.H.; Shan, Y.-S.; Wu, S.-Y. Fucoidan Inhibits Radiation-Induced Pneumonitis and Lung Fibrosis by Reducing Inflammatory Cytokine Expression in Lung Tissues. Mar. Drugs 2018, 16, 392. [Google Scholar] [CrossRef]
- Bai, X.; Li, M.; Wang, X.; Chang, H.; Ni, Y.; Li, C.; He, K.; Wang, H.; Yang, Y.; Tian, T.; et al. Therapeutic potential of fucoidan in the reduction of hepatic pathology in murine schistosomiasis japonica. Parasites Vectors 2020, 13, 1–14. [Google Scholar] [CrossRef]
- Li, J.; Chen, K.; Li, S.; Liu, T.; Wang, F.; Xia, Y.; Lu, J.; Zhou, Y.; Guo, C. Pretreatment with Fucoidan from Fucus vesiculosus Protected against ConA-Induced Acute Liver Injury by Inhibiting Both Intrinsic and Extrinsic Apoptosis. PLoS ONE 2016, 11, e0152570. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Liang, H.; Ji, X.; Liu, Y.; Ge, Y.; Hou, L.; Sun, T. Fucoidan prevent murine autoimmune diabetes via suppression TLR4-signaling pathways, regulation DC/Treg induced immune tolerance and improving gut microecology. Nutr. Metab. 2019, 16, 1–15. [Google Scholar] [CrossRef]
- Ko, W.-S.; Shen, F.-P.; Shih, C.-J.; Chiou, Y.-L. The 25(OH)Vitamin D Status Affected the Effectiveness of Oligo Fucoidan in Patients with Chronic Hepatitis B Virus Infection with Immune Tolerance Phase. Nutrients 2020, 12, 321. [Google Scholar] [CrossRef]
- Myers, S.P.; O’Connor, J.; Fitton, J.H.; Brooks, L.; Rolfe, M.; Connellan, P.; Wohlmuth, H.; Cheras, P.A.; Morris, C. A combined phase I and II open label study on the effects of a seaweed extract nutrient complex on oste-oarthritis. Biol. Targets Ther. 2010, 4, 33–44. [Google Scholar] [CrossRef]
- Takahashi, H.; Kawaguchi, M.; Kitamura, K.; Narumiya, S.; Kawamura, M.; Tengan, I.; Nishimoto, S.; Hanamure, Y.; Majima, Y.; Tsubura, S.; et al. An Exploratory Study on the Anti-inflammatory Effects of Fucoidan in Relation to Quality of Life in Advanced Cancer Patients. Integr. Cancer Ther. 2017, 17, 282–291. [Google Scholar] [CrossRef]
- Peng, F.-H.; Zha, X.-Q.; Cui, S.-H.; Asghar, M.-N.; Pan, L.-H.; Wang, J.-H.; Luo, J.-P. Purification, structure features and anti-atherosclerosis activity of a Laminaria japonica polysaccharide. Int. J. Biol. Macromol. 2015, 81, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Fucoidan attenuates atherosclerosis in LDLR−/− mice through inhibition of inflammation and oxidative stress. Int. J. Clin. Exp. Pathol. 2016, 9, 6896–6904. [Google Scholar]
- Xu, Y.; Xu, J.; Ge, K.; Tian, Q.; Zhao, P.; Guo, Y. Anti-inflammatory effect of low molecular weight fucoidan from Saccharina japonica on atherosclerosis in apoE-knockout mice. Int. J. Biol. Macromol. 2018, 118, 365–374. [Google Scholar] [CrossRef]
- Soin, J.; Kurzejamska, E.; Gaciong, Z.; Henrykowska, G.; Bojakowski, K. Fucoidan Inhibits Vascular Remodeling in Transplant Vasculopathy in Rat. Funct. Foods Health Dis. 2018, 8, 323. [Google Scholar] [CrossRef]
- Zhou, M.; Ding, Y.; Cai, L.; Wang, Y.; Lin, C.; Shi, Z. Low molecular weight fucoidan attenuates experimental abdominal aortic aneurysm through interfering the leukocyte-endothelial cells interaction. Mol. Med. Rep. 2018, 17, 7089–7096. [Google Scholar] [CrossRef]
- Mo, W.; Wang, C.; Li, J.; Chen, K.; Xia, Y.; Li, S.; Xu, L.; Lu, X.; Wang, W.; Guo, C. Fucosterol Protects against Concanavalin A-Induced Acute Liver Injury: Focus on P38 MAPK/NF-kappaB Pathway Activity. Gastroenterol. Res. Pract. 2018, 2018, 2824139. [Google Scholar] [CrossRef] [PubMed]
- Bogie, J.; Hoeks, C.; Schepers, M.; Tiane, A.; Cuypers, A.; Leijten, F.; Chintapakorn, Y.; Suttiyut, T.; Pornpakakul, S.; Struik, D.; et al. Dietary Sargassum fusiforme improves memory and reduces amyloid plaque load in an Alzheimer’s disease mouse model. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Lacy, M.; Atzler, D.; Liu, R.; de Winther, M.; Weber, C.; Lutgens, E. Interactions between dyslipidemia and the immune system and their relevance as putative therapeutic targets in atherosclerosis. Pharmacol. Ther. 2019, 193, 50–62. [Google Scholar] [CrossRef]
- Schulman, I.G. Liver X receptors link lipid metabolism and inflammation. FEBS Lett. 2017, 591, 2978–2991. [Google Scholar] [CrossRef]
- Gonzalez, N.A.; Castrillo, A. Liver X receptors as regulators of macrophage inflammatory and metabolic pathways. Biochim. Biophys. Acta. 2011, 1812, 982–994. [Google Scholar] [CrossRef]
- Irfan, M.; Kwon, T.-H.; Yun, B.-S.; Park, N.-H.; Rhee, M.H. Eisenia bicyclis (brown alga) modulates platelet function and inhibits thrombus formation via impaired P 2 Y 12 receptor signaling pathway. Phytomedicine 2018, 40, 79–87. [Google Scholar] [CrossRef]
- Han, E.J.; Kim, H.S.; Sanjeewa, K.K.A.; Herath, K.; Jeon, Y.J.; Jee, Y.; Lee, J.; Kim, T.; Shim, S.Y.; Ahn, G. Eckol from Ecklonia cava Suppresses Immunoglobulin E-mediated Mast Cell Activation and Passive Cutaneous Anaphylaxis in Mice. Nutrients 2020, 12, 1361. [Google Scholar] [CrossRef] [PubMed]
- Herath, K.H.I.N.M.; Mihindukulasooriya, S.P.; Kim, H.J.; Kim, A.; Kim, H.J.; Jeon, Y.J.; Jee, Y. Oral administration of polyphenol-rich Sargassum horneri suppresses particulate matter exacer-bated airway inflammation in murine allergic asthma: Relevance to the TLR mediated NF-kappa B pathway inhibition. J. Funct. Foods 2020, 71, 103991. [Google Scholar] [CrossRef]
- Sugiura, Y.; Nagayama, K.; Kinoshita, Y.; Tanaka, R.; Matsushita, T. The anti-allergic effect of the ethyl acetate fraction from an Ecklonia kurome extract. Food Agric. Immunol. 2015, 26, 181–193. [Google Scholar] [CrossRef]
- Son, M.; Oh, S.; Choi, J.; Jang, J.T.; Choi, C.H.; Park, K.Y.; Son, K.H.; Byun, K. Attenuation of Inflammation and Leptin Resistance by Pyrogallol-Phloroglucinol-6,6-Bieckol on in the Brain of Obese Animal Models. Nutrients 2019, 11, 2773. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Oh, S.; Choi, J.; Jang, J.T.; Choi, C.H.; Park, K.Y.; Son, K.H.; Byun, K. The Phlorotannin-Rich Fraction of Ecklonia cava Extract Attenuated the Expressions of the Markers Related with Inflammation and Leptin Resistance in Adipose Tissue. Int. J. Endocrinol. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Eo, H.; Jeon, Y.-J.; Lee, M.; Lim, Y. Brown Alga Ecklonia cava Polyphenol Extract Ameliorates Hepatic Lipogenesis, Oxidative Stress, and Inflammation by Activation of AMPK and SIRT1 in High-Fat Diet-Induced Obese Mice. J. Agric. Food Chem. 2014, 63, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-I.; Ahn, J.-H.; Choi, Y.S.; Choi, J.-H. Brown algae phlorotannins enhance the tumoricidal effect of cisplatin and ameliorate cisplatin nephrotoxicity. Gynecol. Oncol. 2015, 136, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, S.-I.; Kim, G.-H.; Imm, J.-Y. Anti-Inflammatory Effect of Ecklonia cava Extract on Porphyromonas gingivalis Lipopolysaccharide-Stimulated Macrophages and a Periodontitis Rat Model. Nutrients 2019, 11, 1143. [Google Scholar] [CrossRef]
- Kim, E.A.; Kim, S.Y.; Ye, B.R.; Kim, J.; Ko, S.C.; Lee, W.W.; Kim, K.N.; Choi, I.W.; Jung, W.K.; Heo, S.J. Anti-inflammatory effect of Apo-9′-fucoxanthinone via inhibition of MAPKs and NF-kB signaling pathway in LPS-stimulated RAW 264.7 macrophages and zebrafish model. Int. Immunopharmacol. 2018, 59, 339–346. [Google Scholar] [CrossRef]
- Kang, K.; Park, Y.; Hwang, H.J.; Kim, S.H.; Lee, J.G.; Shin, H.-C. Antioxidative properties of brown algae polyphenolics and their perspectives as chemopreventive agents against vascular risk factors. Arch. Pharmacal. Res. 2003, 26, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, E.A.; Kang, M.C.; Lee, J.H.; Yang, H.W.; Lee, J.S.; Lim, T.I.; Jeon, Y.J. Polyphenol-rich fraction from Ecklonia cava (a brown alga) processing by-product reduces LPS-induced in-flammation in vitro and in vivo in a zebrafish model. Algae 2014, 29, 165–174. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Kim, H.S.; Sanjeewa, K.K.A.; Oh, J.Y.; Jeon, Y.J.; Lee, W.W. Inhibition of inflammatory responses elicited by urban fine dust particles in keratinocytes and macro-phages by diphlorethohydroxy-carmalol isolated from a brown alga Ishige okamurae. Algae 2017, 32, 261–273. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Zhang, M.; Chen, Y.; Zhu, T.; Wang, J. Protective Effect of Eckol against Acute Hepatic Injury Induced by Carbon Tetrachloride in Mice. Mar. Drugs 2018, 16, 300. [Google Scholar] [CrossRef]
- Sugiura, Y.; Usui, M.; Katsuzaki, H.; Imai, K.; Kakinuma, M.; Amano, H.; Miyata, M. Orally Administered Phlorotannins from Eisenia arborea Suppress Chemical Mediator Release and Cy-clooxygenase-2 Signaling to Alleviate Mouse Ear Swelling. Mar. Drugs 2018, 16, 267. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Oh, S.; Son, M.; Byun, K. Pyrogallol-Phloroglucinol-6,6-Bieckol Alleviates Obesity and Systemic Inflammation in a Mouse Model by Reducing Expression of RAGE and RAGE Ligands. Mar. Drugs 2019, 17, 612. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Oh, S.; Lee, H.S.; Chung, D.-M.; Jang, J.T.; Jeon, Y.-J.; Choi, C.H.; Park, K.Y.; Son, K.H.; Byun, K. Ecklonia Cava Extract Attenuates Endothelial Cell Dysfunction by Modulation of Inflammation and Brown Adipocyte Function in Perivascular Fat Tissue. Nutrients 2019, 11, 2795. [Google Scholar] [CrossRef]
- Goc, A.; Gehring, G.; Baltin, H.; Niedzwiecki, A.; Rath, M. Specific composition of polyphenolic compounds with fatty acids as an approach in helping to reduce spirochete burden in Lyme disease: In vivo and human observational study. Ther. Adv. Chronic Dis. 2020, 11. [Google Scholar] [CrossRef]
- Park, E.Y.; Kim, E.H.; Kim, M.H.; Seo, Y.W.; Lee, J.I.; Jun, H.S. Polyphenol-Rich Fraction of Brown Alga Ecklonia cava Collected from Gijang, Korea, Reduces Obesity and Glucose Levels in High-Fat Diet-Induced Obese Mice. Evid. Based Complement Altern. Med. 2012, 2012, 418912. [Google Scholar] [CrossRef]
- Zhao, Z.; Bao, X.Q.; Zhang, Z.; Liu, H.; Zhang, D. Phloroglucinol derivative compound 21 attenuates cuprizone-induced multiple sclerosis mice through pro-moting remyelination and inhibiting neuroinflammation. Sci. China Life Sci. 2020, 63, 905–914. [Google Scholar] [CrossRef]
- Zhao, Z.; Bao, X.-Q.; Zhang, Z.; Li, F.; Liu, H.; Zhang, D. Novel phloroglucinol derivative Compound 21 protects experimental autoimmune encephalomyelitis rats via inhibiting Th1/Th17 cell infiltration. Brain Behav. Immun. 2020, 87, 751–764. [Google Scholar] [CrossRef]
- Miyashita, K.; Hosokawa, M. Carotenoids as a Nutraceutical Therapy for Visceral Obesity. In Nutrition in the Prevention and Treatment of Abdominal Obesity; Watson, R.R., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 329–340. [Google Scholar]
- Sugawara, T.; Baskaran, V.; Tsuzuki, W.; Nagao, A. Brown Algae Fucoxanthin Is Hydrolyzed to Fucoxanthinol during Absorption by Caco-2 Human Intestinal Cells and Mice. J. Nutr. 2002, 132, 946–951. [Google Scholar] [CrossRef]
- Maeda, H.; Kanno, S.; Kodate, M.; Hosokawa, M.; Miyashita, K. Fucoxanthinol, Metabolite of Fucoxanthin, Improves Obesity-Induced Inflammation in Adipocyte Cells. Mar. Drugs 2015, 13, 4799–4813. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J. Fucoxanthin and Its Metabolite Fucoxanthinol in Cancer Prevention and Treatment. Mar. Drugs 2015, 13, 4784–4798. [Google Scholar] [CrossRef]
- Menna, M.; Imperatore, C.; D’Aniello, F.; Aiello, A. Meroterpenes from Marine Invertebrates: Structures, Occurrence, and Ecological Implications. Mar. Drugs 2013, 11, 1602–1643. [Google Scholar] [CrossRef]
- Gwon, W.-G.; Joung, E.-J.; Shin, T.; Utsuki, T.; Wakamatsu, N.; Kim, H.-R. Meroterpinoid-rich fraction of the ethanol extract from Sargassum serratifolium suppresses TNF-α-induced monocytes adhesion to vascular endothelium and vascular inflammation in high cholesterol-fed C57BL/6J mice. J. Funct. Foods 2018, 46, 384–393. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A Promising Medicinal and Nutritional Ingredient. Evid. Based Complement. Altern. Med. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Kong, Z.-L.; Sudirman, S.; Hsu, Y.-C.; Su, C.-Y.; Kuo, H.-P. Fucoxanthin-Rich Brown Algae Extract Improves Male Reproductive Function on Streptozoto-cin-Nicotinamide-Induced Diabetic Rat Model. Int. J. Mol. Sci. 2019, 20, 4485. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.-L.; Kao, N.-J.; Hu, J.-Y.; Wu, C.-S. Fucoxanthin-Rich Brown Algae Extract Decreases Inflammation and Attenuates Colitis-associated Colon Cancer in Mice. J. Food Nutr. Res. 2016, 4, 137–147. [Google Scholar]
- Wang, P.-T.; Sudirman, S.; Hsieh, M.-C.; Hu, J.-Y.; Kong, Z.-L. Oral supplementation of fucoxanthin-rich brown algae extract ameliorates cisplatin-induced testicular damage in hamsters. Biomed. Pharmacother. 2020, 125, 109992. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Tan, C.-P.; Hou, Y.-H. First Evidence for the Anti-inflammatory Activity of Fucoxanthin in High-Fat-Diet-Induced Obesity in Mice and the Antioxidant Functions in PC12 Cells. Inflammation 2013, 37, 443–450. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Murakami-Funayama, K.; Miyashita, K. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol. Med. Rep. 2009, 2, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Kinoshita, Y.; Usui, M.; Tanaka, R.; Matsushita, T.; Miyata, M. The Suppressive Effect of a Marine Carotenoid, Fucoxanthin, on Mouse Ear Swelling through Regulation of Activities and mRNA Expression of Inflammation-associated Enzymes. Food Sci. Technol. Res. 2016, 22, 227–234. [Google Scholar] [CrossRef]
- Kwon, M.; Lim, S.-J.; Joung, E.-J.; Lee, B.; Oh, C.-W.; Kim, H.-R. Meroterpenoid-rich fraction of an ethanolic extract from Sargassum serratifolium alleviates obesity and non-alcoholic fatty liver disease in high fat-fed C57BL/6J mice. J. Funct. Foods 2018, 47, 288–298. [Google Scholar] [CrossRef]
- Cheng, I.C.; Weng, S.-Y.; Wu, M.-S.; Suk, F.-M.; Lien, G.-S.; Chen, C.-N. Low-molecular-weight fucoidan and high-stability fucoxanthin decrease serum alanine transaminase in patients with nonalcoholic fatty liver disease—A double-blind, randomized controlled trial. Adv. Dig. Med. 2019, 6, 116–122. [Google Scholar] [CrossRef]
- Kurylowicz, A.; Jonas, M.; Lisik, W.; Jonas, M.; Wicik, Z.A.; Wierzbicki, Z.; Chmura, A.; Puzianowska-Kuznicka, M. Obesity is associated with a decrease in expression but not with the hypermethylation of thermogene-sis-related genes in adipose tissues. J. Transl. Med. 2015, 13, 31. [Google Scholar] [CrossRef]
- Borzouie, S.; Rathgeber, B.M.; Stupart, C.M.; MacIsaac, J.; MacLaren, L.A. Effects of Dietary Inclusion of Seaweed, Heat Stress and Genetic Strain on Performance, Plasma Biochemical and Hematological Parameters in Laying Hens. Animals 2020, 10, 1570. [Google Scholar] [CrossRef]
- Kawauchi, S.; Horibe, S.; Sasaki, N.; Tanahashi, T.; Mizuno, S.; Hamaguchi, T.; Rikitake, Y. Inhibitory Effects of Sodium Alginate on Hepatic Steatosis in Mice Induced by a Methionine- and Choline-deficient Diet. Mar. Drugs 2019, 17, 104. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Cho, Y.J.; Yu, D.; Chung, D.; Kim, G.-H.; Kang, H.; Cho, H. Undaria pinnatifida Fucoidan-Rich Extract Induces Both Innate and Adaptive Immune Responses. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Wang, W.; Lu, J.-B.; Wang, C.-S.; Zhang, H.-H.; Li, C.-Y.; Qian, G.-Y. Effects of Sargassum fusiforme polysaccharides on antioxidant activities and intestinal functions in mice. Int. J. Biol. Macromol. 2013, 58, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, T.; Kadena, K.; Tomori, M.; Nakajima, K.; Iha, M. Activation of NK cells in male cancer survivors by fucoidan extracted from Cladosiphon okamuranus. Mol. Clin. Oncol. 2019, 12, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Irhimeh, M.R.; Fitton, J.H.; Lowenthal, R.M. Fucoidan ingestion increases the expression of CXCR4 on human CD34+ cells. Exp. Hematol. 2007, 35, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Negishi, H.; Mori, M.; Mori, H.; Yamori, Y. Supplementation of Elderly Japanese Men and Women with Fucoidan from Seaweed Increases Immune Responses to Seasonal Influenza Vaccination. J. Nutr. 2013, 143, 1794–1798. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Cheng, C.-Y.; Liu, C.-T.; Sue, Y.-M.; Chen, T.-H.; Hsu, Y.-H.; Huang, N.-J.; Chen, C.-H. Combined protective effects of oligo-fucoidan, fucoxanthin, and L-carnitine on the kidneys of chronic kidney disease mice. Eur. J. Pharmacol. 2021, 892, 173708. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Nomura, K.; Nagashima, M.; Kamimura, N. Fucoidan alleviates high-fat diet-induced dyslipidemia and atherosclerosis in ApoE(shl) mice deficient in apolipoprotein E expression. J. Nutr. Biochem. 2016, 32, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T.; Murakami, K.; Nishimura, I.; Nakano, T.; Obata, A. A sulfated polysaccharide, fucoidan, enhances the immunomodulatory effects of lactic acid bacteria. Int. J. Mol. Med. 2012, 29, 447–453. [Google Scholar] [CrossRef]
- Hwang, E.; Park, S.-Y.; Shin, H.-S.; Lee, D.-G.; Yi, T.H. Effect of oral administration of fucosterol from Hizikia fusiformis on DNCB-induced atopic dermatitis in NC/Nga mice. Food Sci. Biotechnol. 2014, 23, 593–599. [Google Scholar] [CrossRef]
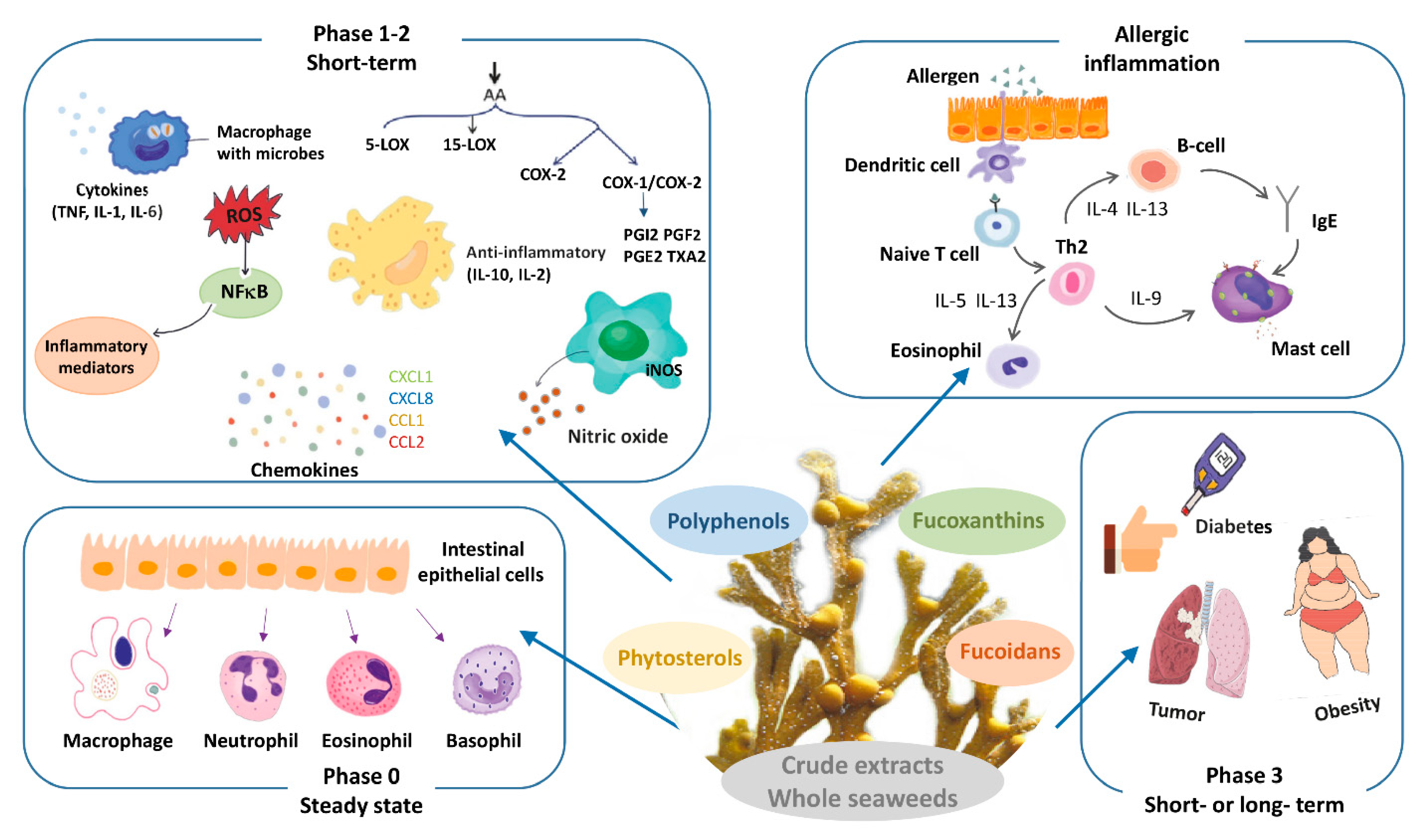
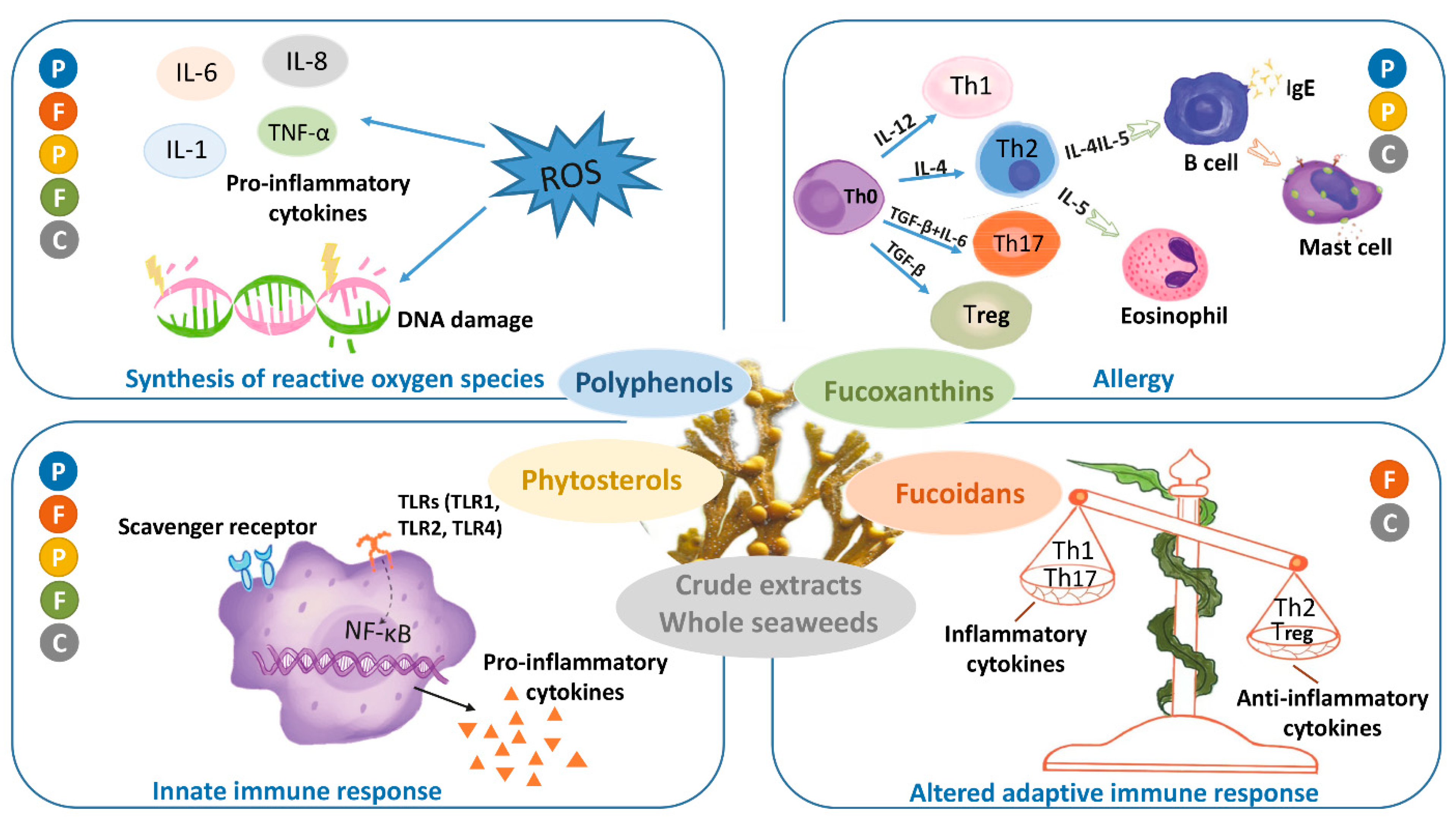
| Phase | Description | Characteristic Read-Outs |
|---|---|---|
| 0 steady state | homeostatic condition |
|
| 1 short-term | initiation of inflammation as response to triggering by damage- or infection-related molecules |
|
| 2 short-term | amplification and regulation of inflammation; initiation of adaptive immunity |
|
| 3 short- or long-term | consequences of severe or chronic inflammation |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olsthoorn, S.E.M.; Wang, X.; Tillema, B.; Vanmierlo, T.; Kraan, S.; Leenen, P.J.M.; Mulder, M.T. Brown Seaweed Food Supplementation: Effects on Allergy and Inflammation and Its Consequences. Nutrients 2021, 13, 2613. https://doi.org/10.3390/nu13082613
Olsthoorn SEM, Wang X, Tillema B, Vanmierlo T, Kraan S, Leenen PJM, Mulder MT. Brown Seaweed Food Supplementation: Effects on Allergy and Inflammation and Its Consequences. Nutrients. 2021; 13(8):2613. https://doi.org/10.3390/nu13082613
Chicago/Turabian StyleOlsthoorn, Simone E. M., Xi Wang, Berend Tillema, Tim Vanmierlo, Stefan Kraan, Pieter J. M. Leenen, and Monique T. Mulder. 2021. "Brown Seaweed Food Supplementation: Effects on Allergy and Inflammation and Its Consequences" Nutrients 13, no. 8: 2613. https://doi.org/10.3390/nu13082613
APA StyleOlsthoorn, S. E. M., Wang, X., Tillema, B., Vanmierlo, T., Kraan, S., Leenen, P. J. M., & Mulder, M. T. (2021). Brown Seaweed Food Supplementation: Effects on Allergy and Inflammation and Its Consequences. Nutrients, 13(8), 2613. https://doi.org/10.3390/nu13082613








